Abstract
In order to study primate lentivirus evolution in the Colobinae subfamily, in which only one simian immunodeficiency virus (SIV) has been described to date, we screened additional species from the three different genera of African colobus monkeys for SIV infection. Blood was obtained from 13 West African colobids, and HIV cross-reactive antibodies were observed in 5 of 10 Piliocolobus badius, 1 of 2 Procolobus verus, and 0 of 1 Colobus polykomos specimens. Phylogenetic analyses of partial pol sequences revealed that the new SIVs were more closely related to each other than to the other SIVs and especially did not cluster with the previously described SIVcol from Colobus guereza. This study presents evidence that the three genera of African colobus monkeys are naturally infected with an SIV and indicates also that there was no coevolution between virus and hosts at the level of the Colobinae subfamily.
Simian immunodeficiency viruses (SIVs) are found naturally in an extensive number of African primate species, and serological and/or molecular evidences for SIVs have been reported in at least 30 African nonhuman primates (13, 25). The sequence similarity of fully characterized viruses allows the classification of SIVs into six approximately equidistant phylogenetic lineages: (i) SIVcpz, from chimpanzees (Pan troglodytes) (together with human immunodeficiency virus type 1 [HIV-1]), (ii) SIVsm, from sooty mangabeys (Cercocebus atys) (together with HIV-2), (iii) SIVagm, from African green monkeys (members of the Chlorocebus aethiops superspecies), (iv) SIVsyk, from Sykes' monkeys (Cercopithecus mitis), (v) SIVlhoest/SIVsun, from l'Hoest (Cercopithecus lhoesti) and Sun-tailed monkeys (Cercopithecus solatus), and (vi) SIVcol, from a guereza colobus (Colobus guereza) (2, 5, 7, 8, 11, 14, 15, 16, 28).
Phylogenetic studies of primate lentiviruses provide several evidences that some SIV lineages have coevolved with their hosts, like SIVagm in the four African green monkey species and SIVlhoest/SIVsun within the Cercopithecus lhoesti superspecies (1, 2, 21). But there are also multiple examples of cross-species transmissions from simians to humans and between different simian species (4, 18, 30). HIV-1 and HIV-2 are of zoonotic origin, with their closest simian relatives in the common chimpanzee (Pan troglodytes) and the sooty mangabey (Cercocebus atys), respectively (11, 13, 16). Patas monkeys in West Africa and chacma baboons in South Africa are infected with an SIV from the local sympatric African green monkey species (4, 30). In addition, full-length genome sequencing of SIVs from sabaeus monkeys (SIVsab) (17), red-cap mangabeys (SIVrcm) (3), mandrills (SIVmnd2) (26), and greater spot-nosed monkeys (SIVgsn) (9) revealed a possible recombinant structure of their genomes. These observations suggest that both cross-species transmission and coinfection with highly divergent viral strains have existed since the beginning of the evolution of primate lentiviruses.
With the exception of SIVcpz, from chimpanzees, all SIVs identified to date originate from African primates belonging to the Cercopithecidae, or Old World monkeys. Cercopithecidae are subdivided into two distinct subfamilies, Colobinae and Cercopithecinae (10). SIVcol, isolated from Colobus guereza, is the only SIV obtained from a representative from the Colobinae subfamily and is very divergent from all known SIVs, possibly reflecting a divergence of the host lineages. Colobids separated from the other Old World monkeys at least 11 million years ago (24) and are subdivided into an African and an Asian group. The living African colobids are represented by three genera, namely, Colobus (or black and white colobus), Piliocolobus (or red colobus), and Procolobus (or olive colobus) (12). All contemporary species of the African colobids are restricted to the tropical and mountain forest belt of Africa.
In order to study primate lentivirus evolution in the Colobinae subfamily, we screened additional samples from the different genera of the African group for SIV infection.
We studied West African colobids from the Taï National Park, located in southwestern Ivory Coast near the border with Liberia; blood was obtained from the animals studied between 1997 and 2000. This park is the largest remaining area of primary forest in West Africa. Following the isolation of a new strain of Ebola virus in Taï Forest, in November 1994, the World Health Organization (WHO) conducted a collective study in the Taï National Park in order to identify the natural reservoir and vectors of the Ebola virus. During this project, samples were also collected from nonhuman primates using two methods. (i) Blood was obtained from live animals after they were darted by using teleinjection rifles (Telinject GUT 50) and a mixture of ketamine and médétomidine antagonized by atipamezole. Once the monkeys were fully anesthetized, two blood smears were obtained and a blood specimen was collected on a dry tube (Vacutainer). The monkeys were then marked on the tail with peroxidase, numbered (M001 for the first one caught, M002 for the second, and so on), and then resuscitated with atipamezole. (ii) The bodies from nonhuman primates that were found dead on the forest floor by sanitary surveillance patrols or by primatologists working in the Tai National Park were collected by the WHO staff and transported to a field laboratory in order to conduct a complete autopsy. Kidney, spleen, lung, liver, and lymph node samples and intestinal tissue were collected for histological examination and virological and serological studies. The sera and tissue samples were initially stored in liquid nitrogen and were later stored at −70°C. The identification of the monkeys was done in the field and confirmed by analysis of the skulls.
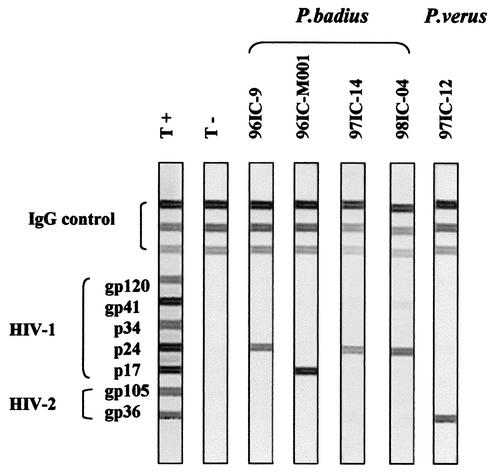
From 1996 until 2001, 43 nonhuman primates were sampled in the Tai National Park, of which 22 were Colobinae. Blood samples were obtained from three different species, representing the three genera: Western red colobus (Piliocolobus badius; n = 10), Western black and white colobus (Colobus polykomos; n = 1), and olive colobus (Procolobus verus; n = 2). Sera were tested for the presence of HIV and SIV antibodies by using the INNO-LIA HIV confirmation test (Innogenetics, Ghent, Belgium) as previously described (25). This test configuration includes HIV-1 and HIV-2 recombinant proteins and synthetic peptides that are coated as discrete lines on a nylon strip. Five (50%) of the 10 western red colobus (P. badius) samples reacted strongly with HIV core antigens, four did so with p24, and one did so with p17; one olive colobus (P. verus) sample cross-reacted strongly with gp36, the HIV-2 transmembrane protein, but the Western black and white colobus (C. polykomos) had no HIV cross-reactive antibodies (Fig. 1).
FIG. 1.
Detection of HIV-1 and HIV-2 cross-reactive antibodies in sera from Piliocolobus badius and Procolobus verus by a line immunoassay (INNO-LIA HIV Confirmation; Innogenetics, Ghent, Belgium). The five HIV-1 antigens include synthetic peptides for the exterior envelope glycoprotein (sgp120) as well as recombinant proteins for the transmembrane envelope glycoprotein (gp41), integrase (p31), core (p24), and matrix (p17) proteins. The HIV-2 antigens include synthetic peptides for the exterior envelope glycoprotein (sgp120) as well as recombinant gp36 protein. All assays were performed in accordance with the manufacturer's instructions, with alkaline phosphatase-labeled goat anti-human immunoglobulin G used as the secondary antibody. Plasma samples were scored as positive if they recognized at least one HIV antigen with an intensity equal to or greater than the assay's cutoff; samples which exhibited weaker but still visible reactivities with at least two HIV antigens were scored as indeterminant; samples were scored as negative if they yielded no reactivity or only a single band of less intensity than the assay's cutoff. Plasma samples from HIV-1- and HIV-2-negative and -positive individuals are shown as controls on the left. The 3+, 1+, and ± bands (respectively, the topmost, middle, and lower bands labeled IgG control) that are evident on the top portions of all test strips control for sample addition (presence of plasma immunoglobulin) and test performance (binding of secondary antibody).
PCR was performed to examine the samples with HIV cross-reactive antibodies for the presence of SIV sequences. DNA was isolated from whole blood or peripheral blood mononuclear cells by using a Qiagen DNA extraction kit (Qiagen, Courtaboeuf, France), and viral RNA was extracted from plasma by using a QIAamp viral RNA kit (Qiagen). We first amplified a fragment of 650 bp in the pol region by using DR1 and PolOR for the first round and Polis4/UNIPOL2 for the second round of amplification, with PCR conditions as previously reported (8). These highly cross-reactive pol primer pairs have been previously shown to amplify sequences from a wide variety of divergent HIV and SIV strains (25).
We were able to amplify a 650-bp fragment in four of the five cross-reactive P. badius samples as well as from the one P. verus sample. The P. badius sample for which no virus could be amplified was subjected to a single round of PCR using primers designed to amplify introns 4 and 5 of the nuclear glucose-6-phosphate dehydrogenase (G6PD) gene (1,450 bp) as previously described; this failed to yield a G6PD amplification product, suggesting DNA degradation and/or the presence of PCR inhibitors in this sample (25).
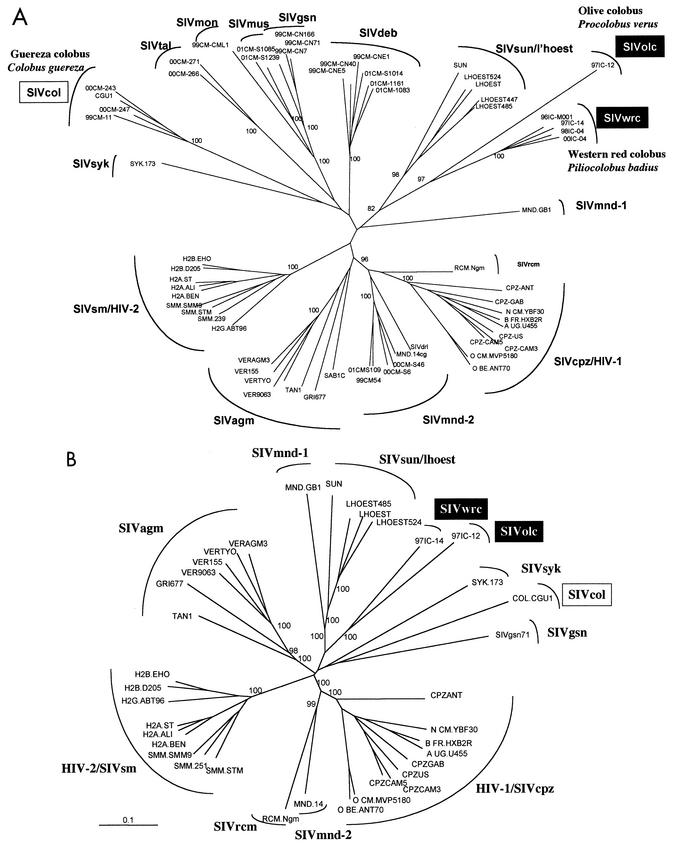
PCR products obtained with the HIV and SIV primers were cloned into the pGEM-TEasy vector (Promega) and subsequently sequenced by using cycle sequencing and dye terminator methodologies (ABI PRISM Big Dye terminator cycle sequencing ready reaction kit with AmpliTaq FS DNA polymerase [PE Biosystems, Warrington, England]) on an automated sequencer (ABI 373, stretch model; Applied Biosystems). Newly derived SIV nucleotide sequences were aligned with reference sequences representing the different SIV lineages and other partial SIV sequences available in the pol region by using CLUSTAL W with minor manual adjustments, bearing in mind the protein sequences (27). Gaps and ambiguous regions in the alignment were omitted from further analyses. A phylogenetic tree was constructed by using the neighbor joining method, and the reliability of branching orders was tested by using the bootstrap approach (27). Sequence distances were calculated by using Kimura's two-parameter method to correct for superimposed hits (19). Phylogenetic analysis of the 650-bp fragment confirmed SIV infection (Fig. 2A) and showed that viral sequences from P. badius (SIVwrc) and P. verus (SIVolc) each formed species-specific monophyletic clusters. The new SIVs obtained from two different genera in the Colobinae subfamily were more closely related to each other than to the other SIVs. Interestingly, the new sequences were not at all related to the SIVcol strain obtained from a guereza colobus (C. guereza) from Cameroon.
FIG. 2.
Phylogenetic tree analyses of the new SIV sequences SIVwrc, from a western red colobus (Piliocolobus badius), and SIVolc, from an olive colobus (Procolobus verus). A 650-bp (A) fragment and a 2,000-bp (B) fragment were amplified in pol, sequenced, and subjected to phylogenetic tree analysis by using the neighbor joining method. The positions of the SIV sequences that were derived from the present study (boxed in black) are shown in relation to HIV and SIV reference sequences representing the different known SIV sequences available in this region of the genome. Branch lengths are drawn to scale (the bar for panel B indicates 10% divergence). The numbers at the nodes indicate the percent bootstrap values supporting the cluster to the right (only values of >80% are shown).
A 2,000-bp fragment, corresponding to part of the reverse transcriptase and integrase of the pol gene and amplified with DR1 and PolOR as the outer primers and with DR4 and specific primers designed on the basis of the 600-bp fragment as the inner primers, was then sequenced for representatives of SIVwrc (SIVwrc-97CI14) and SIVolc (SIVolc-97CI12) (6, 8). The phylogenetic tree analysis shows that the two new SIVs form a separate well supported cluster, although they are only distantly related to each other (Fig. 2B). Amino acid identities between SIVwrc-97CI14 and SIVolc-97CI12, as well as to representatives of the other primate lentivirus lineages, were calculated (Table 1). SIVwrc-97CI14 and SIVolc-97CI12 showed 59.8% amino acid identity, which is close to the identities observed with the majority of the other primate lentiviruses. Surprisingly, the lowest homology was seen with the SIVcol strain, with only 50.8% amino acid identity.
TABLE 1.
Percent amino acid identities in the pol region (661 aa) between SIVwrc (SIVwrc-971C-14) and SIVolc (SIVolc-971C-12) to representatives of other SIV lineages
| SIV lineage | % identity with:
|
|
|---|---|---|
| SIVwrc | SIVolc | |
| SIVolc | 59.8 | |
| SIVwrc | 59.8 | |
| SIVcol | 50.8 | 50.8 |
| SIVlhoest | 59.5 | 57.1 |
| SIVsun | 58.7 | 56.3 |
| SIVmnd-1 | 61.9 | 54.1 |
| SIVsmPBj | 59.3 | 54.2 |
| SIVcpzGAB | 58.9 | 54.8 |
| SIVcpzANT | 58.9 | 55.2 |
| SIVagmVER | 56.3 | 52.5 |
| SIVagmGRI677 | 55.7 | 51.2 |
| SIVagmTAN | 55.5 | 53 |
| SIVsyk | 53.2 | 50.5 |
| SIVgsn-99CM71 | 54.7 | 50.2 |
| SIVmnd-2 | 59 | 56.8 |
| SIVrcmNig | 60.2 | 56.7 |
This study presents evidence that in the Colobinae subfamily, the three African genera are naturally infected with an SIV. At least one representative from each genus is infected, namely, Colobus guereza in Cameroon and Piliocolobus badius and Procolobus verus in Ivory Coast. If we consider that all of these viruses have the same rate of evolution in their respective hosts, it seems that these viruses have not evolved in a host-dependent fashion at the level of the Colobinae subfamily, since representative viruses from the three genera do not cluster together in the region studied. However full-length genome sequencing of the new SIVolc and SIVwrc sequences is necessary to see to what extent they are pure or recombinant SIVs. We also have to take into account the geographical origin of the SIV-harboring species which we are comparing. It is important to note that the African colobid species are reflected by their geographic distribution; e.g., the olive colobus is a relict species confined to the forest of West Africa, and the red colobus (Piliocolobus spp.) once ranged all over the forested areas from Africa, but their regional differentiation shows that their scattered distribution is of long standing.
SIVcol from the guereza colobus from Cameroon is divergent from all known SIVs. This could suggest an ancient infection and a different evolution of SIVcol in its host species, perhaps reflecting a more pure lineage relative to other SIV lineages. On the other hand, the clustering of SIVwrc with SIVolc could also suggest a cross-species transmission between them or infection from a common source. Colobus monkeys share habitats with Cercopithecus species and with mangabeys; therefore, an exchange of ancestral SIVs between these species could have been possible in the past. Monkeys living in tropical forests often form aggregations consisting of multiple species, i.e., polyspecific associations. This has been particularly well documented in the Taï National Park, where associations between olive colobus and diana monkeys (Cercopithecus diana) and associations between red colobus and diana monkeys are frequent (22). Moreover, SIV seropositivity has been previously described in diana monkeys (20). Importantly, such associations cannot be extrapolated to similar primate populations in another location; e.g., by contrast, a study on the same species complex on Tiwai Island in Sierra Leone reveals no associations between red colobus and diana monkeys (23). This illustrates that data on primate behavior are necessary to understand primate lentivirus evolution and to determine between which primate species cross-species transmission and superinfection have or have not been possible (29).
In order to understand the evolution of SIVs in the Colobinae subfamily, it will be important to identify and compare SIVs from Colobus and Piliocolobus species from West, Central, and East Africa to find out whether coevolution between viruses and hosts occurred or whether hosts became infected along with other cohabiting monkeys in the past. Screening of the other sympatric species from the Cercopithecidinae and Colobinae subfamilies (e.g., Cercopithecus diana and Colobus polykomos from the Taï forest) is also required.
Nucleotide sequence accession numbers.
The GenBank accession numbers for the sequences in this study are as follows: AY138265 (SIVwrc-96IC-M001), AY138266 (SIVwrc-98IC-04), AY138267 (SIVwrc-00IC-04), AY138268 (SIVwrc-97IC-14), and AY138269 (SIVolc-97IC-12).
REFERENCES
- 1.Allan, J. S., M. Short, M. E. Taylor, S. Su, V. M. Hirsch, P. R. Johnson, G. M. Shaw, and B. H. Hahn. 1991. Species-specific diversity among simian immunodeficiency viruses from African green monkeys. J. Virol. 65:2816-2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beer, B. E., E. Bailes, R. Goeken, G. Dapolito, C. Coulibaly, S. G. Norley, R. Kurth, J. P. Gautier, A. Gautier-Hion, D. Vallet, P. M. Sharp, and V. M. Hirsch. 1999. Simian immunodeficiency virus (SIV) from sun-tailed monkeys (Cercopithecus solatus): evidence for host-dependent evolution of SIV within the C. lhoesti superspecies. J. Virol. 73:7734-7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beer, B. E., B. T. Foley, C. L. Kuiken, Z. Tooze, R. M. Goeken, C. R. Brown, J. Hu, M. S. Claire, B. T. Korber, and V. M. Hirsch. 2001. Characterization of novel simian immunodeficiency viruses from red-capped mangabeys from Nigeria (SIVrcmNG409 and -NG411). J. Virol. 75:12014-12027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bibollet-Ruche, F., A. Galat-Luong, G. Cuny, P. Sarni-Manchado, G. Galat, J. P.Durand, X. Pourrut, and F. Veas. 1996. Simian immunodeficiency virus infection in a patas monkey (Erythrocebus patas): evidence for cross-species transmission from African green monkeys (Cercopithecus aethiops sabaeus) in the wild. J. Gen. Virol. 77:773-781. [DOI] [PubMed] [Google Scholar]
- 5.Chen, Z., P. Telfier, A. Gettie, P. Reed, L. Zhang, D. D. Ho, and P. A. Marx. 1996. Genetic characterization of new West African simian immunodeficiency virus SIVsm: geographic clustering of household-derived SIV strains with human immunodeficiency virus type 2 subtypes and genetically diverse viruses from a single feral sooty mangabey troop. J. Virol. 70:3617-3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clewley, J. P., J. C. Lewis, D. W. Brown, and E. L. Gadsby. 1998. A novel simian immunodeficiency virus (SIVdrl) pol sequence from the drill monkey, Mandrillus leucophaeus. J. Virol. 72:10305-10309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corbet, S., M. C. Muller-Trutwin, P. Versmisse, S. Delarue, A. Ayouba, J. Lewis, S. Brunak, P. Martin, F. Brun-Vezinet, F. Simon, F. Barre-Sinoussi, and P. Mauclere. 2000. env sequences of simian immunodeficiency viruses from chimpanzees in Cameroon are strongly related to those of human immunodeficiency virus group N from the same geographic area. J. Virol. 74:529-534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Courgnaud, V., X. Pourrut, F. Bibollet-Ruche, E. Mpoudi-Ngole, A. Bourgeois, E. Delaporte, and M. Peeters. 2001. Characterization of a novel simian immunodeficiency virus from guereza colobus monkeys (Colobus guereza) in Cameroon: a new lineage in the nonhuman primate lentivirus family. J. Virol. 75:857-866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Courgnaud, V., M. Salemi, X. Pourrut, E. Mpoudi-Ngole, B. Abela, P. Auzel, F. Bibollet-Ruche, B. Hahn, A. Vandamme, E. Delaporte, and M. Peeters. 2002. The characterization of a novel simian immunodeficiency virus with a vpu gene from greater spot-nosed monkeys (Cercopithecus nictitans) provides new insights into the simian/human immunodeficiency virus phylogeny. J. Virol. 76:8298-8309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Disotell, T. 1996. The phylogeny of Old World monkeys. Evol. Anthropol. 5:18-24. [Google Scholar]
- 11.Gao, F., E. Bailes, D. L. Robertson, Y. Chen, C. M. Rodenburg, S. F. Michael, L. B. Cummins, L. O. Arthur, M. Peeters, G. M. Shaw, P. M. Sharp, and B. H. Hahn. 1999. Origin of HIV-1 in the chimpanzee Pan troglodytes troglodytes. Nature 397:436-441. [DOI] [PubMed] [Google Scholar]
- 12.Groves, C. 2001. Primate taxonomy; Smithsonian series in comparative evolutionary biology. Smithsonian Institution Press, Washington, D.C.
- 13.Hahn, B. H., G. M. Shaw, K. M. De Cock, and P. M. Sharp. 2000. AIDS as a zoonosis: scientific and public health implications. Science 287:607-614. [DOI] [PubMed] [Google Scholar]
- 14.Hirsch, V. M., B. J. Campbell, E. Bailes, R. Goeken, C. Brown, W. R. Elkins, M. Axthelm, M. Murphey-Corb, and P. M. Sharp. 1999. Characterization of a novel simian immunodeficiency virus (SIV) from L'Hoest monkeys (Cercopithecus l'hoesti): implications for the origins of SIVmnd and other primate lentiviruses. J. Virol. 73:1036-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirsch, V. M., G. A. Dapolito, S. Goldstein, H. McClure, P. Emau, P. N. Fultz, M. Isahakia, R. Lenroot, G. Myers, and P. R. Johnson. 1993. A distinct African lentivirus from Sykes' monkeys. J. Virol. 67:1517-1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirsch, V. M., R. A. Olmsted, M. Murphey-Corb, R. H. Purcell, and P. R. Johnson. 1989. An African primate lentivirus (SIVsm) closely related to HIV-2. Nature 339:389-392. [DOI] [PubMed] [Google Scholar]
- 17.Jin, M. J., H. Hui, D. L. Robertson, M. C. Muller, F. Barre-Sinoussi, V. M. Hirsch, J. S. Allan, G. M. Shaw, P. M. Sharp, and B. H. Hahn. 1994. Mosaic genome structure of simian immunodeficiency virus from West African green monkeys. EMBO J. 13:2935-2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jin, M. J., J. Rogers, J. E. Phillips-Conroy, J. S. Allan, R. C. Desrosiers, G. M. Shaw, P. M. Sharp, and B. H. Hahn. 1994. Infection of a yellow baboon with simian immunodeficiency virus from African green monkeys: evidence for cross-species transmission in the wild. J. Virol. 68:8454-8460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kimura, M. 1983. The neutral theory of molecular evolution. Cambridge University Press, Cambridge, United Kingdom.
- 20.Lowenstine, L. J., N. C. Pedersen, J. Higgins, K. C. Pallis, A. Uyeda, P. Marx, N. W. Lerche, R. J. Munn, and M. B. Gardner. 1986. Seroepidemiologic survey of captive Old-World primates for antibodies to human and simian retroviruses, and isolation of a lentivirus from sooty mangabeys (Cercocebus atys). Int. J. Cancer 38:563-574. [DOI] [PubMed] [Google Scholar]
- 21.Muller, M. C., N. K. Saksena, E. Nerrienet, C. Chappey, V. M. Herve, J. P. Durand, P. Legal-Campodonico, M. C. Lang, J. P. Digoutte, A. J. Georges, et al. 1993. Simian immunodeficiency viruses from central and western Africa: evidence for a new species-specific lentivirus in tantalus monkeys. J. Virol. 67:1227-1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Noë, R., and R. Bshary. 1997. The formation of red colobus-diana monkey associations under predation pressure from chimpanzees. Proc. R. Soc. Lond. B 264:253-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oates, J. F., and G. H. Whitesides. 1990. Association between olive colobus (Procolobus verus), diana guenons (Cercopithecus diana), and other forest monkeys in Sierra Leone. Am. J. Primatol. 21:129-146. [DOI] [PubMed] [Google Scholar]
- 24.Page, S. L., C. Chiu, and M. Goodman. 1999. Molecular phylogeny of Old World monkeys (Cercopithecidae) as inferred from gamma-globin DNA sequences. Mol. Phylogenet. Evol. 13:348-359. [DOI] [PubMed] [Google Scholar]
- 25.Peeters, M., V. Courgnaud, B. Abela, P. Auzel, X. Pourrut, F. Bibollet-Ruche, S. Loul, F. Liegeois, C. Butel, D. Koulagna, E. Mpoudi-Ngole, G. M. Shaw, B. H. Hahn, and E. Delaporte. 2002. Risk to human health from a plethora of simian immunodeficiency viruses in primate bushmeat. Emerg. Infect. Dis. 8:451-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Souquiere, S., F. Bibollet-Ruche, D. L. Robertson, M. Makuwa, C. Apetrei, R. Onanga, C. Kornfeld, J.-C. Plantier, F. Gao, K. Abernethy, L. J. T. White, W. Karesh, P. Telfer, E. J. Wickings, P. Mauclere, P. A. Marx, F. Barre-Sinoussi, B. H. Hahn, M. C. Müller-Trutwin, and F. Simon. 2001. Wild Mandrillus sphinx are carriers of two types of lentivirus. J. Virol. 75:7086-7096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thompson, J., D. Higgins, and T. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 11:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsujimoto, H., A. Hasegawa, N. Maki, M. Fukasawa, T. Miura, S. Speidel, R. W. Cooper, E. N. Moriyama, T. Gojobori, and M. Hayami. 1989. Sequence of a novel simian immunodeficiency virus from a wild-caught African mandrill. Nature 341:539-541. [DOI] [PubMed] [Google Scholar]
- 29.Tutin, C. E. 2000. Ecology and social organization of African tropical forest primates: aid in understanding retrovirus transmission. Bull. Soc. Pathol. Exot. 93:157-161. [PubMed] [Google Scholar]
- 30.van Rensburg, E. J., S. Engelbrecht, J. Mwenda, J. D. Laten, B. A. Robson, T. Stander, and G. K. Chege. 1998. Simian immunodeficiency viruses (SIVs) from eastern and southern Africa: detection of a SIVagm variant from a chacma baboon. J. Gen. Virol. 79:1809-1814. [DOI] [PubMed] [Google Scholar]