Abstract
A unique DNA curvature, the CIT, has been found in the 5′-upstream region of the psbA2 gene, which exhibits basal, light-responsive and circadian rhythmic transcription, in a unicellular photosynthetic cyanobacterium, Microcystis aeruginosa K-81. In this study, we report the universality of curvatures found in 5′-upstream regions in the psbA family and the function of the curvature in gene expression. Intrinsic curvatures were identified within 1000 bp upstream from the psbA genes in another cyanobacterium, a red alga and in plants (monocot and dicot). Mutagenized curvatures were constructed and confirmed to have disrupted architecture by gel electrophoresis and atomic force microscopy. Relatively small amounts but light-responsive transcripts of psbA2 were observed in cyanobacterial transformants harboring the mutagenized curvature under light/dark and light/high-light conditions. This shows that the curvature is important for basal transcription. In vitro primer extension and DNA mobility shift assay revealed that factors which might bind to the region upstream from the bending center contribute to the effective basal transcription of psbA2.
INTRODUCTION
It has generally been accepted that the ancestors of cyanobacteria gave rise to plant chloroplasts through endosymbiotic events, thereby conferring the ability for photosynthesis to algae and plants. Members of the genus Microcystis, categorized as the same group as Synechocystis, are cyanobacteria (blue–green algae) which perform oxygenic photosynthesis involving two photosystems, PS I and PS II, as do higher plants. One such member, the unicellular colony-forming cyanobacterium Microcystis aeruginosa strain K-81 (hereafter referred to as K-81), contains a psbA2 gene encoding a D1 homolog as a core protein in PS II (1). In higher plants, a psbA gene is present in the chloroplast genome. In contrast, cyanobacteria have multi-gene families of psbA in chromosomal DNA, the transcription of which is differentially expressed in response to changes in light intensity (photon flux density). For example, the transcript of the psbAI gene, which encodes the Form I type of D1 protein in the unicellular cyanobacterium Synechococcus elongatus PCC 7942 (hereafter referred to as PCC 7942) is predominantly expressed under low light intensity, whereas transcripts of the psbAII and III genes, which encode the Form II type of D1 protein, rapidly increase in number upon a shift to high-light intensity, along with a correlative decrease in the psbAI transcript (2). The K-81 strain also possesses multiple psbA homologs (3). The psbA2 gene is one of them, which encodes the Form II type (as defined for PCC 7942) D1 homolog (1).
Although light-dependent and circadian rhythmic gene expression is unique to phototrophic organisms, studies of DNA architectures and response regulators for transcription have not been sufficient in cyanobacteria. Important 5′-upstream cis-acting elements of the K-81 psbA2 gene, involving its unique promoter and an AU-box, have been characterized for basal, light-responsive and circadian rhythmic transcription (1,4–7). The light-dependent expression may occur through basal transcription modified by cis- and trans-acting factors. Previous studies showed that the AU-box motif (+26 to +33) in the 5′-upstream untranslated leader region (5′-UTR, +1 to +52) confers mRNA instability in darkness for the light-dependent expression (7). The light-dependent expression is therefore controlled at the post-transcriptional level at least. It has been reported that a deletion mutant of the –35 promoter produces abundant psbA2 transcripts with an almost constant pattern in light and dark conditions (8). This implies that light-dependent expression is also modified at the transcriptional level (8).
On the other hand, it is accepted that the tertiary structures of DNA affect gene expression, and sequence-directed (intrinsic, static) or protein-induced DNA bends refer to changes in the DNA double helix conformation. In the former, regular runs of several adenine·thymine (A·T) base pairs occur on one face of the DNA helix, while in the latter, a profound influence on DNA–protein interaction causes the bending (9,10). These flexures are known to be associated with replication, transcription and recombination (11–13). We have found and characterized a novel curved DNA, the CIT (changeable bending center sites of an intrinsic curvature under temperature conditions), located in the 5′-upstream region of psbA2 (4). However, the biological importance for gene expression has not been made clear. In this article, we report the universality of the curved DNA structure in psbA genes of another cyanobacterium, a red alga and in plants and also show the roles of the curvature in basal transcription in a cyanobacterium. Potential factors contributing to psbA2 basal transcription with the CIT curvature are also discussed.
MATERIALS AND METHODS
Bacterial strains and media
The wild-type and recombinants of S.elongatus PCC 7942 (AG406, AG431, AD501 and AD502) were cultivated in BG-11 liquid medium (14) containing spectinomycin sulfate (40 µg/ml). The cells were grown in a 500 ml flask to the optimal cell density (0.4–0.5 at 750 nm) under constant white light (35 µE/m2/s), then exposed to dark or high-light (125 µE/m2/s) conditions following the standard lighting condition. Cells of Cyanidium caldarium were incubated in BG-11 liquid medium adjusted to pH 2.5 with H2SO4 at 20°C under continuous white-light illumination (40 µE/m2/s).
Oligonucleotides
The synthesized oligonucleotides are as follows: GKA5, 5′-TTGCCCCGGGGAATCCACACCTCATC-3′, 26mer, italics indicate a SmaI site; GKA6, 5′-GCGCCCCGGGAAGTAAA AGTTATGAC-3′, 26mer, SmaI site; GKA10, 5′-GCGGAAG ATCTATAATTTCTGCTATG-3′, 26mer, BglII site; lacZ-R1, 5′-AGTTGGGTAACGCCAGGGTTTTCCCA-3′, 26mer; PSBA-Cyan-F2, 5′-ACCCCGGGCAGTTTGGCAAAA TGCTAAACG-3′, 26mer, SmaI site; PSBA-Cyan-R, 5′-GAAGATCTACGTCTTTCTAAAGTAACAG-3′, 26mer, BglII site); AraPSBA-F2, 5′-GAGATATCGTAAAGCAA CCTGGACGGATTC-3′, 30mer, EcoRV site; AraPSBA-R, 5′-GAAGATCTCTTTCGCTTTCGCGTCTCTC-3′, 28mer, BglII site; K81-CITmut, 5′-CGGTTCTAGGTCAGCGAC ACTGAGGCCTGACTCCTTCTTCGCTTCCAATAGTCGTGCGGTACGCTCGGGTATCATTGCTGCACAGTGGAGACGCTGTCTGCTTCTAGTTTACCTG-3′, 115mer; 35BIOT-R, reverse primer; 5′-biotin-GCGAAGATCTTAGTTATGT AAAGGGG-3′, 26mer; M4, 5′-GTTTTCCCAGTCACGAC-3′, 17mer; RV, 5′-CAGGAAACAGCTATGAC-3′, 17mer.
Site-directed mutagenesis
An EcoRI–HindIII fragment was isolated from pHNL7-up (4), carrying the psbA2 5′-upstream region (–404/+113; Fig. 1) and inserted into the EcoRI and HindIII sites of the vector pKF18k (TaKaRa, Tokyo, Japan) to create pKF500. Site-directed mutagenesis was performed with a Mutan-Super Express Km kit (TaKaRa) and the oligonucleotide K81-CITmut for pKF501 and pKF502 (15).
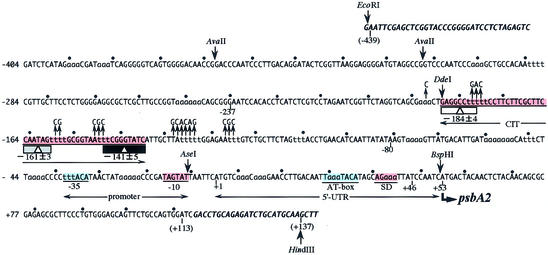
Figure 1.
The 5′-upstream nucleotide sequence of M.aeruginosa K-81 psbA2. The transcription start point is indicated as +1. One pitch (10.5 bp), a turn of the DNA helix, is represented as dots at positions –404 to +113. The center (–184 at 50°C, –161 at 30°C, –141 at 4°C) of the DNA curvature, CIT (changeable bending center sites of an intrinsic curvature under temperature conditions, from –188 to –136), is shown as a rectangle with a triangle (4). Mutagenized sequences around the CIT in pAD501 or pAD502 are represented by upward arrows. The nucleotide sequence of the vector, pUC118B (4), is shown in bold and italic. Color boxes represent positive (red) or negative (blue) cis elements for psbA2 expression. Positions of restriction enzymes are also shown.
Plasmids and recombinant cyanobacteria
For deletion constructs of the CIT curvature, fragments of the K-81 psbA2 5′-upstream region were amplified by PCR with pHNL7-up and primers as follows: for pAG431 (–237/+46), primers GKA5 and GKA10; for pAG406 (–80/+46), primers GKA6 and GKA10. The PCR fragments were digested with SmaI and BglII and introduced into the SmaI and BglII sites of pAM990 (16). For CIT disruption constructs, DNA fragments were amplified by PCR with pKF501 or pKF502 (–237/+46) and primers GKA5 and GKA10. The PCR fragments were digested with SmaI and BglII and introduced into the SmaI and BglII sites of pAM990 to make pAD501 and pAD502, respectively. These plasmids (pAG431, pAG406, pAD501 and pAD502) were transformed into the genome of S.elongutas PCC 7942 by a double cross-over reaction to obtain AG406, AG431, AD501 and AD502 (17). The recombinant PCC 7942 strains were selected on BG-11 plates containing spectinomycin sulfate (40 µg/ml). Genomic DNA was isolated from the transformants, then recombination was confirmed by Southern analysis as previously described (6).
RNA isolation and in vivo primer extension
Cells were sequentially harvested from the BG-11 culture (200 ml) of recombinant PCC 7942, and total RNA was isolated by a hot phenol method (1). Primer extension was performed as follows (18). Total RNA (10 µg) was annealed with the lacZ-R1 primer 5′-end-labeled with [γ-32P]ATP (5000 Ci/mmol). After the extension reaction, samples denatured at 95°C for 3 min with Stop solution (95% formamide, 20 mM EDTA·2Na, 0.05% bromophenol blue, 0.05% xylene cyanol FF) were quickly chilled on ice for 2 min and then 3 µl was loaded on a 6% polyacrylamide gel containing 8 M urea. Electrophoresis was performed with 0.5× TBE buffer at a constant 2500 V for 70 min followed by X-ray exposure at –80°C for 1–5 days. The signal intensity of the band corresponding to the psbA2 transcripts on the original gels was measured by ultraviolet BIOPROFIL (Vilber Lourmat, Marne La Vallée, France) and the relative abundances were plotted.
mRNA synthesis and in vitro primer extension
For in vitro mRNA synthesis, template DNA (pAG431 or pAD501, 3 µg) and RNA polymerase holoenzyme (19), reconstituted with Escherichia coli core (1 pmol) and K-81 principal sigma factor (3 pmol) or *EσA1 [partially purified RNA polymerase (RNAP) fraction containing K-81 σA1, 5 µl (∼2 pmol RNAP)], Mix I (50 mM Tris–HCl, pH 7.9, 1 M NaCl, 10 mM MgCl2, 0.5 mM DTT and 0.1 mM spermidine) and Mix II (0.1 mM ATP, 0.1 mM CTP, 0.1 mM GTP and 0.05 mM UTP) were mixed as a total volume of 40 µl and then incubated at 30°C for 20 min. After incubation, synthesized mRNA was extracted and directly used for in vitro primer extension (5,8). After the extension reaction, samples were denatured at 95°C for 3 min with Stop solution, then quickly chilled on ice for 2 min before 3 µl was loaded on a 6% polyacrylamide gel containing 8 M urea. Electrophoresis was performed with 0.5× TBE buffer at a constant 2500 V for 70 min followed by X-ray exposure at room temperature for 1 day.
Atomic force microscopy (AFM)
PCR fragments (–404/–24) were amplified with plasmid DNA [pAG500 (–404/+113, wtCIT) (8) or pKF501 (–404/+111, cdCIT)] and the set of primers GKA38 (forward primer) (7) and 35BIOT-R (reverse primer). The resultant fragment was purified and subjected to AFM observation (20,21).
DNA mobility shift assay
The RNAP fraction of M.aeruginosa K-81 containing a principal sigma factor (K-81 *EσA1) was partially purified from cells showing exponential growth under continuous light (19). The binding of RNAP to DNA was carried out in 10 µl of reaction mixture [10 mM Tris–HCl, pH 7.9, 50 mM KCl, 1 mM EDTA·2Na, pH 8.0, 5% v/v glycerol, 0.0001% w/v salmon sperm DNA (0.01 µg) and 0.0001% w/v bovine serum albumin (0.01 µg)] containing 1 pmol (1 µl) of DNA fragments end-labeled with [γ-32P]ATP (Amersham) and different RNAP holoenzymes of K-81, *EσA1, Ec + σA1 or E.coli Eσ70. The DNA template was added to the reaction mixture on ice and preincubated at 37°C for 5 min. The protein was then added and incubation was continued at 37°C for 10 min. After incubation, 2 µl of sample buffer, which contains 50% (v/v) glycerol, 0.1% (w/v) xylene cyanol FF and 0.1% (w/v) bromophenol blue, was added, then this mixture was immediately loaded onto a polyacrylamide slab gel containing 4% (w/v) acrylamide, 0.1% (w/v) bisacrylamide, 40 mM Tris–HCl pH 7.9, 5 mM sodium acetate, 0.1% (w/v) ammonium persulfate and 0.2% (v/v) TEMED. Electro phoresis was performed at a constant current (40 mA) with TSE buffer containing 40 mM Tris–HCl pH 7.9, 5 mM sodium acetate and 1 mM EDTA·2Na pH 8.0. Following electrophoresis, the gels were subjected to autoradiography using Fuji RX film.
RESULTS
K-81 psbA2 upstream structure
The psbA2 5′-upstream architecture of the cyanobacterium M.aeruginosa strain K-81 is unique and has been characterized (7,8). Previous experiments indicated that the area upstream possesses positive and negative cis elements for psbA2 expression at the transcriptional or post-transcriptional level. As shown in Figure 1, a novel DNA curvature, the CIT, found as the first case in light-responsive promoters, lies around the –140 to –180 region. Its center of curvature moves with changing temperature, however, its function in cyanobacteria has been unclear.
psbA curvatures found in photosynthetic organisms
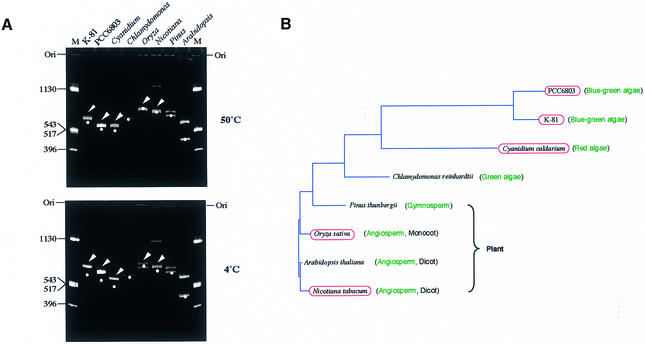
We first assessed whether some of the psbA genes of photosynthetic organisms contain intrinsic DNA curvatures by measuring the electrophoretic mobility of fragments containing an upstream sequence (Fig. 2). An electrophoretic mobility shift was observed in the case of M.aeruginosa K-81, Synechocystis sp. strain PCC 6803, C.caldarium (red alga), Oryza sativa (rice) and Nicotiana tabacum (tobacco) (Fig. 2A, arrowheads) at the low temperature of 4°C. This anomalous migration slightly remained even at a temperature of 50°C. This suggests that the static DNA curvatures were partially conserved in the 5′-upstream region of the psbA family (Fig. 2B).
Figure 2.
Universality of intrinsic curvatures in the 5′-upstream regions of the psbA family. (A) Polyacrylamide gel electrophoresis (6%) at 4 and 50°C was performed with psbA fragments prepared as follows: M.aeruginosa K-81 psbA2 (–404/+111, transcription start point as +1), a 639 bp PCR fragment amplified by M4 and RV primers (these primers were used to amplify an insert DNA cloned at the multiple cloning site in pUC derivatives) with pHNL7-up (4); Synechocystis PCC 6803 psbA2 (–451/+79, transcription start point as +1), a 629 bp PCR fragment amplified by the M4 and RV primers with pSBA2 (Amano et al., unpublished results); C.caldarium psbA (–474/+24, A of the start codon ATG as +1), a 498 bp PCR fragment amplified by PSBA-Cyan-F and PSBA-Cyan-R primers with the genomic DNA; Chlamydomonas reinhardtii psbA (an ∼1000 bp upstream region), pR14 (38) was digested with HindIII, isolated as a small segment, then the fragment digested with DdeI (635 bp HindIII–DdeI and 365 bp DdeI–HindIII fragments appeared); Pinus thunbergii psbA (a 1.3 kb BamHI–PstI fragment, –1040/+260, A of the start codon ATG as +1), a PCR fragment, amplified by the M4 and RV primers with pSBA-B (a pUC progenitor, Matsuo et al., unpublished results), was digested with AseI (707 and 656 bp fragments appeared); O.sativa psbA (a 1.6 kb BamHI–PvuII fragment, –1100/+460, A of the start codon ATG as +1), a PCR fragment amplified by the M4 and RV primers with pSBA-R (a pUC progenitor, Matsuo et al., unpublished results), was digested with AccI (770 and 900 bp fragments appeared); Arabidopsis thaliana psbA (a 1.0 kb fragment, –960/+35, A of the start codon ATG as +1), an EcoRV–BglII PCR fragment amplified by AraPSBA-F2 and AraPSBA-R primers with pMAH2 (39) was digested with AccI (550 and 450 bp fragments appeared); N.tabacum psbA (a 1.37 kb BamHI–PstI fragment, –1100/+267, A of the start codon ATG as +1), a PCR fragment amplified by the M4 and RV primers with pSBA-T (a pUC progenitor, Matsuo et al., unpublished results) was digested with DraI (741 and 731 bp fragments appeared; a small amount of the undigested PCR fragment was also observed as contamination). The positions of moleculer size markers (pUC119 DNA digested by HinfI) are indicated in lane M. Bands shown by arrowheads contain intrinsic DNA curvature. Dots indicate the position corresponding to the expected size of each psbA fragment. Positions of the gel origin (Ori) are presented. (B) A phylogenetic tree of amino acid sequences of the D1 (PsbA) proteins and conservation of the DNA curvature. The red ellipses indicate strains containing an intrinsic curvature in the region 5′-upstream of the psbA gene. The phylogenetic tree was calculated by the neighbor joining method (GENETYX-MAC software; Software Development Co., Japan).
Mutagenized CIT curvatures
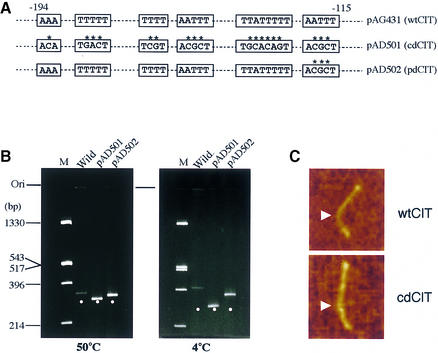
The CIT curvature harbors regular runs of several A·T tracts on one face of the DNA helix (Fig. 1). It has also been reported that the nucleotide sequence around the bending center contains several T tracts, which seem to be a pivotal determinant for the CIT (4). As a first step to addressing the function of the CIT curvature, we tried to obtain curvature-mutagenized A·T tracts (Fig. 3A). We eventually obtained two constructs, pAD501 and pAD502, derived from pAG431 (K-81 psbA2 region –237/+46). Each 289 bp fragment derived from pAG431, pAD501 or pAD502 was subjected to PAGE (Fig. 3B). Normal, partially normal and anomalously slow mobilities were observed when the fragment from pAD501, pAD502 and pAG431 was resolved, respectively (Fig. 3B). Based on previous reports (22,23), we could therefore confirm the architecture of wtCIT (wild-type CIT in pAG431), cdCIT (almost completely disrupted CIT in pAD501) and pdCIT (partially disrupted CIT in pAD502).
Figure 3.
Architecture of the CIT and its mutants. (A) The sequences of the mutations. (B) Wild-type and mutagenized fragments (289 bp) of which pAD501 (almost completely disrupted CIT, cdCIT) and pAD502 (partially disrupted CIT, pdCIT) were digested with SmaI and BglII, purified and subjected to 6% PAGE at 4 and 50°C. Positions of the 289 bp fragments are shown as dots. (C) AFM observation of the CIT fragment. PCR fragments (K-81 psbA2 5′-region, –404/–24) derived from pAG500 (–404/+113, wtCIT) or pKF501 (cdCIT) were observed. Photographs of wtCIT and cdCIT, respectively, shown in the direction from top (–404) to bottom (–24). Arrows indicate the position corresponding to the center locus (approximately –141 at 4°C) of the CIT.
We also observed the wtCIT and cdCIT structures by AFM (Fig. 3C). PCR-amplified fragments (K-81 psbA2 upstream region –404/–24) were labeled with biotin by the reverse primer (Materials and Methods). Therefore, the center of CIT was located at around position –141 when the DNA fragment was fixed to mica with aminopropyltriethoxy silane at 4°C (Fig. 1). This also means that the ratio of length from the 5′-end (–404) to the curved center site (–141) against that from the center (–141) to the 3′-end (–24) in one molecule is 2:1. We observed 512 wtCIT fragments in a window on the 2 µm scale in the AFM observation (data not shown). We randomly selected 17 curved molecules (n = 17), then calculated the angle at a position two-thirds (2:1, as an error range from 2.3:1 to 1.7:1) from the 5′-end. The average value of the angle was 63.8° at 4°C. One of the wtCIT molecules is shown in Figure 3C. This value coincides well with that estimated by gel electrophoresis (4). Twenty samples (n = 20) of cdCIT molecules were also chosen and the angle at this position was 6.1° at 4°C. A cdCIT molecule is shown in Figure 3C. We again confirmed the architecture of wtCIT and cdCIT. These curvatures were used in later experiments.
Effects of mutagenized curvatures on psbA expression in cyanobacteria
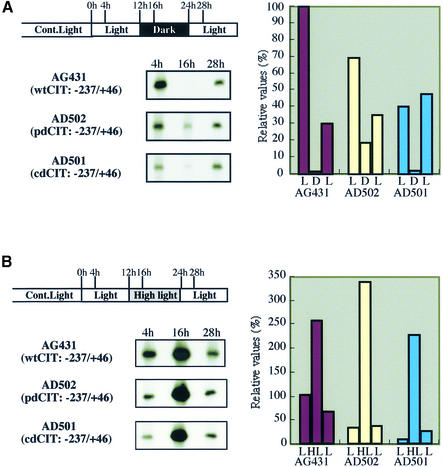
A heterologous system in a unicellular cyanobacterium, PCC 7942, has been established for in vivo analyses of K-81 gene expression (6–8,24). We investigated the influence of the altered curved structure on K-81 psbA2 expression in the cyanobacterium. After obtaining PCC 7942 recombinant cells carrying the K-81 psbA2 upstream region with the mutagenized curvature, primer extension was examined for the psbA2 transcripts. Signals referring to the transcription start point (+1) of psbA2 are shown as observed in recombinants which were cultivated under a light/dark/light (L/D/L) or light/high light/light (L/HL/L) cycle for light-responsive psbA expression (Fig. 4). Clear patterns of light-responsive transcripts of psbA2 were observed in AG431 (wtCIT, –237/+46) under both conditions. In contrast, while AD502 (pdCIT, –237/+46) and AD501 (cdCIT, –237/+46) still exhibited light-responsive transcripts, the basal level (see 4 h data) of the transcripts apparently decreased less than that of AG431. When we used a deletion mutant of the CIT curvature, pAG406 (no CIT, –80/+46), the expression pattern under the same conditions was similar to that of AD501 (data not shown). These results suggest that the CIT curvatures do not affect light-responsive expression but do affect basal transcription of psbA2.
Figure 4.
Effect of the mutagenized DNA curvature on in vivo transcription of K-81 psbA2. Recombinant S.elongatus PCC 7942 cells, which carry the K-81 psbA2 5′-upstream region from –237 to +46 containing the CIT, were cultivated under continuous white light, then entrained into a cycle of L/D/L or L/HL/L. Total RNA was isolated and primer extension was performed using the lacZ-R1 primer (7). The signal intensity on X-ray film (left) is shown as a relative value (right).
In vitro mRNA synthesis by RNAPs and factors contributing to the transcription
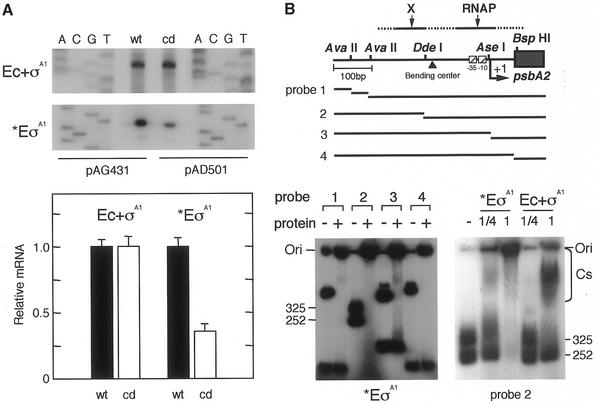
We investigated whether the mutagenized CIT curvature influences psbA2 basal transcription in vitro by RNAPs. The reconstituted RNAP (E.coli core and K-81 σA1 = Ec + σA1) or a partially purified RNAP fraction (*EσA1) containing K-81 σA1 from K-81 cells was subjected to in vitro primer extension (Fig. 5A, top). These RNAPs can specifically recognize the K-81 psbA2 promoter (5,8). Results showed that signal intensities transcribed by Ec + σA1 from psbA2 with wtCIT and cdCIT were almost the same, indicating that these mutagenized nucleotides and the mutagenized curvature have no effect on basal transcription if the RNAP fraction contains no other trans-acting factors. In contrast, transcription by K-81 *EσA1 from the wtCIT promoter was approximately three times higher than that from cdCIT (Fig. 5A, bottom). We furthermore tested RNAP binding to the upstream region (Fig. 5B, top). First, binding by heterologous E.coli core + K-81 σA1 RNAP or K-81*EσA1 (the binding site to the psbA2 upstream could not be determined previously) was compared (Fig. 5B, bottom right). When an approximately equal volume of the RNAPs was used in the assay, both 325 and 252 bp fragments shifted up with *EσA1, but not the 252 bp AvaII–DdeI fragment with Ec + σA1 (Fig. 5B, bottom right). The same result in the case of Ec + σA1 was observed when purified E.coli Eσ70 (the binding site to the psbA2 upstream was –115 to +23) (8) was used (data not shown). Cyanobacterial core enzymes as well as K-81σA1 (the amino acid residues of conserved regions 1.2, 2, 3 and 4 in σA1 were 64% identical to those of σ70) exhibit a high degree of structural similarity to those of E.coli RNAP subunits (24). This strongly suggests that the manner of DNA binding by RNAPs between E.coli Eσ70 and Ec + σA1 are similar, but not between K-81*EσA1 and Ec + σA1. In other words, the interactions of the E.coli core with σA1 and σ70 are similar to each other, and the differences in transcription using *EσA1 in Figure 5A might depend on RNAP holoenzyme conformation with K-81 core + σA1 and/or other factors. Results of the gel-shift assay imply that some trans-acting factor, X, can bind to the 252 bp fragment which contains the sequence just upstream from the center site of the CIT. Secondly, the results with *EσA1 in the upstream region again suggested that two differential binding sites for RNAP and factor X exist in the fraction of K-81 *EσA1. For example, both fragments were shifted using probe 2, whereas only the longest segment was shifted using probes 1, 3 and 4 (Fig. 5B, bottom left). This may indicate that factors outside the RNAP holoenzyme in the K-81*EσA1 fraction positively contribute to basal transcription of psbA2.
Figure 5.
In vitro psbA2 basal transcription and factors contributing to the transcription. (A) In vitro mRNA synthesis by two different RNAPs from the mutagenized CIT promoter. Using the RNAP of Ec + σA1 (1 pmol purified E.coli core enzyme + 3 pmol K-81 principal sigma factor σA1) or *EσA1 [partially purified RNAP fraction containing K-81 σA1; 15 µl (∼2 pmol RNAP)], in vitro mRNA synthesis was carried out from the DNA template pAG431 or pAD501 (3 µg). The synthesized mRNA was examined by in vitro primer extension with the primer lacZ-R1 (7). Relative values of synthesized mRNA (%) according to the signal intensity on X-ray film are shown. (B) DNA binding of RNAP components to the psbA2 upstream region. (Top) A schematic representation of template DNAs. A 576 bp EcoRI (E)–HindIII (H) fragment (Fig. 1) isolated from pHNL7-up (the –404 to +113 psbA2 region) (7) was digested with the respective restriction enzymes (see Fig. 1). These fragments were end-labeled, then the mixture was subjected to the assay. DNA fragments (1 µl, 1 pmol) were mixed with (left) K-81 *EσA1 (0.25 or 1 µl RNAP at 0.4 pmol/µl) or (right) Ec + σA1 (0.25 or 1 µl at 0.4 pmol/µl). The gel profile for no addition of protein is indicated as –. The positions of the DNA fragments, DNA–protein complexes (Cs) and gel origin (Ori) on the gel are shown.
DISCUSSION
In this study, the results showed that the CIT architecture acts to enhance psbA2 basal transcription. Not only the CIT structure but also other functional cis-acting elements in the psbA2 upstream region have been characterized and are presented in Figure 1. These elements seem to affect basal transcription in light-responsive psbA2 expression. For example, the –10 promoter, but not the –35 sequence, is essential for basal transcription. Basal transcription, which is conserved even in non-photosynthetic bacteria, might occur both in light and dark conditions. psbA2 mRNA instability was caused by the AU-box element with trans-acting factors, which is located just upstream from a Shine–Dalgarno sequence in the 5′-UTR, for light-responsive expression (7,8). The curved center of the CIT is ∼100–160 bp upstream from the –35 promoter. We found such intrinsic DNA curvatures at least within 1000 bp upstream of the psbA genes in phototrophic cyanobacteria, alga and plants (Fig. 2). However, no apparent curved DNA structures were identified in green algae or gymnosperms in this study. Rapid migration in PAGE is known for some genomic DNA fragments of many organisms (25). Also known is the phenomenon that the rapid migration property can conceal the presence of DNA curvature (26). Thus, this might have been the case for green algae and gymnosperms, or reflected in a variation in the DNA architecture during the process of evolution. Further experiments, using other organisms and/or the further upstream (>1000 bp) region of psbA, will reveal whether DNA curvatures exist and function. Of note, apparent DNA curvatures have also been identified in the 5′-upstream regions of not only M.aeruginosa K-81 psbA2/3 but also Synechocystis PCC 6803 psbA2/3 (unpublished data). This indicates that curved DNAs also exist in these Form II-type (see Introduction) psbA genes in cyanobacteria. It is still unclear whether the CIT type exists in other cyanobacteria or plants. Functional analyses of DNA curvatures located upstream from light-responsive promoters have not been performed in photosynthetic organisms.
The positions of curvatures have been known to vary in prokaryotic genes. These positions often exist in the region –40 to –240 from the transcriptional start point (+1), and these static DNA bends can modulate transcription (12). It has been reported that right-handed coils are often located just upstream from promoters (27). An RNAP-induced change in the sense of the superhelix from right-handed to left-handed might cause a local unwinding of DNA to form an open complex during transcription (27,28). A previous study presented evidence in vivo and in vitro that right-handed superhelical curvatures immediately upstream of the β-lactamase promoter clearly facilitate transcription (29,30). Because the α-subunit of the RNAP holoenzyme may recognize the DNA configuration in the region just upstream from the promoter (31), the interaction between the RNAP and DNA writhe is of interest and should be important to transcription initiation. In eukaryotes, the following hypothesis has been proposed: bent DNA that has a left-handed superhelical writhe (which can mimic the negative supercoil found in nucleosomes) or a right-handed writhe (analogous to a positive supercoil) organizes local chromatin in the infrastructure in order to help the interaction between cis-DNA elements and trans-acting factors (32,33). In cyanobacteria, we also found a sequence-directed DNA curvature with an AT-rich sequence just 5′-upstream from rpoD1 Promoter 2, the sequence of which is categorized as an E.coli type. Insertions of 2–21 bp into the curved center changed the gross geometry of the original curved DNA structure (24), like the cases heretofore reported (34,35). This curved DNA structure seems to play a significant role in effective K-81 rpoD1 transcription.
On the other hand, effective cis elements located far upstream (>300 bp) from promoters can modulate the transcription associated with trans-acting factors, by DNA looping (36). In the case of the psbA2 5′-upstream curvature, its locus is also relatively far from the promoter (Fig. 1). The CIT structure might be a plane-type curved DNA because we could not observe S- or reverse S-shaped curvatures on the mica plate in AFM (data not shown). It has been generally accepted that a 3-dimensional type curvature has often been taken as an S- or reverse S-shaped molecule on mica plates. A computer analysis (37) for the CIT also suggested the plane type (data not shown). Furthermore, when we used Ec + σA1, the transcription efficacies were almost the same between the template with wtCIT and that with cdCIT (Fig. 5). These phenomena reinforce the suggestion that the CIT curvature might be different in shape and function from those found just upstream from promoters (24,30,34,35). Gene regulation with DNA looping generally involves trans-acting factors, such as Fis or NtrC (36). In this study, the RNAP holoenzyme fraction (*EσA1), partially purified from M.aeruginosa strain K-81 cultivated under light (19), contained factors that contribute to psbA2 basal transcription (Fig. 5A). The factors might associate with the region spanning from the AvaII (–312) to the DdeI (–188) site, which locates just upstream from the curved center of CIT (Figs 1 and 5B). The identification and functional analysis of possible factors in the *EσA1 fraction will be needed to clarify the mechanism of psbA2 basal transcription.
AFM was useful for structural analyses of the wild-type and mutagenized DNA curvature (Fig. 3). The angle of the curvature coincided well with that previously estimated by gel electrophoresis (4). The CIT curvature and factors for psbA2 expression are summarized in Figure 6. Taking all things into consideration, gene regulation might consist of two steps. Step 1 is driven at the transcriptional level with cis elements (promoter, CIT and others) and trans-acting factors (RNAP and others). Basal transcription is constitutive, however, it might be modified by response factors. When transcription is over, mRNA instability occurs in darkness in Step 2. What factors are essential and/or conserved in the regulation of basal and light-responsive psbA expression in photosynthetic organisms? Functional analyses of cis- and trans-acting factors will provide clues as to the role of psbA expression.
Figure 6.
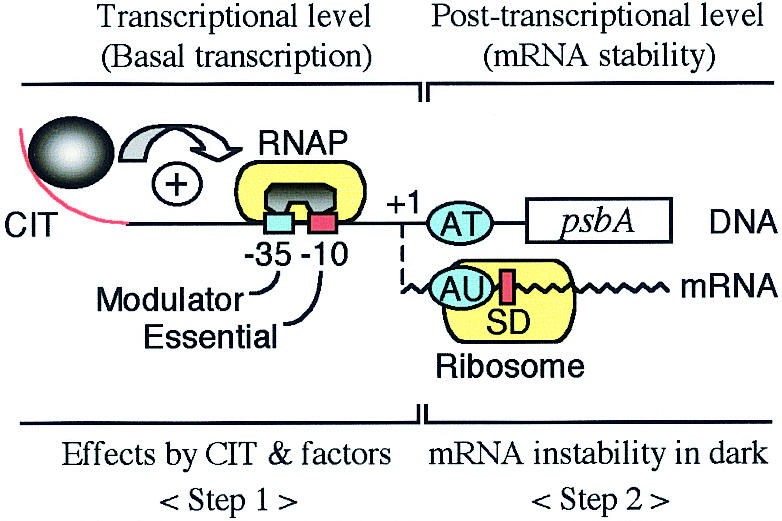
Schematic psbA2 expression with the CIT curvature and factors. The positive (red) and negative (blue) cis-acting elements corresponding to Figure 1 are presented. Details are described in the text.
Acknowledgments
ACKNOWLEDGEMENTS
We thank Dr T. Okada (Research Institute of Biomolecule Metrology) and RIBM members for AFM. We also thank Dr S. Tabata (Kazusa DNA Research Institute) and Dr Y. Takahashi (Okayama University) for pMAH2 and pR14, respectively. This work was supported by a scientific grant from Ibaraki University to M.A.
REFERENCES
- 1.Sato M., Shibato,J., Aida,T., Asayama,M. and Shirai,M. (1996) Light-responsive and rhythmic gene expression of psbA2 in cyanobacterium Microcystis aeruginosa K-81. J. Gen. Appl. Microbiol., 42, 381–391. [Google Scholar]
- 2.Schaefer M.R. and Golden,S.S. (1989) Differential expression of members of a cyanobacterial psbA gene family in response to light. J. Bacteriol., 171, 3973–3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asayama M., Iijima,O., Shirai,M., Sato,A., Aida,T. and Nakano,M. (1992) Gene structure and characterization of rpoD and psbA in cyanobacteria. In Murata,N. (ed.), Proceedings of the IXth International Congress on Photosynthesis Research in Photosynthesis. Kluwer Academic, Dordrecht, The Netherlands, Vol. III, pp. 397–400.
- 4.Agrawal G.K., Asayama,M. and Shirai,M. (1997) A novel bend of DNA CIT: changeable bending-center sites of an intrinsic curvature under temperature conditions. FEMS Microbiol. Lett., 147, 139–145. [DOI] [PubMed] [Google Scholar]
- 5.Shibato J., Asayama,M. and Shirai,M. (1998) Specific recognition of the cyanobacterial psbA promoter by RNA polymerases containing principal sigma factors. Biochim. Biophys. Acta, 1441, 296–303. [DOI] [PubMed] [Google Scholar]
- 6.Agrawal G.K., Asayama,M. and Shirai,M. (1999) Light-dependent and rhythmic psbA transcripts in homologous/heterologous cyanobacterial cells. Biochem. Biophys. Res. Commun., 255, 47–53. [DOI] [PubMed] [Google Scholar]
- 7.Agrawal G.K., Kato,H., Asayama,M. and Shirai,M. (2001) An AU-box motif upstream of the SD sequence of light-dependent psbA transcripts confers mRNA instability under darkness in cyanobacteria. Nucleic Acids Res., 29, 1835–1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shibato J., Agrawal,G.K., Kato,H., Asayama,M. and Shirai,M. (2002) The 5′-upstream cis-sequences of cyanobacterial psbA: analysis of their roles in basal, light-dependent and circadian rhythmic transcription. Mol. Genet. Genomics, 267, 684–694. [DOI] [PubMed] [Google Scholar]
- 9.Wu H.-M. and Crothers,D.M. (1984) The locus of sequence-directed and protein-induced DNA bending. Nature, 308, 509–513. [DOI] [PubMed] [Google Scholar]
- 10.Van der Vliet P.C. and Verrijzer,C.P. (1988) Bending of DNA by transcription factors. Bioessays, 15, 25–32. [DOI] [PubMed] [Google Scholar]
- 11.Zahn K. and Blattner,F.R. (1993) Sequence-induced DNA curvature at the bacteriophage λ origin of replication. Nature, 317, 451–453. [DOI] [PubMed] [Google Scholar]
- 12.Perez-Martin J., Rojo,F. and Lorenzo,V.D. (1994) Promoters responsive to DNA bending: a common theme in prokaryotic gene expression. Microbiol. Rev., 58, 268–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Milot E., Belmaaza,A., Wallenburg,J.C., Gusew,N., Bradley,W.E. and Chartrand,P. (1992) Chromosomal illegitimate recombination in mammalian cells is associated with intrinsically bent DNA elements. EMBO J., 17, 3845–3853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rippka R., Deruelles,J., Waterbury,J.B., Herdman,M. and Stanier,R.Y. (1979) Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J. Gen. Microbiol., 111, 1–61. [Google Scholar]
- 15.Kunkel T.A. (1985) Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc. Natl Acad. Sci. USA, 82, 488–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li R. and Golden,S.S. (1993) Enhancer activity of light-responsive regulatory elements in the untranslated leader regions of cyanobacterial psbA genes. Proc. Natl Acad. Sci. USA, 90, 11678–11682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Golden S.S. and Sherman,L.A. (1984) Optimal conditions for genetic transformation of the cyanobacterium Anacystis nidulans R2. J. Bacteriol., 158, 36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Asayama M., Tanaka,K., Takahashi,H., Sato,A., Aida,T. and Shirai,M. (1996) Cloning, sequencing and characterization of the gene encoding a principal sigma factor homolog from the cyanobacterium Microcystis aeruginosa K-81. Gene, 181, 213–217. [DOI] [PubMed] [Google Scholar]
- 19.Asayama M., Suzuki,H., Sato,A., Aida,T., Tanaka,K., Takahashi,H. and Shirai,M. (1996) The rpoD1 gene product is a principal sigma factor of RNA polymerase in Microcystis aeruginosa K-81. J. Biochem., 120, 752–758. [DOI] [PubMed] [Google Scholar]
- 20.Hansma P.K., Elings,V.B., Marti,O. and Bracker,C.E. (1988) Scanning tunneling microscopy and atomic force microscopy: application to biology and technology. Science, 242, 209–216. [DOI] [PubMed] [Google Scholar]
- 21.Uchihashi T. and Okada,T. (1999) Self-assembled monolayer of adenine base on graphite studied by noncontact atomic force microscopy. Phys. Rev., 60, 8309–8313. [Google Scholar]
- 22.Calladine C.R., Drew,H.R. and McCall,M.J. (1988) The intrinsic curvature of DNA in solution. J. Mol. Biol., 201, 127–137. [DOI] [PubMed] [Google Scholar]
- 23.Diekmann S. (1987) Temperature and salt dependence of the gel migration anomaly of curved DNA fragments. Nucleic Acids Res., 15, 247–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Asayama M., Hayasaka,Y., Kabasawa,M., Shirai,M. and Ohyama,T. (1999) An intrinsic DNA curvature found in the cyanobacterium Microcystis aeruginosa K-81 affects the promoter activity of rpoD1 encoding a principal sigma factor. J. Biochem., 125, 460–468. [DOI] [PubMed] [Google Scholar]
- 25.Fitzgerald D.J., Dryden,G.L., Bronson,E.C., Williams,J.S. and Anderson,J.N. (1994) Conserved patterns of bending in satellite and nucleosome positioning DNA. J. Biol. Chem., 269, 21303–21314. [PubMed] [Google Scholar]
- 26.Ohyama T., Tsujibayashi,H., Tagashira,H., Inano,K., Ueda,T., Hirata,Y. and Hashimato,K. (1998) Suppression of electrophoretic anomaly of bent DNA segments by the structural property that causes rapid migration. Nucleic Acids Res., 26, 4811–4817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Travers A.A. (1990) Why bend DNA? Cell, 60, 177–180. [DOI] [PubMed] [Google Scholar]
- 28.Amouyal M. and Buc,H. (1987) Topological unwinding of strong and weak promoters by RNA polymerase: a comparison between the lac wild-type and the UV5 sites of Escherichia coli. J. Mol. Biol., 195, 795–808. [DOI] [PubMed] [Google Scholar]
- 29.Ohyama T., Nagumo,M., Hirota,Y. and Sakuma,S. (1992) Alteration of the curved helical structure located in the upstream region of the β-lactamase promoter of plasmid pUC19 and its effect on transcription. Nucleic Acids Res., 20, 1617–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hirota Y. and Ohyama,T. (1995) Adjacent upstream superherical writhe influences an Escherichia coli promoter as measured by in vivo strength and in vitro open complex formation. J. Mol. Biol., 254, 566–578. [DOI] [PubMed] [Google Scholar]
- 31.Ross W., Gosink,K.K., Salomon,J., Igarashi,K., Zou,C., Ishihama,A., Severinov,K. and Gourse,R.L. (1993) A third recognition element in bacterial promoters: DNA binding by the a subunit of RNA polymerase. Science, 262, 1407–1413. [DOI] [PubMed] [Google Scholar]
- 32.Ohyama T. (1996) Bent DNA in the human adenovirus type 2 E1A enhancer is an architectural element for transcription stimulation. J. Biol. Chem., 271, 27823–27828. [PubMed] [Google Scholar]
- 33.Ohyama T. (2001) Intrinsic DNA bends: an organizer of local chromatin structure for transcription. Bioessays, 23, 708–715. [DOI] [PubMed] [Google Scholar]
- 34.Bracco L., Kotlarz,D., Kolb,A., Diekmann,S. and Buc,H. (1989) Synthetic curved DNA sequences can act as transcriptional activators in Escherichia coli. EMBO J., 13, 4289–4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McAllister C.F. and Achberger,E.C. (1989) Rotational orientation of upstream curved DNA affects promoter function in Bacillus subtilis. J. Biol. Chem., 264, 10451–10456. [PubMed] [Google Scholar]
- 36.Matthews K.S. (1992) DNA looping. Microbiol. Rev., 56, 123–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dlakic M. and Harrington,R.E. (1998) DIAMOD: display and modeling of DNA bending. Bioinformatics, 14, 326–331. [DOI] [PubMed] [Google Scholar]
- 38.Erickson J.M., Rahire,M. and Rochaix,J.D. (1984) Chlamydomonas reinhardii gene for the 32000 mol. wt. protein of photosystem II contains four large introns and is located entirely within the chloroplast inverted repeat. EMBO J., 3, 2753–2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu Y.-G., Mitsukawa,N., Vazquez-Tello,A. and Whittier,R.F. (1995) Generation of a high-quality P1 library of Arabidopsis suitable for chromosome walking. Plant J., 7, 351–358. [Google Scholar]