Abstract
The properties of three variants of cloned simian immunodeficiency virus strain 239 (SIV239) were compared. One strain (M5) lacked five sites for N-linked carbohydrate attachment in variable regions 1 and 2 (V1 and V2) of the gp120 envelope protein, one strain (ΔV1-V2) completely lacked V1 and V2 sequences, and another (316) had nine mutations in the envelope that impart high replicative capacity for tissue macrophages. All three strains were capable of significant levels of fusion independent of CD4, and all three were considerably more sensitive to antibody-mediated neutralization than the parent strain from which they were derived. Upon experimental infection of rhesus monkeys, these three variant strains replicated to viral loads at peak height around day 14 that were indistinguishable from or only slightly less than those observed in monkeys infected with the parental SIV239 strain. Viral loads at the set point 20 to 50 weeks after infection, however, were more than 400- to 10,000-fold lower with the variant strains. Depletion of B cells around the time of infection with M5 resulted in less effective immunological control and much higher viral loads at the set point in two of three monkeys. The differences between SIV239 infection, where there is not effective immunological control, and SIVM5 infection, where there is effective immunological control, cannot be easily explained by differences in the inherent replicative capacity of the viruses; rather, they are more readily explained by differences in the effectiveness of the antibody response. These results suggest that resistance of SIV239 to antibody-mediated neutralization is very important for evading effective immunological control, for allowing continuous viral replication, for maintenance of moderate-to-high viral loads at set point, and for disease progression.
Human immunodeficiency virus type 1 (HIV-1) replication is continuous and unrelenting throughout the prolonged course of infection (14, 35, 46). Although antibody and cytotoxic T-lymphocyte responses may serve to limit the extent of viral replication somewhat, it is clear that these immune responses are ineffective in most cases since the vast majority of infected individuals show signs of disease progression in the absence of therapeutic intervention. HIV uses a variety of immune evasion strategies to allow continuous, unrelenting replication (8, 11, 20).
Rare examples of long-term nonprogression importantly illustrate that HIV-1 infection can sometimes be controlled by effective host immune responses. A variety of factors may contribute to these rare cases, including a genetic predisposition to restrict HIV-1 replication (5, 7, 15, 24, 28, 41, 43, 48), infection by attenuated forms of HIV-1 (2, 6, 22), or unusually effective immune responses (40). Strains of simian immunodeficiency virus (SIV) that normally cause AIDS in rhesus monkeys can be effectively controlled by host immune responses when deletions of specific genetic elements are intentionally introduced (9, 13). Effective control of HIV replication by immune responses has also been observed following the early initiation of antiviral therapy after infection (39).
Despite the existence of these examples of immunological control, it is presently not known what the key, or minimal, elements are for an effective, controlling immune response. The question is an important one if we are to learn how to make a prophylactic vaccine. Much of the early work on vaccine development for HIV focused on the elicitation of antibodies, but emphasis more recently has been placed on the importance of eliciting cellular responses. The appearance of cytotoxic T lymphocytes in natural infection is coincident with the decline in viral load from the peak level (25). Depletion of CD8 cells in SIV-infected monkeys results in increases in viral load (17, 42). And the presence of virus-specific CD4 proliferative responses correlates with more effective host control of the infection (40). In this report we show that diverse changes to the SIV envelope that result in increased sensitivity to antibody-mediated neutralization result in more effective control by host immune responses.
MATERIALS AND METHODS
Viruses used in this study.
SIVmac239, SIVmac316, SIV-M5, and SIVmac239ΔV1V2 have all been previously described (19, 21, 31-33, 37, 38) and are referred to in this report in abbreviated form as SIV239, SIV316, SIVM5, and SIVΔV1V2, respectively. Briefly, SIV239 is a molecularly cloned, pathogenic, primary viral isolate and SIV316 is a macrophagetropic variant of SIV239. SIVM5 was engineered to contain mutations eliminating five N-glycan attachment sites from the SIV239 env gene, and SIVΔV1V2 was derived from SIV239 by deletion of 100 amino acids encompassing the first two variable loops of gp120. Stocks of all four viruses were generated by transfection of cultured cell lines with cloned viral DNA as previously described (9, 16, 37, 38). SIVmac251 is an uncloned, pathogenic viral stock.
Neutralization assays.
Sensitivity of viral variants to neutralization by pooled sera from SIV-infected rhesus macaques was measured using CEMx174SIV-SEAP indicator cells. The assay has been described in detail elsewhere (29). Briefly, serial dilutions of pooled sera were incubated with a fixed concentration of test virus for 1 h at room temperature and then the mixture was added to CEMx174SIV-SEAP cells. At 3 to 7 days postinfection, secreted alkaline phosphatase (SEAP) activity in the culture supernatants was measured using the Phospha-Light assay kit (Applied Biosystems) and infectivity was calculated as the percentage of induced SEAP activity relative to that of viruses treated with SIV-negative control serum.
Viral RNA measurements.
Viral RNA loads in the plasma of SIV-infected rhesus monkeys were measured by quantitative real-time RT-PCR as previously described (27, 44)
Survival curves.
Accumulated survival data for 85 rhesus monkeys experimentally infected at the New England Regional Primate Research Center were analyzed using the Kaplan-Meier method and GraphPad Prism data-analysis software (GraphPad Software, Inc., San Diego, Calif.).
B-cell depletion and inoculation of rhesus monkeys.
The depletion of B cells from rhesus monkeys was accomplished by intravenous injection of monoclonal mouse-human chimeric anti-human CD20 antibody (Rituxan; IDEC Pharmaceutical Corp., San Diego, Calif.) at 20 mg/kg of body weight once a week for 3 weeks as described elsewhere (41a). Control animals were treated in the same way but with an isotype-matched control monoclonal antibody. B-cell depletion was confirmed by flow cytometry. All animals were infected by intravenous inoculation with SIVM5 virus stocks prepared from the supernatant of CEMx174 cells transfected with cloned proviral DNA.
Whole-virus ELISA.
The whole-virion enzyme-linked immunosorbent assay (ELISA) procedure was performed as previously described (49). Briefly, SIV grown in tissue culture was concentrated by ultracentrifugation and by Sepharose chromatography (Pharmacia). Concentrated, purified virions were then disrupted in Triton X-100 detergent and used to coat flat-bottom 96-well plates. Coated wells were incubated with serially diluted serum samples, and bound antibodies were detected with an alkaline-phosphatase-conjugated secondary antibody (49).
RESULTS
Three variant strains are neutralization sensitive.
SIV239 is a molecularly cloned, neutralization-resistant, primary isolate that replicates persistently in rhesus monkeys and consistently induces moderate-to-high viral loads and disease progression (1, 21). SIV strain M5 was derived from SIV239 by mutating five sites for N-linked carbohydrate attachment in the linear sequence that contains variable regions 1 and 2 (V1 and V2) of the gp120 envelope glycoprotein (37, 38). For each site, an asparagine codon was changed to one for glutamine such that two nucleotide changes would be required in each codon to get reversion. The remaining 10,269 nucleotides in the genome of M5 are identical to those in SIV239. The V1-V2 deletion mutant of SIV239 is missing 100 amino acids encompassing the entire V1-V2 region yet, amazingly, is still replication competent (19). SIV316 is a derivative of SIV239 with nine amino acid changes in envelope (32). These nine amino acid changes impart high replicative capacity to the virus for tissue macrophages which express CD4 only at low or undetectable levels (4, 33).
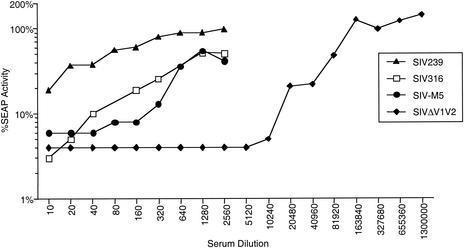
SIV316 and SIVΔV1V2 have previously been shown to be capable of infecting cells in the complete absence of CD4 through the use of one or more chemokine receptors (4, 19, 30, 36), although both strains infect cells better when CD4 is present on the surface of the target cell. Like the double carbohydrate attachment mutants g45, g46, and g56 (38), the quintuple mutant SIVM5 (missing the 5th, 6th, 8th, 12th, and 13th sites for carbohydrate attachment on the gp120 peptide backbone) can also infect cells independently of CD4 (36). These three variant strains are also significantly more sensitive to antibody-mediated neutralization than the parent SIV239 strain (19, 30, 38). This increased sensitivity to antibody-mediated neutralization has been observed by using pools of plasma from SIV-infected monkeys (Fig. 1), plasma from individual SIV-infected monkeys, and individual monoclonal antibodies of various specificities (data not shown). Plasma from the infected animals described in this report also neutralized the variant virus strains much more effectively than the parental, difficult-to-neutralize SIV239 strain (data not shown).
FIG. 1.
Comparative neutralization of SIV239 and three variants. The indicated viruses were incubated with increasing dilutions of pooled sera from SIV-infected rhesus monkeys prior to infection of CEMx174SIV-SEAP target cells. Infectivity of each variant in the presence of SIV-positive serum was calculated as the percentage of Tat-inducible SEAP activity produced in culture supernatants relative to that of the same variant treated with SIV-negative serum.
Phenotype of SIVM5 infection in monkeys.
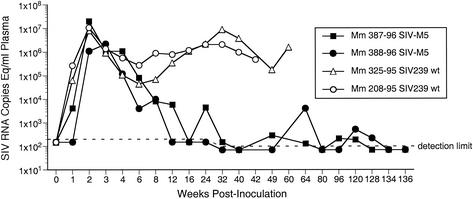
Two monkeys were initially infected with SIVM5 by intravenous inoculation, and the characteristics of the infection were compared to those seen in monkeys infected similarly with the parent SIV239 strain. Consistent with the replicative capacities of SIVM5 and SIV239 in rhesus peripheral blood mononuclear cells in culture (37), early replication of SIVM5 in monkeys was comparable to that of wild-type (WT) SIV239 (Fig. 2). In contrast, viral loads at the set point were markedly lower (Fig. 2).
FIG. 2.
Comparison of viral RNA loads in plasma of rhesus monkeys infected with the parental virus SIV239 (open symbols) or the quintuple glycosylation attachment site mutant SIVM5 (closed symbols).
Viral loads at the peak height around week 2 and at the set point between weeks 20 and 50 were averaged and compared for SIVM5 versus SIV239 (Table 1). We included historical controls from previous studies of intravenous SIV239 infection and we also included two control SIVM5 animals from the B-cell depletion studies described below. At peak height at around week 2 postinfection, the average number of RNA copies per milliliter of plasma for 10 monkeys with SIV239 was 47 × 106 (range, 8 × 106 to 88 × 106). For SIVM5, the average number of RNA copies per milliliter of plasma for four monkeys was 33 × 106 (range, 2.2 × 106 to 80 × 106). Thus, there appears to be no inherent defect in the ability of SIVM5 to replicate in monkeys in the early weeks after infection. However, in contrast to the situation with SIV239, the replication of SIVM5 was significantly controlled over time. Set points with SIV239 averaged 4.2 × 106 RNA copies per ml of plasma (range, 1.6 × 106 to 8 × 106) and with SIVM5 averaged less than 2,000 copies (Table 1).
TABLE 1.
Viral RNA load comparisonsa
| Time of load detection | Viral RNA load (no. of animals) for:
|
|||
|---|---|---|---|---|
| SIV239 | SIVM5 | SIVΔV1V2 | SIV316 | |
| Peak height (∼ wk 2) | 47 × 106 (10) | 33 × 106 (4) | 7.2 × 105 (2) | 84 × 106 (6) |
| Set point (wk 20-50) | 4.2 × 106 (5) | <2,000 (4) | <100 (2) | <10,000 (6) |
Viral loads are expressed as SIV RNA copy equivalents per milliliter of plasma. The numbers denote the averages for the indicated numbers of animals. Two of the 10 SIV239 monkeys used for the peak height averages did not yet have set point data. In addition, three of the SIV239 monkeys were excluded from set point averages because they progressed rapidly to AIDS and death within 6 months and developed soaring (>100 × 106) viral loads. The detection limit for the SIV316 experiments was only 10,000 copies per ml because of the historic nature of the study and the way the samples were stored.
The original two SIVM5-infected monkeys were challenged intravenously at 136 weeks postinfection with pathogenic, uncloned, slightly heterologous SIVmac251. These animals were strongly protected, as evidenced by the absence of any detectable plasma viremia following the challenge (data not shown). The level of protection was as robust as we have seen with any live attenuated strain we have studied to date. The two SIVM5-infected monkeys maintained undetectable viral loads and normal CD4 counts for as long as they were monitored (for 178 weeks after SIVM5 infection). The average time to death with AIDS induced by SIV239 is 56 weeks (47).
Phenotype of SIVΔV1V2 infection in monkeys.
Two rhesus monkeys were infected with SIVΔV1V2 by intravenous inoculation, and the characteristics of the infection were compared to those seen in monkeys infected similarly with the parental SIV239. Early replication of SIVΔV1V2 was slightly reduced compared to that of WT SIV239, by about 1 1/2 logs in viral RNA copies per milliliter of plasma at peak height. In contrast, viral loads at set point were markedly lower (Fig. 3 and Table 1). Using a detection limit of 100 RNA copies per ml of plasma, viral loads were not detectable in either of the SIVΔV1V2-infected animals after 4 to 78 weeks postinfection.
FIG. 3.
Viral RNA loads in plasma of two rhesus monkeys infected with SIVΔV1V2.
Phenotype of SIV316 infection in monkeys.
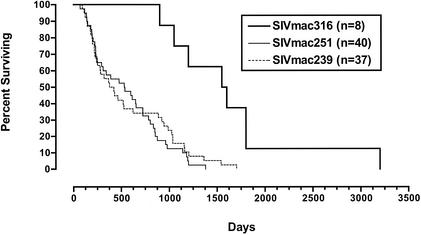
Six rhesus monkeys were infected with SIV316 by intravenous inoculation. Again, consistent with the replication of SIV316 in culture (31, 32), early replication of SIV316 by intravenous inoculation was at least as good as if not better than that of the parental SIV239 strain from which it was derived (Table 1). In contrast, viral loads at set point were markedly lower. Because of the historic nature of this study and the way the samples were stored, the detection limit was only 10,000 copies per ml. However, viral loads at the set point were at least 400-fold lower than with SIV239 (Table 1). Examination of Kaplan-Meier plots, which included animals for which viral loads are not available, revealed prolonged survival with the 316 derivative, suggesting that lower viral load set points with SIV316 translated to slower disease progression (Fig. 4) (47).
FIG. 4.
Kaplan-Meier survival curves of rhesus monkeys experimentally infected with the indicated viruses.
Effects of B-cell depletion.
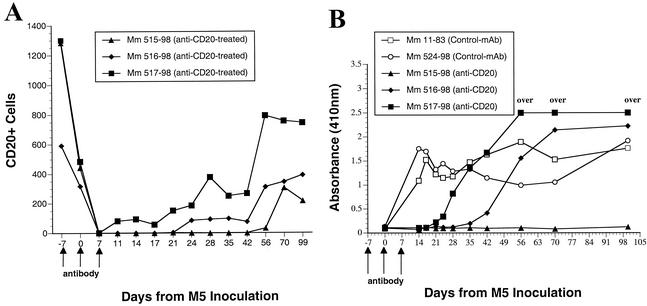
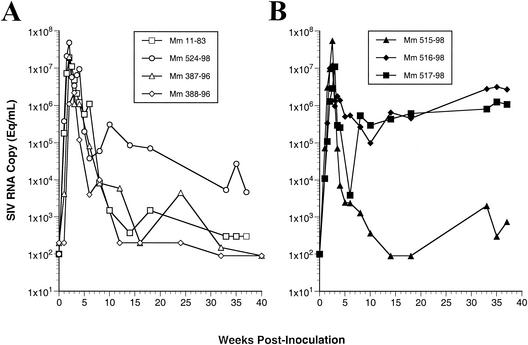
The above results suggested to us that greater effectiveness of antibody responses contributed importantly to the immunological control of the variant strains. We studied in vivo monoclonal antibody-mediated depletion of B cells to examine the extent to which B-cell responses may contribute to the immunological control of SIVM5 (41a). Anti-CD20 antibody was administered to three monkeys (Mm 515-98, Mm 516-98, and Mm 517-98) on days −7, 0, and 7; two monkeys (Mm 11-83 and Mm 524-98) received isotype-matched control antibody at the same times. All five monkeys were inoculated with SIVM5 on day 0. The extent and duration of B-cell depletion varied somewhat among the three animals that got the CD20-depleting antibody (Fig. 5A). Monkeys with CD20 cell depletion exhibited delays in the appearance of anti-SIV antibody responses that exactly paralleled the rank order of B-cell depletion (Fig. 5B). Differences in viral load were only apparent at later time points after week 10, following the decline from peak viremia (Fig. 6). Two of the three monkeys with anti-CD20 treatment and SIVM5 infection exhibited high viral loads at 30 to 40 weeks postinfection (Fig. 6B). This contrasts with four of four control monkeys that exhibited low viral loads with SIVM5 at 30 to 40 weeks postinfection (Fig. 6A). Included in the four controls for this analysis are the two naive monkeys (Mm 387-96 and Mm 388-96) that were infected with SIVM5 previously as described above. Differences in RNA loads between the two groups at these time points approached but did not quite reach statistical significance (P = 0.08 by Mann-Whitney test). Cell-associated virus loads, as measured by quantitative virus recovery from serial dilutions of peripheral blood mononuclear cells (18), also revealed differences between the two groups at weeks 30 to 40. In this case, the differences were statistically significant (P = 0.03 by Mann-Whitney test).
FIG. 5.
Effects of depletion of CD20+ cells on the antibody response to SIV. An anti-human CD20 antibody (anti-CD20) was given to three monkeys (Mm 515-98, Mm 516-98, and Mm 517-98) at the times indicated by arrows on the x axis. Two monkeys (Mm 11-83 and Mm 524-98) received a similar dose of isotype-matched control antibody (Control-mAb) at the same times. Animals were inoculated with SIVM5 on day 0. (A) CD20+ B cells in peripheral blood (measured in number of cells per microliter) relative to the time of anti-CD20 antibody administration. (B) Anti-SIV antibody responses as measured by whole-virus ELISA (see Materials and Methods); “over” indicates an absorbance reading out of the linear range of detection.
FIG. 6.
Effect of depletion of CD20+ cells on viral RNA loads in animals infected with SIVM5. Mm 387-96 and Mm 388-96 were naive, untreated rhesus monkeys infected previously with SIVM5; Mm 11-83 and Mm 524-98 were SIVM5-infected monkeys that received isotype-matched control antibody; and Mm 515-98, Mm 516-98, and Mm 517-98 were monkeys treated with anti-CD20 antibody.
DISCUSSION
These experiments began with an attempt to correlate specific genetic changes in gp120 with sensitivity to antibody-mediated neutralization. Although we did not intentionally set out to achieve similar phenotypic properties with these diverse mutations, we indeed did just that. The similarities in phenotypic properties occurred despite the very different natures of the mutations in the three variant strains. The M5 carbohydrate attachment mutant, the V1-V2 deletion mutant, and the point-mutated 316 derivative are all easy-to-neutralize viruses, they are all less dependent on CD4 for infection than the WT virus, they replicate similarly to the WT virus during the initial weeks following infection of monkeys, and they all exhibit markedly lower viral load set points than those observed with the parent strain from which they were derived. The SIVM5 and SIVΔV1V2 variants are artificial constructs, engineered by site-specific mutagenesis. The nature of the genetic changes make them difficult to revert. The changes in the SIV316 envelope recombinant occurred naturally in infected macrophages in the lung compartment of a monkey infected with SIV239 (32). The decreased dependence of SIV316 on CD4 likely reflects a natural evolutionary process, since tissue macrophages express CD4 only at low or undetectable levels (33). Sequential point mutations can restore CD4 dependence and neutralization resistance to SIV316 (30).
The association of relative CD4 independence with ease of antibody-mediated neutralization is not likely to be coincidental. A number of laboratories have noted an association of relative CD4 independence with ease of antibody-mediated neutralization for both SIV and HIV (10, 23, 30, 36). The ability to infect cells in the complete absence of CD4 probably requires a more open configuration of envelope protein in spikes on the surface of virions to allow direct binding to the chemokine receptor, which precedes the conformational changes leading to fusion. A more open configuration would logically result in increased potential exposure to antibodies. Other explanations are also possible. For example, it is also possible that these changes slow the entry process, thereby allowing more time for certain classes of antibodies to bind and neutralize.
What factors are likely to be principally responsible for the low viral load set points with the variant strains? The results cannot be easily explained by inherent defects in the ability of the variant strains to replicate. SIVM5 and SIV316 replicated as well as the parental SIV239 strain in the initial weeks following monkey infection, and they achieved viral loads at the peak height around week 2 that were as high as those seen with SIV239. Viral loads with SIVΔV1V2 were reduced about 1 1/2 logs at the peak height compared to those with SIV239 but were more than 4 logs lower at subsequent time points. In previous studies of vpr and vpx mutations and NF-κB and Sp1 binding site mutations, subtle effects on early viral replication never translated to major differential effects later (13, 16). One could postulate the presence of a different type of prominent target cell during the persistent infection stage that follows primary infection and that the variant strains may all be deficient for replication in this later-stage target cell. However, the B-cell depletion results are inconsistent with this theoretical argument. The differential effects of the mutations on viral loads at the peak height versus the set point and the results of the B-cell depletion studies are both consistent with an important role for B-cell responses in the immunological control of the variant strains. While both T cells and B cells likely contribute to the immunological control of the variant strains, the difference between SIV239 infection (where there is no effective immunological control) and SIVM5 infection (where there is effective immunological control) appears to be due in large part to the effectiveness of the antibody response.
Other factors may also have contributed to the observed results. It is possible that greater effectiveness of the antibody response in limiting continued viral replication improved cellular responses by helping to preserve CD4 helper cell activity. The monkeys used for these studies were outbred and differed in their major histocompatibility complex types; thus, individual monkeys may have differed in the inherent effectiveness of their cell-mediated immune responses. It is worth noting in this regard that the monkey with the most profound B-cell depletion (Mm 515-98) controlled SIVM5 replication reasonably well. This was the only monkey in the group that was Mamu-A*01 positive. The presence of the Mamu-A*01 class I allele has been correlated with lower viral load set points and an increased ability to control SIVmac replication (34). Finally, transient B-cell depletion could potentially have indirect effects on T-helper-cell memory (3, 45).
B-cell depletion around the time of SIVM5 inoculation had no obvious effect on the initial viral load decline for 1 to 2 weeks after the peak height; effects were only manifest at subsequent times (Fig. 6). It is possible that there is a period of time after 2 to 3 weeks postinfection when the presence of antibodies limits the dissemination of variant viruses and thus the seeding of virus-infected cells throughout the body; the absence of antibodies during this critical period could thus have long-lasting effects on the ability to limit viral loads at later times, even after antiviral antibodies have finally appeared.
Neither antibodies nor cytotoxic T lymphocytes are effective in controlling viral replication during the natural course of HIV-1 infection in humans and SIV239 infection in rhesus monkeys. Our results suggest that the inherent resistance of SIV239 to antibody-mediated neutralization is a critical factor for its ability to maintain high viral loads and to induce reproducible disease progression in rhesus monkeys. Our envelope mutations that had little or no discernible effect on inherent replicative capacity, but which strongly sensitized virus to antibody-mediated neutralization, had strong attenuating effects on viral load set points. These observations suggest that resistance to antibody-mediated neutralization is not a minor player but is a major factor in allowing continuous, high-level SIVmac and HIV-1 replication.
These observations have relevance for attempts to develop a prophylactic AIDS vaccine. There is growing concern that vaccines based principally on the elicitation of cytotoxic T-lymphocyte responses may have limited usefulness against difficult-to-neutralize primary isolates (12, 26). If antibodies with potent neutralizing activity against primary isolates of HIV-1 can be raised and maintained, there is hope that they would be able to contribute importantly to effective control analogous to the control of SIVM5 strain described in this report. The major challenge for vaccine developers will be to define ways to elicit such antibodies with high-titer neutralizing activity against primary HIV-1 isolates. The road to achievement of this goal will be difficult at best but will almost certainly require modifications to the native, difficult-to-neutralize envelope structure.
Acknowledgments
We thank Keith Mansfield and the staff of the Division of Primate Resources at NERPRC for the acquisition of the rhesus monkeys and their sampling and care. We thank Jöern Schmitz and Keith Reimann for their participation in the B-cell depletion studies (supported by National Institutes of Health grant R24 RR16001). We also thank Hannah Sanford and Susan Czajak for technical assistance and Michael Piatek, Jr., for expert assistance with viral load measurements. We also thank Andrew Lackner and Susan Westmoreland for compiling the numbers for the Kaplan-Meier curve shown in Fig. 4.
This work was supported by PHS grants AI35365 and RR00168. This work was also funded in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract N01-C0-12400 (J.D.L.). W.E.J. was supported by an Elizabeth Glaser Pediatric AIDS Foundation 2-year Scholar Award.
REFERENCES
- 1.Alexander, L., L. Denekamp, S. Czajak, and R. C. Desrosiers. 2001. Suboptimal nucleotides in the infectious, pathogenic simian immunodeficiency virus clone SIVmac239. J. Virol. 75:4019-4022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alexander, L., E. Weiskopf, T. C. Greenough, N. C. Gaddis, M. R. Auerbach, M. H. Malim, S. J. O'Brien, B. D. Walker, J. L. Sullivan, and R. C. Desrosiers. 2000. Unusual polymorphisms in human immunodeficiency virus type 1 associated with nonprogressive infection. J. Virol. 74:4361-4376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asano, M. S., and R. Ahmed. 1996. CD8 T cell memory in B cell-deficient mice. J. Exp. Med. 183:2165-2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bannert, N., D. Schenten, S. Craig, and J. Sodroski. 2000. The level of CD4 expression limits infection of primary rhesus monkey macrophages by a T-tropic simian immunodeficiency virus and macrophagetropic human immunodeficiency viruses. J. Virol. 74:10984-10993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. 1981. Pneumocystis pneumonia—Los Angeles. Morb. Mortal. Wkly. Rep. 30:250-252. [PubMed] [Google Scholar]
- 6.Deacon, N. J., A. Tsykin, A. Solomon, K. Smith, M. Ludford-Menting, D. J. Hooker, D. A. McPhee, A. L. Greenway, A. Ellett, C. Chatfield, V. A. Lawson, S. Crowe, A. Maerz, S. Sonza, J. Learmont, J. S. Sullivan, A. Cunningham, D. Dwyer, D. Downton, and J. Mills. 1995. Genomic structure of an attenuated quasi species of HIV-1 from a blood transfusion donor and recipients. Science 270:988-991. [DOI] [PubMed] [Google Scholar]
- 7.Dean, M., M. Carrington, C. Winkler, G. A. Huttley, M. W. Smith, R. Allikmets, J. J. Goedert, S. P. Buchbinder, E. Vittinghoff, E. Gomperts, S. Donfield, D. Vlahov, R. Kaslow, A. Saah, C. Rinaldo, R. Detels, S. J. O'Brien, et al. 1996. Genetic restriction of HIV-1 infection and progression to AIDS by a deletion allele of the CKR5 structural gene. Science 273:1856-1862. [DOI] [PubMed] [Google Scholar]
- 8.Desrosiers, R. C. 1999. Strategies used by human immunodeficiency virus that allow persistent viral replication. Nat. Med. 5:723-725. [DOI] [PubMed] [Google Scholar]
- 9.Desrosiers, R. C., J. D. Lifson, J. S. Gibbs, S. C. Czajak, A. Y. M. Howe, L. O. Arthur, and R. P. Johnson. 1998. Identification of highly attenuated mutants of simian immunodeficiency virus. J. Virol. 72:1431-1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edwards, T. G., T. L. Hoffman, F. Baribaud, S. Wyss, C. C. LaBranche, J. Romano, J. Adkinson, M. Sharron, J. A. Hoxie, and R. W. Doms. 2001. Relationships between CD4 independence, neutralization sensitivity, and exposure of a CD4-induced epitope in a human immunodeficiency virus type 1 envelope protein. J. Virol. 75:5230-5239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Evans, D. T., and R. C. Desrosiers. 2001. Immune evasion strategies of the primate lentiviruses. Immunol. Rev. 183:141-158. [DOI] [PubMed] [Google Scholar]
- 12.Feinberg, M. B., and J. P. Moore. 2002. AIDS vaccine models: challenging challenge viruses. Nat. Med. 8:207-210. [DOI] [PubMed] [Google Scholar]
- 13.Gibbs, J. S., A. A. Lackner, S. M. Lang, M. A. Simon, P. K. Sehgal, M. D. Daniel, and R. C. Desrosiers. 1995. Progression to AIDS in the absence of genes for vpr or vpx. J. Virol. 69:2378-2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ho, D. D., A. U. Neumann, A. S. Perelson, W. Chen, J. M. Leonard, and M. Markowitz. 1995. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature 373:123-126. [DOI] [PubMed] [Google Scholar]
- 15.Huang, Y., W. A. Paxton, S. M. Wolinsky, A. U. Neumann, L. Zhang, T. He, S. Kang, D. Ceradini, Z. Jin, K. Yazdanbakhsh, K. Kunstman, D. Erickson, E. Dragon, N. R. Landau, J. Phair, D. D. Ho, and R. A. Koup. 1996. The role of a mutant CCR5 allele in HIV-1 transmission and disease progression. Nat. Med. 2:1240-1243. [DOI] [PubMed] [Google Scholar]
- 16.Ilyinskii, P. O., M. A. Simon, S. C. Czajak, A. A. Lackner, and R. C. Desrosiers. 1997. Induction of AIDS by simian immunodeficiency virus lacking NF-κB and SP1 binding elements. J. Virol. 71:1880-1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jin, X., D. E. Bauer, S. E. Tuttleton, S. Lewin, A. Gettie, J. Blanchard, C. E. Irwin, J. T. Safrit, J. Mittler, L. Weinberger, L. G. Kostrikis, L. Zhang, A. S. Perelson, and D. D. Ho. 1999. Dramatic rise in plasma viremia after CD8+ T cell depletion in simian immunodeficiency virus-infected macaques. J. Exp. Med. 189:991-998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson, R. P., J. D. Lifson, S. C. Czajak, K. S. Cole, K. H. Manson, R. Glickman, J. Yang, D. C. Montefiori, R. Montelaro, M. Wyand, and R. C. Desrosiers. 1999. Highly attenuated vaccine strains of simian immunodeficiency virus protect against vaginal challenge: inverse relation of degree of protection with level of attenuation. J. Virol. 73:4952-4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson, W. E., J. Morgan, J. Reitter, B. Puffer, S. Czajak, R. Doms, and R. C. Desrosiers. 2002. A replication-competent, neutralization-sensitive variant of simian immunodeficiency virus lacking 100 amino acids of envelope. J. Virol. 76:2075-2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson, W. E., and R. C. Desrosiers. 2002. Viral persistence: HIV's strategies of immune system evasion. Annu. Rev. Med. 53:499-518. [DOI] [PubMed] [Google Scholar]
- 21.Kestler, H., T. Kodama, D. Ringler, M. Marthas, N. Pedersen, A. Lackner, D. Regier, P. Sehgal, M. Daniel, N. King, and R. Desrosiers. 1990. Induction of AIDS in rhesus monkeys by molecularly cloned simian immunodeficiency virus. Science 248:1109-1112. [DOI] [PubMed] [Google Scholar]
- 22.Kirchhoff, F., T. C. Greenough, D. B. Brettler, J. L. Sullivan, and R. C. Desrosiers. 1995. Absence of intact nef sequences in a long-term survivor with nonprogressive HIV-1 infection. N. Engl. J. Med. 332:228-232. [DOI] [PubMed] [Google Scholar]
- 23.Kolchinsky, P., E. Kiprilov, and J. Sodroski. 2001. Increased neutralization sensitivity of CD4-independent human immunodeficiency virus variants. J. Virol. 75:2041-2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kostrikis, L. G., Y. Huang, J. P. Moore, S. M. Wolinsky, L. Zhang, Y. Guo, L. Deutsch, J. Phair, A. U. Neumann, and D. D. Ho. 1998. A chemokine receptor CCR2 allele delays HIV-1 disease progression and is associated with a CCR5 promoter mutation. Nat. Med. 4:350-353. [DOI] [PubMed] [Google Scholar]
- 25.Koup, R. A., J. T. Safrit, Y. Cao, C. A. Andrews, G. McLeod, W. Borkowsky, C. Farthing, and D. D. Ho. 1994. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J. Virol. 68:4650-4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lifson, J. D., and M. A. Martin. 2002. One step forwards, one step back. Nature 415:272-273. [DOI] [PubMed] [Google Scholar]
- 27.Lifson, J. D., J. L. Rossio, M. Piatak, Jr., T. Parks, L. Li, R. Kiser, V. Coalter, B. Fisher, B. M. Flynn, S. Czajak, V. M. Hirsch, K. A. Reimann, J. E. Schmitz, J. Ghrayeb, N. Bischofberger, M. A. Nowak, R. C. Desrosiers, and D. Wodarz. 2001. Role of CD8+ lymphocytes in control of simian immunodeficiency virus infection and resistance to rechallenge after transient early antiretroviral treatment. J. Virol. 75:10187-10199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu, R., W. A. Paxton, S. Choe, D. Ceradini, S. R. Martin, R. Horuk, M. E. MacDonald, H. Stuhlmann, R. Koup, and N. R. Landau. 1996. Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell 86:367-377. [DOI] [PubMed] [Google Scholar]
- 29.Means, R. E., T. Greenough, and R. C. Desrosiers. 1997. Neutralization sensitivity of cell culture-passaged simian immunodeficiency virus. J. Virol. 71:7895-7902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Means, R. E., T. Matthews, J. A. Hoxie, M. H. Malim, T. Kodama, and R. C. Desrosiers. 2001. Ability of the V3 loop of simian immunodeficiency virus to serve as a target for antibody-mediated neutralization: correlation of neutralization sensitivity, growth in macrophages, and decreased dependence on CD4. J. Virol. 75:3903-3915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mori, K., D. J. Ringler, and R. C. Desrosiers. 1993. Restricted replication of simian immunodeficiency virus strain 239 in macrophages is determined by env but is not due to restricted entry. J. Virol. 67:2807-2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mori, K., D. J. Ringler, T. Kodama, and R. C. Desrosiers. 1992. Complex determinants of macrophage tropism in env of simian immunodeficiency virus. J. Virol. 66:2067-2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mori, K., M. Rosenzweig, and R. C. Desrosiers. 2000. Mechanisms for adaptation of simian immunodeficiency virus to replication in alveolar macrophages. J. Virol. 74:10852-10859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pal, R., D. Venzon, N. L. Letvin, S. Santra, D. C. Montefiori, N. R. Miller, E. Tryniszewska, M. G. Lewis, T. C. VanCott, V. Hirsch, R. Woodward, A. Gibson, M. Grace, E. Dobratz, P. D. Markham, Z. Hel, J. Nacsa, M. Klein, J. Tartaglia, and G. Franchini. 2002. ALVAC-SIV-gag-pol-env-based vaccination and macaque major histocompatibility complex class I (A*01) delay simian immunodeficiency virus SIVmac-induced immunodeficiency. J. Virol. 76:292-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pantaleo, G., C. Graziosi, J. F. Demarest, L. Butini, M. Montroni, C. H. Fox, J. M. Orenstein, D. P. Kotler, and A. S. Fauci. 1993. HIV infection is active and progressive in lymphoid tissue during the clinically latent stage of disease. Nature 362:355-358. [DOI] [PubMed] [Google Scholar]
- 36.Puffer, B. A., S. Pöhlmann, A. L. Edinger, D. Carlin, M. D. Sanchez, J. Reitter, D. D. Watry, H. S. Fox, R. C. Desrosiers, and R. W. Doms. 2002. CD4 independence of simian immunodeficiency virus Envs is associated with macrophage tropism, neutralization sensitivity, and attenuated pathogenicity. J. Virol. 76:2595-2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reitter, J. N., and R. C. Desrosiers. 1998. Identification of replication-competent strains of simian immunodeficiency virus lacking multiple attachment sites for N-linked carbohydrates in variable regions 1 and 2 of the surface envelope protein. J. Virol. 72:5399-5407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reitter, J. N., R. E. Means, and R. C. Desrosiers. 1998. A role for carbohydrates in immune evasion in AIDS. Nat. Med. 4:679-684. [DOI] [PubMed] [Google Scholar]
- 39.Rosenberg, E. S., M. Altfeld, S. H. Poon, M. N. Phillips, B. M. Wilkes, R. L. Eldridge, G. K. Robbins, R. T. D'Aquila, P. J. Goulder, and B. D. Walker. 2000. Immune control of HIV-1 after early treatment of acute infection. Nature 407:523-526. [DOI] [PubMed] [Google Scholar]
- 40.Rosenberg, E. S., J. M. Billingsley, A. M. Caliendo, S. L. Boswell, P. E. Sax, S. A. Kalams, and B. D. Walker. 1997. Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science 278:1447-1450. [DOI] [PubMed] [Google Scholar]
- 41.Samson, M., F. Libert, B. J. Doranz, J. Rucker, C. Liesnard, C. M. Farber, S. Saragosti, C. Lapoumeroulie, J. Cognaux, C. Forceille, G. Muyldermans, C. Verhofstede, G. Burtonboy, M. Georges, T. Imai, S. Rana, Y. Yi, R. J. Smyth, R. G. Collman, R. W. Doms, G. Vassart, and M. Parmentier. 1996. Resistance to HIV-1 infection in caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene. Nature 382:722-725. [DOI] [PubMed] [Google Scholar]
- 41a.Schmitz, J. E., M. J. Kuroda, S. Santra, M. A. Simon, M. A. Lifton, W. Lin, R. Khunkhun, M. Piatak, J. D. Lifson, G. Grosschupff, R. S. Gelman, P. Racz, K. Tenner-Racz, K. A. Mansfield, N. L. Letvin, D. C. Montefiori, and K. A. Reimann. Effect of humoral immune responses in controlling viremia during primary infection of rhesus monkeys with simian immunodeficiency virus. J. Virol., in press. [DOI] [PMC free article] [PubMed]
- 42.Schmitz, J. E., M. J. Kuroda, S. Santra, V. G. Sasseville, M. A. Simon, M. A. Lifton, P. Racz, K. Tenner-Racz, M. Dalesandro, B. J. Scallon, J. Ghrayeb, M. A. Forman, D. C. Montefiori, E. P. Rieber, N. L. Letvin, and K. A. Reimann. 1999. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 283:857-860. [DOI] [PubMed] [Google Scholar]
- 43.Smith, M. W., M. Dean, M. Carrington, C. Winkler, G. A. Huttley, D. A. Lomb, J. J. Goedert, T. R. O'Brien, L. P. Jacobson, R. Kaslow, S. Buchbinder, E. Vittinghoff, D. Vlahov, K. Hoots, M. W. Hilgartner, and S. J. O'Brien. 1997. Contrasting genetic influence of CCR2 and CCR5 variants on HIV-1 infection and disease progression. Hemophilia Growth and Development Study (HGDS), Multicenter AIDS Cohort Study (MACS), Multicenter Hemophilia Cohort Study (MHCS), San Francisco City Cohort (SFCC), ALIVE Study. Science 277:959-965. [DOI] [PubMed] [Google Scholar]
- 44.Suryanarayana, K., T. A. Wiltrout, G. M. Vasquez, V. M. Hirsch, and J. D. Lifson. 1998. Plasma SIV RNA viral load by determination by real-time quantification of product generation in reverse transcriptase-polymerase chain reaction. AIDS Res. Hum. Retrovir. 14:183-189. [DOI] [PubMed] [Google Scholar]
- 45.van Essen, D., P. Dullforce, T. Brocker, and D. Gray. 2000. Cellular interactions involved in Th cell memory. J. Immun. 165:3640-3646. [DOI] [PubMed] [Google Scholar]
- 46.Wei, X., S. K. Ghosh, M. E. Taylor, V. A. Johnson, E. A. Emini, P. Deutsch, J. D. Lifson, S. Bonhoeffer, M. A. Nowak, B. H. Hahn, M. S. Sag, and G. M. Shaw. 1995. Viral dynamics in human immunodeficiency virus type 1 infection. Nature 373:117-122. [DOI] [PubMed] [Google Scholar]
- 47.Westmoreland, S. V., E. Halpern, and A. A. Lackner. 1998. Simian immunodeficiency virus encephalitis in rhesus macaques is associated with rapid disease progression. J. Neurovirol. 4:260-268. [DOI] [PubMed] [Google Scholar]
- 48.Winkler, C., W. Modi, M. W. Smith, G. W. Nelson, X. Wu, M. Carrington, M. Dean, T. Honjo, K. Tashiro, D. Yabe, S. Buchbinder, E. Vittinghoff, J. J. Goedert, T. R. O'Brien, L. P. Jacobson, R. Detels, S. Donfield, A. Willoughby, E. Gomperts, D. Vlahov, J. Phair, S. J. O'Brien, et al. 1998. Genetic restriction of AIDS pathogenesis by an SDF-1 chemokine gene variant. Science 279:389-393. [DOI] [PubMed] [Google Scholar]
- 49.Wyand, M. S., K. H. Manson, M. Garcia-Moll, D. Montefiori, and R. C. Desrosiers. 1996. Vaccine protection by a triple deletion mutant of simian immunodeficiency virus. J. Virol. 70:3724-3733. [DOI] [PMC free article] [PubMed] [Google Scholar]