Abstract
Hepatocyte growth factor (HGF) signaling via its receptor, the proto-oncogene Met, alters cell proliferation and motility and has been associated with tumor metastasis. HGF treatment of HepG2 human hepatocellular carcinoma cells induces cell migration concomitant with increased levels of the p27kip1 cyclin-cdk inhibitor. HGF signaling resulted in nuclear export of endogenous p27 to the cytoplasm, via Ser-10 phosphorylation, where it colocalized with F-actin. Introduction of transducible p27 protein (TATp27) was sufficient for actin cytoskeletal rearrangement and migration of HepG2 cells. TATp27 mutational analysis identified a novel p27 C-terminal domain required for cell migration, distinct from the N-terminal cyclin-cyclin-dependent kinase (cdk) binding domain. Loss or disruption of the p27 C-terminal domain abolished both actin rearrangement and cell migration. The cell-scattering activity of p27 occurred independently of its cell cycle arrest functions and required cytoplasmic localization of p27 via Ser-10 phosphorylation. Furthermore, Rac GTPase was necessary for p27-dependent migration but alone was insufficient for HepG2 cell migration. These results predicted a migration defect in p27-deficient cells. Indeed, p27-deficient primary fibroblasts failed to migrate, and reconstitution with TATp27 rescued the motility defect. These observations define a novel role for p27 in cell motility that is independent of its function in cell cycle inhibition.
The presence of tumor metastases is one of the most significant factors affecting survival of cancer patients (18). Metastatic tumor growth is characterized by aberrant cell cycle regulation, loss of cell-cell contact, increased motility, and adherence to and subsequent invasion of the extracellular matrix at a secondary site, followed by uncontrolled proliferation and angiogenesis. However, molecular events that contribute to metastatic progression are presently poorly understood. Several lines of evidence suggest that hepatocyte growth factor (HGF) (also known as scatter factor) signaling contributes to metastasis via Met receptor signaling (10). HGF was independently identified as a growth factor for hepatocytes and an effector of epithelial cell motility. In normal tissues, HGF is secreted by cells of mesenchymal origin to affect epithelial cells expressing the Met receptor tyrosine kinase and is thought to be involved in development and tissue regeneration (49). However, deregulated and/or constitutive HGF and/or Met signaling can contribute to increased cell motility, invasion, and proliferation in the context of tumorigenesis and metastasis (10).
Previously it was reported that HGF signaling in human hepatocellular carcinoma cells results in increased cell migration, actin cytoskeletal rearrangement, and elevated levels of the p27 cyclin-cyclin-dependent kinase (cdk) inhibitor tumor suppressor protein (28). High levels of p27 protein have also been reported in several other human malignancies (25, 57). p27, a member of the Cip/Kip family of cell cycle inhibitors, is a nuclear protein that negatively regulates G1 cell cycle progression by sequestering and inactivating cyclin E or A-cdk2 complexes (19, 46). Although p27 is characterized as a tumor suppressor, inactivating point mutations with loss of heterozygosity are rarely observed in human cancer. In contrast, alteration of the machinery regulating p27 protein stability has been observed in numerous tumor cells (9, 47). Processes that regulate p27 protein degradation involve Thr-187 phosphorylation by cyclin E-cdk2 complexes followed by SCFSkp2/Cks1 complex-mediated ubiquitination and degradation by the 26S proteosome (12, 15, 48). Recently, p27 degradation has been reported to occur in both the cytoplasm and nucleus of cells (40) and can occur independently of Thr-187 phosphorylation (27).
Here we investigated HGF regulation of p27 protein and the role of p27 in motility of HepG2 human hepatocellular carcinoma cells. We found that HGF treatment resulted in phosphorylation of endogenous p27 on Ser-10 coupled with nuclear export of p27 to the cytoplasm, where it colocalized with F-actin. These results supported the hypothesis that HGF signaling events position p27 for a function in cell migration. Indeed, we identified a novel p27 C-terminal scatter domain required for both actin cytoskeletal rearrangement and cell motility. The activity of the p27 scatter domain was distinct from the cell cycle inhibitory function of the N-terminal cyclin-cdk binding domain. These results predicted a migration defect in p27-deficient cells. Indeed, p27-deficient primary fibroblasts failed to migrate, and reconstitution with TATp27 rescued the motility defect. Taken together, our observations define a novel role for p27 in cell motility that is independent of its function in cell cycle inhibition.
MATERIALS AND METHODS
Generation of recombinant TATp27-transducing proteins.
PCR-based strategies were used to generate truncation mutants of the human p27 cDNA (33) from the pTAT-HA-p27 expression vector (28) at amino acid residues 158 and 118. Similarly, we generated a Ser-10-Ala point mutant (p27-S10A) and a quadruple-point mutant, p27-QM (S140A, Q141A, A149E, and I151E). The coding region of each plasmid was PCR amplified, with NcoI and EcoRI restriction endonuclease recognition sites added at the N and C termini, respectively, and was cloned into the pTAT-HA expression vector (28). Rho GTPases were cloned into the pTAT-HA expression vector as described earlier (2, 6). All constructs were verified by DNA sequencing. All TAT fusion proteins contain an N-terminal, six-His purification tag, 11-residue (YGRKKRRQRRR) TAT protein transduction domain, and hemagglutinin (HA) epitope tag. Fusion proteins were purified as previously described (2, 28). Briefly, bacterial lysates containing recombinant TAT fusion proteins were sonicated in 8 M urea, passed over a Ni-nitrilotriacetic acid resin (Qiagen), eluted with imidazole, loaded in 4 M urea onto a Mono S column attached to a fast protein liquid chromatography apparatus (Amersham-Pharmacia), eluted with 1 M NaCl, and desalted into phosphate-buffered saline (PBS) on a Sephadex G-25 exchange column (Amersham-Pharmacia). TAT-GTPase protein fractions from the Ni-nitrilotriacetic acid resin were purified directly into cell culture media on the buffer exchange column. All TAT fusion proteins were sterile filtered and stored in 10% glycerol at −80°C.
Cell culture, protein transduction, and cell cycle analysis.
Human HepG2 hepatocellular carcinoma cells and synchronized primary diploid human fibroblasts were maintained in Dulbecco's minimal essential medium (DMEM) plus 5 and 10% fetal bovine serum, respectively, as described earlier (13, 28). p27-deficient and strain-matched wild-type (WT) murine embryonic fibroblasts (MEFs) were immortalized with a 3T3 protocol and were maintained in DMEM plus 10% fetal bovine serum as described earlier (16). Cells were transduced by addition of purified TAT fusion proteins directly to cell culture media. Transduction efficiency was verified by immunoblotting for the HA epitope contained in all TAT fusion proteins. HepG2 cells were treated with 100 nM TATp27 proteins for 6 h and trypsinized 15 min to degrade extracellular and external membrane-bound protein. Cell pellets were washed three times with PBS and immunoblotted with anti-HA antibodies (1:1,000; BabCo) as described earlier (13). Where indicated, band signal intensity on immunoblots was quantified using a Storm optical scanner (Molecular Dynamics). The functionality of TATp27 fusion proteins was determined by cell cycle analysis of synchronized primary diploid human fibroblasts as described earlier (13). Briefly, 48-h high-density, contact-inhibited, G1-arrested synchronized primary diploid human fibroblasts were replated at low density, treated with 200 nM TATp27 fusion proteins, and analyzed for cell cycle position by propidium iodide staining and flow cytometry.
Immunocytochemistry and leptomycin B assays.
Intracellular localization of TATp27 fusion proteins was determined by immunostaining for the HA epitope (present in the N-terminal leader on all TAT fusion proteins); endogenous proteins (p27, cyclin A, cyclin B, and cdk2) were visualized by direct immunofluorescence. HepG2 cells were plated into eight-well chamber slides (Nunc Nalgene), allowed to adhere overnight, and then treated with PBS, 20 ng of HGF (Sigma)/ml, or 100 nM TAT fusion proteins in fresh media. At given time points, cells were washed extensively with PBS, fixed in 3.7% paraformaldehyde, permeabilized with ice-cold 100% ethyl alcohol, and blocked with 1% bovine serum albumin for 10 min. Cells were incubated for 30 min at 42°C with anti-HA (1:1,000; BabCo), anti-p27 (1:1,000; Transduction Labs), anti-cyclin A (1:500, C-19; Santa Cruz) monoclonal antibodies, or rabbit polyclonal anti-cdk2 antibody (1:500, kindly provided by Helen Piwnica-Worms) (58). To control for antibody specificity, cells were incubated with nonimmune serum or PBS. Cells were then washed and incubated with either fluorescein isothiocyanate (FITC)-conjugated anti-mouse immunoglobulin G (IgG) or tetramethyl rhodamine isocyanate (TRITC)-conjugated anti-rabbit IgG (Sigma) for 20 min at 42°C and mounted in the presence of Slowfade reagent (Molecular Probes). Where indicated, actin filaments were visualized by staining with TRITC-conjugated phalloidin (100 ng/ml; Sigma) for 30 min to 1 h at room temperature. In noted cases, cell nuclei were counterstained with 0.2 μg of 4,′6′-diamidino-2-phenylindole (DAPI; Sigma)/ml.
Leptomycin B assays were performed by plating HepG2 cells into eight-well chamber slides and treated with either PBS or 20 ng of HGF/ml for 20 h. Culture media were exchanged, and 10 ng of leptomycin B/ml was added with or without HGF for 3 h at 37°C followed by immunocytochemistry as described above using monoclonal antibodies to p27 (1:500) or cyclin B (1:200; Santa Cruz). Randomly selected fields were analyzed, and individual cells were scored for nuclear, cytoplasmic, or nuclear-cytoplasmic staining.
Analysis of p27 phosphorylation status.
HepG2 cells were treated with either PBS or 10 ng of HGF/ml for 16 h, starved of phosphate for 45 min, and then labeled with 3.75 mCi of 32PO4 for 4 h in the presence of either HGF or PBS. Cells were lysed in immunoprecipitation buffer (250 mM NaCl, 50 mM HEPES, 2 mM EDTA, 0.5% NP-40, including dithiothretiol, aprotinin, phenylmethylsulfonyl fluoride, and leupeptin), precleared with zysorbin (Zymed), and immunoprecipitated overnight with anti-p27 monoclonal antibody plus protein G Sepharose.Immunoprecipitates were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to nitrocellulose. The same membrane was used to detect both 32P-labeled p27 by phosphorimager analysis and total p27 protein levels by immunoblotting using polyclonal anti-p27 (1:1,000). Phospho-specific antibodies to Thr-187 and Ser-10 of p27 were used to detect phosphorylation status of respective residues (5). Lysates from HepG2 cells that were treated with HGF or PBS control for 24 h were immunoprecipitated using monoclonal antibody to p27, followed by immunoblotting using anti-p27 (1:1,000), anti-phospho-Thr-187 (1:200), and anti-phospho-Ser-10 (1:500) polyclonal antibodies.
HepG2 cell migration assay.
HepG2 cells were plated at low density to form discrete 5- to 10-cell colonies and were allowed to adhere overnight. PBS, 5 ng of HGF/ml, or 100 nM TAT fusion proteins were added to fresh cell culture media for indicated periods. Colonies were considered positive for scattering if cell-cell contact was lost and if the distance between cells increased upon microscopic observation. Cells were fixed and stained with hematoxylin, and digital images were used to quantify cell distances. The distance between colony cell nuclei was measured at 0 h and subtracted from that at 8 or 24 h to yield the average total distance of cell migration.
PI3-K inhibition.
HepG2 cells were plated at low density and allowed to adhere overnight. To assess the role of phosphatidylinositol 3-kinase (PI3-K) in early events in p27-dependent migration, cells were pretreated for 20 min with 30 nM wortmannin and were then treated with either 5 ng of HGF/ml or 100 nM TATp27 protein. For analysis of later events cells were treated with either 5 ng of HGF/ml or 100 nM TATp27 protein, after which 30 nM wortmannin was added at 8 h. In both cases, HepG2 cell migration assays were performed 24 h after addition of HGF or TATp27.
MEF and 3T3 migration assay.
Cell migration of p27−/− MEFs was compared to that of MEFs from matched WT embryos by using a migration assay on a confluent monolayer (54). Briefly, subconfluent MEFs in six-well plates were incubated overnight with DMEM plus 0.1 to 0.5% FCS. The confluent monolayers were scraped with either a rubber policeman or the edge of a sterile microtome blade (model 818; Leica Instruments GmbH) in an area measuring approximately 1.5 × 0.3 cm. To measure cell migration, the cultures were incubated at 37°C for 24 h, washed with PBS, fixed with absolute methanol, and stained with hematoxylin. Cells that had migrated into the denuded area were observed by using an Axiovert 25 microscope (Carl Zeiss, Inc.) (×100 magnification). Random fields were observed along the wound edge in duplicate culture wells. Reconstitution of p27 was performed by addition of 100 nM TATp27 proteins at the time of denuding. Cells were fixed, stained with hematoxylin, and photographed 24 h posttreatment.
RESULTS
HGF signals nuclear export of endogenous p27 that is mediated by Ser-10 phosphorylation.
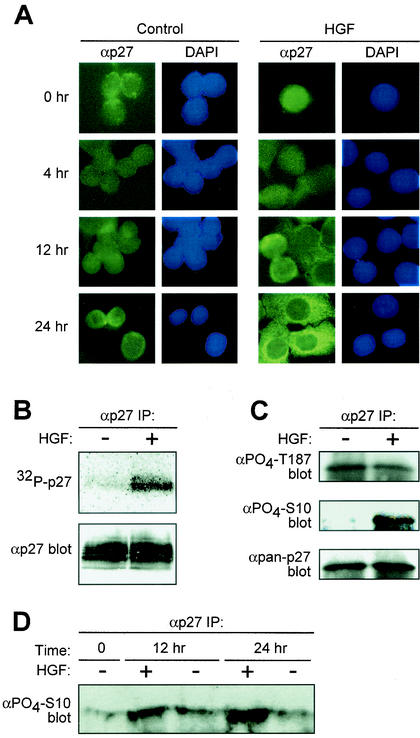
HGF treatment of HepG2 human hepatocellular carcinoma cells results in elevated p27 protein levels (28). The majority of p27 regulation occurs via posttranslational mechanisms, including phosphorylation and subcellular localization, events that are often deregulated in cancer cells (47). Therefore, we investigated the effects of Met receptor activation by HGF on the subcellular distribution of p27 protein over time by immunocytochemistry. p27 remained nuclear in control PBS-treated cells throughout the time course as determined by counterstaining with DAPI (Fig. 1A, left panels). In contrast, HGF treatment of HepG2 cells resulted in redistribution of p27 from the nucleus to the cytoplasm in a time-dependent fashion (Fig. 1A, right panels). p27 was first detected in the cytoplasm of HGF-treated cells at 12 h, and by 24 h, the majority of p27 was present in the cytoplasm (Fig. 1A, right panels). Inhibition of Crm1-dependent nuclear export by leptomycin B prevented cytoplasmic accumulation of endogenous p27 in HGF-treated cells (data not shown), suggesting that HGF signaling specifically regulates p27 nuclear export.
FIG. 1.
HGF signaling induces nuclear export of p27. (A) Analysis of p27 localization. Human HepG2 hepatocellular carcinoma cells treated with PBS control (left panels) or HGF (20 ng/ml; right panels) were analyzed for endogenous p27 localization by immunocytochemistry. At indicated time points, cells were stained with anti-p27 antibodies and FITC-conjugated anti-mouse IgG (green). Corresponding cell nuclei were visualized by DAPI (blue). Results represent a minimum of four independent observations. (B) p27 phosphorylation status. HepG2 cells treated with HGF for 20 h were metabolically labeled with [32P]orthophosphate followed by anti-p27 immunoprecipitation (IP) (top panel). Total p27 protein levels were determined by anti-p27 immunoblotting (bottom panel). (C) HGF-dependent Ser-10 phosphorylation. p27 was immunoprecipitated from lysates of control and HGF-treated HepG2 cells with pan-anti-p27 antibodies followed by immunoblot analysis with anti-phospho-Thr-187 (top panel), anti-phospho-Ser-10 (middle panel), and pan-anti-p27 (bottom panel) antibodies. (D) Time course of HGF-dependent Ser-10 phosphorylation. HepG2 cells were treated in the presence of HGF (+) or PBS control (−). At indicated time points, cells were lysed and total p27 was immunoprecipitated. Relative levels of phospho-Ser-10 were determined by Western blotting using the anti-phospho-Ser-10 antibodies.
p27 protein stability and localization are regulated by phosphorylation events (8, 47). Therefore, we examined the phosphorylation status of p27 in HGF-treated cells by [32P]orthophosphate metabolic labeling followed by p27 immunoprecipitation. HGF-treated cells, containing predominantly cytoplasmic p27, showed a significant level of endogenous p27 phosphorylation compared to the level of p27 from control cells (Fig. 1B).
We next determined the phosphorylation status of specific p27 residues in response to HGF signaling. Phosphorylation of p27 on Thr-187 initiates ubiquitin-mediated degradation of p27 (12, 53). Ser-10 was identified as a predominant site of p27 phosphorylation that both increases protein stability and mediates nuclear export of p27 to the cytoplasm in a Crm1-dependent manner (21, 40). We performed p27 immunoblot analyses using phospho-specific antibodies to Ser-10 and Thr-187 (5, 40). Total p27 was immunoprecipitated from lysates of HepG2 cells grown in 5% serum plus or minus HGF for 24 h, followed by immunoblotting. Low levels of Thr-187 phosphorylation were equally detected in HGF and control-treated cells (Fig. 1C, top panel). In contrast, Ser-10 phosphorylation was increased in HGF-treated cells compared to that in control cells (Fig. 1C, middle panel). To establish temporal coordination of p27 phosphorylation and nuclear export, Ser-10 was measured over a 24-h time course in the presence of HGF or PBS control. Ser-10 phosphorylation remained consistently low in control PBS-treated cells over 24 h (Fig. 1D). In contrast, HGF treatment increased p27 Ser-10 phosphorylation at 12 h, with maximal levels achieved by 24 h (Fig. 1D). These data correlate with the appearance of p27 in the cytoplasm of HGF- treated cells.
Cytoplasmic p27 is not associated with cyclin-cdk complexes but colocalizes with actin in HGF-treated cells.
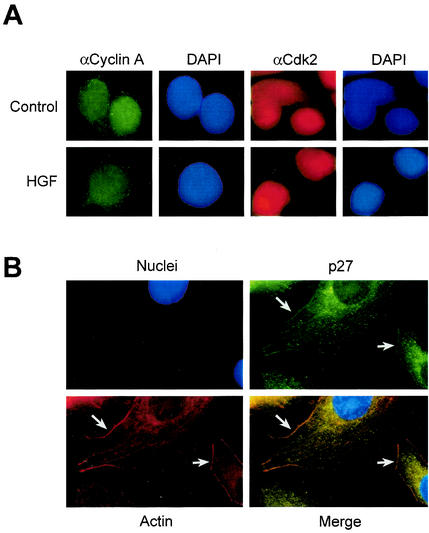
p27 binds cyclin A-cdk2 complexes to inhibit their activity (3, 37). Therefore, to determine whether nuclear export of p27 involved concomitant export of cyclin-cdk2 complexes, HGF- and control-treated HepG2 cells were analyzed by immunocytochemistry for endogenous cyclin A and cdk2 localization. Although p27 was exported to the cytoplasm in HGF-treated cells, both cyclin A and cdk2 remained localized to the nucleus after 24 h of HGF or control treatment with little or no detectable difference between either group (Fig. 2A). These observations demonstrate that HGF-dependent nuclear export of p27 to the cytoplasm does not involve accompanying export of cyclin A-cdk2 complexes.
FIG. 2.
p27 localizes with actin, and cyclin A-cdk2 complexes remain nuclear. (A) Cyclin A and cdk2 localization in HGF-treated cells. HepG2 cells treated with control PBS or HGF for 24 h were analyzed by immunocytochemistry for cyclin A (FITC; green) and cdk2 (TRITC; red) localization. Corresponding nuclei were counterstained with DAPI (blue). (B) p27 colocalizes with F-actin. HepG2 cells treated with HGF for 24 h were analyzed by immunocytochemistry for endogenous p27 (FITC; green) and F-actin (TRITC; red) localization. Corresponding nuclei were counterstained with DAPI (blue). Arrows indicate areas in merged image with colocalized (yellow) cytoplasmic p27 and F-actin.
Cellular responses to HGF treatment include increased motility associated with extensive rearrangement of the actin cytoskeleton, including filopodium and lamellipodium formation (49). Therefore, we investigated endogenous p27 localization with respect to F-actin. HepG2 cells were treated for 24 h with HGF and triply stained for endogenous p27 (green), F-actin (red), and nuclei or DNA (blue) (Fig. 2B). HGF-treated cells contained a substantial amount of endogenous p27 colocalized with F-actin at both the membrane and in areas of organized actin filaments (Fig. 2B, bottom right panel). Taken together, these observations demonstrate that HGF signaling results in the specific export of endogenous nuclear p27 to the cytoplasm, where it colocalizes with F-actin, exclusive of cyclin A-cdk2 complexes.
Identification of a novel p27 C-terminal domain necessary for cell migration.
The kinetics of p27 nuclear export and colocalization with F-actin in the cytoplasm of HGF-treated cells suggested that p27 may be playing a role in cell migration. Previously, it was established that transduction of full-length TATp27-WT protein into HepG2 cells was sufficient for both cell migration and filopodium formation (28). In contrast, transduction of TATp27-N-terminal protein (residues 1 to 103), which retained the cyclin-cdk binding domain, was insufficient to induce either phenotype, suggesting that p27 C-terminal elements are required to mediate cell migration.
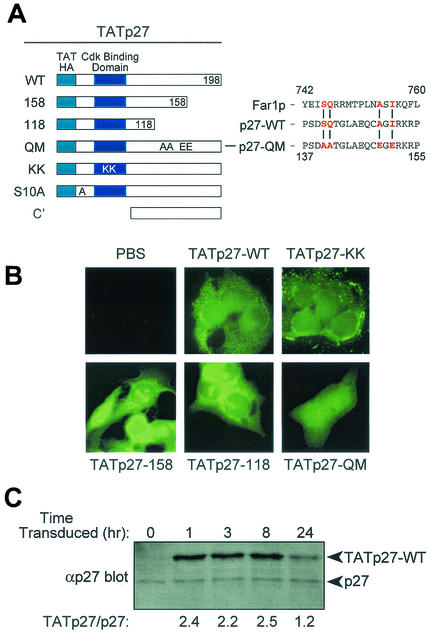
Although the C-terminal portion of p27 outside the cyclin-cdk binding domain is evolutionarily conserved, little is known about its structure or function. To determine the C-terminal p27 structural elements required for cell migration, we generated a series of transducible TATp27 C-terminal truncation proteins and point mutations in full-length TATp27 protein (Fig. 3A). All TATp27 fusion proteins were expressed in bacteria, purified under similar conditions, and contained an N-terminal leader containing the 11-amino-acid TAT protein transduction domain, six-His purification tag, and HA epitope tag.
FIG. 3.
Generation of transducible TATp27 fusion proteins. (A) Schematic representation of TATp27 fusion proteins. N-terminal leader contains the 11-amino-acid TAT protein transduction domain, HA epitope tag, and six-histidine purification tag. CBD, cyclin-cdk binding domain of p27. TATp27-WT represents full-length WT p27 protein, whereas TATp27-158 and TATp27-118 designate terminal truncations at respective residues. TATp27-QM contains four single-point mutations in the actin-interacting domain of the Far1p-like motif. TATp27-KK contains two inactivating point mutations that disrupt p27 binding to cyclin-cdk complexes. TATp27-S10A contains an alanine substitution for serine at residue 10. (B) Intracellular localization of TATp27 proteins. HepG2 cells were treated with control PBS or transducible TATp27 proteins and visualized by immunocytochemistry 1 h after transduction with anti-HA antibody and FITC-conjugated secondary antibody. (C) Immunoblot comparison of TATp27 and endogenous p27 protein levels in transduced cells. Lysates from HepG2 cells transduced with 100 nM TATp27-WT were immunoblotted for total p27 at indicated time points, and band intensity was quantified. The largest increase of TATp27 over endogenous p27 at any time point was 2.5-fold.
To assess the transduction efficiency of TATp27 fusion proteins, HepG2 cells were treated with an equal concentration of each protein for 1 h, washed extensively, fixed, and analyzed by anti-HA immunocytochemistry. Consistent with other transducible TAT fusion proteins (55), all TATp27 fusion proteins transduced into cells and were visualized equally in the cytoplasm and nucleus of cells 1 h after addition to the media (Fig. 3B). In addition, immunoblotting for the HA epitope in lysates from HepG2 cells treated with TATp27 fusion proteins and trypsinized to remove any extracellular TATp27 protein independently confirmed transduction of TATp27 proteins into cells (data not shown).
The altered molecular weight of TAT fusion proteins allowed for assessment of intracellular TATp27 protein levels relative to endogenous p27 protein over time. Cells transduced with TATp27-WT protein were trypsinized at indicated time points, resuspended in cell lysis buffer, and immunoblotted for total p27 protein. Quantification of band intensity indicated that the maximal level of intracellular TATp27-WT protein was 2.5-fold above that of endogenous p27 within the same cell populations (Fig. 3C). Furthermore, TATp27-WT protein was detectable 24 h after treatment (Fig. 3C). All Tatp27-fusion proteins that retained an intact N-terminal cyclin-cdk binding domain (TATp27-WT, TATp27-158, TATp27-118, and TATp27-QM) induced a G1 cell cycle arrest, whereas TATp27 fusions containing a mutation in the cdk binding domain (TATp27-KK) and the C terminus alone (TATp27-C′) failed to induce a cell cycle arrest (data not shown). Taken together, these observations confirm that TATp27-fusion proteins transduce into cells and maintain intracellular biological activity to induce G1 cell cycle arrest.
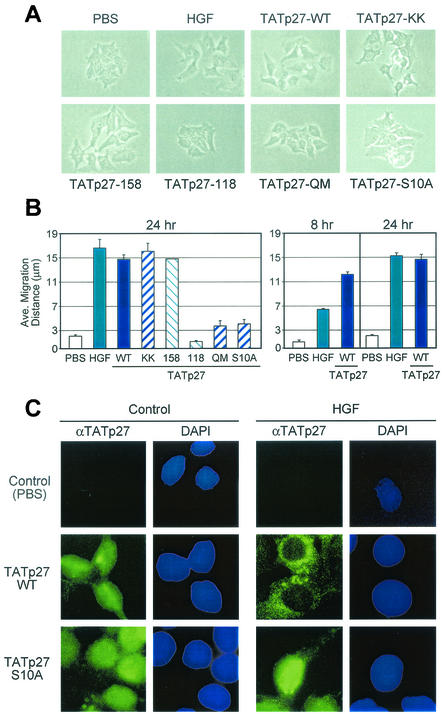
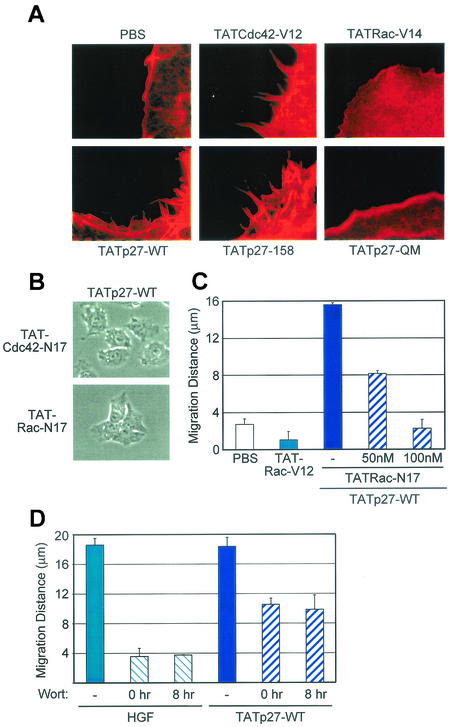
To directly test the involvement of p27 in cell migration, small colonies of 5 to 10 HepG2 cells were treated with PBS control, HGF, or TATp27-WT protein and observed over a 24-h period. Cell migration was defined by loss of cell-cell contact and increased distance between cells in a colony. Control PBS-treated cells retained cell-cell contacts and maintained discrete colonies with average distances between cell nuclei of less than 3 μm (Fig. 4A and B). HGF treatment resulted in disruption of cell-cell contacts and accumulation of significant distances between colony cell nuclei in excess of 15 μm (Fig. 4A and B). Treatment of cells with TATp27-WT protein induced cell migration to distances nearly identical to those caused by HGF treatment (Fig. 4A and B). TATp27-WT protein induced a greater extent of cell scattering than did HGF treatment at 8 h, whereas the two treatments resulted in similar migration distances by 24 h (Fig. 4B, right panel), indicating that direct transduction of TATp27-WT protein accelerates the rate of cell migration.
FIG. 4.
p27-dependent cell migration requires a C-terminal scatter domain and Ser-10 phosphorylation-dependent nuclear export. (A) Effect of TATp27 proteins on cell migration. HepG2 cells were treated for 24 h with control PBS, HGF, or TATp27 fusion protein as indicated and observed for cell scattering. The scattering phenotype is characterized by loss of cell-cell contact and increased distance between neighboring cells. (B) Quantification of cell migration. Cells treated for panel A above were stained with hematoxylin and recorded by digital microscopy at 0, 8, and 24 h posttreatment. Distance between colony nuclei measured at 0 h was subtracted from distance at 8 or 24 h to calculate average distance of migration. Data represent a minimum of 50 random fields and 300 measurements per treatment for each of three separate experiments. (C) TATp27 protein localization in response to HGF. HepG2 cells were treated with control PBS (left panels) or HGF (right panels) for 20 h, followed by treatment with control PBS, TATp27-WT protein, or TATp27-S10A protein for an additional 3 h. TATp27 proteins were visualized by immunostaining with anti-HA antibodies (HA epitope tag is present in the N-terminal leader of all TAT fusion proteins) and FITC-anti-mouse IgG (green). Corresponding nuclei were counterstained with DAPI (blue).
Transduction of the N-terminal half of p27 containing the functional cyclin-cdk binding domain fails to induce cell migration (28). Therefore, to delineate the region of p27 required for cell migration, we examined the ability of TATp27-158 and TATp27-118 C-terminal truncation proteins to induce scattering. TATp27-158 protein induced cell migration to distances similar to those of HGF and TATp27-WT protein treatments (Fig. 4A and B). In contrast, TATp27-118 C-terminal truncation protein failed to disrupt cell-cell contacts and maintained cell-cell distances of less than 3 μm (Fig. 4A and B). These data indicate that p27 contains a scatter domain between residues 118 to 158 that is required for p27-induced cell motility.
Functional similarities between p27 and Far1p.
Analogous to HGF treatment of mammalian cells, α-factor treatment of yeast cells results in nuclear export of Far1p, a cyclin-cdk inhibitor, to the cytoplasm followed by reorientation of the actin cytoskeleton into a shmoo structure (4, 36). Therefore, we compared the sequence of the p27 scatter domain (residues 118 to 158) to the C terminus of Far1p involved in shmoo formation. Although there is limited sequence identity between the two distantly related and functionally analogous proteins, an SQx7AxI motif present within the p27 scatter domain (residues 140 to 151) was found in the Far1p C-terminal Cdc42p/Bem1p/Cdc24p-binding region (Fig. 3A).
To test the requirement of this motif for p27-mediated cell migration, we generated a transducible full-length p27 protein that contained a mutation of each residue, termed TATp27- QM (Fig. 3A). TATp27-QM protein efficiently transduced into cells and induced a G1 cell cycle arrest (Fig. 3B and data not shown). However, TATp27-QM protein treatment failed to induce migration of HepG2 cells (Fig. 4A and B). In addition, while TATp27-WT localized to areas of dynamic actin rearrangement, both TATp27-QM and TATp27-118 proteins, which fail to induce cell migration but are exported to the cytoplasm, localized to perinuclear regions and were absent from actin-based structures at the cell membrane (data not shown). These observations indicate that the SQx7AxI motif present in the p27 scatter domain is critical for the ability of p27 to induce cell migration and to interact with actin-remodeling complexes at the cell membrane.
p27-induced scattering is independent of cell cycle arrest and requires cytoplasmic localization.
Although the scatter domain is necessary for p27-dependent migration, transduction of the p27 C-terminal scatter domain alone (residues 108 to 198) was insufficient for cell scattering (data not shown). p27 was originally identified as an inhibitor of G1 cell cycle progression (19, 46); therefore, we tested whether p27 must retain its cell cycle arrest function in order to induce cell migration. HepG2 cells were transduced with TATp27-KK protein containing a mutation in the cyclin binding domain (26), which fails to mediate G1 cell cycle arrest. TATp27-KK protein induced cell scattering to a distance comparable to that caused by HGF and TATp27-WT protein treatments (Fig. 4A and B). Thus, the ability of p27 to mediate cell cycle arrest is not a requirement for its function in cell migration.
We next examined the requirement of p27 Ser-10 phosphorylation for cell migration (22, 40). A transducible full-length p27 protein was generated containing a Ser-to-Ala mutation at position 10 (TATp27-S10A) (Fig. 3A). TATp27-S10A protein transduced into cells and induced a G1 cell cycle arrest (data not shown). However, TATp27-S10A protein failed to induce migration of HepG2 cells (Fig. 4A and B), suggesting that Ser-10 phosphorylation is required for p27-mediated cell migration.
To confirm that cytoplasmic localization of p27 is necessary for function of the scatter domain, we examined the intracellular localization of TATp27 proteins in response to HGF treatment. Both TATp27-WT and TATp27-S10A proteins were present in the nucleus of control PBS-treated cells 3 h after transduction (Fig. 4C, left panels). Consistent with HGF-dependent cytoplasmic localization of endogenous p27 (Fig. 1A), TATp27-WT protein was exported from the nucleus to the cytoplasm in HGF-treated cells (Fig. 4C, right panels). TATp27-158, TATp27-118, and TATp27-QM proteins were also exported from the nucleus in an HGF-dependent fashion (data not shown). In contrast, TATp27-S10A protein remained in the nuclei of HGF-treated cells (Fig. 4C, right panels). Taken together, these observations establish that p27-mediated cell migration requires Ser-10 phosphorylation-dependent cytoplasmic localization of p27 bearing an intact C-terminal scatter domain (residues 118 to 158) but not a functional cyclin-cdk binding domain.
The p27 scatter domain induces actin cytoskeletal rearrangement in a Rac-dependent manner.
Rearrangement of the actin cytoskeleton during cell migration is mediated by the Rho family of GTPases, specifically Rho, Rac, and Cdc42 (17, 32). In particular, the activities of Rac and Cdc42 are coordinated at the leading edge of cells to form lamellipodia and filopodia, respectively (38). HGF induces cytoskeletal rearrangement that is required for motility of a number of cell lines (20, 39, 52) and specifically induces formation of filopodia (28) and membrane ruffling (data not shown) in HepG2 cells. Therefore, we examined the dependency of cytoskeletal rearrangement on the p27 scatter domain. Formation of both filopodia and lamellipodia was observed in cells transduced with TATp27-WT and TATp27-158 proteins bearing an intact scatter domain but not in cells transduced with TATp27-118 or TATp27-QM protein (Fig. 5A). Transduction of constitutively active forms of Cdc42 (TATCdc42-V12), Rac (TATRac-V12), and Rho (TATRho-V14) induces formation of filopodia, lamellipodia, and stress fibers, respectively, in less than 15 min (2, 6) (Fig. 5A; data not shown). However, similar to HGF-induced cytoskeletal rearrangement, TATp27-WT- and TATp27-158-induced formation of lamellipodia and filopodia occurred in a time course consistent with induction of cell migration. These observations suggest that, while the scatter domain is required for p27-dependent cytoskeletal rearrangement, p27 does not activate Rho family GTPases directly upon transduction into cells.
FIG. 5.
Rac GTPase is required for p27-dependent cell migration. (A) TATp27 induces formation of both filopodia and lamellipodia. HepG2 cells were treated with 100 nM TATp27, TATCdc42-V12, or TATRac-V12 fusion proteins as indicated, and F-actin polymerization was visualized by TRITC-conjugated phalloidin. Formation of actin microspikes (filopodia) and membrane ruffling (lamellipodia) were observed only in cells transduced with control TATCdc42-V12 or TATRac-V12 fusion proteins and TATp27-fusion proteins containing an intact scatter domain. (B) Rac dominant-negative blocks p27-mediated scattering. HepG2 cells were treated simultaneously with 100 nM TATCdc42-N17 or TATRac-N17 dominant-negative proteins (as indicated) plus 100 nM TATp27-WT protein. Cell migration was scored at 24 h. (C) Quantification of inhibitory effect of Rac dominant negative. HepG2 cells were treated as for panel B with TATp27-WT protein and various concentrations of TATRac-N17 dominant-negative protein or 100 nM constitutively active TATRac-V12 protein alone. Cell migration was scored at 24 h. (D) Effect of wortmannin (Wort) on cell migration. HepG2 cells were treated with 5 ng of HGF/ml or 100 nM TATp27-WT. Thirty nanomolar wortmannin was added at either 0 or 8 h, and cell migration was quantified 24 h after transduction.
We next used transducible forms of Rho GTPases to determine the specific family member(s) involved in p27-mediated HepG2 cell migration. Although dominant-negative TATCdc42-N17 blocked p27-induced formation of filopodia (data not shown), it failed to inhibit p27-dependent migration (Fig. 5B and data not shown). In contrast, dominant-negative TATRac-N17 inhibited p27-mediated migration in a dose-dependent fashion (Fig. 5B and C), suggesting that activated Rac is necessary for p27-dependent migration. However, although transduction of constitutively active TATRac-V12 alone induced lamellipodia, it was not sufficient for induction of cell migration (Fig. 5C), suggesting that p27 provides signals necessary for Rac-mediated migration.
PI3-K inactivation partially inhibits p27-dependent migration.
PI3-K is required for HGF-mediated motility of some epithelial cell types in cell culture and can activate Rac (10). To test the involvement of PI3-K activation in p27-dependent migration, we used wortmannin to inhibit PI3-K activity. Wortmannin was added to cells either before or 8 h after treatment with HGF or TATp27-WT protein. Consistent with previously published reports, wortmannin inhibited HGF-induced cell migration at both time points (Fig. 5D). However, p27-dependent migration was only partially inhibited in cells either pretreated with wortmannin or treated with wortmannin at 8 h (Fig. 5D), suggesting that some aspects of p27-mediated migration were downstream and/or independent of PI3-K signaling.
p27-deficient cells fail to migrate.
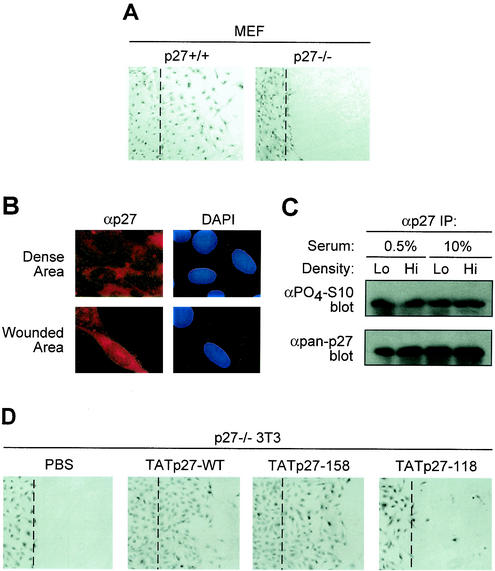
To genetically confirm the placement of p27 in a cell motility pathway, we examined the ability of WT and p27-deficient primary MEFs to migrate in a standard cell culture migration assay. MEFs isolated from WT mice efficiently migrated into the denuded region in 24 h (Fig. 6A, left panel). In contrast, p27-deficient MEFs from strain-matched mice failed to migrate (Fig. 6A, right panel). These observations demonstrated that nonepithelial cells were also dependent on p27 function for migration under these conditions.
FIG. 6.
Reconstitution of p27 rescues a migration defect of p27−/− fibroblasts. (A) Migration analysis of WT and p27-deficient MEFs. A wound area was mechanically induced by a single passage of a microtome blade across culture plate surface to confluent MEF monolayers. After 24 h, cells were fixed and stained with hematoxylin. The experiment was repeated three times with indistinguishable results. The wound edge is noted as a digitally drawn line over the image. (B) p27 is localized to the cytoplasm of NIH 3T3 cells. WT NIH 3T3 cells were subject to the standard cell migration assay and immunostained for p27 localization (left panels). Corresponding nuclei were stained with DAPI (right panels). (C) p27 is constitutively phosphorylated on Ser-10 in NIH 3T3 cells. Total p27 was immunoprecipitated (IP) from lysates of WT cells grown under indicated conditions. Immunoprecipitates were blotted for phospho-Ser-10 and total p27 antibodies. (D) p27 protein rescues cell migration defect. Confluent p27-deficient 3T3 cells were subjected to the wound migration assay described for panel A, by single passage of a rubber policeman followed by treatment with transducible TATp27 proteins. p27-WT and p27-158 proteins rescued the wound migration defect, whereas mock treatment (PBS) and TATp27-118 protein (lacking the p27 scatter domain) failed to induce cell migration. The wound edge is noted as a digitally drawn line over the image.
To determine if basal migratory activity of fibroblasts was related to p27 status, WT NIH 3T3 fibroblasts were examined for p27 localization and phosphorylation. NIH 3T3 cells efficiently migrated into a denuded region in the standard cell migration assay (data not shown), suggesting that the immortalization process does not interfere with this pathway. Immunocytochemical analysis of NIH 3T3 cells demonstrated that p27 is localized to the cytoplasm of cells in both dense and denuded regions of the plate (Fig. 6B). Consistent with this result, p27 cytoplasmic localization has been observed in Swiss 3T3 fibroblasts (56). The phosphorylation status of Ser-10 was determined in NIH 3T3 cells grown at high and low densities in the presence of high and low serum concentrations. p27 was constitutively phosphorylated on Ser-10 under all conditions (Fig. 6C).
We next examined the ability of TATp27-WT protein to restore migration of p27-deficient 3T3 fibroblasts. Consistent with nonimmortalized MEFs, p27-deficient immortalized 3T3 derivates also failed to migrate (Fig. 6D, left panel), demonstrating that p27 was essential for cell migration after immortalization. Both TATp27-WT and TATp27-158 proteins complemented the migration defect in p27-deficient 3T3 cells (Fig. 6D). In contrast, TATp27-118 protein containing a deleted C terminus failed to rescue the migration defect in p27-deficient 3T3 cells (Fig. 6D). Taken together, these observations demonstrate a role for p27 in a cell motility pathway that functions in both epithelial and fibroblast cells that is independent of G1 cell cycle arrest functions of p27.
DISCUSSION
Metastatic tumor growth is one of the most important factors in predicting the survival of cancer patients. HGF/c-Met signaling mediates phenotypes characteristic of tumor progression and enhanced metastatic potential (23, 41). HGF treatment of hepatocellular carcinoma cells results in increased cell motility, actin cytoskeletal rearrangement, and, paradoxically, stabilization of the p27 tumor suppressor protein. Although several studies have correlated a poor patient outcome with loss or low levels of p27 protein in multiple human malignancies (9, 47), high levels of p27 tumor suppressor protein have been reported in a subset of human malignancies, including esophageal, colon, breast, and small-cell lung carcinoma (11, 14, 45, 57). Recently, a subset of invasive breast carcinomas were characterized with high p27 protein levels that correlated with lymph node metastasis (25). Consistent with this observation, cytoplasmic localization of p27 has been associated with anchorage-independent growth in culture and aggressive tumor progression (7, 30, 31, 43). These observations suggest the potential for alternative functions of p27 in addition to its role in cell cycle inhibition. Indeed, here we define a novel role for p27 in cell motility in response to HGF signaling that is independent of the G1 cell cycle arrest functions of p27.
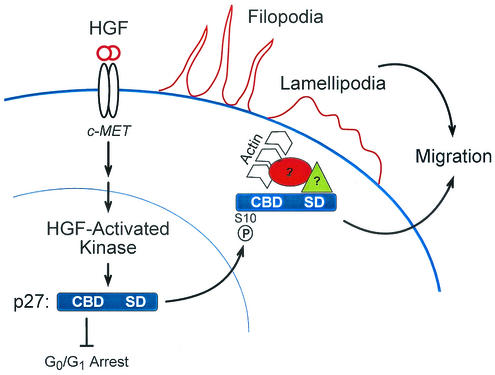
The data presented here support a model in which an HGF-activated kinase phosphorylates p27 on Ser-10, resulting in nuclear export of p27 to the cytoplasm (Fig. 7). These events position p27 for its function in cell migration and cytoskeletal rearrangement. Consistent with these observations, we detected cytoplasmic p27 in areas of dynamic cytoskeletal rearrangement. Importantly, the ability of p27 to induce cell migration mapped to a C-terminal scatter domain (residues 118 to 158) that was independent of previously described cell cycle arrest functions of p27 that reside in the N terminus. Loss or disruption of the C-terminal p27 scatter domain abolishes both actin rearrangement and cell scattering. However, the scatter domain alone is insufficient for cell migration because p27 must be exported to the cytoplasm via Ser-10 phosphorylation. This conclusion is further supported by the observation that TATp27-C′ (residues 108 to 198), which does not induce cell scattering, is retained in the nucleus of transduced cells (unpublished observation).
FIG. 7.
Model of HGF signaling through p27 to mediate cell migration. HGF binding to the Met receptor activates HGF-activated kinase (HACK), which phosphorylates p27 on Ser-10. Phosphorylation of Ser-10 is required for export of p27 from the nucleus to the cytoplasm in a Crm1-dependent fashion. In the cytoplasm, p27 interacts directly or indirectly with actin-remodeling proteins, thereby inducing cytoskeletal rearrangement accompanied by Rac-dependent cell migration. p27-dependent cell migration requires Ser-10 phosphorylation and the p27 scatter domain (residues 118 to 158) but not a functional cyclin-cdk binding domain.
The involvement of p27 in actin cytoskeletal rearrangement is evolutionarily reminiscent of α-factor pheromone signaling in yeast. Far1p, the yeast cell cycle inhibitor, mediates both G1 cell cycle arrest and shmoo formation, which orients the actin cytoskeleton toward an opposite mating partner (35). In response to α-factor, Far1p translocates from the nucleus to the cytoplasm and interacts with cdc42p (4). Consistent with this functional analogy, mutation of a Far1p-like sequence motif present in the p27 scatter domain inactivates both cell migration and actin rearrangement but preserves the cell cycle arrest functions of p27.
In a manner similar to HGF treatment, transduced p27 protein induces formation of both lamellipodia and filopodia, suggesting that activation of Rac and/or Cdc42 is a downstream event in a p27-dependent pathway. Transduction or microinjection of constitutively active Rac or Cdc42 protein into cells results in the expected phenotype in less than 10 min (2, 29). However, the first signs of membrane ruffling and cell migration in TATp27- or HGF-treated cells are not detectable until >4 h posttreatment, even though TATp27 reaches an intracellular maximum concentration in <15 min. Therefore, it is unlikely that unmodified p27 is capable of immediately activating Rac or Cdc42 directly. Rate-limiting events such as p27 posttranslational modification or assembly into macromolecular complexes may be required during the initiation of p27-dependent cell migration in order for p27 to activate Rho GTPases.
Migratory responses to Met activation proceed in two stages: an early stage that occurs during the first 6 h after treatment and involves cytoskeletal extension, spreading, and detachment of cell-cell contacts and a later stage involving cell scattering that is not observed until 16 to 20 h after treatment (34, 39, 50). Importantly, the relatively long period (8 to 24 h) before the onset of TATp27-mediated scattering is consistent with the time course of HGF-mediated migration described in MDCK cells (34, 39) and human colon cancer cell lines (24). This type of prolonged signaling is also observed in normal human hepatocytes and HepG2 cells, where HGF-Met activation is sustained up to 28 h (44). The time at which endogenous p27 is observed in the cytoplasm of HGF-treated cells (12 to 24 h) and at which TATp27-WT is observed predominantly in the cytoplasm of transduced cells correlates precisely with the appearance of cell migration.
PI3-K is involved in early scatter responses to HGF, including dissociation of adherens junctions (34, 42) and activation of Rac (10). In the present study, PI3-K inactivation completely inhibited HGF-mediated scattering but only partially inhibited p27-dependent migration. Furthermore, we demonstrated that p27 accelerates the rate of cell migration compared to HGF at the 8-h time point. Taken together, these data suggest that p27 has a role in both early and late migration events that both involves PI3-K signaling and also appears independent of PI3-K.
Our model therefore predicts a motility defect in p27-deficient cells. Indeed, p27-deficient fibroblasts failed to migrate in culture. Rescue of the cell migration defect in p27-deficient MEFs and 3T3 cells by p27 reconstitution genetically places p27 in a cell motility pathway independently of its cell cycle arrest functions. Furthermore, these data broaden the importance of p27-dependent motility, as hepatocytes are epithelial cells that express the Met receptor, whereas fibroblasts do not express Met (51), suggesting that p27 can mediate cell migration independently of Met receptor signaling. Indeed, development and tissue regeneration following injury are biological processes that require specific regulation of cell migration and the cell cycle. Consistent with this notion, rodent models of partial hepatectomy show significantly increased levels of p27 that are detected in both nuclear and cytoplasmic fractions (1). Taken together, these observations suggest a novel role for p27 in cell migration that is independent of cell cycle arrest function with potential implications in wound healing and metastasis.
Acknowledgments
We thank J. Cooper, H. Piwnica-Worms, T. Bashir, P. Mignatti, M. Roussel, C. Der, L. Yamasaki, and A. Vocero for reagents and critical input.
M.P. is a recipient of the Irma T. Hirschl Scholarship. This work was supported by the Human Frontier Science Program Organization (RG0229 [M.P.]), the National Institutes of Health (R01-CA76584 and R01-GM57587 [M.P.] and RO1-CA96098 [S.F.D.]), and the Howard Hughes Medical Institute (S.F.D.). S.F.D. is an Associate Investigator of the Howard Hughes Medical Institute.
REFERENCES
- 1.Albrecht, J. H., B. M. Rieland, C. J. Nelsen, and C. L. Ahonen. 1999. Regulation of G(1) cyclin-dependent kinases in the liver: role of nuclear localization and p27 sequestration. Am. J. Physiol. 277:G1207-G1216. [DOI] [PubMed] [Google Scholar]
- 2.Becker-Hapak, M., S. S. McAllister, and S. F. Dowdy. 2001. TAT-mediated protein transduction into mammalian cells. Methods 24:247-256. [DOI] [PubMed] [Google Scholar]
- 3.Blain, S. W., E. Montalvo, and J. Massague. 1997. Differential interaction of the cyclin-dependent kinase (Cdk) inhibitor p27Kip1 with cyclin A-Cdk2 and cyclin D2-Cdk4. J. Biol. Chem. 272:25863-25872. [DOI] [PubMed] [Google Scholar]
- 4.Butty, A. C., P. M. Pryciak, L. S. Huang, I. Herskowitz, and M. Peter. 1998. The role of Far1p in linking the heterotrimeric G protein to polarity establishment proteins during yeast mating. Science 282:1511-1516. [DOI] [PubMed] [Google Scholar]
- 5.Carrano, A. C., E. Eytan, A. Hershko, and M. Pagano. 1999. SKP2 is required for ubiquitin-mediated degradation of the CDK inhibitor p27. Nat. Cell Biol. 1:193-199. [DOI] [PubMed] [Google Scholar]
- 6.Chellaiah, M. A., N. Soga, S. Swanson, S. McAllister, U. Alvarez, D. Wang, S. F. Dowdy, and K. A. Hruska. 2000. Rho-A is critical for osteoclast podosome organization, motility, and bone resorption. J. Biol. Chem. 275:11993-12002. [DOI] [PubMed] [Google Scholar]
- 7.Ciaparrone, M., H. Yamamoto, Y. Yao, A. Sgambato, G. Cattoretti, N. Tomita, T. Monden, H. Rotterdam, and I. B. Weinstein. 1998. Localization and expression of p27KIP1 in multistage colorectal carcinogenesis. Cancer Res. 58:114-122. [PubMed] [Google Scholar]
- 8.Ciarallo, S., V. Subramaniam, W. Hung, J.-H. Lee, R. Kotchetkov, C. Sandhu, A. Milic, and J. M. Slingerland. 2002. Altered p27Kip1 phosphorylation, localization, and function in human epithelial cells resistant to transforming growth factor β-mediated G1 arrest. Mol. Cell. Biol. 22:2993-3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clurman, B. E., and P. Porter. 1998. New insights into the tumor suppression function of P27(kip1). Proc. Natl. Acad. Sci. USA 95:15158-15160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Comoglio, P. M., and C. Boccaccio. 2001. Scatter factors and invasive growth. Semin. Cancer Biol. 11:153-165. [DOI] [PubMed] [Google Scholar]
- 11.Doki, Y., M. Imoto, E. K. Han, A. Sgambato, and I. B. Weinstein. 1997. Increased expression of the P27KIP1 protein in human esophageal cancer cell lines that over-express cyclin D1. Carcinogenesis 18:1139-1148. [DOI] [PubMed] [Google Scholar]
- 12.Ekholm, S. V., and S. I. Reed. 2000. Regulation of G(1) cyclin-dependent kinases in the mammalian cell cycle. Curr. Opin. Cell Biol. 12:676-684. [DOI] [PubMed] [Google Scholar]
- 13.Ezhevsky, S. A., H. Nagahara, A. M. Vocero-Akbani, D. R. Gius, M. C. Wei, and S. F. Dowdy. 1997. Hypo-phosphorylation of the retinoblastoma protein (pRb) by cyclin D:Cdk4/6 complexes results in active pRb. Proc. Natl. Acad. Sci. USA 94:10699-10704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fredersdorf, S., J. Burns, A. M. Milne, G. Packham, L. Fallis, C. E. Gillett, J. A. Royds, D. Peston, P. A. Hall, A. M. Hanby, D. M. Barnes, S. Shousha, M. J. O'Hare, and X. Lu. 1997. High level expression of p27(kip1) and cyclin D1 in some human breast cancer cells: inverse correlation between the expression of p27(kip1) and degree of malignancy in human breast and colorectal cancers. Proc. Natl. Acad. Sci. USA 94:6380-6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ganoth, D., G. Bornstein, T. K. Ko, B. Larsen, M. Tyers, M. Pagano, and A. Hershko. 2001. The cell-cycle regulatory protein Cks1 is required for SCFSkp2-mediated ubiquitinylation of p27. Nat. Cell Biol. 3:321-324. [DOI] [PubMed] [Google Scholar]
- 16.Groth, A., J. D. Weber, B. M. Willumsen, C. J. Sherr, and M. F. Roussel. 2000. Oncogenic Ras induces p19ARF and growth arrest in mouse embryo fibroblasts lacking p21Cip1 and p27Kip1 without activating cyclin D-dependent kinases. J. Biol. Chem. 275:27473-27480. [DOI] [PubMed] [Google Scholar]
- 17.Hall, A. 1998. Rho GTPases and the actin cytoskeleton. Science 279:509-514. [DOI] [PubMed] [Google Scholar]
- 18.Hanahan, D., and R. A. Weinberg. 2000. The hallmarks of cancer. Cell 100:57-70. [DOI] [PubMed] [Google Scholar]
- 19.Hengst, L., and S. I. Reed. 1998. Inhibitors of the Cip/Kip family. Curr. Top. Microbiol. Immunol. 227:25-41. [DOI] [PubMed] [Google Scholar]
- 20.Hordijk, P. L., J. P. ten Klooster, R. A. van der Kammen, F. Michiels, L. C. Oomen, and J. G. Collard. 1997. Inhibition of invasion of epithelial cells by Tiam1-Rac signaling. Science 278:1464-1466. [DOI] [PubMed] [Google Scholar]
- 21.Ishida, N., T. Hara, T. Kamura, M. Yoshida, K. Nakayama, and K. I. Nakayama. 2002. Phosphorylation of p27Kip1 on serine 10 is required for its binding to CRM1 and nuclear export. J. Biol. Chem. 277:14355-14358. [DOI] [PubMed] [Google Scholar]
- 22.Ishida, N., M. Kitagawa, S. Hatakeyama, and K. Nakayama. 2000. Phosphorylation at serine 10, a major phosphorylation site of p27Kip1, increases its protein stability. J. Biol. Chem. 275:25146-25154. [DOI] [PubMed] [Google Scholar]
- 23.Jeffers, M., S. Rong, and G. F. Vande Woude. 1996. Hepatocyte growth factor/scatter factor-Met signaling in tumorigenicity and invasion/metastasis. J. Mol. Med. 74:505-513. [DOI] [PubMed] [Google Scholar]
- 24.Jiang, W., S. Hiscox, K. Matsumoto, and T. Nakamura. 1999. Hepatocyte growth factor/scatter factor, its molecular, cellular and clinical implications in cancer. Crit. Rev. Oncol. Hematol. 29:209-248. [DOI] [PubMed] [Google Scholar]
- 25.Kouvaraki, M., V. G. Gorgoulis, G. Z. Rassidakis, P. Liodis, C. Markopoulos, J. Gogas, and C. Kittas. 2002. High expression levels of p27 correlate with lymph node status in a subset of advanced invasive breast carcinomas. Cancer 94:2454-2465. [DOI] [PubMed] [Google Scholar]
- 26.Luo, Y., J. Hurwitz, and J. Massague. 1995. Cell-cycle inhibition by independent CDK and PCNA binding domains in p21Cip1. Nature 375:159-161. [DOI] [PubMed] [Google Scholar]
- 27.Malek, N. P., H. Sundberg, S. McGrew, K. Nakayama, T. R. Kyriakidis, and J. M. Roberts. 2001. A mouse knock-in model exposes sequential proteolytic pathways that regulate p27Kip1 in G1 and S phase. Nature 413:323-327. [DOI] [PubMed] [Google Scholar]
- 28.Nagahara, H., A. M. Vocero-Akbani, E. L. Snyder, A. Ho, D. G. Latham, N. A. Lissy, M. Becker-Hapak, S. A. Ezhevsky, and S. F. Dowdy. 1998. Transduction of full-length TAT fusion proteins into mammalian cells: TAT-p27Kip1 induces cell migration. Nat. Med. 4:1449-1452. [DOI] [PubMed] [Google Scholar]
- 29.Nobes, C. D., and A. Hall. 1995. Rho, rac and cdc42 GTPases: regulators of actin structures, cell adhesion and motility. Biochem. Soc. Trans. 23:456-459. [DOI] [PubMed] [Google Scholar]
- 30.Orend, G., T. Hunter, and E. Ruoslahti. 1998. Cytoplasmic displacement of cyclin E-cdk2 inhibitors p21Cip1 and p27Kip1 in anchorage-independent cells. Oncogene 16:2575-2583. [DOI] [PubMed] [Google Scholar]
- 31.Piva, R., I. Cancelli, P. Cavalla, S. Bortolotto, J. Dominguez, G. F. Draetta, and D. Schiffer. 1999. Proteasome-dependent degradation of p27/kip1 in gliomas. J. Neuropathol. Exp. Neurol. 58:691-696. [DOI] [PubMed] [Google Scholar]
- 32.Pollard, T. D., L. Blanchoin, and R. D. Mullins. 2001. Actin dynamics. J. Cell Sci. 114:3-4. [DOI] [PubMed] [Google Scholar]
- 33.Polyak, K., M. H. Lee, H. Erdjument-Bromage, A. Koff, J. M. Roberts, P. Tempst, and J. Massague. 1994. Cloning of p27Kip1, a cyclin-dependent kinase inhibitor and a potential mediator of extracellular antimitogenic signals. Cell 78:59-66. [DOI] [PubMed] [Google Scholar]
- 34.Potempa, S., and A. J. Ridley. 1998. Activation of both MAP kinase and phosphatidylinositide 3-kinase by Ras is required for hepatocyte growth factor/scatter factor-induced adherens junction disassembly. Mol. Biol. Cell 9:2185-2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pruyne, D., and A. Bretscher. 2000. Polarization of cell growth in yeast. J. Cell Sci. 113:571-585. [DOI] [PubMed] [Google Scholar]
- 36.Pruyne, D., and A. Bretscher. 2000. Polarization of cell growth in yeast. I. Establishment and maintenance of polarity states. J. Cell Sci. 113:365-375. [DOI] [PubMed] [Google Scholar]
- 37.Reynisdottir, I., and J. Massague. 1997. The subcellular locations of p15(Ink4b) and p27(Kip1) coordinate their inhibitory interactions with cdk4 and cdk2. Genes Dev. 11:492-503. [DOI] [PubMed] [Google Scholar]
- 38.Ridley, A. J., W. E. Allen, M. Peppelenbosch, and G. E. Jones. 1999. Rho family proteins and cell migration. Biochem. Soc. Symp. 65:111-123. [PubMed] [Google Scholar]
- 39.Ridley, A. J., P. M. Comoglio, and A. Hall. 1995. Regulation of scatter factor/hepatocyte growth factor responses by Ras, Rac, and Rho in MDCK cells. Mol. Cell. Biol. 15:1110-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rodier, G., A. Montagnoli, L. Di Marcotullio, P. Coulombe, G. F. Draetta, M. Pagano, and S. Meloche. 2001. p27 cytoplasmic localization is regulated by phosphorylation on Ser10 and is not a prerequisite for its proteolysis. EMBO J. 20:6672-6682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rong, S., S. Segal, M. Anver, J. H. Resau, and G. F. Vande Woude. 1994. Invasiveness and metastasis of NIH 3T3 cells induced by Met-hepatocyte growth factor/scatter factor autocrine stimulation. Proc. Natl. Acad. Sci. USA 91:4731-4735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Royal, I., and M. Park. 1995. Hepatocyte growth factor-induced scatter of Madin-Darby canine kidney cells requires phosphatidylinositol 3-kinase. J. Biol. Chem. 270:27780-27787. [DOI] [PubMed] [Google Scholar]
- 43.Sánchez-Beato, M., F. I. Camacho, J. C. Martínez-Montero, A. I. Sáez, R. Villuendas, L. Sánchez-Verde, J. F. García, and M. A. Piris. 1999. Anomalous high p27/KIP1 expression in a subset of aggressive B-cell lymphomas is associated with cyclin D3 overexpression. p27/KIP1-cyclin D3 colocalization in tumor cells. Blood 94:765-772. [PubMed] [Google Scholar]
- 44.Schaper, F., E. Siewert, M. J. Gomez-Lechon, P. Gatsios, M. Sachs, W. Birchmeier, P. C. Heinrich, and J. Castell. 1997. Hepatocyte growth factor/scatter factor (HGF/SF) signals via the STAT3/APRF transcription factor in human hepatoma cells and hepatocytes. FEBS Lett. 405:99-103. [DOI] [PubMed] [Google Scholar]
- 45.Sgambato, A., Y. J. Zhang, N. Arber, H. Hibshoosh, Y. Doki, M. Ciaparrone, R. M. Santella, A. Cittadini, and I. B. Weinstein. 1997. Deregulated expression of p27(Kip1) in human breast cancers. Clin. Cancer Res. 3:1879-1887. [PubMed] [Google Scholar]
- 46.Sherr, C. J., and J. M. Roberts. 1999. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 13:1501-1512. [DOI] [PubMed] [Google Scholar]
- 47.Slingerland, J., and M. Pagano. 2000. Regulation of the cdk inhibitor p27 and its deregulation in cancer. J. Cell. Physiol. 183:10-17. [DOI] [PubMed] [Google Scholar]
- 48.Spruck, C., H. Strohmaier, M. Watson, A. P. Smith, A. Ryan, T. W. Krek, and S. I. Reed. 2001. A CDK-independent function of mammalian Cks1: targeting of SCF(Skp2) to the CDK inhibitor p27Kip1. Mol. Cell 7:639-650. [DOI] [PubMed] [Google Scholar]
- 49.Stella, M. C., and P. M. Comoglio. 1999. HGF: a multifunctional growth factor controlling cell scattering. Int. J. Biochem. Cell Biol. 31:1357-1362. [DOI] [PubMed] [Google Scholar]
- 50.Stoker, M., E. Gherardi, M. Perryman, and J. Gray. 1987. Scatter factor is a fibroblast-derived modulator of epithelial cell mobility. Nature 327:239-242. [DOI] [PubMed] [Google Scholar]
- 51.Stuart, K. A., S. M. Riordan, S. Lidder, L. Crostella, R. Williams, and G. G. Skouteris. 2000. Hepatocyte growth factor/scatter factor-induced intracellular signalling. Int. J. Exp. Pathol. 81:17-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Takaishi, K., T. Sasaki, H. Kotani, H. Nishioka, and Y. Takai. 1997. Regulation of cell-cell adhesion by rac and rho small G proteins in MDCK cells. J. Cell Biol. 139:1047-1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tomoda, K., Y. Kubota, and J. Kato. 1999. Degradation of the cyclin-dependent-kinase inhibitor p27Kip1 is instigated by Jab1. Nature 398:160-165. [DOI] [PubMed] [Google Scholar]
- 54.Tsuboi, K., S. Yamaoka, M. Maki, G. Ohshio, T. Tobe, and M. Hatanaka. 1990. Soluble factors including proteinases released from damaged cells may trigger the wound healing process. Biochem. Biophys. Res. Commun. 168:1163-1170. [DOI] [PubMed] [Google Scholar]
- 55.Wadia, J. S., and S. F. Dowdy. 2002. Protein transduction technology. Curr. Opin. Biotechnol. 13:52-56. [DOI] [PubMed] [Google Scholar]
- 56.Wang, G., R. Miskimins, and W. K. Miskimins. 1999. The cyclin-dependent kinase inhibitor p27Kip1 is localized to the cytosol in Swiss/3T3 cells. Oncogene 18:5204-5210. [DOI] [PubMed] [Google Scholar]
- 57.Weinstein, I. B. 2000. Disorders in cell circuitry during multistage carcinogenesis: the role of homeostasis. Carcinogenesis 21:857-864. [DOI] [PubMed] [Google Scholar]
- 58.Xu, M., K.-A. Sheppard, C.-Y. Peng, A. S. Yee, and H. Piwnica-Worms. 1994. Cyclin A/CDK2 binds directly to E2F-1 and inhibits the DNA-binding activity of E2F-1/DP-1 by phosphorylation. Mol. Cell. Biol. 14:8420-8431. [DOI] [PMC free article] [PubMed] [Google Scholar]