Abstract
SALL/Sall is a mammalian homolog of the Drosophila region-specific homeotic gene spalt (sal), and heterozygous mutations in SALL1 in humans lead to Townes-Brocks syndrome. We earlier reported that mice deficient in Sall1 die in the perinatal period and that kidney agenesis or severe dysgenesis are present. We have now generated mice lacking Sall2, another Sall family gene. Although Sall2 is expressed mostly in an overlapping fashion versus that of Sall1, Sall2-deficient mice show no apparent abnormal phenotypes. Morphology and gene expression patterns of the mutant kidney were not affected. Mice lacking both Sall1 and Sall2 show kidney phenotypes comparable to those of Sall1 knockout, thereby demonstrating the dispensable roles of Sall2 in embryonic and kidney development.
Drosophila sal is the region-specific homeotic gene characterized by unique multiple double zinc finger motifs (10). sal was first identified by its capacity to promote terminal differentiation, and it is expressed in anterior and posterior compartments of Drosophila (5). Mutations in sal cause head and tail segments to develop trunk structures. sal also plays a critical role in wing development (4, 13). sal is expressed at the anterior-posterior boundary of wing imaginal disks, and its expression is controlled by dpp (BMP-4 ortholog), the expression of which is highest at the boundary and which is in turn controlled by hedgehog expressed in the posterior compartment.
Humans and mice have at least three sal-related genes, respectively (SALL1, -2, and -3 for humans and Sall1, -2, and -3 for mice) (2, 6-8, 15). SALL1 is located on chromosome 16q12.1, and heterozygous mutations of SALL1 lead to Townes-Brocks syndrome, an autosomal-dominant disease with features of dysplastic ears, preaxial polydactyly, imperforate anus and, less commonly, kidney and heart anomalies (9). Mice deficient in Sall1 die in the perinatal period, and kidney agenesis or severe dysgenesis are present (14). Sall1 is expressed in the metanephric mesenchyme surrounding ureteric bud, and homozygous deletion of Sall1 results in an incomplete ureteric bud outgrowth and failure of tubule formation in the mesenchyme. Therefore, Sall1 is essential for ureteric bud invasion, the initial key step for metanephros development.
Another Sall family gene, SALL2 is located on human chromosome 14q12, possibly overlapping a region of loss of heterozygosity in ovarian cancers (1). Mouse Sall2 binds to polyomavirus large T antigen and is proposed to be a potential tumor suppressor (11). Although mouse Sall2 was reported to be expressed during development and abundantly in the adult brain (6), precise expression patterns and the physiological function of Sall2 have remained unknown. We now report generation of Sall2-deficient mice, and in these animals we found that Sall2 is dispensable for normal developmental processes. We also present phenotypes of mice lacking both Sall1 and Sall2.
MATERIALS AND METHODS
Cloning of Sall2 genome.
PCR was done by using as a template for fetal kidney cDNA obtained 14.5 days postcoitus (dpc) to clone Sall2. The resulting 188-bp product was used to screen the 129SvJ genomic library (Stratagene).
Generation of Sall2-deficient mice.
The targeting vector was constructed by incorporating the 5′ BamHI-EcoRI 6.0-kb fragment and the 3′ SmaI-BamHI 1.7-kb fragment into a vector that contained the neomycin-resistant (Neor) gene (pMC1-NeopolyA) and a diphtheria toxin A subunit (pMC1-DTA) in tandem. The 5′ fragment was subcloned into a NotI-XhoI site 5′ of the Neor gene, and the 3′ fragment was cloned into an EcoRV site 3′ of the Neor gene. The construct was linearized with NotI.
E14.1 embryonic stem cells were plated on mitomycin C-treated primary embryonic fibroblasts and clones resistant to G418 (400 μg/ml) were screened by using Southern blots. The genomic DNA from clones was digested with EcoRI, electrophoresed through 0.7% agarose, transferred to nylon membrane (HybondN+; Amersham-Pharmacia), and hybridized to a radioactive probe. The probe used to screen the samples was a BamHI-BamHI 0.6-kb fragment downstream of the 3′ homology (probe B). The samples were also digested with SpeI and XhoI and then hybridized with the 5′ probe (probe A) to confirm the correct homologous recombination. A probe corresponding to the Neor sequence was also used to verify that only one copy of the vector was integrated into the genome. Of 120 clones, 6 were correctly targeted.
Recipient blastocysts were from C57BL/6J mice. Chimeric animals were bred with C57BL/6J females. Mutant animals studied were of F2 and F3 generations. Mice were genotyped by using Southern blots or genomic PCR. The primer sequences used for PCR were as follows: CACATTTCGTGGGTCACAAG, CTCAGAGCTGTTTTCCTGGG , and GCGTTGGCTACCCGTGATAT (188 bp for the wild-type Sall2 allele and 380 bp for the mutated allele). To screen Sall1 mutants, we used AGCTAAAGCTGCCAGAGTGC, CAACTTGCGATTGCCATAAA, and GCGTTGGCTACCCGTGATAT (288 bp for the wild-type Sall1 allele,and 350 bp for the mutated allele).
The probes used for Northern blots were as follows: the Sall1-XhoI Sall1 fragment (2.5 kb), the EcoRI-SmaI N-terminal Sall2 fragment (2.0 kb), the SmaI-HindIII C-terminal Sall2 fragment (1.4 kb), and the N-terminal Sall3 fragment (2.0 kb).
Histological examination and in situ hybridization.
Samples were fixed in 10% formalin and processed for paraffin-embedded sections (6-μm thick), followed by double staining with hematoxylin and eosin.
In situ hybridization was done with digoxigenin-labeled antisense riboprobes as described previously (14). A 1-kb fragment of Sall2 cDNA corresponding C-terminal three zinc fingers was amplified by using PCR, subcloned into pCRII (Invitrogen), and sequenced. Antisense transcript was generated with SP6 polymerase. Other probes were as described previously (14). None of the sense probes yielded signals.
Peripheral blood count and renal parameter measurement.
Cardiac puncture was done in 12-week-old mice, and samples were processed with Celltac α (Nihon Koden, Japan) for peripheral blood counts.
Next, 10- to 14-month-old mice were used to measure blood urea nitrogen and creatinine in serum with an automatic analyzer 7150 (Hitachi, Tokyo, Japan). Urinary protein was measured by using Pretest (Wako, Tokyo, Japan) containing tetrabromophenol blue.
RESULTS AND DISCUSSION
Generation of Sall2-deficient mice.
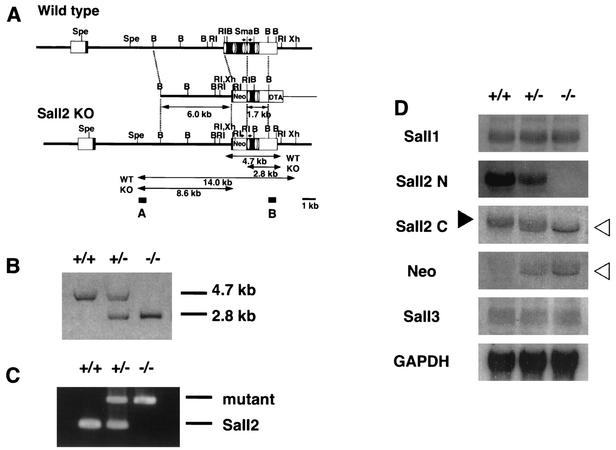
To examine developmental functions, we inactivated Sall2 in the mouse by using embryonic stem cells (Fig. 1A). The Sall2 gene consists of two exons, the intron being ca. 12 kb. All eight zinc finger domains are located in exon 2. We generated a targeting construct, which deleted the N-terminal five zinc fingers. Chimeras from two independent homologous recombinants transmitted the mutations through the germ line. Mice were genotyped by using Southern blots and genomic PCR (Fig. 1B and C). Northern blots confirmed that full-length Sall2 transcript was indeed absent in Sall2-deficient mice, with either the N-terminal region or the C-terminal region of Sall2 as a probe (Fig. 1D). Probing with the C-terminal region of Sall2, however, showed a slightly shorter transcript in heterozygous and homozygous mice and was expressed more abundantly in the latter. This transcript was also evident with a Neor probe, indicating that it may be a transcript that read through the poly(A) addition signal of Neor and fused to C-terminal Sall2 (Fig. 1D). We isolated this cDNA from Sall2-deficient mice and found that this was indeed the case. There were several stop codons in the junction and the Sall2 region was out of frame (data not shown). There were no other irregular transcripts indicative of aberrant Sall2 molecules. Therefore, it is unlikely that the functional C-terminal protein of Sall2 was expressed in the mutant mice. Expression of Sall1 and Sall3 was not altered in the absence of Sall2, as determined by using Northern blots (Fig. 1D).
FIG. 1.
Generation of Sall2-deficient mice. (A) Targeting strategy of Sall12 locus. Positions of the zinc finger motifs are indicated by ovals. Restriction sites: B, BamHI; RI, EcoRI; Spe, SpeI; Sma, SmaI; Xh, XhoI. (B) Southern blot analysis of wild-type (+/+), heterozygous (+/−), and homozygous (−/−) Sall2-deficient mice. Tail DNA was digested with EcoRI and hybridized with probe B. (C) Genomic PCR of wild-type (+/+), heterozygous (+/−), and homozygous (−/−) Sall2-deficient mice. The 388-bp band was amplified from the mutant allele, and the 188-bp band was amplified from the wild-type Sall2 genome. The positions of the PCR primers are indicated by arrows in panel A. (D) Northern blotting analysis of Sall genes in Sall2-deficient embryos at 13.5 dpc. Note that N-terminal Sall2 probe gave no signal in Sall2-deficient mice. In the case of the C-terminal Sall2 probe, the Sall2 band was absent in Sall2-deficient mice (solid arrowhead), but a shorter band appeared that also hybridized with Neor probe (open arrowheads).
Expression patterns of Sall2.
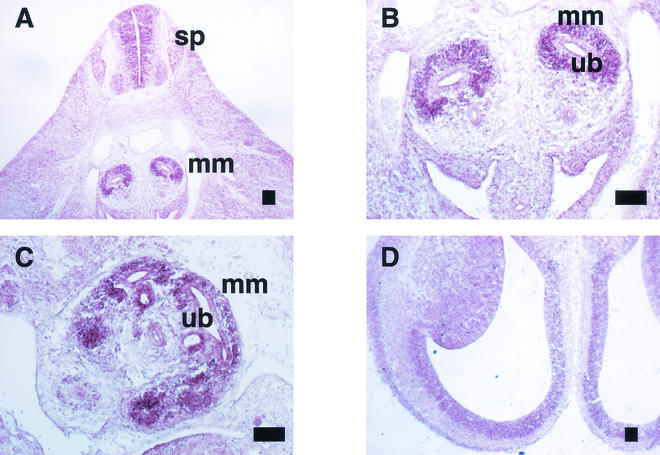
Transverse sections obtained 11.5 dpc showed Sall2 expression in the metanephric mesenchyme surrounding the ureteric bud and subventricular region of the spinal cord (Fig. 2A,B). At 13.5 dpc, Sall2 expression was observed in the mesenchyme around the ureteric buds in the cortical regions of the developing kidney (Fig. 2C). Sall2 was also expressed in the subventricular zone of the brain at 14.5 dpc (Fig. 2D). This expression pattern partly overlaps that of mouse Sall1 (3, 14, 16).
FIG. 2.
Expression of Sall2 in developing embryos. (A) Metanephros and spinal cord at 11.5 dpc (sp, spinal cord; mm, metanephric mesenchyme); (B) metanephros at 11.5 dpc (ub, ureteric bud; mm, metanephric mesenchyme); (C) metanephros at 13.5 dpc; (D) brain at 14.5 dpc. Scale bar, 100 μm.
Normal phenotypes in Sall2-deficient mice.
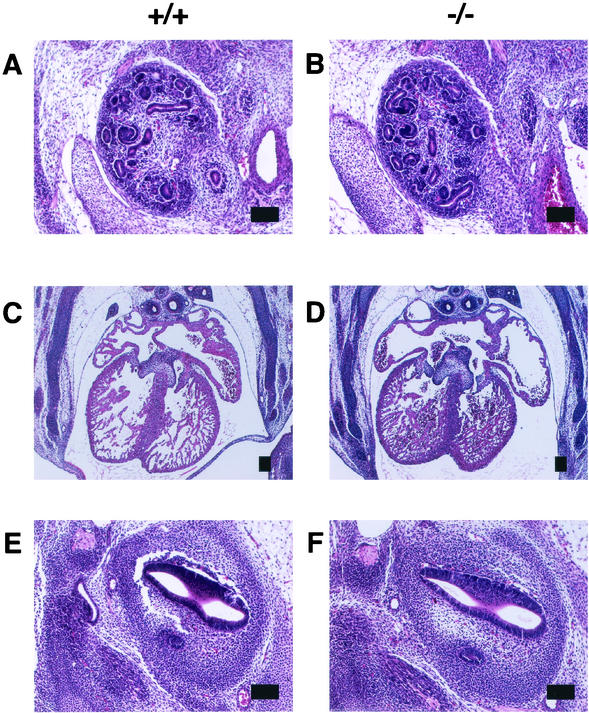
No obvious phenotype was observed in the heterozygous mutants. When heterozyotes were intercrossed, the homozygous mice were of Mendelian frequency (Table 1), they had a normal appearance, and both male and female homozygotes were fertile. We found no abnormalities despite extensive anatomical examinations. Figure 3A to F show an almost-normal histology of the Sall2 mutant kidney, heart, and ears at 13.5 dpc. The hematological parameters of peripheral blood samples were also normal (Table 2).
TABLE 1.
Genotype analysis of mice from Sall2 heterozygous intercrossesa
| Mouse | n | % of total |
|---|---|---|
| +/+ | 26 | 27.7 |
| +/− | 46 | 48.9 |
| −/− | 22 | 23.4 |
DNA was extracted from the tails of 3-week-old mice and analyzed by using PCR as for Fig. 1C.
FIG. 3.
Histology in Sall2-deficient mice at 13.5 dpc. (A and B) Kidneys in wild-type (A) and Sall2-deficient mice (B); (C and D) hearts in wild-type (C) and Sall2-deficient mice (D); (E and F) inner ears in wild-type (E) and Sall2-deficient mice (F). Scale bars, 100 μm.
TABLE 2.
Peripheral blood counts of Sall2-deficient mice
| Mouse (n) | Mean amt (SD)
|
||||
|---|---|---|---|---|---|
| Leukocytes (103/μl) | Erythrocytes (106/μl) | Hemoglobin (g/dl) | Hematocrit (%) | Platelets (103/μl) | |
| +/+ (9) | 8.0 (2.4) | 8.7 (2.9) | 16.1 (1.6) | 52.9 (6.8) | 920 (248) |
| +/− (6) | 10.6 (5.5) | 9.5 (1.4) | 16.6 (1.5) | 51.7 (7.0) | 821 (86) |
| −/− (8) | 9.9 (3.8) | 10.1 (0.6) | 17.1 (0.8) | 54.9 (2.7) | 819 (117) |
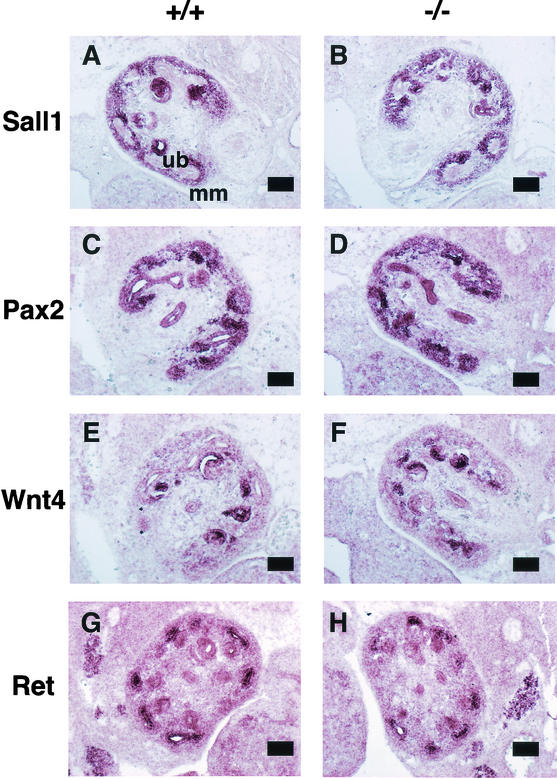
The expression patterns of well-characterized molecular markers of either metanephric mesenchyme or ureteric bud-derived cells were also examined.
Sall1 is expressed in the metanephric mesenchyme, and the expression of Sall1 was not altered in the absence of Sall2, findings consistent with the data in Fig. 1D (Fig. 4A and B).
FIG. 4.
In situ hybridization of molecular markers in 13.5-dpc metanephros of wild-type (left panels [A, C, E, and G]) and Sall2-deficient mice (right columns [B, D, F, and H]). Scale bars, 100 μm. (A and B) Sall1; (C and D) Pax2; (E and F) Wnt4; (G and H) Ret.
Pax2-deficient mice do not develop mesonephric tubules and lack ureteric buds (21). In metanephros at 13.5 dpc, Pax2 is expressed both in the ureteric bud and in the condensed mesenchyme surrounding the ureteric bud (Fig. 4C). In Sall2 mutant mice, the expression of Pax2 was unaltered in both these locations (Fig. 4D).
Wnt4 is required for epithelialization of the induced mesenchyme but not for the initial induction by the ureter (20). Wnt4 is expressed in mesenchymal cells on the sides of the ureteric bud and correlates to the site where the first pretubular aggregates form (Fig. 4E). Sall2-deficient mice showed unaltered Wnt4 expression (Fig. 4F).
Mice deficient in the tyrosine-kinase type receptor, Ret, show a failure of ureteric bud invasion and subsequent failure of mesenchymal differentiation (12, 17-19). Ret was expressed in the ureteric bud in the wild type, and its expression in Sall2 mutant mice was unaltered (Fig. 4G and H). These results indicate that markers of metanephric mesenchyme and ureteric bud were not affected in the absence of Sall2 and that Sall2 is not required for normal kidney development.
There was no limb deformity, anorectal anomaly, or ear anomaly, all of which are characteristic of Townes-Brocks syndrome, which is caused by SALL1 mutation. We suggest that Sall2 is not essential for development and that Sall2 absence may be compensated for by other Sall genes, the expression of which overlaps with that of Sall2.
Human SALL2 is located on chromosome 14q12, possibly overlapping a region of loss of heterozygosity in ovarian cancers (1). In addition, mouse Sall2 binds to polyomavirus large T antigen and was proposed to be a potential tumor suppressor (11). Sall2 mutant mice, however, did not show spontaneous tumor formation for more than 1 year after birth. Tumor formation upon virus inoculation will be required to test the hypothesis that Sall2 is a tumor suppressor.
Kidney defects in mice lacking both Sall1 and Sall2.
Mouse Sall1 is essential for the initial step for metanephros formation: ureteric bud attraction. Hence, kidney agenesis or severe dysgenesis was present in Sall1-deficient mice. Other organs, however, were not affected, although heterozygous mutations of human SALL1 lead to Townes-Brocks syndrome, with features of dysplastic ears, preaxial polydactyly, imperforate anus, and heart anomalies in addition to kidney anomalies. The relative importance of SALL1 over SALL2 and -3 may be higher in humans than in mice, and Sall1 deficiency may be compensated for by Sall2 and -3 in mice.
To address this question, we crossed Sall1 and Sall2 mutants and generated mice lacking both genes. Some pups from a double heterozygous cross were dead perinatally and had kidney abnormalities; most were Sall1 single mutants, but we did find some double homozygotes. To further confirm the phenotypes, we set up pairs of Sall1+/− Sall2−/− mice and found that double mutants were born at a Mendelian frequency (Table 3). All of the double mutants, as well as the Sall1 mutants, were dead perinatally, and they all had kidney abnormalities. Of 12 double mutants (25.0%), 3 had no kidneys or ureters bilaterally. Four mice (33.3%) had unilateral kidney agenesis and hypoplasia on the other side. Five mice (41.7%) had two small remnant kidneys. Histological examination of all of the residual kidneys in the double mutant newborn showed size reduction and multiple cysts, which are comparable to the Sall1 mutants (Fig. 5A to F). At 12.5 dpc, size reduction and impaired ureteric branching were observed in Sall1/2-null mutants, which is also comparable to findings in the Sall1 mutants (Fig. 5G and H and data not shown). Thus, the severity of the kidney impairment of the double mutants was comparable to that of Sall1 single mutants reported earlier (14), indicating that Sall2 absence does not exacerbate the kidney defects caused by Sall1 mutation.
TABLE 3.
Genotype analysis of mice from Sall1+/− Sall2−/− intercrossesa
| Sall1 | Sall2 | n | % of total |
|---|---|---|---|
| +/+ | −/− | 12 | 23.1 |
| +/− | −/− | 28 | 53.8 |
| −/− | −/− | 12 | 23.1 |
DNA was extracted from the tails of newborn mice and analyzed by using PCR.
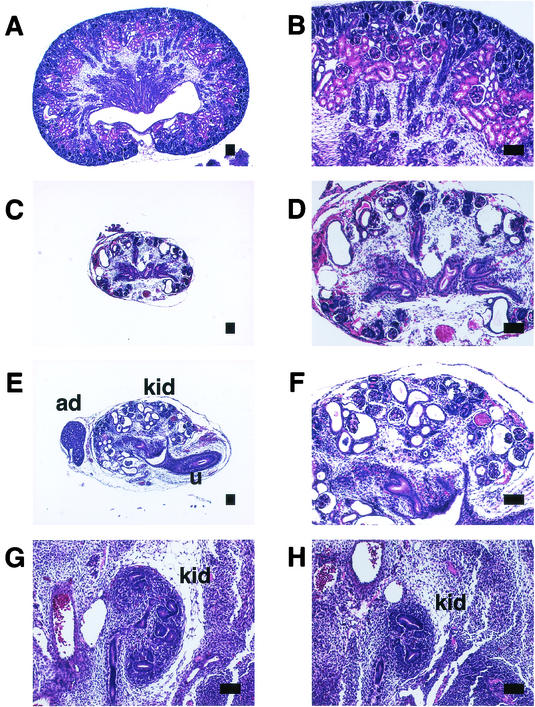
FIG. 5.
Kidney development in Sall1/2 double deficient mice. (A and B) Kidney of wild-type newborn. (C and D) Kidney of Sall1/2 double deficient mice. The kidney is small and contains multiple cysts. (E and F) Kidney of Sall1-deficient mice, which shows a similar histology to Sall1/2 doubly deficient mice. (G) Metanephros in wild-type mice at 12.5 dpc. Branching is evident. (H) Metanephros in Sall1/2 doubly deficient mice at 12.5 dpc. Kidney size and ureteric branching are reduced. kid, kidney; ad, adrenal gland; u, ureter. Scale bars, 100 μm.
To address the role of Sall genes in maintaining renal function in adults, several parameters were examined in aged Sall1+/− Sall2−/− mutants, as well as in Sall1+/− Sall2+/− mutants, Sall2 single mutants, and wild-type control (Table 4). Blood urea nitrogen and creatinine levels in serum were not significantly different among the four groups. Urinary protein was undetectable in all animals tested. Although these parameters are not sensitive enough for detecting minor renal malfunction, the data do suggest that the absence of Sall2 or reducing Sall1 dosage upon Sall2 mutant background does not lead to overt kidney diseases in adult mice.
TABLE 4.
Renal function in adult animalsa
| Sall1 | Sall2 | n | Mean level (SD)
|
|
|---|---|---|---|---|
| BUNb (mg/dl) | Creatinine (mg/dl) | |||
| +/+ | +/+ | 5 | 27.3 (2.4) | 0.30 (0.03) |
| +/− | +/− | 8 | 28.7 (3.4) | 0.29 (0.04) |
| +/+ | −/− | 8 | 24.5 (4.3) | 0.31 (0.04) |
| +/− | −/− | 7 | 24.0 (4.1) | 0.31 (0.03) |
Ten- to fourteen-month-old mice were used.
BUN, blood urea nitrogen.
Furthermore, the double mutants showed no phenotypes of Townes-Brocks syndrome, such as dysplastic ears, preaxial polydactyly, and imperforate anus. These data suggest that the discrepancy of the mutant phenotypes of human SALL1 and mouse Sall1 cannot be explained by compensation by Sall2 in mice. Generation of mice lacking all of the Sall genes will be necessary in order to address the developmental roles of Sall genes.
Acknowledgments
The Division of Stem Cell Regulation is supported by Amgen Limited.
REFERENCES
- 1.Bandera, C. A., H. Takahashi, K. Behbakht, P. C. Liu, V. A. LiVolsi, I. Benjamin, M. A. Morgan, S. A. King, S. C. Rubin, and J. Boyd. 1997. Deletion mapping of two potential chromosome 14 tumor suppressor gene loci in ovarian carcinoma. Cancer Res. 57:513-515. [PubMed] [Google Scholar]
- 2.Buck, A., L. Archangelo, C. Dixkens, and J. Kohlhase. 2000. Molecular cloning, chromosomal localization, and expression of the murine SALL1 ortholog Sall1. Cytogenet. Cell Genet. 89:150-153. [DOI] [PubMed] [Google Scholar]
- 3.Buck, A., A. Kispert, and J. Kohlhase. 2001. Embryonic expression of the murine homologue of SALL1, the gene mutated in Townes-Brocks syndrome. Mech. Dev. 104:143-146. [DOI] [PubMed] [Google Scholar]
- 4.de Celis, J. F., R. Barrio, and F. C. Kafatos. 1996. A gene complex acting downstream of dpp in Drosophila wing morphogenesis. Nature 381:421-424. [DOI] [PubMed] [Google Scholar]
- 5.Jurgens, G. 1988. Head and tail development of the Drosophila embryos involves spalt, a novel homeotic gene. EMBO J. 7:189-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kohlhase, J., M. Altmann, L. Archangelo, C. Dixkens, and W. Engel. 2000. Genomic cloning, chromosomal mapping, and expression analysis of msal-2. Mamm. Genome 11:64-68. [DOI] [PubMed] [Google Scholar]
- 7.Kohlhase, J., S. Hausmann, G. Stojmenovic, C. Dixkens, K. Bink, W. Schulz-Schaeffer, M. Altmann, and W. Engel. 1999. SALL3, a new member of the human spalt-like gene family, maps to 18q23. Genomics 62:216-222. [DOI] [PubMed] [Google Scholar]
- 8.Kohlhase, J., R. Schuh, G. Dowe, R. P. Kuhnlein, H. Jackle, B. Schroeder, W. Schulz-Schaeffer, H. A. Kretzschmar, A. Kohler, U. Muller, M. Raab-Vetter, E. Burkhardt, W. Engel, and R. Stick. 1996. Isolation, characterization, and organ-specific expression of two novel human zinc finger genes related to the Drosophila gene spalt. Genomics 38:291-298. [DOI] [PubMed] [Google Scholar]
- 9.Kohlhase, J., A. Wischermann, H. Reichenbach, U. Froster, and W. Engel. 1998. Mutations in the SALL1 putative transcription factor gene cause Townes-Brocks syndrome. Nat. Genet. 18:81-83. [DOI] [PubMed] [Google Scholar]
- 10.Kuhnlein, R. P., G. Frommer, M. Friedrich, M. Gonzalez-Gaitan, A. Weber, J. F. Wagner-Bernholz, W. J. Gehring, H. Jackle, and R. Schuh. 1994. spalt encodes an evolutionarily conserved zinc finger protein of novel structure which provides homeotic gene function in the head and tail region of the Drosophila embryo. EMBO J. 13:168-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li, D., K. Dower, Y. Ma, Y. Tian, and L. T. Benjamin. 2001. A tumor host range selection procedure identifies p150sal2 as a target of polyoma virus large T antigen. Proc. Natl. Acad. Sci. USA 98:14619-14624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moore, M. W., R. D. Klein, I. Farinas, H. Sauer, M. Armanini, H. Phillips, L. F. Reichardt, A. M. Ryan, K. Carver-Moore, and A. Rosenthal. 1996. Renal and neuronal abnormalities in mice lacking GDNF. Nature 382:76-79. [DOI] [PubMed] [Google Scholar]
- 13.Nellen, D., R. Burke, G. Struhl, and K. Basler. 1996. Direct and long-range action of a DPP morphogen gradient. Cell 85:357-368. [DOI] [PubMed] [Google Scholar]
- 14.Nishinakamura, R., Y. Matsumoto, K. Nakao, K. Nakamura, A. Sato, G. N. Copeland, J. D. Gilbert, A. N. Jenkins, S. Scully, L. D. Lacey, M. Katsuki, M. Asashima, and T. Yokota. 2001. Murine homolog of Sall1 is essential for ureteric bud invasion in kidney development. Development 128:3105-3115. [DOI] [PubMed] [Google Scholar]
- 15.Ott, T., K. H. Kaestner, A. P. Monaghan, and G. Schutz. 1996. The mouse homolog of the region specific homeotic gene spalt of Drosophila is expressed in the developing nervous system and in mesoderm-derived structures. Mech. Dev. 56:117-128. [DOI] [PubMed] [Google Scholar]
- 16.Ott, T., M. Parrish, K. Bond, A. Schwaeger-Nickolenko, and A. P. Monaghan. 2001. A new member of the spalt like zinc finger protein family, Msal3, is expressed in the CNS and sites of epithelial/mesenchymal interaction. Mech. Dev. 101:203-207. [DOI] [PubMed] [Google Scholar]
- 17.Pichel, J. G., L. Shen, H. Z. Sheng, A. C. Granholm, J. Drago, A. Grinberg, E. J. Lee, S. P. Huang, M. Saarma, B. J. Hoffer, H. Sariola, and H. Westphal. 1996. Defects in enteric innervation and kidney development in mice lacking GDNF. Nature 382:73-76. [DOI] [PubMed] [Google Scholar]
- 18.Sanchez, M. P., I. Silos-Santiago, J. Frisen, B. He, S. A. Lira, and M. Barbacid. 1996. Renal agenesis and the absence of enteric neurons in mice lacking GDNF. Nature 382:70-73. [DOI] [PubMed] [Google Scholar]
- 19.Schuchardt, A., V. D'Agati, L. Larsson-Blomberg, F. Costantini, and V. Pachnis. 1994. Defects in the kidney and enteric nervous system of mice lacking the tyrosine kinase receptor Ret. Nature 367:380-383. [DOI] [PubMed] [Google Scholar]
- 20.Stark, K., S. Vainio, G. Vassileva, and A. P. McMahon. 1994. Epithelial transformation of metanephric mesenchyme in the developing kidney regulated by Wnt-4. Nature 372:679-683. [DOI] [PubMed] [Google Scholar]
- 21.Torres, M., E. Gomez-Pardo, G. R. Dressler, and P. Gruss. 1995. Pax-2 controls multiple steps of urogenital development. Development 121:4057-4065. [DOI] [PubMed] [Google Scholar]