Abstract
Binding of anti-herpes simplex virus (HSV) immunoglobulin G (IgG) to HSV type 1 (HSV-1)-infected HEL and HEp-2 cells causes changes in surface viral glycoprotein distribution, resulting in a capping of all viral glycoproteins towards one pole of the cell. This occurs in a gE-dependent manner. In HEL cells, low concentrations of anti-HSV IgG also enhance cell-to-cell spread of wild-type HSV-1 but not of gE deletion mutant HSV-1. These observations raised the possibility that gE-dependent mechanisms exist that allow some HSV-1-infected cells to respond to the presence of extracellular antibodies by enhancing the antibody-resistant mode of virus transmission.
The glycoproteins gE and gI of alphaherpesviruses are type I transmembrane proteins found on the virion surface as well as on the surface of infected cells. These glycoproteins are termed nonessential as they are not required for virus replication in cultured cells (for example, see reference 29). However, in animal models of virus infections, viral mutants lacking gE and gI have markedly reduced virulence and spread poorly from initial sites of infection (2, 3, 6, 7, 24, 25, 33). gE and gI form stable complexes (17, 18, 34, 35, 37), and gE-gI complexes of some alphaherpesviruses function as Fc receptors (FcRs) specific for immunoglobulin G (IgG) (12, 18, 21). The FcR activity of herpes simplex virus type 1 (HSV-1) gE-gI complexes has been shown to reduce the efficacy of antibody-mediated immune responses in vitro and in vivo (8, 28).
An important in vitro phenotype of mutant viruses lacking gE or gI is the formation of small plaques, relative to those formed by wild-type virus, in many cell types (2, 6, 23, 26, 31, 36). The small-plaque phenotype arises due to impaired cell-to-cell spread of gE deletion mutant (gE−) viruses. In healthy human fibroblasts, the small-plaque phenotype of the gE− virus was shown to correlate with a reduced ability of gE− HSV-1, compared with that of wild-type HSV-1, to replicate in the presence of neutralizing antibodies (6). Whereas in the absence of neutralizing antibodies, the yields of cell-associated gE− and gI− viruses in the fibroblasts were reduced only slightly relative to that of the wild type, 100- to 200-fold reductions in gE− virus yields have been reported when neutralizing antibodies were present in the culture medium (6). These previous observations suggested that plaque formation (cell-to-cell spread) involves a mode of virus transmission whereby virions are sequestered from contact with extracellular antibodies. In HSV-1 infections of cultured cell monolayers, plaque formation is induced either by the presence of extracellular antibodies or by the presence of a semisolid matrix such as carboxymethyl cellulose (CMC). Thus, antibodies are not essential for the induction of cell-to-cell spread. However, the question of whether antibody binding induces specific responses that enhance the extent of cell-to-cell spread has not previously been explored.
In polarized epithelial cells, HSV virions have been suggested to be preferentially targeted, via a gE-mediated function, to lateral junctions rather than the apical surface (19). Because virus particles are directed away from the apical surface, it has been suggested that HSV avoids contact with extracellular antibodies (19). However, in nonpolarized cells, such as fibroblasts, which are also targets of HSV infections in vivo, virions reaching the cell surface would be accessible to extracellular antibodies. A relevant question, therefore, is whether mechanisms exist in such cells for the virus to sense and thus respond to the presence of extracellular antibodies. We studied this possibility in four HSV-1-infected cell lines grown under nonpolarizing conditions. In HSV-1-infected human embryonic lung fibroblasts (HEL), we noted two distinct effects of the presence of extracellular anti-HSV antibodies: (i) capping of cell surface viral glycoproteins and (ii) enhancement of cell-to-cell spread. Both responses were dependent on gE and on the presence of a polyclonal mix of anti-HSV antibodies. While it is possible that the two phenomena are mechanistically unrelated, the gE and antibody dependence of both phenomena raises the possibility that capping results in alterations of the functional properties of one or more HSV glycoproteins, which in turn influence the extent of cell-to-cell spread. Ultimately, the existence of such a mechanism would enhance virus survival and propagation in the face of an antibody response.
Antibodies induce glycoprotein capping in HSV-1-infected HEL and HEp-2 cells.
Binding of anti-pseudorabies virus (PRV) antibodies to PRV-infected swine kidney cells induced a redistribution of cell surface PRV glycoproteins towards one pole of the cell, reminiscent of mammalian receptor capping (13). The capping was a concerted process that involved all surface-expressed viral glycoproteins and was significantly enhanced by the presence of viral gE (12, 13).
To investigate the occurrence of antibody-dependent glycoprotein capping in HSV-1-infected cells, we used the following cell lines: HEL, Vero (Cercopithecus aethiops African green monkey kidney cells), HEp-2 (human larynx epidermoid carcinoma), ARPE-19 (human retinal pigment epithelial cells), swine kidney cells that express the HveA entry receptor (SK; obtained from Oveta Fuller) (27), and HeLa (human cervix epithelioid carcinoma). The antibodies used in this study were human IgG (hIgG) (Gammagard; Baxter Health Care Corporation), which contains antibodies against various HSV glycoproteins; rabbit anti-HSV IgG (Scytek Laboratories); normal rabbit IgG (nonimmune IgG; Jackson Immunoresearch); a murine anti-gD monoclonal antibody (III-174) (30); a murine gI- and gE-gI-specific antibody, 2E9 (32); and three commercial antibodies with specificities for gE, gB, and gC (1108 [anti-gE], 1122 [anti-gB], and 1125 [anti-gC]; Goodwin Institute).
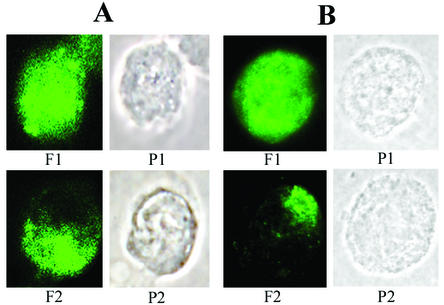
Cells were infected with wild-type HSV-1 or gE− HSV-1 (construct R7032 [25]) at a multiplicity of infection (MOI) of 5. The gE− virus was on the same background as the wild type (strain F). After a 12-h infection, cells were detached and 0.5 × 106 cells were incubated for 2 h at 37°C in buffer containing 1 mg of hIgG/ml. The buffer used for all labeling steps was phosphate-buffered saline containing 3% bovine serum albumin. After the incubation, cells were fixed with 0.4% formaldehyde, followed by an incubation with fluorescently conjugated secondary antibodies (fluorescein isothiocyanate [FITC]-conjugated donkey anti-human IgG; Jackson Immunoresearch). In control experiments, cells were treated for 2 h at 37°C with buffer containing anti-gD, fixed, further incubated with hIgG for 30 min at 4°C, and subsequently stained with FITC-conjugated donkey anti-human IgG. Cells were applied to slides coated with polylysine, mounted with Prolong Antifade (Molecular Probes), and visualized by immunofluorescence microscopy with a Leitz Orthoplan microscope with a Spot II digital camera. Cells were scored for the presence of a capped phenotype in which viral glycoproteins were clustered towards one pole of the cell. This phenotype is illustrated in Fig. 1, panels F2, for HEL and HEp-2 cells.
FIG. 1.
Effect of antibodies on glycoprotein distribution in HSV-1-infected HEL (A) and HEp-2 (B) cells. HSV-1-infected cells were incubated with hIgG for 2 h at 37°C, fixed, stained with FITC-labeled secondary antibody, and processed for visualization of HSV-1 surface glycoproteins. The cells were classified according to whether the viral glycoprotein distribution showed uniform staining (1), i.e., the fluorescent label exhibited a uniform cell surface distribution, or was capped (2), i.e., viral glycoproteins formed a large aggregate at one end of the cell. The images are shown in fluorescent (F) and phase-contrast (P) views.
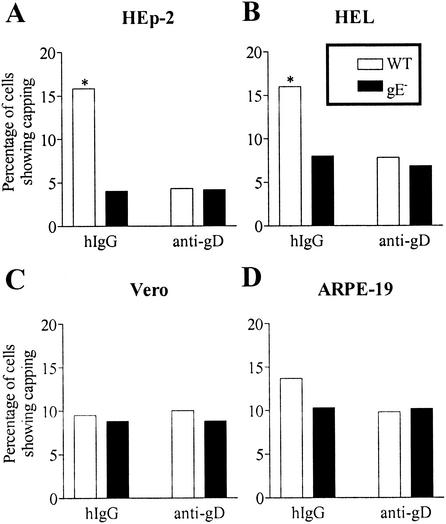
A small background level of glycoprotein capping was observed in all cell types even in the absence of antibody treatment. hIgG-mediated enhancement of glycoprotein capping was observed in two of the cell lines investigated, HEL and HEp-2 (Fig. 2A and B). In both of these cell lines, the extent of capping was enhanced by incubation with hIgG but not anti-gD (Fig. 2A and B). Furthermore, the extent of capping in infections with wild-type virus was enhanced relative to that in infections with gE− virus (Fig. 2A and B). These observations indicated that the enhancement in glycoprotein capping in the presence of antibodies was gE dependent. Other cell lines (Vero and ARPE-19 [Fig. 2C and D] and SK and HeLa [data not shown]) that were screened for glycoprotein capping did not show a gE dependence or antibody enhancement above the observed background level. We investigated the possibility that the lower level of cell surface expression of gE-gI in Vero and ARPE-19 cells compared with that in HEL or HEp-2 cells might be responsible for the absence or observation of capping. This was a possibility as, in some cell types, gE has been reported to be found exclusively in the perinuclear region, whereas in other cell types, gE has been found both at the cell surface and intracellularly (1). For these analyses, cells were infected as described above. At 12 h postinfection, cells were incubated with gE- or gI-specific antibody (1108 or 2E9, respectively), followed by incubation with FITC-conjugated goat anti-mouse secondary antibody and fluorescence-activated cell sorting analyses. We found that the mean fluorescence values corresponding to gE and gI expression were lower for HEp-2 and Vero cells than for ARPE-19 and HEL cells (data not shown). Thus, no correlation was apparent between the cell surface expression levels of gE-gI and the observation of glycoprotein capping.
FIG. 2.
Cell type specificity and gE dependence of glycoprotein capping in HSV-1-infected HEp-2 (A), HEL (B), Vero (C), and ARPE-19 (D) cells. The glycoprotein capping observed upon treatment of the indicated HSV-1-infected cells with hIgG or anti-gD at 37°C was quantified by counting, in a blind fashion, of 200 cells per experimental condition analyzed. Data are results from a single experiment but are representative of at least three experiments with each cell line. Asterisks indicate statistical significance at a probability level of 0.05.
Trends similar to those shown in Fig. 2A and B were also observed when HEL and HEp-2 cells were incubated with purified rabbit anti-HSV IgG (ScyTek Laboratories) but not when they were incubated with nonimmune rabbit IgG, which can bind to viral gE-gI complexes via the Fc domain (data not shown). This observation suggested that capping required interactions of antibody variable domains with viral glycoproteins. The mixture of antibodies directed against many glycoproteins is also likely to effect the observed glycoprotein capping. With HEp-2 cells, we tested whether murine antibodies directed against individual glycoproteins could mediate capping. We found that antibodies directed against gE, gB, or gC could not induce a concerted capping of cell surface glycoproteins, although clustering of the individual proteins could be observed in some cases (data not shown). These observations suggested that the capping of all of the surface glycoproteins, as shown in Fig. 1, requires antibodies directed against multiple glycoproteins, as would be present in an immune response against HSV. The binding of antibodies to their respective epitopes appears to induce cross-linking of the different HSV proteins to one another in a gE-dependent manner. The most likely explanation for this phenomenon is the occurrence of antibody Fc-mediated bridging of glycoproteins to gE-gI, apparently by a mechanism previously described as antibody bipolar bridging (15).
In PRV-infected swine kidney cells, antibody-induced capping of PRV glycoproteins was followed by detachment and extrusion of membranes containing the viral glycoproteins, such that viral proteins were subsequently not detectable on the infected cell surface by immunofluorescence microscopy (13). The phenomenon was suggested to be an immune evasion strategy designed to inhibit detection of cell surface glycoproteins by components of the immune system. Detaching membrane vesicles containing viral glycoprotein were not visualized in HSV-1-infected HEp-2 or HEL cells, indicating that glycoprotein extrusion as a consequence of antibody binding either did not occur in these cells or was a rare event. These observations led us to investigate the possibility that glycoprotein capping induced signaling events that enhanced viral evasion of the antibody response. Thus, we investigated the effects of antibody binding on direct cell-to-cell spread of HSV-1.
hIgG and rabbit anti-HSV IgG induce an enhancement in cell-to-cell spread of HSV-1 in HEL cells.
To investigate the effects of antibody binding on direct cell-to-cell spread of virus, we first determined the occurrence and extent of this mode of virus spread in different cell lines. HEL and HEp-2 cells (4 × 106 to 8 × 106) in six-well plates were infected with wild-type or gE− HSV-1 at an MOI of 0.0001. Ninety minutes after virus adsorption, the inoculum was removed. Cells were prepared for measurement of plaque size (Fig. 3A) or estimation of viral titers (Fig. 3B). For measurement of plaque size, cells were overlaid with 5 ml of medium containing 1% CMC. Forty-eight hours postinfection, the monolayers were fixed with methanol and stained with Giemsa stain (for HEL) or immunostained (for HEp-2). Plaque images were captured with a digital camera (Sony DKC 5000) attached to an inverted microscope and quantified using NIH Image. For cell-associated virus titer estimations, 5 ml of fresh medium containing no antibody or 50 mg of hIgG (1% hIgG) was added following virus adsorption. At 24 to 48 h postinfection, monolayers were detached and cell-associated virus titers were estimated by plaque assays with Vero cells.
FIG. 3.
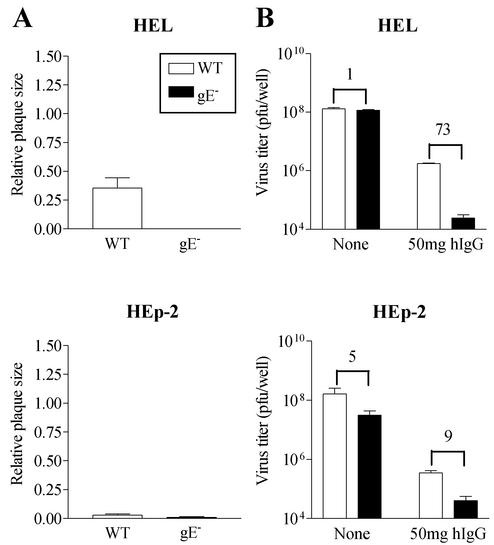
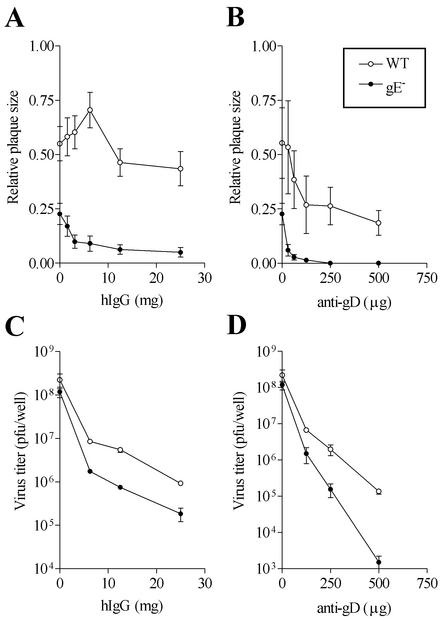
gE dependence of cell-to-cell spread and HSV-1 replication in the presence of antibodies. (A) Plaques formed by wild-type or gE− HSV-1 in HEL and Hep-2 cells were measured 48 h postinfection. No antibodies were used in the overlaying medium. Twenty to thirty plaques were used to estimate average plaque size under each condition. (B) HEL and HEp-2 cells were infected with wild-type HSV-1 or gE− HSV-1 and subsequently cultured in the absence of antibody or in the presence of 50 mg hIgG (1% hIgG) for 48 h, after which the total cell-associated virus yields were estimated. Each value is the average virus titer obtained from duplicate or triplicate measurements. The numbers above the columns indicate the wild-type HSV-1/gE− virus yield ratio in the absence of antibody (1 and 5) or in the presence of hIgG (73 and 9) in the indicated cell lines. Results are representative of two independent sets of analyses.
Wild-type HSV-1 forms relatively large plaques in HEL cells, whereas plaques formed by the gE− virus were significantly reduced in size and almost undetectable 48 h postinfection (Fig. 3A). In the absence of antibody in HEL cells, infections with viruses at equivalent inputs resulted in very similar virus titers for gE− and wild-type HSV-1 (Fig. 3B, upper panel). However, in the presence of 1% hIgG, the yields of the gE− virus were reduced more than 70-fold relative to those of wild-type HSV-1 (Fig. 3B, lower panel). Thus, the small-plaque phenotype of gE− virus in HEL cells correlated with a significant growth impairment of the gE− virus in these cells in the presence of neutralizing antibodies. These observations implied that plaque dimensions in HEL cells indicated the extent of an antibody-resistant mode of HSV-1 transmission. In HEp-2 cells, both wild-type and gE− viruses formed very small plaques, which required immunostaining in order to be visualized (Fig. 3A). In correlation with this observation, the antibody resistance of the gE− virus in HEp-2 cells was only slightly different from that of wild-type HSV-1 (Fig. 3B). An approximately fivefold reduction in gE− virus yield relative to that of the wild type was observed in HEp-2 cells in the absence of antibody. The presence of 1% hIgG increased the difference slightly, resulting in a ninefold reduction in gE− virus yield relative to that of the wild type. Furthermore, whereas the yields of wild-type HSV-1 in HEL and HEp-2 cells were approximately equal in the absence of antibody, the yield from HEp-2 cells was reduced by approximately 1 log when 1% hIgG was present. Taken together, these observations suggested that antibody-resistant spread was relatively nonpermissive in HEp-2 cells.
HEL cells were used for further analysis of possible correlations between the occurrence of antibody binding and enhancement of cell-to-cell spread. ARPE-19 and Vero cells were also used in these analyses. In both ARPE-19 and Vero cells, wild-type HSV-1 formed relatively large plaques compared with those formed by gE− viruses (three- and fourfold differences in size, respectively, for plaques in APRE-19 and Vero cells). The ratios of wild-type/gE− virus yields in the absence and presence of 1% hIgG were 3 and 22, respectively, for ARPE-19 cells and 2 and 38, respectively, for Vero cells (data not shown). Thus, cell-to-cell spread is permissive in both cell types, although its extent appears to be reduced compared with that in HEL cells.
To investigate the correlations between antibody binding and enhancement in cell-to-cell spread, HEL, Vero, and ARPE-19 cells were infected with wild-type or gE− HSV-1 at an MOI of 0.0001. Ninety minutes after virus adsorption, the inoculum was removed and cells were overlaid with medium containing CMC alone or different concentrations of hIgG or anti-gD. At 48 to 72 h postinfection, the monolayers were fixed and processed for quantification of plaque size as described above.
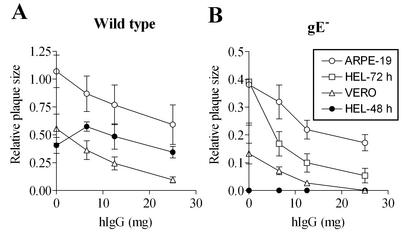
In HEL, ARPE-19, and Vero cells, plaque formation by gE− virus was significantly diminished at relatively low concentrations of hIgG (Fig. 4), consistent with the progressive reduction in gE− virus yields with increasing antibody concentrations. Under conditions in which very small plaques or no plaques were observed with gE− viruses (Fig. 4B), wild-type HSV-1 produced relatively large plaques (Fig. 4A). The extent of cell-to-cell spread can be influenced by the total amount of cell-associated virus that is available. Since the presence of antibodies reduced the total amount of cell-associated virus (Fig. 3), cell-to-cell spread was expected to be correspondingly reduced. Indeed, in ARPE-19 and Vero cells, a gradual increase in the antibody concentration resulted in a progressive decrease in the size of plaques formed by wild-type HSV-1 (Fig. 4). Surprisingly, with HEL cells, we consistently found that at low concentrations of hIgG, the plaques formed by wild-type virus were larger than those formed under conditions that lacked antibody (Fig. 4A). Furthermore, no enhancement of cell-to-cell spread was observed in the presence of hIgG in HEL cells infected with gE− virus, and plaques were not observable 48 h postinfection but were observable when the infections were allowed to proceed for 72 h (Fig. 4B).
FIG. 4.
Effects of antibodies on cell-to-cell spread of wild-type (A) and gE− (B) HSV-1 in different cell types. The indicated cells were infected with virus and subsequently cultured for 48 h in the presence of the indicated concentration of hIgG. In HEL cells infected with gE− HSV-1, no plaques were discernible at 48 h postinfection in the absence of antibody but were visualized and quantifiable at 72 h postinfection. Plaque measurements were made at 48 h postinfection unless indicated as 72 h. Twenty to thirty plaques were used to estimate average plaque size under each condition. Results shown are representative of several independent sets of analyses.
We undertook a more detailed analysis of the effects of various antibody concentrations on HSV-1 replication and plaque formation in HEL cells (Fig. 5). In several different analyses, enhancement in cell-to-cell spread (in cells infected with wild-type HSV-1) was apparent at low concentrations of hIgG but was reversed as the hIgG concentrations were increased (Fig. 5A). Enhancement in cell-to-cell spread was observed with multiple batches of commercial hIgG, although the exact concentration at which maximal enhancement was observed varied slightly between different batches (maximal enhancement was in the range of 6.5 to 15 mg in the three different batches of hIgG used). A similar enhancement was not apparent in infections with gE− HSV-1 even at very low hIgG concentrations (Fig. 5A). Additionally, such an enhancement was not observed in the presence of anti-gD in infections with either wild-type or gE− HSV-1 over the entire range of concentrations examined (Fig. 5B). In contrast to what was observed with cell-to-cell spread, yields of cell-associated HSV-1 were progressively reduced with increasing hIgG or anti-gD concentrations (Fig. 5C and D). As expected, the reductions were more pronounced in infections with gE− HSV-1 than in those with wild-type HSV-1. Thus, in HEL cells, although the presence of hIgG decreased the overall yield of wild-type HSV-1 (by neutralizing extracellular virus), an enhancement in cell-to-cell spread was apparent at low concentrations of hIgG. At higher antibody concentrations, the large accompanying decrease in total virus yields due to neutralization of extracellular virus must have an opposing effect on cell-to-cell spread, thus masking the enhancement that is apparent at the lower antibody concentrations.
FIG. 5.
Effects of antibodies on cell-to-cell spread and replication of wild-type and gE− HSV-1 in HEL cells. (A and B) Infected cells were processed for quantification of plaque size after being cultured in the presence of the indicated concentrations of hIgG or anti-gD as described in the legend to Fig. 4. Wild-type HSV-1 plaque size measurements were made at 48 h postinfection, whereas gE− HSV-1 plaque size measurements were made at 72 h postinfection. Results shown are representative of several independent sets of analyses. (C and D) Cells were infected with wild-type or gE− HSV-1 and subsequently cultured in the presence of the indicated concentrations of antibodies. Cell-associated virus titers were estimated 48 h postinfection by plaque assays with Vero cells. Results are representative of two independent sets of analyses.
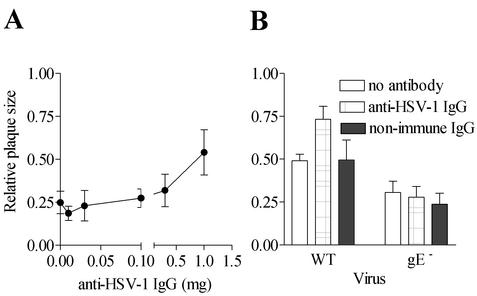
A similar gE-dependent enhancement in cell-to-cell spread was also observed when protein G-purified rabbit anti-HSV IgG rather than hIgG was used in the overlays (Fig. 6). In correlation with the observation that nonimmune rabbit IgG did not enhance glycoprotein clustering in HSV-1-infected cells, we also found that nonimmune rabbit IgG did not significantly enhance cell-to-cell spread in cells infected with either wild-type or gE− HSV-1 (Fig. 6B). Thus, Fc binding alone is not sufficient to effect glycoprotein clustering or enhance the extent of cell-to-cell spread.
FIG. 6.
Effect of immune and nonimmune IgG on cell-to-cell spread of wild-type and gE− HSV-1 in HEL cells. HEL cells were infected with wild-type or gE− HSV-1 and subsequently cultured in the presence of protein G-purified rabbit anti-HSV IgG or rabbit nonimmune IgG. Plaque sizes were determined at 48 h for the wild-type HSV-1 infections or at 72 h for the gE− HSV-1 infections. (A) Plaque dimensions were measured as a function of rabbit anti-HSV IgG concentration. (B) Sizes of plaques formed by wild-type and gE− HSV-1 were determined in the absence of antibody, in the presence of 1 mg of rabbit anti-HSV IgG, or in the presence of 1 mg of nonimmune rabbit IgG. For each condition depicted, 20 to 30 plaques were used to estimate the average plaque size. Results are representative of one (A) or two (B) independent sets of analyses.
Effects of monoclonal antibodies against individual glycoproteins on cell-to-cell spread.
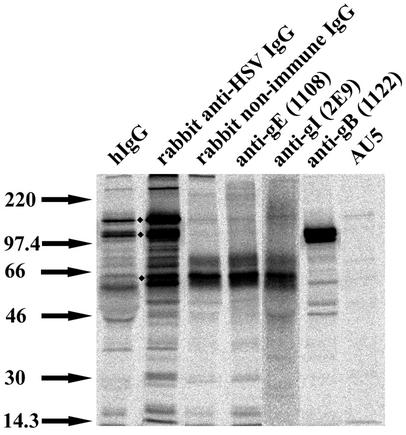
The observations of hIgG-induced and rabbit anti-HSV IgG-induced enhancements in cell-to-cell spread in HEL cells raised the question of what the underlying mechanisms might be. To obtain further insights into which HSV-1 glycoproteins are predominantly recognized by antibodies present in the hIgG and rabbit anti-HSV antibody mixes, we examined the profiles of proteins that were immunoprecipitated by hIgG and rabbit anti-HSV IgG (Fig. 7, lanes 1 and 2 from left). For these analyses, cells were infected with wild-type HSV-1 at an MOI of 5. Twelve hours postinfection, the medium was replaced with RPMI 1640 medium containing 10 μCi of [35S]methionine-cysteine (ICN Biomedicals), and cells were labeled for 5 h. Following this, cells were lysed in buffer containing 1% NP-40, 20 mM Tris, 100 mM NaCl, 10 mM EDTA (pH 7.5), and protease inhibitors. HSV glycoproteins were immunoprecipitated with the indicated antibodies by using a previously described protocol (32).
FIG. 7.
HSV-1-encoded protein recognized by anti-HSV antibodies. Lysates from metabolically labeled cells infected with wild-type HSV-1 were immunoprecipitated with 1 mg of hIgG (lane 1 from left), 40 μg of rabbit anti-HSV IgG (lane 2), 40 μg of rabbit nonimmune IgG (lane 3), 10 μg of anti-gE (lane 4), 70 μg of anti-gI (lane 5), 10 μg of anti-gB (lane 6), or 2.5 μl of a control antibody, AU5 ascites fluid (Covance Scientific) (lane 7), that is not specific for any HSV glycoprotein. Immunoprecipitated proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, the gels were dried, and labeled proteins were visualized by phosphorimaging analyses.
Proteins with molecular masses of approximately 65, 120, and 150 kDa appeared to be major epitopes for antibodies contained in both the rabbit IgG and hIgG mixes (Fig. 7, lanes 1 and 2 from left). The identity of the 150-kDa protein is currently unknown, but the 65- and 120-kDa proteins likely correspond to gI and gB, respectively, based on parallel immunoprecipitation analyses with antibodies that specifically recognize these proteins (Fig. 7, lanes 3 to 5 from left for gI and lane 6 for gB). In previously described experiments, we showed that the rabbit anti-HSV IgG was capable of immunoprecipitating truncated gE and gI constructs lacking FcR activity from Chinese hamster ovary cells expressing these constructs (32). These observations indicated the presence of antibodies with Fab region specificity for gE and gI in the rabbit anti-HSV IgG mix. We also verified the presence of gB-specific antibodies in the rabbit anti-HSV antisera by immunoprecipitation of HSV-1-infected or uninfected cells with the sera, followed by immunoblotting analyses with the gB-specific antibody (data not shown).
The gE dependence of antibody-induced enhancement of cell-to-cell spread (Fig. 4 to 6) and the indication that antibodies with Fab specificities for gE, gI, and gB were well represented in the anti-HSV IgG mix (Fig. 7) (32) raised the question of whether cross-linking of gE-gI or gB by use of monoclonal antibodies could influence the extent of cell-to-cell spread. Indeed, both gE-gI and gB are important for mediating antibody-induced glycoprotein redistribution in PRV-infected cells (11, 13), and mutations in the cytoplasmic domain of gB have also been shown recently to influence the efficiency of cell-to-cell spread (14).
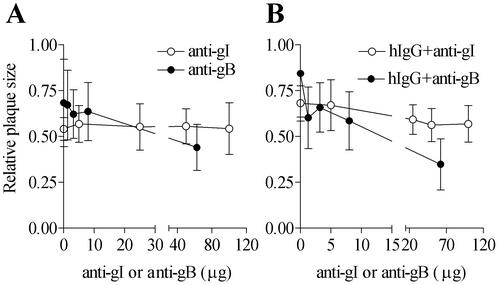
The gI (and gE-gI)-specific antibody 2E9 and the gB-specific antibody 1122 were used for further investigation of the effects of gE-gI and gB cross-linking on cell-to-cell spread. The presence of 2E9 alone did not significantly influence plaque dimensions at 2E9 amounts up to 100 μg (Fig. 8A). This observation is consistent with our result showing that 2E9 was non-neutralizing or weakly neutralizing under conditions in which the anti-gD antibody was strongly neutralizing (data not shown). The presence of 1122 caused a significant reduction in the average plaque size at higher antibody concentrations, consistent with its documented virus-neutralizing potential (Goodwin Institute). Based on these results, it appears that antibody-induced cross-linking of gE-gI or gB per se was not sufficient to effect a significant enhancement in cell-to-cell spread.
FIG. 8.
Effects of gE-gI- and gB-specific antibodies on cell-to-cell spread of wild-type HSV-1 in HEL cells. HEL cells were infected with wild-type HSV-1 and subsequently cultured in the presence of the indicated antibodies over the specified range of concentrations. Plaque sizes were determined 48 h postinfection. (A) Cells were cultured postinfection in the presence of different concentrations of 2E9 or 1122 antibody. (B) Cells were cultured postinfection in the presence of hIgG in the absence or presence of the indicated amounts of 2E9 or 1122. Results are representative of a single analysis. For each condition depicted, 20 to 30 plaques were used to estimate average plaque size.
Interestingly, we observed that when combined with hIgG, both 2E9 and 1122 inhibited the plaque-enhancing effect of hIgG in a dose-dependent manner (Fig. 8B). The inhibition was much more pronounced with 1122 than with 2E9, with very low concentrations of 1122 being sufficient to cause inhibition. The inhibition profile with 2E9 was more gradual, with higher 2E9 concentrations being required to cause significant inhibition. Increased HSV neutralization by hIgG-1122 combinations compared with that by hIgG alone might account for the pronounced inhibitory effects of the 1122 antibody. However, we believe that this is unlikely because 1122 by itself has a much less potent plaque-inhibitory effect at lower concentrations. Rather, since murine antibodies cannot bind to gE-gI via their Fc domains (4, 16), it is possible that 1122, a murine monoclonal antibody, exerts its inhibitory effect at least partially by interfering with hIgG(Fc)-mediated cross-linking of gB to gE-gI. Similarly, by binding to gE-gI, 2E9 could interfere with Fc-mediated cross-linking of gB or other glycoproteins to gE-gI. These observations suggest that enhancement in cell-to-cell spread might involve a coclustering of multiple glycoproteins, as observed in the capping response.
Among the three cell lines that were permissive for cell-to-cell spread (HEL, ARPE-19, and Vero), antibody-induced enhancement in cell-to-cell spread was observed only in HEL cells (Fig. 4). We used fluorescence-activated cell sorting analyses with gE- or gI-specific antibody (1108 and 2E9, respectively) to determine whether the extent of cell-to-cell spread, or the occurrence of antibody-induced enhancement in cell-to-cell spread, correlated with cell surface gE-gI levels in the different cell types. Under the infection conditions of the cell-to-cell spread analyses (MOI, 0.0001), the mean fluorescence values corresponding to gE-gI expression in ARPE-19 and HEL cells were quite similar but were reduced in Vero cells (when analyzed 20 to 24 h postinfection) (data not shown). Thus, as with glycoprotein capping, no specific correlation between the expression levels of cell surface gE-gI and the observation of antibody-induced enhancement in cell-to-cell spread was apparent in the different cell types. However, in the different cell types, correlations were observed between the occurrence of antibody-induced glycoprotein capping and antibody-induced enhancement in cell-to-cell spread (compare data shown in Fig. 2 and 4 for HEL, ARPE-19, and Vero cells). Absence of capping in ARPE-19 and Vero cells translated to an absence of antibody-induced enhancement in cell-to-cell spread. By drawing an analogy to immune receptor capping that precedes signal transduction in leukocytes (10), we suggest that antibody-induced glycoprotein capping in HSV-1-infected HEL cells could initiate signal transduction pathways that culminate in an enhancement in cell-to-cell spread.
Based on observations of cell-type-specific capping of viral glycoproteins in other systems, it has been suggested that antibody-induced mobility of viral glycoproteins on cell membranes may require host cell-dependent factors as well as virus-specific factors (22). By analogy, it is possible that in ARPE-19 and Vero cells, viral glycoproteins are defective in their ability to interact with either the cytoskeleton or membrane-associated cellular proteins, which accounts for the absence of antibody-induced lateral mobility (capping) of HSV glycoproteins in these cells. Lack of glycoprotein coclustering in ARPE-19 and Vero cells could subsequently translate to an inability to transduce signaling responses that are required for enhancement in cell-to-cell spread.
In addition to the cell-type-specific correlations between the occurrence of antibody-induced glycoprotein capping and enhancement in cell-to-cell spread, we note that both processes are gE dependent and that neither phenomenon was observed in the presence of nonimmune IgG or induced by antibodies directed against individual glycoproteins. Additional investigations with viral mutants that lack the cytoplasmic domains of specific viral glycoproteins will generate further mechanistic insights into the molecular basis of antibody-induced glycoprotein capping and antibody-induced enhancement in cell-to-cell spread.
We have argued that antibody-induced enhancement in cell-to-cell spread might be correlated with glycoprotein capping, yet glycoprotein capping was observed in HEp-2 cells that are not significantly permissive for cell-to-cell spread. It is possible that other distinct responses that can counteract the antiviral effects of antibodies are induced in HEp-2 cells upon glycoprotein capping, allowing the virus to persist in the face of an antibody response. Further study will be required to address this possibility. The interplay between antibodies and HSV might be similar to the better characterized interplay between the type I interferon pathway and HSV (reviewed in reference 20), in which the antiviral effects of interferon-stimulated genes are countered by viral proteins, thereby facilitating viral persistence in infected cells.
To achieve its long-term survival in a host, HSV-1 appears to have evolved multiple gE-dependent strategies for countering a host antibody response. First, the HSV-1 FcR (with high affinity for human Fc) (4) can inhibit the initiation of effector responses such as antibody-dependent cell-mediated cytotoxicity (9). Second, the existence of a gE-dependent antibody-resistant mode of viral spread in some cells allows higher levels of virus propagation in the presence of HSV-1-neutralizing antibodies. We present in this study evidence for enhanced antibody-resistant spread that is induced in the presence of anti-HSV antibodies and that correlates with the occurrence of antibody-induced glycoprotein capping. Although anti-HSV antibodies reduce total virus yields by neutralizing extracellular virus, hIgG-induced enhancement in cell-to-cell spread would ultimately result in greater virus yields compared with those found under conditions in which antibody-induced enhancement in cell-to-cell spread is not observed. The combination of the gE-dependent processes described here and the Fc binding function of HSV-1-infected cells could account for the observation that HSV infections frequently recur in patients with high levels of HSV-neutralizing antibodies (5). The present study also raises the intriguing possibility of the existence of other response mechanisms in HSV-1-infected cells that counteract the antiviral effects of antibodies.
Acknowledgments
We thank Jennifer Reuer for early contributions to the project, Oveta Fuller for various cell lines and HSV stocks, Bernard Roizman for the gE− mutant virus, Pat Spear for the gD-specific antibody, and the University of Michigan Hybridoma Core for ascites production. We are grateful to David Knipe, Oveta Fuller, Kathy Collins, and Wes Dunnick for ideas and many helpful suggestions during the progress of this work.
This work was supported by a grant from the American Heart Association (to M.R.) and a Rheumatic Diseases Core Center grant to the University of Michigan (AR48310-02).
REFERENCES
- 1.Alconada, A., U. Bauer, B. Sodeik, and B. Hoflack. 1999. Intracellular traffic of herpes simplex virus glycoprotein gE: characterization of the sorting signals required for its trans-Golgi network localization. J. Virol. 73:377-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balan, P., N. Davis-Poynter, S. Bell, H. Atkinson, H. Browne, and T. Minson. 1994. An analysis of the in vitro and in vivo phenotypes of mutants of herpes simplex virus type 1 lacking glycoproteins gG, gE, gI or the putative gJ. J. Gen. Virol. 75:1245-1258. [DOI] [PubMed] [Google Scholar]
- 3.Card, J. P., M. E. Whealy, A. K. Robbins, and L. W. Enquist. 1992. Pseudorabies virus envelope glycoprotein gI influences both neurotropism and virulence during infection of the rat visual system. J. Virol. 66:3032-3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chapman, T. L., I. You, I. M. Joseph, P. J. Bjorkman, S. L. Morrison, and M. Raghavan. 1999. Characterization of the interaction between the herpes simplex virus type I Fc receptor and immunoglobulin G. J. Biol. Chem. 274:6911-6919. [DOI] [PubMed] [Google Scholar]
- 5.Corey, L., and P. G. Spear. 1986. Infections with herpes simplex viruses (1). N. Engl. J. Med. 314:686-691. [DOI] [PubMed] [Google Scholar]
- 6.Dingwell, K. S., C. R. Brunetti, R. L. Hendricks, Q. Tang, M. Tang, A. J. Rainbow, and D. C. Johnson. 1994. Herpes simplex virus glycoproteins E and I facilitate cell-to-cell spread in vivo and across junctions of cultured cells. J. Virol. 68:834-845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dingwell, K. S., L. C. Doering, and D. C. Johnson. 1995. Glycoproteins E and I facilitate neuron-to-neuron spread of herpes simplex virus. J. Virol. 69:7087-7098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dubin, G., N. O. Fishman, R. J. Eisenberg, G. H. Cohen, and H. M. Friedman. 1992. The role of herpes simplex virus glycoproteins in immune evasion. Curr. Top. Microbiol. Immunol. 179:111-120. [DOI] [PubMed] [Google Scholar]
- 9.Dubin, G., E. Socolof, I. Frank, and H. M. Friedman. 1991. Herpes simplex virus type 1 Fc receptor protects infected cells from antibody-dependent cellular cytotoxicity. J. Virol. 65:7046-7050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dykstra, M., A. Cherukuri, and S. K. Pierce. 2001. Rafts and synapses in the spatial organization of immune cell signaling receptors. J. Leukoc. Biol. 70:699-707. [PubMed] [Google Scholar]
- 11.Favoreel, H. W., H. J. Nauwynck, H. M. Halewyck, P. Van Oostveldt, T. C. Mettenleiter, and M. B. Pensaert. 1999. Antibody-induced endocytosis of viral glycoproteins and major histocompatibility complex class I on pseudorabies virus-infected monocytes. J. Gen. Virol. 80:1283-1291. [DOI] [PubMed] [Google Scholar]
- 12.Favoreel, H. W., H. J. Nauwynck, and M. B. Pensaert. 1999. Role of the cytoplasmic tail of gE in antibody-induced redistribution of viral glycoproteins expressed on pseudorabies-virus-infected cells. Virology 259:141-147. [DOI] [PubMed] [Google Scholar]
- 13.Favoreel, H. W., H. J. Nauwynck, P. Van Oostveldt, T. C. Mettenleiter, and M. B. Pensaert. 1997. Antibody-induced and cytoskeleton-mediated redistribution and shedding of viral glycoproteins, expressed on pseudorabies virus-infected cells. J. Virol. 71:8254-8261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Favoreel, H. W., G. Van Minnebruggen, H. J. Nauwynck, L. W. Enquist, and M. B. Pensaert. 2002. A tyrosine-based motif in the cytoplasmic tail of pseudorabies virus glycoprotein B is important for both antibody-induced internalization of viral glycoproteins and efficient cell-to-cell spread. J. Virol. 76:6845-6851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frank, I., and H. M. Friedman. 1989. A novel function of the herpes simplex virus type 1 Fc receptor: participation in bipolar bridging of antiviral immunoglobulin G. J. Virol. 63:4479-4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johansson, P. J., E. B. Myhre, and J. Blomberg. 1985. Specificity of Fc receptors induced by herpes simplex virus type 1: comparison of immunoglobulin G from different animal species. J. Virol. 56:489-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson, D. C., and V. Feenstra. 1987. Identification of a novel herpes simplex virus type 1-induced glycoprotein which complexes with gE and binds immunoglobulin. J. Virol. 61:2208-2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson, D. C., M. C. Frame, M. W. Ligas, A. M. Cross, and N. D. Stow. 1988. Herpes simplex virus immunoglobulin G Fc receptor activity depends on a complex of two viral glycoproteins, gE and gI. J. Virol. 62:1347-1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson, D. C., M. Webb, T. W. Wisner, and C. Brunetti. 2001. Herpes simplex virus gE/gI sorts nascent virions to epithelial cell junctions, promoting virus spread. J. Virol. 75:821-833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katze, M. G., Y. He, and M. Gale. 2002. Viruses and interferon: a fight for supremacy. Nat. Rev. Immunol. 2:675-687. [DOI] [PubMed] [Google Scholar]
- 21.Litwin, V., W. Jackson, and C. Grose. 1992. Receptor properties of two varicella-zoster virus glycoproteins, gpI and gpIV, homologous to herpes simplex virus gE and gI. J. Virol. 66:3643-3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lydy, S. L., S. Basak, and R. W. Compans. 1990. Host cell-dependent lateral mobility of viral glycoproteins. Microb. Pathog. 9:375-386. [DOI] [PubMed] [Google Scholar]
- 23.Mallory, S., M. Sommer, and A. M. Arvin. 1998. Analysis of the glycoproteins I and E of varicella-zoster virus (VZV) using deletional mutations of VZV cosmids. J. Infect. Dis. 178(Suppl. 1):S22-S26. [DOI] [PubMed] [Google Scholar]
- 24.Matsumura, T., T. Kondo, S. Sugita, A. M. Damiani, D. J. O'Callaghan, and H. Imagawa. 1998. An equine herpesvirus type 1 recombinant with a deletion in the gE and gI genes is avirulent in young horses. Virology 242:68-79. [DOI] [PubMed] [Google Scholar]
- 25.Meignier, B., R. Longnecker, P. Mavromara-Nazos, A. E. Sears, and B. Roizman. 1988. Virulence of and establishment of latency by genetically engineered deletion mutants of herpes simplex virus 1. Virology 162:251-254. [DOI] [PubMed] [Google Scholar]
- 26.Mijnes, J. D., B. C. Lutters, A. C. Vlot, E. van Anken, M. C. Horzinek, P. J. Rottier, and R. J. de Groot. 1997. Structure-function analysis of the gE-gI complex of feline herpesvirus: mapping of gI domains required for gE-gI interaction, intracellular transport, and cell-to-cell spread. J. Virol. 71:8397-8404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Montgomery, R. I., M. S. Warner, B. J. Lum, and P. G. Spear. 1996. Herpes simplex virus-1 entry into cells mediated by a novel member of the TNF/NGF receptor family. Cell 87:427-436. [DOI] [PubMed] [Google Scholar]
- 28.Nagashunmugam, T., J. Lubinski, L. Wang, L. T. Goldstein, B. S. Weeks, P. Sundaresan, E. H. Kang, G. Dubin, and H. M. Friedman. 1998. In vivo immune evasion mediated by the herpes simplex virus type 1 immunoglobulin G Fc receptor. J. Virol. 72:5351-5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neidhardt, H., C. H. Schroder, and H. C. Kaerner. 1987. Herpes simplex virus type 1 glycoprotein E is not indispensable for viral infectivity. J. Virol. 61:600-603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Para, M. F., M. L. Parish, A. G. Noble, and P. G. Spear. 1985. Potent neutralizing activity associated with anti-glycoprotein D specificity among monoclonal antibodies selected for binding to herpes simplex virions. J. Virol. 55:483-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rebordosa, X., J. Pinol, J. A. Perez-Pons, J. Lloberas, J. Naval, X. Serra-Hartmann, E. Espuna, and E. Querol. 1996. Glycoprotein E of bovine herpesvirus type 1 is involved in virus transmission by direct cell-to-cell spread. Virus Res. 45:59-68. [DOI] [PubMed] [Google Scholar]
- 32.Rizvi, S. M., and M. Raghavan. 2001. An N-terminal domain of herpes simplex virus type I gE is capable of forming stable complexes with gI. J. Virol. 75:11897-11901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Whealy, M. E., J. P. Card, A. K. Robbins, J. R. Dubin, H. J. Rziha, and L. W. Enquist. 1993. Specific pseudorabies virus infection of the rat visual system requires both gI and gp63 glycoproteins. J. Virol. 67:3786-3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Whitbeck, J. C., A. C. Knapp, L. W. Enquist, W. C. Lawrence, and L. J. Bello. 1996. Synthesis, processing, and oligomerization of bovine herpesvirus 1 gE and gI membrane proteins. J. Virol. 70:7878-7884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yao, Z., W. Jackson, B. Forghani, and C. Grose. 1993. Varicella-zoster virus glycoprotein gpI/gpIV receptor: expression, complex formation, and antigenicity within the vaccinia virus-T7 RNA polymerase transfection system. J. Virol. 67:305-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zsak, L., F. Zuckermann, N. Sugg, and T. Ben-Porat. 1992. Glycoprotein gI of pseudorabies virus promotes cell fusion and virus spread via direct cell-to-cell transmission. J. Virol. 66:2316-2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zuckermann, F. A., T. C. Mettenleiter, C. Schreurs, N. Sugg, and T. Ben-Porat. 1988. Complex between glycoproteins gI and gp63 of pseudorabies virus: its effect on virus replication. J. Virol. 62:4622-4626. [DOI] [PMC free article] [PubMed] [Google Scholar]