Abstract
Food-borne transmission of prions can lead to infection of the gastrointestinal tract and neuroinvasion via the splanchnic and vagus nerves. Here we report that the transmission of transmissible mink encephalopathy (TME) is 100,000-fold more efficient by inoculation of prions into the tongues of hamsters than by oral ingestion. The incubation period following TME agent (hereinafter referred to as TME) inoculation into the lingual muscles was the shortest among the five nonneuronal routes of inoculation, including another intramuscular route. Deposition of the abnormal isoform of the prion protein, PrPSc, was first detected in the tongue and submandibular lymph node at 1 to 2 weeks following inoculation of the tongue with TME. PrPSc deposits in the tongue were associated with individual axons, and the initial appearance of TME in the brain stem was found in the hypoglossal nucleus at 2 weeks postinfection. At later time points, PrPSc was localized to brain cell groups that directly project to the hypoglossal nucleus, indicating the transneuronal spread of TME. TME PrPSc entry into the brain stem preceded PrPSc detection in the rostral cervical spinal cord. These results demonstrate that TME can replicate in both the tongue and regional lymph nodes but indicate that the faster route of brain invasion is via retrograde axonal transport within the hypoglossal nerve to the hypoglossal nucleus. Topical application of TME to a superficial wound on the surface of the tongue resulted in a higher incidence of disease and a shorter incubation period than with oral TME ingestion. Therefore, abrasions of the tongue in livestock and humans may predispose a host to oral prion infection of the tongue-associated cranial nerves. In a related study, PrPSc was detected in tongues following the intracerebral inoculation of six hamster-adapted prion strains, which demonstrates that prions can also travel from the brain to the tongue in the anterograde direction along the tongue-associated cranial nerves. These findings suggest that food products containing ruminant or cervid tongue may be a potential source of prion infection for humans.
Prion diseases are fatal neurodegenerative diseases of humans, livestock, and cervids. The majority of prion diseases have an infectious etiology, and food-borne infection has been linked to the transmission of transmissible mink encephalopathy (TME), bovine spongiform encephalopathy (BSE), and kuru in humans (21, 23, 58). Indirect evidence suggests that oral infection is involved in the transmission of other prion diseases, such as scrapie in sheep, chronic wasting disease (CWD) in deer and elk, and variant Creutzfeldt-Jakob disease in humans (2, 13, 26, 47, 52). The experimental ingestion of high doses of scrapie agent (hereinafter referred to as scrapie) has been used to determine the sites of scrapie replication in peripheral tissues and the routes by which the disease spreads to the peripheral and central nervous systems (24, 33, 36, 38, 54).
The disease-specific isoform of the prion protein, PrPSc, is found in the enteric nervous system of the submucosal and myenteric plexus and the gut-associated lymphoid tissue following oral scrapie ingestion (6, 24, 36, 38). Prion spread from these sites to the central nervous system can occur by axonal transport within the parasympathetic nervous system (e.g., from the vagus nerve to the dorsal motor nucleus of the vagus) and the sympathetic nervous system (e.g., from the splanchnic nerve to the intermediolateral cell column of the spinal cord) (36, 38). The distribution of PrPSc in the tissues of subclinically infected sheep with scrapie and deer with CWD is consistent with spread along these pathways (2, 47, 52, 53). The additional spread of prions within a host can occur within the lymphoreticular system (LRS) and can result in systemic prion infection of secondary lymphoid organs. There is no consensus on the cell type(s) involved in prion replication and accumulation in the LRS. Although PrPSc deposition is associated with follicular dendritic cells in the germinal centers of the secondary lymphoid organs (31, 37), recent studies using immunodeficient mice indicated that mature follicular dendritic cells are not required for prion infection and neuroinvasion (34, 40, 42). Macrophage subsets located in the marginal zones of secondary lymphoid tissues appear to be necessary for prion propagation in the LRS (34, 42).
The view that LRS infection must be established prior to the spread of prions to the nervous system has been challenged by several studies. Prion infectivity and PrPSc are not detected outside of the nervous system in animals with natural BSE (11), even though early PrPSc deposition in the brain stem has been reported to take place in the dorsal motor nucleus of the vagus and the nucleus of the solitary tract (44). Oral ingestion of high doses of mouse-adapted scrapie can also result in neuroinvasion and disease in the absence of LRS infection (43). In one study, peripheral scrapie inoculation was performed on transgenic mice (with a PrP knockout genetic background) that had restricted expression of Syrian hamster PrPC in a subset of neuronal cells (i.e., gene expression was controlled by the neuron-specific enolase promoter) and no expression of PrPC in secondary lymphoid organs. In these transgenic mice, infection with the 263K strain of scrapie was not found in the LRS due to the lack of PrPC expression, but the mice were susceptible to hamster-adapted 263K scrapie by intraperitoneal (i.p.) inoculation and oral ingestion (43). These findings indicate that peripheral prion infection and neuroinvasion can be LRS independent and suggest that direct infection of the nervous system is an alternate route of infection. This conclusion is supported by additional studies in which peripheral scrapie, of immunodeficient mice (e.g., muMT and RAG-1 knockout mice, which lack functional germinal centers and are unable to replicate scrapie in the LRS) resulted in scrapie infection of the brain (20).
In the present study, we investigated the ability of the HY strain of the TME agent (hereinafter referred to as HY TME) TME to establish disease in hamsters following oral infection by ingestion, inoculation of the lingual muscles, or topical application to the surface of the tongue in the presence and absence of a superficial wound. We demonstrate that tongue infection is a more efficient route of prion neuroinvasion than ingestion and that HY TME can directly spread to the brain from the tongue via the hypoglossal nerve. We propose that the exposure of nerve endings in the tongue or oral cavity, possibly due to lesions or microbial infections, may increase the risk of prion infection and may serve as an alternate route of infection following oral prion exposure. In addition, we demonstrate that six hamster-adapted prion strains can spread to the tongue following intracerebral (i.c.) inoculation. This finding has implications for public health, since livestock tongue is used in food products and may be a potential source of prion infection in humans.
MATERIALS AND METHODS
Strains of hamster TME and scrapie agents.
Biological clones of HY and DY TME were isolated as previously described (9) and maintained by i.c. inoculation into weanling male outbred Golden Syrian hamsters (Harlan Sprague Dawley, Indianapolis, Ind.) as described below. Scrapie strains 139H, 22AH, 22CH, and Me7H were isolated upon serial passage in hamsters and were a gift from Richard Rubenstein (New York State Institute for Basic Research in Developmental Disabilities, Staten Island, N.Y.).
Animal inoculations.
All procedures involving animals were approved by the Creighton University Institutional Animal Care and Use Committee and are in compliance with the Guide for the Care and Use of Laboratory Animals (39). Hamsters were inoculated with 5 to 100 μl of a 1% (wt/vol) brain homogenate from an HY TME-infected hamster containing 107.5 median (50%) lethal doses (LD50) per ml. Intrasciatic nerve (i.n.), i.c., and i.p. inoculations were performed as previously described (4, 9). Intratongue (i.t.) inoculations were performed by the bilateral inoculation of 20 μl of HY TME into the intrinsic muscles of the tongue. In a second study (see Fig. 5), the tongues of hamsters were unilaterally inoculated with 5 μl of HY TME. For intramuscular inoculations, hamsters received injections in the right femoral biceps. Intravenous inoculations were performed by injecting HY TME into the penile vein. For oral ingestion studies, inoculum was dried on a food pellet and subsequently fed to hamsters. To produce a superficial wound on the tongue, hamsters were anesthetized with a ketamine and xylazine mixture and the tip of a 30-gauge needle was used to cut the dorsal surface of the tongue. Each hamster received a 3-mm-long wound that penetrated through the epithelium. HY TME inoculum was directly applied to the wound before each animal regained consciousness. Following inoculation of HY TME, hamsters were observed daily for the onset of clinical symptoms. The incubation period was determined based on the initial onset and early progression of symptoms characteristic of HY TME, which included hyperactivity in response to touch and sound, a tremor of the head and body, and ataxia.
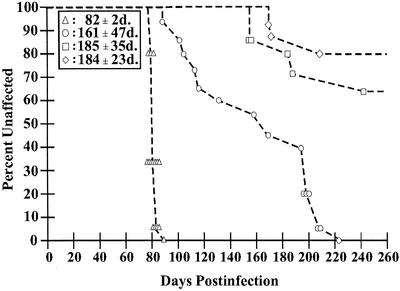
FIG. 5.
Incubation period of HY TME following oral infection. Syrian hamsters were exposed to HY TME by four different oral routes of inoculation. The percentage of unaffected animals in each group versus the incubation periods of individual affected hamsters following inoculation of 105.2 LD50 of HY TME was plotted for each route. Groups of 15 hamsters were inoculated by (i) unilateral injection into the lingual muscles (triangles; 15 affected of 15 inoculated), (ii) topical application to a superficial wound on the dorsal surface of the tongue (circles; 15 affected of 15 inoculated), (iii) topical application to the dorsal surface of a normal tongue (squares; 4 affected of 14 inoculated), or (iv) oral ingestion (diamonds; 4 affected of 15 inoculated). The mean incubation period in days (d.) ± the standard error of the mean for each route of inoculation is indicated in the boxed area.
Efficiency of the i.t. route.
Endpoint titration of HY TME by the i.c. and i.t. routes of inoculation was performed by injecting groups of five hamsters with consecutive, serial 10-fold dilutions of HY TME-infected brain. The titer was calculated by the method of Kärber. Differences in titer were used to determine the efficiency of i.t. inoculation relative to that of i.c. inoculation in establishing TME infection as previously described for rodent-adapted scrapie (12, 29).
Tissue collection for PrPSc analysis.
To study the route of TME spread following i.t. inoculation of HY TME, three to five hamsters were sacrificed each week postinfection for 10 consecutive weeks. The brains, spinal cords, tongues, spleens, and submandibular and cervical lymph nodes were collected for PrPSc analysis by Western blotting and immunohistochemistry.
Tissue preparation for PrPSc Western blotting.
PrPSc was enriched from tissue prior to Western blotting. Briefly, 2 to 100 mg of tissue was homogenized to 20% (wt/vol) in Tris-HCl (pH 7.4) buffer containing 5 mM MgCl2. Benzonase nuclease (Novagen, Inc., Madison, Wis.) was added to a concentration of 100 U per ml, and the reaction mixture was incubated at 37°C for 1 h with constant shaking. An equal volume of 20% (wt/vol) N-lauroylsarcosine in 10 mM Tris-HCl (pH 7.4)-133 mM NaCl-1 mM EDTA was added, and the tissue homogenates were incubated for 30 min at room temperature with constant shaking. The tissue homogenates were further subjected to a series of ultracentrifugation procedures and a proteinase K digestion step in order to enrich for PrPSc as previously described (4). The PrP-enriched pellet was resuspended in a sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample loading buffer.
Western blot analysis.
SDS-PAGE and Western blot analysis were performed as previously described with monoclonal antibody 3F4 hybridoma (28) (a gift of Victoria Lawson, National Institutes of Health Rocky Mountain Laboratories, Hamilton, Mont.) (4, 8). Quantification of PrPSc bands from Western blots was performed with a Storm PhosphorImager (Molecular Dynamics, Sunnyvale, Calif.) and ImageQuant software as previously described (4).
PrPSc immunohistochemistry.
Immunostaining of brain tissue for PrPSc was performed as previously described (8) or by using the method of Wilson and McBride (59). Briefly, tissues were immersion fixed in neutral buffered formalin or animals were perfused with McLean's paraformaldehyde-lysine-periodate (PLP) fixative, after which tissues were postfixed in PLP. Paraffin-embedded tissue sections (7 μm) were subjected to antigen retrieval by either hydrolytic autoclaving (1 to 3 mM HCl) or pretreatment with formic acid for 20 min. We found that the procedure using PLP and short fixation times produced more consistent results and was less disruptive to tissue morphology. A minimum of 2 serial sections for every 20 tissue sections were examined for PrPSc by immunohistochemistry analysis. In the brain, the region between the first segment of the cervical spinal cord and the midbrain at the level of the inferior colliculus was analyzed each week postinfection. Tissues were incubated with monoclonal 3F4 hybridoma antibody (1:600 dilution) or ascites fluid (1:2,000 dilution) (the latter was a gift from Richard Kascsak, Institute for Basic Research in Developmental Disabilities, Staten Island, N.Y.). The ABC-HRP Elite (Vector Laboratories, Burlingame, Calif.) method was used for anti-PrP antibody signal amplification, and PrPSc was visualized with 3-amino-9-ethylcarbazole in 50 mM sodium acetate (pH 5.0)-0.03% H2O2. For immunofluorescence, rabbit anti-mouse Alexa Fluor 488 (Molecular Probes, Portland, Oreg.) was used at a 1:200 dilution. Adjacent tissue sections were stained with cresyl violet to aid in the identification of brain and brain stem nuclei.
RESULTS
TME infection by neuronal and nonneuronal routes of inoculation.
The length of the incubation period following inoculation of HY TME was investigated with neuronal and nonneuronal routes of inoculation. i.c. inoculation directly established TME infection in the brain and resulted in an incubation period of 59 ± 1 day, which was 42 days shorter than the incubation period resulting from i.p. inoculation (Table 1). i.n. inoculation directly established infection of the peripheral nervous system and resulted in an incubation period 8 days longer than that associated with i.c. inoculation but 33 days shorter than than that associated with i.p. inoculation (Table 1). Among the five nonneuronal peripheral routes of inoculation, i.t. inoculation into the lingual muscles resulted in the shortest incubation period, at 79 ± 5 days. The incubation period of the i.t. route of inoculation was statistically significantly longer (P < 0.01) than those of the i.c. and i.n. routes but statistically significantly shorter (P < 0.01) than those of oral ingestion and i.p., intravenous, and intramuscular inoculations of HY TME (Table 1).
TABLE 1.
Incubation period of HY TME in hamsters following inoculation by the neuronal and nonneuronal routes
| Routea | Incubation period (days)b | No. affected/no. inoculated |
|---|---|---|
| Cerebrum | 59 ± 1 | 5/5 |
| Sciatic nerve | 68 ± 2 | 14/14 |
| Tongue | 79 ± 5 | 16/16 |
| Peritoneum | 101 ± 7 | 5/5 |
| Vein | 118 ± 33 | 8/8 |
| Muscle | 142 ± 14 | 5/5 |
| Oral ingestion | 191 | 1/5 |
An LD50 of ∼105.5 prions was inoculated by each route.
Means ± standard errors of the means.
The efficiency of the i.t. route of TME infection was determined by calculating the TME titer by endpoint dilution for both the i.t. and i.c. routes. All of the hamsters that were i.c. inoculated with the 10−8 dilution (wt/vol) of brain homogenate developed TME, while none of the hamsters in the group inoculated with the 10−9 dilution of brain homogenate developed clinical TME by 400 days postinfection (Table 2). Of the i.t.-inoculated animals, 20% of those in the 10−7 dilution group developed TME and none developed clinical symptoms of TME at higher dilutions of brain inoculum. Based on this data, the LD50 per gram of brain were 109.5 and 108.4 for the i.c. and i.t. routes of inoculation, respectively (Table 2). These findings demonstrated that the i.t. route of inoculation was 10- to 100-fold less efficient in transmitting disease than i.c. inoculation of HY TME. In contrast, only 20% of the hamsters that received the 10−2 dilution of brain inoculum by oral ingestion developed clinical TME (Table 1). This percentage of clinically affected animals was similar to that found for those receiving the 10−7 dilution of brain inoculum following i.t. inoculation. Based on this comparative analysis, the estimated titer of HY TME following oral ingestion would be 103.4 LD50 per g of brain. These results indicate that the i.t. route of TME inoculation was 100,000-fold more efficient in transmitting disease than oral ingestion of HY TME.
TABLE 2.
i.c. and i.t. HY TME inoculationa
| Brain inoculum dilutiona | i.c. inoculation
|
i.t. inoculation
|
||||
|---|---|---|---|---|---|---|
| Incubation period (days)b | No. affected/no. inoculated | Incubation period (days) | No. affected/no. inoculated | |||
| 10−2 | 59 ± 1 | 5/5 | 77 ± 4 | 5/5 | ||
| 10−3 | ND | 0/0 | ND | 0/0 | ||
| 10−4 | ND | 0/0 | 105 ± 45 | 5/5 | ||
| 10−5 | ND | 0/0 | 182 ± 23 | 5/5 | ||
| 10−6 | 107 ± 1 | 5/5 | 272 ± 47 | 5/5 | ||
| 10−7 | 154 ± 22 | 5/5 | 270 | 1/5 | ||
| 10−8 | 190 ± 22 | 5/5 | >417 | 0/5 | ||
| 10−9 | >400 | 0/5 | >417 | 0/5 | ||
| Titer | 109.5LD50/g | 108.3LD50/g | ||||
Twenty microliters (i.t.) or 50 μl (i.c.) was inoculated into each hamster.
Means ± standard errors of the means. ND, not done.
Temporal accumulation of PrPSc following i.t. inoculation of HY TME.
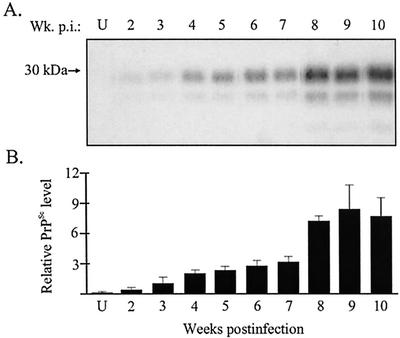
The chronological deposition of PrPSc was investigated in order to determine the route of HY TME neuroinvasion following i.t. inoculation. Hamsters were mock infected or i.t. inoculated with HY TME, and three animals per group were sacrificed each week postinfection. Brains, spinal cords, tongues, spleens, and submandibular and cervical lymph nodes were collected for PrPSc analysis. PrPSc deposition was detected in the tongue from PrPSc-enriched preparations (25-mg tissue equivalents) beginning at 2 weeks postinfection (Fig. 1A). The amount of PrPSc in the tongue gradually increased each week until 8 weeks postinfection. There was approximately a twofold increase in the amount of PrPSc found in the tongue at 8 weeks postinfection compared to the levels measured at 7 weeks postinfection (Fig. 1). From 8 to 10 weeks postinfection, PrPSc levels in the tongue reached a plateau. In the first half of the incubation period, PrPSc was localized to nerve fascicles in the tongue and was associated with individual axons (Fig. 2A). These findings indicate that TME can replicate in the tongue at early stages of infection and that axons are potential sites for PrPSc formation or accumulation.
FIG. 1.
Temporal deposition of PrPSc in the tongue following i.t. inoculation of HY TME. Tongue homogenates were enriched for PrPSc by detergent extraction and proteinase K digestion as described in Materials and Methods. Shown are the results of Western blot analysis (A) and quantification (B) of PrPSc (25-mg tissue equivalent) in tongue between 2 and 10 weeks postinfection (Wk. p.i.). The amount of PrPSc in each PrP-enriched preparation was expressed relative to the PrPSc signal from an HY TME-infected brain (0.25-mg brain equivalent) when the animal was terminally ill. The PrPSc signal was measured with a Storm PhosphorImager and ImageQuant software. Western blots from individual hamsters (A) and the averages of the relative PrPSc signal intensities from three animals (B) at each week postinfection are shown. Uninfected (U) tongue controls and standard error bars are indicated.
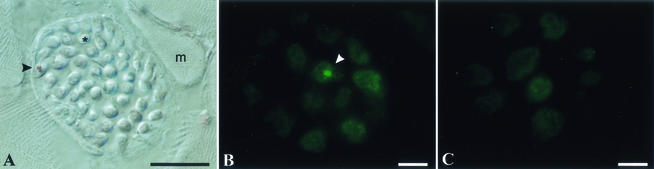
FIG. 2.
Immunodetection of PrPSc in the tongue. Tongues from HY TME-infected hamsters sacrificed at 6 (A) and 12 (B) weeks postinfection and mock-infected Syrian hamsters (C) were immunostained for PrPSc, and the results were visualized by differential interference contrast microscopy (A) and fluorescence microscopy (B and C). Hamsters were inoculated in the tongue (A) or the brain (B) with HY TME as described in the text. In panel A, the arrowhead indicates the perineurium surrounding a nerve fascicle; within the fascicle is an axon containing PrPSc. The asterisk indicates a cross section of an individual axon. A muscle cell (m) is adjacent to the nerve fascicle. In panel B, the white arrowhead indicates an individual axon that contains a PrPSc deposit. The bars in the lower right corners represent 25 μm.
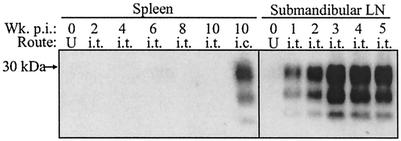
PrPSc was not detected in comparable amounts of spleen (25-mg tissue equivalents) between 1 and 10 weeks postinfection, suggesting that a systemic infection of the LRS did not occur following i.t. inoculation of HY TME (Fig. 3). However, PrPSc was detected in the submandibular lymph node (25-mg tissue equivalents) and cervical lymph node (2-mg tissue equivalents) at 1 through 10 weeks postinfection (Fig. 3 and data not shown). The PrPSc levels in the submandibular lymph node peaked by 3 to 4 weeks postinfection and, at this time, were present at higher levels than those found in the tongue. These findings indicated that HY TME established a regional infection of the LRS following i.t. inoculation.
FIG. 3.
Temporal deposition of PrPSc in secondary lymphoid tissues after i.t. inoculation of HY TME. Spleen and submandibular lymph node (LN) homogenates were enriched for PrPSc and analyzed by Western blotting at the indicated week postinfection (Wk. p.i.) as described in the legend to Fig. 1. Twenty-five-milligram tissue equivalents from animals that were inoculated by either the i.t. or the i.c. route were analyzed in each lane. Uninfected (U) spleen and lymph node controls are indicated.
PrPSc deposition in the central nervous system following i.t. inoculation of HY TME.
PrPSc immunohistochemistry was performed on the spinal cord and brain stem at weekly intervals postinfection to determine whether HY TME enters the brain stem by rostral spread in the spinal cord following i.t. inoculation of TME. PrPSc was initially found in the brain stem at 2 weeks postinfection but was not present in the first segment of the cervical spinal cord (C1) until 6 weeks postinfection (Table 3). At 6 weeks postinfection, PrPSc deposits were not detected in segments below C1. These results suggested that TME spread directly from the tongue into the brain stem and that entry was not a result of rostral transport from the spinal cord. The entry of PrPSc into C1 at 6 weeks postinfection was likely due to the caudal spread of TME from the brain stem to the spinal cord.
TABLE 3.
Temporal PrPSc distribution in the brain stem and spinal cord following HY TME inoculation into the lingual muscles
| Brain cell group | PrpSc staining at week postinfectiona:
|
|||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| First-order neurons, hypoglossal nucleus | 0 | + | + | + | ++ | +++ |
| Second-order cell groups | ||||||
| Reticular formationb | ||||||
| Lateral | 0 | 0 | 0 | 0 | + | ++ |
| Medial | 0 | 0 | 0 | + | + | ++ |
| Intermediate | 0 | 0 | 0 | 0 | + | +++ |
| Trigeminal nucleus (V) | ||||||
| Spinal nucleus of V | 0 | 0 | 0 | 0 | 0 | + |
| Principal sensory root of V | 0 | 0 | 0 | 0 | 0 | + |
| Motor root of V | 0 | 0 | 0 | 0 | 0 | + |
| Nucleus of the solitary tract | 0 | 0 | 0 | 0 | 0 | + |
| Spinal cord, first cervical segment | 0 | 0 | 0 | 0 | 0 | + |
0 indicates no PrPSc staining, + indicates staining of 0 to 5 cells, ++ indicates staining of 5 to 20 cells, and +++ indicates staining >20 cells per brain cell group per slide.
The lateral reticular formation includes the parvicellular reticular nucleus and the dorsal medullary field; the medial reticular formation includes the gigantocellular reticular nuclei, the ventral medullary field, and the raphe fields; and the intermediate reticular formation includes the intermediate reticular nucleus and the subcoeruleus nucleus.
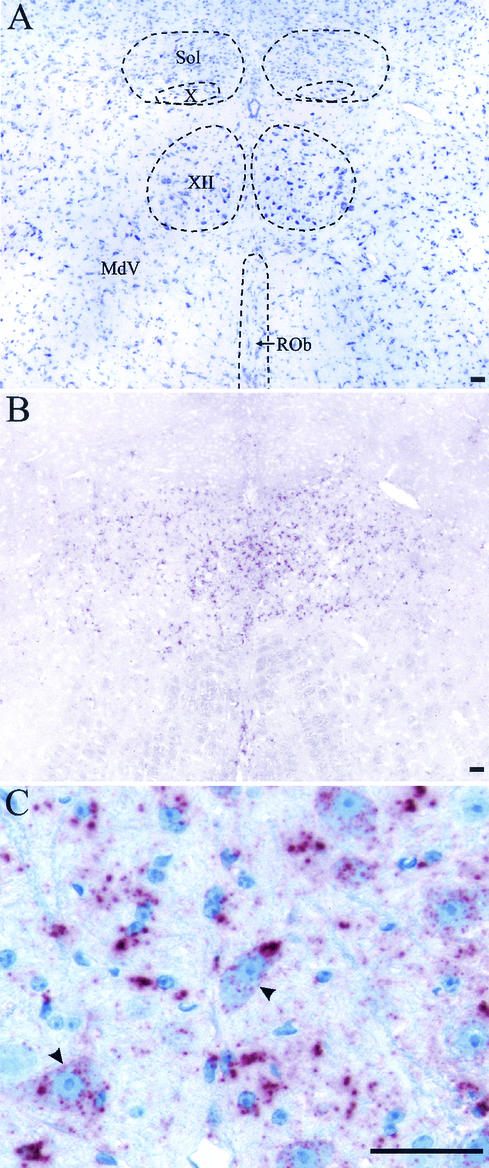
The sites of PrPSc deposition in the brain stem were investigated to determine the possible route(s) of neuroinvasion following i.t. inoculation of HY TME. PrPSc was initially found in the hypoglossal nucleus (XII nucleus) at 2 weeks postinfection, and the intensity and distribution of PrPSc immunostaining in the XII nucleus increased between 2 and 6 weeks postinfection (Table 3 and Fig. 4A and B). PrPSc deposits were found in additional areas of the brain beginning at 4 weeks postinfection. Prominent PrPSc accumulation was detected in specific areas of the reticular formation and to a lesser degree in the sensory trigeminal nucleus and the nucleus of the solitary tract (Table 3). These findings indicate that HY TME can spread to the brain stem via axonal transport within the hypoglossal nerve (cranial nerve XII [CN XII]) following i.t. inoculation. Subsequent spread of HY TME in the brain is consistent with transsynaptic TME spread and axonal transport to brain cell groups that project to the XII nucleus (i.e., second-order neurons). PrPSc deposition was also found in the dorsal motor nucleus of the vagus (X nucleus) at 6 weeks postinfection, but these deposits were not located in the somata of neurons.
FIG. 4.
PrPSc deposition in hypoglossal nucleus following i.t. inoculation of HY TME. Shown are cresyl violet staining (A) and PrPSc immunostaining (B) of adjacent brain stem sections containing hypoglossal nucleus (XII), dorsal motor nucleus of the vagus (X), nucleus of the solitary tract (Sol), ventral medullary reticular nucleus (MdV), and raphe obscurus nucleus (ROb) from an HY TME-infected hamster at 6 weeks postinfection. (C) Higher magnification of PrPSc immunostaining (red pattern) in the XII nucleus illustrating PrPSc deposition in the neuropil and in the cell bodies of motoneurons (arrowheads). The bar represents 50 μm.
Punctate cytoplasmic and perinuclear PrPSc deposits were found in motoneurons beginning at the earliest detection of TME in the XII nucleus, at 2 weeks postinfection. The number of PrPSc deposits varied within the cytoplasm, and initially these deposits had a small, uniform size, but at later times large heterogeneous PrPSc deposits were also found in the cytoplasm (Fig. 4C). The intracellular accumulation of PrPSc in the motoneurons of the XII nucleus is consistent with retrograde axonal transport from the axons located in the tongue to the cell bodies located in the brain stem.
Tongue lesion model of prion infection.
To investigate the role of an injury to the tongue in establishing prion infection, we tested the hypothesis that a wound on the surface of the tongue will enhance prion entry following oral prion exposure. A 30-gauge needle was used to make a 3-mm-long superficial cut in the dorsal epithelium of the tongue, and HY TME inoculum was topically applied to the surface of the tongue. The incubation period for the tongue lesion group was 161 ± 47 days (15 affected of 15 inoculated), and the first six hamsters in this group to develop TME had an average incubation period of 110 ± 15 days, which was statistically significantly different (P < 0.001) than the incubation periods of the i.t.-inoculated group (82 ± 2 days; 15 affected of 15 inoculated) and the oral ingestion group (184 ± 23 days; 3 affected of 15 inoculated) (Fig. 5). A fourth group of hamsters received a topical application of HY TME to the dorsal surface of the tongue in the absence of a lesion. In this group, the mean incubation period and the percentage of animals that developed clinical TME (185 ± 35 days; 5 affected of 14 inoculated) were similar to those of the oral ingestion group (Fig. 5). These findings indicate that a lesion on the surface of the tongue can increase the likelihood of prion infection following oral exposure.
Prion transport from the brain to the tongue following i.c. inoculation.
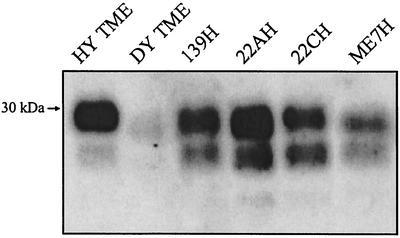
To investigate whether prion infection in the brain can spread to the tongue, hamsters were i.c. inoculated with HY TME and the tongue was examined for PrPSc deposition. After the onset of clinical symptoms of HY TME, PrPSc was found in a PrP-enriched preparation of the tongue (25-mg equivalents) upon analysis by Western blotting (Fig. 6). With the use of immunohistochemistry, PrPSc was found to be associated with axons in the nerve fascicles of the tongue in hamsters that were i.c. inoculated with HY TME (Fig. 2B and C). To determine if the spread of PrPSc to the tongue was a property of additional prion strains, we examined the tongue for PrPSc deposition in hamsters that had been i.c. inoculated with DY TME and scrapie strains 139H, 22AH, 22CH, and Me7H. These five prion strains have distinct phenotypes that are defined by incubation period, clinical symptoms, and brain neuropathology, as previously reported. For each prion strain, PrPSc was found in the tongue at the onset of clinical disease (Fig. 6), indicating that prion infection of the tongue is a common outcome following prion infection of the brain. These findings demonstrate that prion infection can spread to skeletal muscle, and specifically to the tongue, from a prion infection that originates in the brain.
FIG. 6.
PrPSc accumulation in the tongue following i.c. inoculation of TME or scrapie prions. Hamsters were i.c. inoculated with distinct TME or scrapie strains, and animals were sacrificed during the early stages of clinical disease. PrPSc was purified from the tongue and analyzed by Western blotting as described in the legend to Fig. 1. Each lane contains 25-mg tissue equivalents.
DISCUSSION
Our findings indicate that the inoculation of HY TME into the lingual muscles results in TME replication in the tongue and regional lymph nodes and direct HY TME transport within CN XII to motoneurons in the XII nucleus. HY TME infection of the tongue resulted in the shortest prion incubation period reported for Golden Syrian hamsters following inoculation by a nonneuronal route. PrPSc deposition in the XII nucleus at 2 weeks postinfection is also the shortest amount of time in which prions have been demonstrated to enter the brain following peripheral inoculation in any experimental model, including direct ocular and i.n. inoculations. There was a 6-week delay in the entry of PrPSc into the brain stem following i.n. inoculation of HY TME (4), while infectivity was not found in the brain for 7 weeks following the intraocular inoculation of hamsters with scrapie strain 263K (a strain with properties similar to those of HY TME) (30). An 8- to 10-week delay in the increase in titer in the brain was found following intraocular inoculation of murine scrapie (19, 45), but other studies report a minimum of 2 weeks for scrapie to reach the brain via the optic nerve even though no scrapie infectivity was detected at this time point (46). HY TME infectivity should be found in the XII nucleus by the prion animal bioassay in less than 2 weeks following i.t. inoculation, since bioassay is a more sensitive method for measuring prions than PrPSc immunohistochemistry (5). Our findings suggest that the inoculation of TME into peripheral tissues that are innervated by cranial nerves that project to the brain stem, such as the skeletal muscle of the tongue, can result in rapid direct prion neuroinvasion of the brain.
The present study indicates that prion infection of the tongue may be an alternate route of prion neuroinvasion following oral exposure. The transport of TME to the XII nucleus following i.t. inoculation was rapid, and the efficiency of the i.t. route of inoculation was 100,000-fold greater than that of oral ingestion of HY TME. A previous study also reported a low efficiency of infection in hamsters following oral ingestion of 263K scrapie (16). Our findings indicate that a low dose of prions, which is more likely to exemplify a natural infection, is unable to cause disease when the prions are orally ingested but may cause disease when they are inoculated into the tongue. We also found a higher incidence of TME infection (100%) following topical application of HY TME to a superficial wound on the tongue than that resulting from a similar dose delivered by oral TME ingestion (20%), suggesting that TME neuroinvasion proceeds by different pathways in these groups. In the hamster tongue lesion group, the incubation periods of three animals ranged from 88 to 104 days postinfection, which may have been due to prion infection of the tongue, but these incubation times are not consistent with neuroinvasion via the splanchnic and vagus nerves following oral ingestion (6). Reduced access to prion replication sites in the tongue or lower prion doses delivered to these sites may account for the long and highly variable incubation periods in the tongue lesion group compared to those in the i.t. inoculation group. Prion infection via a lesion on the tongue is a more representative route of natural infection than i.t. inoculation, especially in grazing and foraging animal species such as ruminants and cervids. Prior studies report that scrapie inoculation into the tooth pulp of hamsters (25) and scrapie exposure via gingival scarification (14) in mice can cause disease, but i.t. inoculation results in a significantly shorter incubation period than intradental inoculation (82 ± 2 versus 156 ± 16 days). We propose that prion infection by an alternate route can occur when a host has an infection or minor wound on the tongue and that, under these conditions, greater access to the tongue-associated cranial nerves may result in prion infection and direct prion transport to the brain stem.
The detection of PrPSc within axons in the tongue following the inoculation of HY TME into the lingual muscles and the localization of PrPSc to the XII nucleus indicates that movement of HY TME to the brain stem was via retrograde axonal transport within CN XII. Although HY TME was also detected in the submandibular and cervical lymph nodes at 1 week postinfection, the absence of PrPSc in the spinal cord at 5 weeks postinfection is inconsistent with TME neuroinvasion of the brain stem via the sympathetic nervous system. HY TME entry into CN XII may occur at the neuromuscular junction since PrPC is localized to subsynaptic areas of the postsynaptic and presynaptic cells (3, 22). It is possible that PrPSc can bind to PrPC at the neuromuscular junction and allow PrPSc entry into the nerve terminal. In the present study, PrPSc was localized to individual axons in nerve fascicles but we were unable to determine the spatial location of PrPSc in the neuromuscular junction. The detection of PrPSc in the tongue at 2 weeks postinfection and the subsequent increase in PrPSc levels indicate that HY TME can replicate in the tongue. Previous studies described TME replication in the skeletal muscles of mink (35) and scrapie replication in the muscles of transgenic mice that express elevated levels of PrPC in myocytes (10). The lingual tonsils located at the root of the tongue may also serve as a site for HY TME replication.
Our findings on the location of PrPSc in the brain stem following i.t. inoculation are consistent with those of previous studies that used viral transneuronal tracers to identify brain cell groups involved in higher-order afferent control of the lingual muscles (18, 32, 49, 51). The distribution of PrPSc in the brain stem outside of the XII nucleus at 4 through 6 weeks postinfection is consistent with the transsynaptic spread and retrograde axonal transport of HY TME to second-order brain stem neurons. Each of the brain cell groups with PrPSc deposits has been reported to project its axons to the XII nucleus (18, 32, 49-51). PrPSc deposition was also found in the X nucleus in hamsters at earlier times postinfection than has been previously reported following experimental prion ingestion (38), but immunostaining was not localized to neuronal cell bodies in our study. The dorsal motor nucleus of the vagus is the primary site of prion entry into the brain stem following oral prion ingestion and in natural cases of scrapie and CWD (2, 7, 38, 47, 52). In the present study, PrPSc localization to the X nucleus may have been due to the accumulation of PrPSc in the dendrites of motoneurons of the XII nucleus that extend into the X nucleus, since a previous study reported extranuclear dendritic projections from the XII nucleus (1). Several brain cell groups project to both the X and XII nuclei, including the areas of the reticular formation and the nucleus of the solitary tract, both of which contain PrPSc deposits during the early stages of brain neuroinvasion following oral scrapie ingestion (7) and i.t. inoculation of HY TME. These patterns of PrPSc overlap indicate that examining the brain stem pathology and the distribution of PrPSc in the brains of ruminants and cervids naturally infected with prion diseases is an unreliable approach for determining the route of neuroinvasion. One study reports that intraneuronal PrPSc deposition in the XII nucleus was greater than that in the X nucleus in sheep after experimental oral BSE agent (hereinafter referred to as BSE) ingestion even though stronger PrPSc deposition is expected in the X nucleus if neuroinvasion is via the vagus nerve (27).
A second potential route of spread for HY TME from the tongue to the brain stem is by the sensory pathways, which include the general somatic afferents (CN V and IX) and specialized afferents (CN VII and IX). In this scenario, retrograde axonal transport within these cranial nerves followed by transsynaptic spread to brain stem nuclei would result in initial PrPSc deposition in the spinal trigeminal nucleus and the nucleus of the solitary tract. Since PrPSc deposition in these locations occurred after PrPSc was found in the XII nucleus following i.t. inoculation, and the spinal trigeminal and solitary tract nuclei are known to project to the XII nucleus, axonal transport of HY TME via these sensory nerves does not appear to be the primary route of neuroinvasion following HY TME inoculation of the lingual muscles.
The accumulation of PrPSc in the hamster tongue following i.c. inoculation of two hamster-adapted TME strains and four hamster-adapted scrapie strains indicates that the spread of prions to the tongue may be a common event in prion diseases. The detection of PrPSc in axons of the tongue after i.c. inoculation suggests that one possible route involved in the establishment of tongue infection is axonal transport of HY TME from the brain to the tongue. In this case, TME transport may be via the motor efferent or sensory afferent pathways of the tongue. Prion infection of the XII nucleus, the spinal trigeminal nucleus, or the nucleus of the solitary tract would be necessary for prions to have access to and be transported within the tongue-associated cranial nerves. In cases of both natural and experimental oral infection of ruminants with scrapie and BSE, as well as in infection of deer with the CWD agent, there is evidence for the infection of the tongue-associated brain stem nuclei (27, 48, 56, 57). Experimental oral ingestion of scrapie and BSE in sheep results in PrPSc deposition in the XII nucleus, and the relative amount of PrPSc deposition was greater in the XII nucleus than in the X nucleus in BSE-infected sheep but not in scrapie-infected sheep (27). The latter observation may indicate that CN XII has a more prominent role than the vagus nerve in neuroinvasion following oral BSE infection of sheep. In natural cases of infection with BSE, PrPSc deposition has also been reported to occur in the XII nucleus and spongiform lesions are found in the nucleus of the solitary tract (56, 57). The spongiform lesion distribution in the brain stems of animals with BSE is remarkably uniform, and a high lesion score is found for the spinal trigeminal nucleus (56, 57). Furthermore, BSE infectivity is present in the trigeminal ganglia of cattle (11), which suggests that BSE can spread within axons from the spinal trigeminal nucleus to the trigeminal ganglion or, perhaps, in the reverse direction. In either case, BSE transport to the tongue may proceed via the general somatic afferents of the trigeminal ganglion. There are no reports describing the presence or absence of PrPSc deposition in the tongues of cattle with BSE. Inoculation of mice with a tongue homogenate from cattle with BSE did not result in detectable prion infectivity, but the mouse prion bioassay cannot detect levels of BSE below 104.1 LD50 per g of tissue (17, 55). The findings of the present study, and the ability of BSE to target brain stem regions that are synaptically connected to the tongue, indicate that the Specified Risk Material Regulations (15), which do not completely exclude tongue from human consumption, need to be reevaluated in order to minimize human exposure to BSE and other prion diseases through ingestion of food products containing tongue.
Acknowledgments
This work was supported by NIH grant number RR15635 from the COBRE Program of the National Center for Research Resources and by USDA grant number 98352046409 (R.A.B.) and USDA postdoctoral fellowship number 00352049228 (J.C.B.) from the National Research Initiative Competitive Grants Program.
Special thanks go to Maria Christensen and Emily Hansen for excellent technical assistance.
REFERENCES
- 1.Altschuler, S. M., X. Bao, and R. R. Miselis. 1994. Dendritic architecture of hypoglossal motoneurons projecting to extrinsic tongue musculature in the rat. J. Comp. Neurol. 342:538-550. [DOI] [PubMed] [Google Scholar]
- 2.Andreoletti, O., P. Berthon, D. Marc, P. Sarradin, J. Grosclaude, L. van Keulen, F. Schelcher, J. M. Elsen, and F. Lantier. 2000. Early accumulation of PrP(Sc) in gut-associated lymphoid and nervous tissues of susceptible sheep from a Romanov flock with natural scrapie. J. Gen. Virol. 81:3115-3126. [DOI] [PubMed] [Google Scholar]
- 3.Askanas, V., M. Bilak, W. K. Engel, A. Leclerc, and F. Tome. 1993. Prion protein is strongly immunolocalized at the postsynaptic domain of human normal neuromuscular junctions. Neurosci. Lett. 159:111-114. [DOI] [PubMed] [Google Scholar]
- 4.Bartz, J. C., A. E. Kincaid, and R. A. Bessen. 2002. Retrograde transport of transmissible mink encephalopathy within descending motor tracts. J. Virol. 76:5759-5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beekes, M., E. Baldauf, and H. Diringer. 1996. Sequential appearance and accumulation of pathognomonic markers in the central nervous system of hamsters orally infected with scrapie. J. Gen. Virol. 77:1925-1934. [DOI] [PubMed] [Google Scholar]
- 6.Beekes, M., and P. A. McBride. 2000. Early accumulation of pathological PrP in the enteric nervous system and gut-associated lymphoid tissue of hamsters orally infected with scrapie. Neurosci. Lett. 278:181-184. [DOI] [PubMed] [Google Scholar]
- 7.Beekes, M., P. A. McBride, and E. Baldauf. 1998. Cerebral targeting indicates vagal spread of infection in hamsters fed with scrapie. J. Gen. Virol. 79:601-607. [DOI] [PubMed] [Google Scholar]
- 8.Bessen, R. A., and R. F. Marsh. 1994. Distinct PrP properties suggest the molecular basis of strain variation in transmissible mink encephalopathy. J. Virol. 68:7859-7868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bessen, R. A., and R. F. Marsh. 1992. Identification of two biologically distinct strains of transmissible mink encephalopathy in hamsters. J. Gen. Virol. 73:329-334. [DOI] [PubMed] [Google Scholar]
- 10.Bosque, P. J., C. Ryou, G. Telling, D. Peretz, G. Legname, S. J. DeArmond, and S. B. Prusiner. 2002. Prions in skeletal muscle. Proc. Natl. Acad. Sci. USA 99:3812-3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bradley, R. 1999. BSE transmission studies with particular reference to blood. Dev. Biol. Stand. 99:35-40. [PubMed] [Google Scholar]
- 12.Bruce, M. E. 1985. Agent replication dynamics in a long incubation period model of mouse scrapie. J. Gen. Virol. 66:2517-2522. [DOI] [PubMed] [Google Scholar]
- 13.Bruce, M. E., R. G. Will, J. W. Ironside, I. McConnell, D. Drummond, A. Suttie, L. McCardle, A. Chree, J. Hope, C. Birkett, S. Cousens, H. Fraser, and C. J. Bostock. 1997. Transmissions to mice indicate that ′new variant' CJD is caused by the BSE agent. Nature 389:498-501. [DOI] [PubMed] [Google Scholar]
- 14.Carp, R. I. 1982. Transmission of scrapie by oral route: effect of gingival scarification. Lancet i:170-171. [DOI] [PubMed] [Google Scholar]
- 15.Department for Environment, Food and Rural Affairs. 1997-2002, posting date. Guidance notes on the Specified Risk Material Regulations 1997. [Online.] Department for Environment, Food and Rural Affairs. http://www.defra.gov.uk/animalh/bse/public-health/srm-scheme-lit.html.
- 16.Diringer, H., J. Roehmel, and M. Beekes. 1998. Effect of repeated oral infection of hamsters with scrapie. J. Gen. Virol. 79:609-612. [DOI] [PubMed] [Google Scholar]
- 17.European Commission for Food Safety. 2002. Scientific steering committee: opinion on TSE infectivity distribution in ruminant tissues, December 2001. European Commission Health & Consumer Protection Directorate-General, Brussels, Belgium.
- 18.Fay, R. A., and R. Norgren. 1997. Identification of rat brainstem multisynaptic connections to the oral motor nuclei using pseudorabies virus. III. Lingual muscle motor systems. Brain Res. Rev. 25:291-311. [DOI] [PubMed] [Google Scholar]
- 19.Fraser, H., and A. G. Dickinson. 1985. Targeting of scrapie lesions and spread of agent via the retino-tectal projection. Brain Res. 346:32-41. [DOI] [PubMed] [Google Scholar]
- 20.Frigg, R., M. A. Klein, I. Hegyi, R. M. Zinkernagel, and A. Aguzzi. 1999. Scrapie pathogenesis in subclinically infected B-cell-deficient mice. J. Virol. 73:9584-9588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gajdusek, D. C. 1977. Unconventional viruses and the origin and disappearance of kuru. Science 197:943-960. [DOI] [PubMed] [Google Scholar]
- 22.Gohel, C., V. Grigoriev, F. Escaig-Haye, C. I. Lasmezas, J. P. Deslys, J. Langeveld, M. Akaaboune, D. Hantai, and J. G. Fournier. 1999. Ultrastructural localization of cellular prion protein (PrPc) at the neuromuscular junction. J. Neurosci. Res. 55: 261-267. [DOI] [PubMed] [Google Scholar]
- 23.Hartsough, G. R., and D. Burger. 1965. Encephalopathy of mink. I. Epizootiologic and clinical observations. J. Infect. Dis. 115: 387-392. [DOI] [PubMed] [Google Scholar]
- 24.Heggebo, R., C. M. Press, G. Gunnes, K. I. Lie, M. A. Tranulis, M. Ulvund, M. H. Groschup, and T. Landsverk. 2000. Distribution of prion protein in the ileal Peyer's patch of scrapie-free lambs and lambs naturally and experimentally exposed to the scrapie agent. J. Gen. Virol. 81:2327-2337. [DOI] [PubMed] [Google Scholar]
- 25.Ingrosso, L., F. Pisani, and M. Pocchiari. 1999. Transmission of the 263K scrapie strain by the dental route. J. Gen. Virol. 80:3043-3047. [DOI] [PubMed] [Google Scholar]
- 26.Ironside, J. W. 1998. Neuropathological findings in new variant CJD and experimental transmission of BSE. FEMS Immunol. Med. Microbiol. 21:91-95. [DOI] [PubMed] [Google Scholar]
- 27.Jeffrey, M., S. Martin, L. Gonzalez, S. J. Ryder, S. J. Bellworthy, and R. Jackman. 2001. Differential diagnosis of infections with the bovine spongiform encephalopathy (BSE) and scrapie agents in sheep. J. Comp. Pathol. 125:271-284. [DOI] [PubMed] [Google Scholar]
- 28.Kascsak, R. J., R. Rubenstein, P. A. Merz, M. Tonna-DeMasi, R. Fersko, R. I. Carp, H. M. Wisniewski, and H. Diringer. 1987. Mouse polyclonal and monoclonal antibody to scrapie-associated fibril proteins. J. Virol. 61:3688-3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kimberlin, R. H., and C. Walker. 1977. Characteristics of a short incubation model of scrapie in the golden hamster. J. Gen. Virol. 34:295-304. [DOI] [PubMed] [Google Scholar]
- 30.Kimberlin, R. H., and C. A. Walker. 1986. Pathogenesis of scrapie (strain 263K) in hamsters infected intracerebrally, intraperitoneally or intraocularly. J. Gen. Virol. 67:255-263. [DOI] [PubMed] [Google Scholar]
- 31.Kitamoto, T., T. Muramoto, S. Mohri, K. Doh-Ura, and J. Tateishi. 1991. Abnormal isoform of prion protein accumulates in follicular dendritic cells in mice with Creutzfeldt-Jakob disease. J. Virol. 65:6292-6295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuypers, H. G., and G. Ugolini. 1990. Viruses as transneuronal tracers. Trends Neurosci. 13:71-75. [DOI] [PubMed] [Google Scholar]
- 33.Maignien, T., C. I. Lasmezas, V. Beringue, D. Dormont, and J. P. Deslys. 1999. Pathogenesis of the oral route of infection of mice with scrapie and bovine spongiform encephalopathy agents. J. Gen. Virol. 80:3035-3042. [DOI] [PubMed] [Google Scholar]
- 34.Manuelidis, L., I. Zaitsev, P. Koni, Z. Y. Lu, R. A. Flavell, and W. Fritch. 2000. Follicular dendritic cells and dissemination of Creutzfeldt-Jakob disease. J. Virol. 74:8614-8622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marsh, R. F., D. Burger, and R. P. Hanson. 1969. Transmissible mink encephalopathy: behavior of the disease agent in mink. Am. J. Vet. Res. 30:1637-1642. [PubMed] [Google Scholar]
- 36.McBride, P. A., and M. Beekes. 1999. Pathological PrP is abundant in sympathetic and sensory ganglia of hamsters fed with scrapie. Neurosci. Lett. 265:135-138. [DOI] [PubMed] [Google Scholar]
- 37.McBride, P. A., P. Eikelenboom, G. Kraal, H. Fraser, and M. E. Bruce. 1992. PrP protein is associated with follicular dendritic cells of spleens and lymph nodes in uninfected and scrapie-infected mice. J. Pathol. 168:413-418. [DOI] [PubMed] [Google Scholar]
- 38.McBride, P. A., W. J. Schulz-Schaeffer, M. Donaldson, M. Bruce, H. Diringer, H. A. Kretzschmar, and M. Beekes. 2001. Early spread of scrapie from the gastrointestinal tract to the central nervous system involves autonomic fibers of the splanchnic and vagus nerves. J. Virol. 75:9320-9327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.National Research Council. 1996. Guide for the care and use of laboratory animals. National Academy Press, Washington, D.C.
- 40.Oldstone, M. B. A., R. Race, D. Thomas, H. Lewicki, D. Homann, S. Smelt, A. Holz, P. Koni, D. Lo, B. Chesebro, and R. Flavell. 2002. Lymphotoxin-α- and lymphotoxin-β-deficient mice differ in susceptibility to scrapie: evidence against dendritic cell involvement in neuroinvasion. J. Virol. 76:4357-4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parker, R. C. 1959. Methods of tissue culture, 3rd ed., p. 245-266. Pitman Medical Publishing, London, England.
- 42.Prinz, M., F. Montrasio, M. A. Klein, P. Schwarz, J. Priller, B. Odermatt, K. Pfeffer, and A. Aguzzi. 2002. Lymph nodal prion replication and neuroinvasion in mice devoid of follicular dendritic cells. Proc. Natl. Acad. Sci. USA 99:919-924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Race, R., M. Oldstone, and B. Chesebro. 2000. Entry versus blockade of brain infection following oral or intraperitoneal scrapie administration: role of prion protein expression in peripheral nerves and spleen. J. Virol. 74:828-833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schulz-Schaeffer, W. J., R. Fatzer, M. Vandevelde, and H. A. Kretzschmar. 2000. Detection of PrP(Sc) in subclinical BSE with the paraffin-embedded tissue (PET) blot. Arch. Virol. Suppl. 16:173-180. [DOI] [PubMed] [Google Scholar]
- 45.Scott, J. R., D. Davies, and H. Fraser. 1992. Scrapie in the central nervous system: neuroanatomical spread of infection and Sinc control of pathogenesis. J. Gen. Virol. 73:1637-1644. [DOI] [PubMed] [Google Scholar]
- 46.Scott, J. R., and H. Fraser. 1989. Enucleation after intraocular scrapie injection delays the spread of infection. Brain Res. 504:301-305. [DOI] [PubMed] [Google Scholar]
- 47.Sigurdson, C. J., T. R. Spraker, M. W. Miller, B. Oesch, and E. A. Hoover. 2001. PrP(CWD) in the myenteric plexus, vagosympathetic trunk and endocrine glands of deer with chronic wasting disease. J. Gen. Virol. 82:2327-2334. [DOI] [PubMed] [Google Scholar]
- 48.Spraker, T. R., R. R. Zink, B. A. Cummings, M. A. Wild, M. W. Miller, and K. I. O'Rourke. 2002. Comparison of histological lesions and immunohistochemical staining of proteinase-resistant prion protein in a naturally occurring spongiform encephalopathy of free-ranging mule deer (Odocoileus hemionus) with those of chronic wasting disease of captive mule deer. Vet. Pathol. 39:110-119. [DOI] [PubMed] [Google Scholar]
- 49.Travers, J. B., N. Montgomery, and J. Sheridan. 1995. Transneuronal labeling in hamster brainstem following lingual injections with herpes simplex virus-1. Neuroscience 68:1277-1293. [DOI] [PubMed] [Google Scholar]
- 50.Travers, J. B., and R. Norgren. 1983. Afferent projections to the oral motor nuclei in the rat. J. Comp. Neurol. 220:280-298. [DOI] [PubMed] [Google Scholar]
- 51.Ugolini, G. 1995. Specificity of rabies of virus as a transneuronal tracer of motor networks: transfer from hypoglossal motoneurons to connected second-order and higher-order central nervous system cell groups. J. Comp. Neurol. 356:457-480. [DOI] [PubMed] [Google Scholar]
- 52.van Keulen, L. J., B. E. Schreuder, M. E. Vromans, J. P. Langeveld, and M. A. Smits. 2000. Pathogenesis of natural scrapie in sheep. Arch. Virol. Suppl. 16:57-71. [DOI] [PubMed] [Google Scholar]
- 53.van Keulen, L. J., M. E. Vromans, and F. G. van Zijderveld. 2002. Early and late pathogenesis of natural scrapie infection in sheep. APMIS 110:23-32. [DOI] [PubMed] [Google Scholar]
- 54.van Keulen, L. J. M., B. E. C. Schreuder, R. H. Meloen, G. Mooij-Harkes, M. E. W. Vromans, and J. P. M. Langeveld. 1996. Immunohistochemical detection of prion protein in lymphoid tissues of sheep with natural scrapie. J. Clin. Microbiol. 34:1228-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wells, G. A., S. A. Hawkins, R. B. Green, A. R. Austin, I. Dexter, Y. I. Spencer, M. J. Chaplin, M. J. Stack, and M. Dawson. 1998. Preliminary observations on the pathogenesis of experimental bovine spongiform encephalopathy (BSE): an update. Vet. Rec. 142:103-106. [DOI] [PubMed] [Google Scholar]
- 56.Wells, G. A., and J. W. Wilesmith. 1995. The neuropathology and epidemiology of bovine spongiform encephalopathy. Brain Pathol. 5:91-103. [DOI] [PubMed] [Google Scholar]
- 57.Wells, G. A., J. W. Wilesmith, and I. S. McGill. 1991. Bovine spongiform encephalopathy: a neuropathological perspective. Brain Pathol. 1:69-78. [DOI] [PubMed] [Google Scholar]
- 58.Wilesmith, J. W., G. A. Wells, M. P. Cranwell, and J. B. Ryan. 1988. Bovine spongiform encephalopathy: epidemiological studies. Vet. Rec. 123:638-644. [PubMed] [Google Scholar]
- 59.Wilson, M. I., and P. A. McBride. 2000. Technical aspects of tracking scrapie infection in orally dosed rodents. J. Cell. Pathol. 5:17-22. [Google Scholar]