Abstract
A classical cellular response to hypoxia is a cessation of growth. Hypoxia-induced growth arrest differs in different cell types but is likely an essential aspect of the response to wounding and injury. An important component of the hypoxic response is the activation of the hypoxia-inducible factor 1 (HIF-1) transcription factor. Although this transcription factor is essential for adaptation to low oxygen levels, the mechanisms through which it influences cell cycle arrest, including the degree to which it cooperates with the tumor suppressor protein p53, remain poorly understood. To determine broadly relevant aspects of HIF-1 function in primary cell growth arrest, we examined two different primary differentiated cell types which contained a deletable allele of the oxygen-sensitive component of HIF-1, the HIF-1α gene product. The two cell types were murine embryonic fibroblasts and splenic B lymphocytes; to determine how the function of HIF-1α influenced p53, we also created double-knockout (HIF-1α null, p53 null) strains and cells. In both cell types, loss of HIF-1α abolished hypoxia-induced growth arrest and did this in a p53-independent fashion. Surprisingly, in all cases, cells lacking both p53 and HIF-1α genes have completely lost the ability to alter the cell cycle in response to hypoxia. In addition, we have found that the loss of HIF-1α causes an increased progression into S phase during hypoxia, rather than a growth arrest. We show that hypoxia causes a HIF-1α-dependent increase in the expression of the cyclin-dependent kinase inhibitors p21 and p27; we also find that hypophosphorylation of retinoblastoma protein in hypoxia is HIF-1α dependent. These data demonstrate that the transcription factor HIF-1 is a major regulator of cell cycle arrest in primary cells during hypoxia.
Mammalian cells have evolved to utilize molecular oxygen for energy production. Cells can respond differently to wide ranges of oxygen through alterations in both their metabolic states and growth rates. In recent years, several lines of evidence have indicated that hypoxia can alter cell proliferation in two distinct ways: via programmed cell death and through growth arrest. In transformed cells, hypoxia can provoke apoptosis via the p53 pathway; ultimately, this can represent a potent mechanism for the selection of p53 mutants in tumor cell populations (30, 34). Nontransformed hypoxic cells, on the other hand, can undergo cell cycle arrest at the G1/S interface without any alteration in their long-term viability (10).
It has been proposed that hypoxically induced cell cycle arrest is caused by inactivation of enzymes responsible for nucleotide synthesis, ultimately inhibiting DNA replication (19, 39). However, inhibition of nucleotide synthesis occurs only under severe hypoxia (0.01% oxygen) or anoxia, but not under moderate hypoxia (0.1 to ∼1% oxygen) (10).
In the moderately hypoxic microenvironment, various biological reactions show significant changes relative to normoxia. Numerous studies on moderate hypoxia have indicated that hypoxia-induced G1 arrest is associated with a decreased activity of certain cyclin-CDK complexes, leading to hypophosphorylation of retinoblastoma protein (Rb) and inhibition of cell cycle progression (10, 16). Although these studies have demonstrated an increase in cyclin-dependent kinase inhibitors, such as p27, and a decrease in cyclin-CDK components, such as cyclin D, cyclin E, and CDK4, most of this data has come from experiments using transformed and/or immortalized cells in which the cell cycle machinery has already been modified (12, 17).
The hypoxia-inducible factor 1 (HIF-1) transcription factor is a major regulator of the hypoxic response. It consists of two distinct basic-helix-loop-helix-PAS transcription factors, HIF-1α and HIF-1β (40-42). HIF-1α is sensitive to decreased oxygen levels and is degraded rapidly by the ubiquitin proteasomal pathway under normoxic conditions. Hypoxia results in an altered availability of HIF-1α to the von Hippel-Lindau protein and ubiquitination, ultimately blocking its degradation (7, 23, 38). This in turn results in nuclear accumulation of the protein and enhancement of its transcriptional activity through binding to enhancer elements in target genes that include vascular endothelial growth factor (9, 35), erythropoietin (15, 41), and phosphoglycerate kinase (PGK) (14, 32).
In an attempt to elucidate the role of HIF-1α in hypoxia-induced cell cycle arrest in primary differentiated cells, we have conditionally targeted HIF-1α in a Cre-loxp-based system (27). Because of the high rate of spontaneous growth arrest in embryonic fibroblasts, in this study, we have focused on both embryonic fibroblasts and another easily accessible primary cell type which is much less susceptible to growth arrest in culture, splenic B cells. To determine how this factor interacts with the tumor suppressor gene p53, we have also added this conditionally targeted allele of HIF-1α into a p53 null background by crosses. Our findings indicate that deletion of HIF-1α results in ablation of hypoxia-induced G1 arrest, demonstrating that hypoxia-induced growth arrest is solely dependent on the action of this transcription factor. Further, we show that this is correlated with a HIF-1α-dependent increase in p27 expression which accompanies hypophosphorylation of Rb. Thus, the HIF-1 transcription factor is necessary for control of the cell cycle during hypoxia via its control of cyclin kinase inhibitor (CKI) function and acts independently of p53.
MATERIALS AND METHODS
Isolation of mEFs and splenic B lymphocytes.
Conditional targeting of the HIF-1α locus was performed via Cre-loxp-based technology as described by Ryan et al. (27). Briefly, mouse embryonic fibroblast (mEF) cells were established from 13.5-day-old embryos of HIF-1αf+/f+ mice [mice with the loxP-flanked (+f/+f) HIF-1α allele], which carry a loxp site on both sides of HIF-1α exon 2 in each allele, and were cultured on 15-cm-diameter dishes until 70 to 80% confluent. The cells were infected by adenovirus expressing either Cre recombinase or β-galactosidase, respectively. Splenic B lymphocytes from mice containing a Cre expression transgene linked to the B-cell-specific CD19 promoter (25) were isolated from 8- to 15-week-old mice by depletion, using the MidiMACS system with anti-CD43 (Ly-48) microbeads, according to the manufacturer's protocol. Purity of the collected B lymphocytes was greater than 97% as determined by fluorescence-activated cell sorting (FACS) analysis with phosphatidylethanolamine-conjugated anti-B220 antibody and fluorescein isothiocyanate (FITC)-conjugated anti-CD3 antibody. These primary cells were analyzed to determine their HIF-1α status by Southern blot analysis and PCR as described previously (27). In some experiments, to elucidate the molecular implications of p53 in hypoxia-induced alterations of the cell proliferation and the possible interactions between p53 and HIF-1, we generated primary mEFs and splenic B lymphocytes from p53−/− HIF-1αf+/f+ mice with or without the endogenous CD19 promoter-driven Cre transgene by intercrossing with p53+/− heterozygous mice.
Cell culture and in vitro stimulation.
The fibroblast cells were maintained in Dulbecco modified Eagle medium containing a high level of glucose supplemented with 10% fetal calf serum (FCS), 25 mM HEPES (pH 7.4), and 1 mM sodium pyruvate. For all experiments, 105 cells were plated in 10-cm-diameter dishes 24 h before experiments. The B lymphocytes were cultured in RPMI 1640 supplemented with 10% FCS, 25 mM HEPES (pH 7.4), and 50 μM 2-mercaptoethanol at a density of 2 × 106 cells per ml. These cells were incubated with goat F(ab′)2 fragment (10 μg/ml) to mouse anti-immunoglobulin M (IgM) (μ chain) (ICN Pharmaceuticals, Inc.) with or without 500 pg of recombinant mouse interleukin-4 (IL-4) (Pharmingen) per ml under either 0.5% (hypoxia) or 20% (normoxia) O2 at 37°C for the desired times.
Cell cycle analysis.
S-phase cells were labeled with 10 μM bromodeoxyuridine (BrdU) for the last 30 min of treatment under either hypoxic or normoxic conditions. Cells were collected and then fixed with ice-cold 70% ethanol for at least 24 h before staining. Double staining with propidium iodide and FITC-conjugated anti-BrdU antibody (Becton Dickinson) was performed according to the manufacturer's protocol as described elsewhere (12) and then used in FACS analysis.
[3H]thymidine incorporation.
[3H]thymidine (0.5 μCi) (Amersham Corp.) was added to 200 μl of stimulated B-lymphocyte suspension in 96 wells during the last 12 h of incubation.
Western blotting.
Cells were harvested quickly after either hypoxic or normoxic incubation for the desired time, washed twice with ice-cold phosphate-buffered saline, frozen in liquid N2, and then stored at −80°C for later use in Western blotting and in vitro kinase assays. For HIF-1α protein detection, cells were directly lysed in buffer A (10 mM HEPES [pH 7.8], 10 mM KCl, 0.1 mM EDTA, 1 mM dithiothreitol [DTT], 0.1% Nonidet P-40 [NP-40]) with protease inhibitors (5 μg of aprotinin per ml, 5 μg of pepstatin per ml, 5 μg of leupeptin per ml, 0.5 mM Pefabloc, 1 mM phenylmethylsulfonyl fluoride) and phosphatase inhibitors (10 mM sodium fluoride, 1 mM sodium orthovanadate, and 20 mM β-glycerophosphate), and then the nuclear proteins were extracted with buffer C (50 mM HEPES [pH 7.8], 420 mM KCl, 0.1 mM EDTA, 1 mM DTT, 5 mM MgCl2, 20% glycerol) containing both protease and phosphatase inhibitors. For other protein analyses, whole-cell lysates were prepared using radioimmunoprecipitation assay buffer (20 mM HEPES [pH 7.8], 350 mM NaCl, 0.5 mM EDTA, 0.1 mM EGTA, 1 mM DTT, 1 mM MgCl2, 20% glycerol, 1% NP-40). Protein concentrations were measured by using the Bradford protein assay (Bio-Rad). The proteins were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (7.5 to 15% polyacrylamide), transferred to polyvinylidene difluoride membranes (Amersham), and subjected to Western blot analysis, as described previously (12). The antibodies against Rb and p21 were obtained from Pharmingen, and p27, cyclin E, and cyclin A antibodies were purchased from Santa Cruz.
In vitro kinase assay.
The CDK2-dependent in vitro kinase assay was performed using histone H1 as a substrate as described elsewhere (12). Briefly, stimulated B lymphocytes were lysed in 1 ml of 0.1% NP-40 lysis buffer containing 50 mM HEPES (pH 7.8), 250 mM NaCl, 1 mM EGTA, 1 mM DTT, and 10% glycerol with protease and phosphatase inhibitors. Three hundred micrograms of the cellular lysate was utilized for the kinase assay. The samples were precleared with protein A agarose (Roche) and were incubated with anti-CDK2 antibody (Pharmingen), anti-cyclin E antibody (Upstate Biotechnology), or anti-cyclin A antibody (Upstate Biotechnology) at 4°C for 1 h with rocking. Protein A agarose was added to the lysate, and the mixtures were incubated overnight at 4°C. After three washes with lysis buffer followed by three washes with kinase reaction buffer (50 mM HEPES [pH 7.4], 1 mM DTT, 10 mM MgCl2), the beads were resuspended in 30 μl of the buffer containing 5 μg of histone H1 (Roche), 20 μM ATP, and 10 μ Ci of [γ32P]ATP (Amersham Corp.). The reaction was terminated after 30 min of incubation at 30°C by adding 15 μl of 3× sample buffer. Samples were run on sodium dodecyl sulfate-15% polyacrylamide gels, fixed in 10% acetic acid, dried, and exposed to films.
Quantitative real-time RT-PCR analysis.
Gene expression for p27, p21, and PGK1 was quantified by real-time reverse transcription-PCR (RT-PCR) relative to the endogenous expression level of 18S rRNA, using the following primer sets and probe which is labeled with fluorescent dye (FAM or VIC). For p27, the forward primer was 5′-GGAGCAGTGTCCAGGGATGA-3′), the reverse primer was 5′-TGTTCTGTTGGCCCTTTTGTT-3′), and the probe was 5′-FAM-AGCGACCTGCTGCAGAAGATTCTTCTTCGCAA-BHQ-3′. For p21, the forward primer was 5′-CAACTCGGGATGTGGCTACA-3′, the reverse primer was 5′-CGTGTTCCGGGTCAAAGC-3′, and the probe was 5′-FAM-TTCTCCTACTGAGAATAACACCACTCCGCCAGA-BHQ-3′. For PGK1, the forward primer was 5′-CAAATTTGATGAGAATGCCAAGACT-3′, the reverse primer was 5′-TTCTTGCTGCTCTCAGTACCACA-3′, and the probe was 5′-FAM-TATACCTGCTGGCTGGATGGGCTTGGACT-BHQ-3′.
RESULTS
HIF-1α gene inactivation in B lymphocytes.
Although a number of groups have utilized mEFs as tools to explore primary cell cycling events, mEFs are characterized by rapid senescence, which makes them a poor model for the study of induced cell cycle arrest. To better understand the role of HIF-1 in cell cycle progression during hypoxia, we have deleted the HIF-1α gene in B lymphocytes, an excellent model system for ex vivo study of the primary cell cycle (36).
To this end, we crossed mice with the floxed (+f/+f) HIF-1α allele (HIF-1αf+/+) with CD19cre (CD19cre) transgenic mice (8, 25). The matched control mice were established by breeding CD19cre mice with either C57BL/6 or HIF-1αf+/+ mice. All comparisons in this study were with Cre-expressing cells from littermates lacking conditional alleles to preclude confusion with possible effects of Cre expression on the cell cycle (20).
Cre expression in CD19cre mice confers B-lineage-specific gene inactivation from the point of onset of pro-B-cell maturation (8, 25). FACS analysis to characterize the splenic B-cell population was performed to determine the effect of HIF-1α deletion. We did not observe any significant changes in the number of splenic B lymphocytes and their maturation in HIF-1αf+/f+ CD19cre mice compared to both CD19cre mice and HIF-1αf+/+ CD19cre mice, indicating that Cre expression itself has little effect on B-cell development (data not shown) and that the loss of HIF-1α in this model does not affect B-cell maturation or proliferation (data not shown).
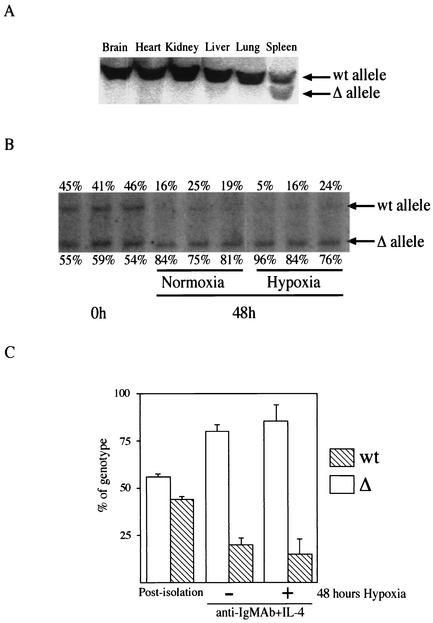
Southern blot analyses showing a partial deletion of the floxed HIF-1α gene in spleen, but not in other tissues, demonstrate the specificity of excision induced by this Cre strain (Fig. 1A). Southern blot analysis and densitometry results showed that the deletion of the floxed gene occurred in ∼56% of purified splenic B lymphocytes from HIF-1αf+/f+ CD19cre mice (Fig. 1B and C). Interestingly, the proportion of B cells with the HIF-1α gene to those with the HIF-1α gene deleted changed over time in culture in response to mitogen; after 48 h, the average number of HIF-1α null cells had increased to 83% (Fig. 1B and C). A similar phenomenon was seen during expansion of B cells with the HIF-1α gene deleted under hypoxic conditions (Fig. 1B and C). This selective advantage for the null cells in the course of mitogenically induced proliferation indicated that HIF-1α is involved in regulating survival and/or cell cycle progression in B cells ex vivo.
FIG. 1.
Deletion of the HIF-1α allele in B cells in vivo and in vitro. (A) Tissue-specific deletion of HIF-1α caused by CD19cre expression. Southern blot analysis of DNA from different organs of HIF-1α null (HIFDFCD19cre) mice was performed. DNA was digested with EcoRI and PstI and probed with a fragment of HIF-1α intron 1 as described elsewhere (27). The positions of DNA with the HIF-1α floxed allele (wt) and DNA in which the HIF-1α allele had been deleted (Δ) are shown to the right of the blot. (B) Increased HIF-1α-deficient B cells in response to mitogen. B cells isolated from HIF-1α null (HIFDFCD19cre) mice were stimulated with anti-IgM antibody (Ab) (10 μg/ml) plus IL-4 (0.5 ng/ml) for 48 h under either normoxia or hypoxia. After the stimulation, DNA was extracted and analyzed as described above for panel A. The data shown are representative results from three different preparations. The values immediately above and below the blot are the percentages of wild-type B cells and HIF-1α null cells, respectively. Note that HIF-1α null cells expanded preferentially in response to mitogen under both normoxia and hypoxia compared to wild-type B cells in culture. (C) Quantification of the proliferative advantage of HIF-1α null B cells in culture. The histogram is a graphic representation of information from panel B. Error bars show standard errors.
Loss of HIF-1α in mEFs results in loss of S-phase arrest during hypoxia.
Although earlier studies on a variety of transformed and nontransformed, immortalized cells have demonstrated that hypoxia induces cell cycle arrest and/or apoptosis (10, 30, 34), it is unclear whether primary cells respond to hypoxia by G1 arrest. This is because previous studies of this phenomenon have utilized mEFs to model primary cell behavior; these cells are very prone to spontaneous growth arrest and so make study of induced G1 arrest difficult (33). Initially, however, we used mEFs conditionally deleted for HIF-1α (27) to determine how hypoxia-induced growth arrest affects the cell cycle in the presence and absence of HIF-1α. This was done via detection of DNA synthesis and simultaneous staining for DNA content.
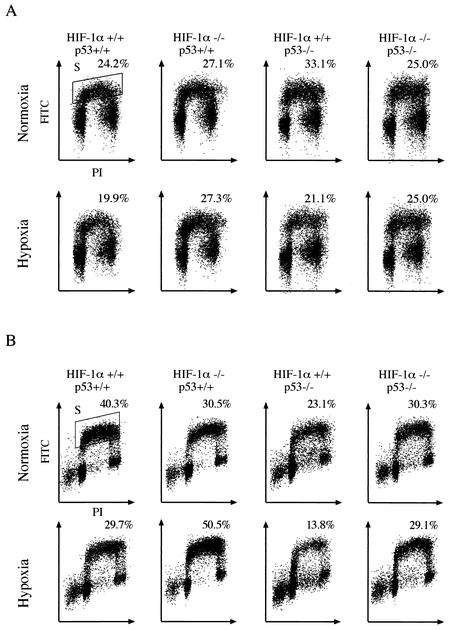
After 24 h of incubation under normoxic conditions, approximately 24% of wild-type mEFs enter S phase. As seen in Fig. 2A, hypoxia (0.5% oxygen) reduced the percentage of cells undergoing the G1/S transition in wild-type cells. Hypoxia-treated HIF-1α null mEFs did not demonstrate any significant change in S-phase entry relative to normoxia-treated mEFs (Fig. 2A). These data clearly indicate that mEFs respond to decreased oxygen concentration through a clear but moderate change in S-phase entrance and that this occurs in a HIF-1α-dependent manner.
FIG. 2.
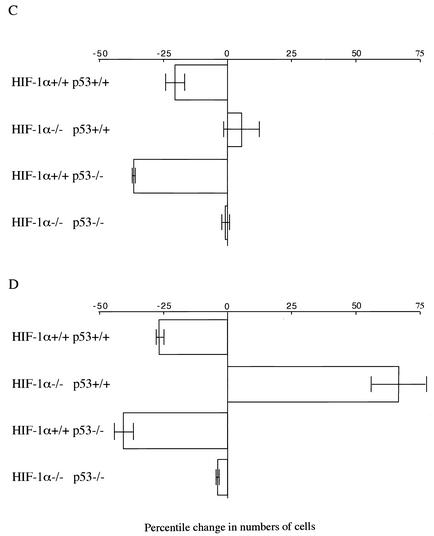
Cell cycle response to hypoxia in HIF-1α null mEFs and B cells. (A) Hypoxic arrest in mEFs. Primary mEF cells from p53 wild-type (p53+/+) and p53 knockout (p53−/−) mice with floxed alleles at the HIF-1α locus were infected with adenovirus expressing either Cre recombinase or β-galactosidase (as a control for adenovirus expression). The cells were incubated first in the presence of either 0.5 or 20% oxygen for 24 h and then with BrdU for 30 min and then subjected to cell cycle analysis. The x axis shows red propidium iodide (PI) fluorescence; the y axis shows green FITC fluorescence associated with anti-BrdU antibody. The percentage of cells positive for BrdU (S-phase cells) is indicated. Note that 0.5% hypoxia induced G1 arrest in wild-type (HIF-1α+/+ p53+/+) mEF cells. A deletion of HIF-1α allowed progress beyond the G1 checkpoint during hypoxia. Primary p53−/− mEF cells demonstrated pronounced hypoxia-induced cell cycle arrest. Inactivation of the HIF-1α gene causes a loss of growth arrest in p53−/− cells similar to that seen in p53+/+ cells. (B) Hypoxic arrest in splenic B cells. B cells isolated from either wild-type (CD19cre) or HIF-1α null (HIFDFCD19cre) mice were incubated with 0.5% (hypoxia) or 20% oxygen (normoxia) for 48 h in the presence of anti-IgM antibody (10 μg/ml) plus IL-4 (0.5 ng/ml) and then were subjected to cell cycle analysis as described above for panel A. The hypoxic responses in p53−/− B cells were also analyzed. The percentage of cells in S phase is indicated. Note that 0.5% hypoxia inhibited G1/S transition in wild-type B cells, while circa 50% of B cells with the HIF-1α gene deleted entered S phase under hypoxia. A deletion of p53 gene enhanced the hypoxia-induced HIF-1α-dependent G1 arrest. However, double-knockout cells do not respond in any fashion to hypoxia. (C and D) Graphic representation of the data shown in panels A and B, respectively. The percentile change in the number of cells in S phase during hypoxia relative to that during normoxia is shown. Error bars show standard errors.
Previous studies have linked hypoxia-induced cell cycle arrest and p53 status (13). To better understand the importance of p53 on the HIF-1-regulated G1/S transition under hypoxia, we generated both double-knockout primary mEFs (p53−/− HIF-1α−/− mEFs) and p53−/− mEFs. The mEF cells nullizygous for p53 still arrest in response to hypoxia, but interestingly, hypoxia-induced G1 arrest was completely absent in HIF-1α−/− p53−/− cells (Fig. 2A). This demonstrates that in these cells, the loss of HIF-1α is the sole determinant of hypoxically induced growth arrest and that p53 is not acting to prevent entry into S phase during hypoxia.
Loss of HIF-1α in B cells results in progression into S phase during hypoxia.
As shown in Fig. 2B, circa 40% of B cells from wild-type mice entered S phase after 48 h of normoxic incubation in response to mitogen. In contrast, hypoxic treatment suppressed S-phase entry of wild-type B cells, and only 30% are in S phase after 48 h of culture in 0.5% oxygen. In HIF-1α null B-cell cultures, lower numbers of cells were seen in S phase during normoxia relative to those of wild-type cells, but strikingly, the number of cells in S phase increased during hypoxia (Fig. 2B). Thus, in B cells, as in mEFs, there is a complete release from hypoxia-induced G1 arrest caused by the deletion of HIF-1α. Again, we evaluated the effects of p53 loss on hypoxia-induced cell cycle alterations, using B cells lacking endogenous p53. In p53 null B cells, hypoxia still causes an increase in growth arrest (Fig. 2B). However, p53 and HIF-1α double-knockout B cells did not display any significant changes in response to hypoxia. Thus, the increase in S phase seen following the loss of HIF-1α under hypoxia is abolished through the deletion of p53. This in turn indicates that p53 is responsible for the regulation of a factor that promotes S-phase entry in the absence of HIF-1α and argues for a synergistic relationship between these two factors during hypoxia-induced G1 arrest. The effects of loss of HIF-1α and p53 alone and together on the cell cycle during hypoxia in mEFs and B cells are summarized in Fig. 2C and D.
Loss of HIF-1α increases proliferation of B lymphocytes under hypoxia.
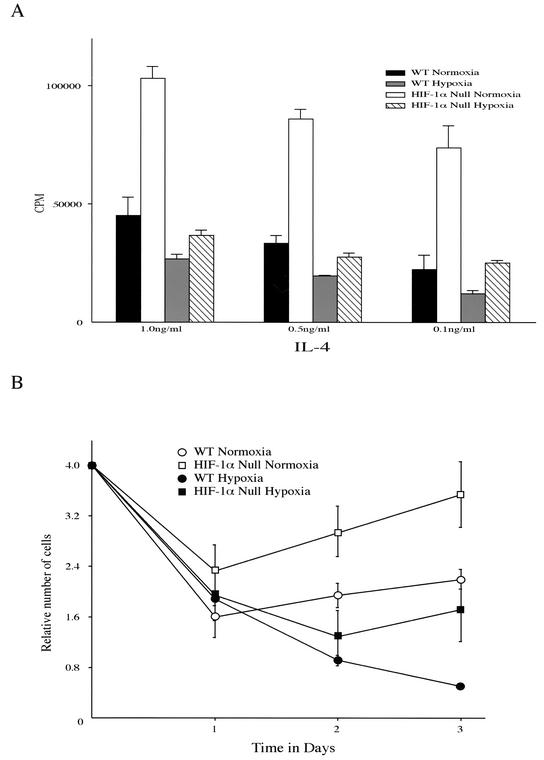
To determine the effect of HIF-1α gene deletion on B-cell proliferation in vitro, isolated splenic B lymphocytes from either control mice or HIF-1α null mice were stimulated with anti-IgM antibody plus IL-4 under either normoxic (20% O2) or hypoxic (0.5% O2) conditions. As seen in Fig. 3A, HIF-1α null B cells showed increased thymidine incorporation compared to wild-type B cells in both environments. As shown in Fig. 3B, cell numbers in all strains began to increase 2 days after isolation under normoxic conditions; B cells lacking HIF-1α were able to grow much more rapidly compared to wild-type cells. Under hypoxic conditions, growth of wild-type cells was completely inhibited; however, HIF-1α null cells continued to proliferate after a 2-day lag. This indicates that loss of HIF-1α is acting to release B cells from a hypoxia-induced growth arrest. These results are consistent with the FACS analysis results described above and in Fig. 2.
FIG. 3.
Enhanced expansion of HIF-1α null B cells in response to mitogen. (A) Thymidine incorporation upon mitogenic stimulation of isolated B cells under either normoxia or hypoxia. B cells isolated from either HIF-1α null (HIFDFCD19cre) or wild-type (WT) (CD19cre) mice were stimulated with anti-IgM antibody (10 μg/ml) plus IL-4 (1, 0.5, or 0.1 ng/ml) under either normoxia or hypoxia for 48 h. The incubation with [3H]thymidine was performed for the last 12 h. The results shown are the average values for two CD19cre and HIFDFCD19cre mice. Note that B cells in which the HIF-1α gene had been deleted (HIF-1α null B cells) incorporated more thymidine than wild-type cells under either normoxia or hypoxia in response to mitogenic stimulation. (B) Proliferation curves of isolated splenic B cells under normoxia and hypoxia. HIF-1α null B cells show increased growth rates during normoxic and hypoxic culture. Isolated B cells were incubated with anti-IgM antibody (10 μg/ml) plus IL-4 (0.5 ng/ml) under either normoxia or hypoxia for 3 days. Viable cells were counted after staining with trypan blue. The results shown are the average values for two wild-type (WT) (CD19cre) and HIF-1α null (HIFDFCD19cre) mice.
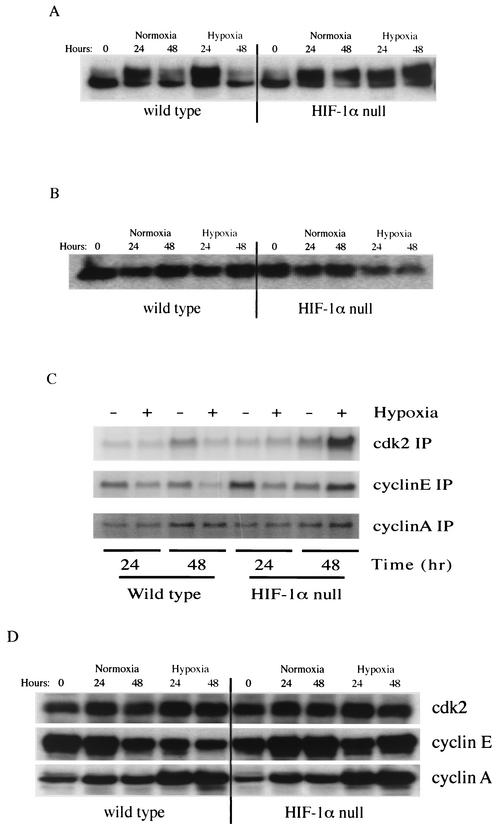
HIF-1α is required for hypoxia-induced inhibition of Rb phosphorylation.
Mitogenic stimulation of B cells under normoxic conditions results in hyperphosphorylation of the retinoblastoma gene product Rb by cyclin-associated kinases. This occurs in both wild-type and HIF-1α null B cells under normoxic conditions and is associated with S-phase entry (Fig. 4A). Data from a number of groups have indicated that hypoxia acts to prevent hyperphosphorylation of Rb and shown that this hypophosphorylation is correlated with growth arrest of hypoxic cells.
FIG. 4.
Hypoxic effects on cell cycle regulatory proteins in primary B cells. (A) Rb hyperphosphorylation in hypoxic HIF-1α null B cells. Isolated B cells were stimulated with anti-IgM antibody (10 μg/ml) plus IL-4 (0.5 ng/ml) under either normoxia or hypoxia and centrifuged, and pellets were collected at the indicated time points after stimulation. Western blotting analysis was performed with antibodies against Rb. As can be seen, at 48 h, hyperphosphorylation occurs in HIF-1α null B cells in hypoxia, but in wild-type cells there is virtually no detectable hyperphosphorylated Rb. (B) Following extended incubation in hypoxic conditions, p27 expression is elevated only in wild-type B cells, not in HIF-1α null B cells. (C) Hypoxia induces cyclin E kinase activity in HIF-1α null B cells after 48 h. Isolated B cells were stimulated, and after stimulation, samples were immunoprecipitated (IP) with the indicated antibodies. Kinase assays were performed as described in Materials and Methods. As can be seen, loss of HIF-1α activity is accompanied by increased cyclin E- and CDK2-associated kinase activity following prolonged hypoxia. (D) Increased cyclin E expression in HIF-1α null B cells under hypoxia. Hypoxia inhibits the mitogen-stimulated accumulation of cyclin E protein in wild-type cells but not in HIF-1α null cells. Cyclin A and CDK2 expression in both cell types is not affected by HIF-1 status.
Under hypoxic conditions in wild-type cells, the hyperphosphorylated form of Rb is not seen (Fig. 4A) and only hypophosphorylated Rb is detected. In HIF-1α null cells, however, hyperphosphorylated Rb protein is still the predominant form of Rb detected under hypoxic conditions (Fig. 4A). This demonstrates that HIF-1α is specifically linked to negative regulation of Rb phosphorylation during hypoxia.
p27 levels are reduced during hypoxia in HIF-1α null cells.
The CKIs p21 (5, 28), p27 (10), and p57Kip2 (29) are up-regulated by hypoxia and may be mediators of hypoxia-induced cell cycle arrest. Consistent with previous reports (36), abundant expression of p27 was detected in samples collected from freshly isolated splenic B lymphocytes irrespective of HIF-1α status, as shown in Fig. 4B. Mitogenic stimulation under normoxia reduced the amount of p27 protein in both wild-type and HIF-1α null B cells. However, hypoxia prevented the mitogenic reduction in p27 levels in wild-type B cells, whereas HIF-1α-deficient cells exhibited significant reductions in p27 levels (Fig. 4B). A similar HIF-1α-dependent reduction of p27 levels was seen in mEFs (data not shown). p21 protein levels were not affected by HIF-1α status (data not shown); both genotypes showed an increase in expression of p21 after 48 h of hypoxia.
Collectively, these data suggest that, under hypoxia, HIF-1 regulates the G1/S transition through an inhibition of cyclin E/CDK2 activity, presumably by an increase in p27 expression that ultimately prevents Rb hyperphosphorylation.
CDK inhibition during hypoxia is lost in HIF-1α null cells.
Rb protein is phosphorylated at multiple sites by cyclin-CDK complexes during cell cycle progression, and CDK2 kinase activity is an important aspect of the transition from late G1 to S phase. CDK2 kinase activity in the wild-type cells under hypoxia is significantly decreased (Fig. 4C), as has been shown by others (9, 11). In contrast, hypoxia-treated HIF-1α null cells displayed a significant increase in CDK activity. Surprisingly, the kinase activity observed in HIF-1α-deficient cells treated under hypoxia for 48 h was higher than that of normoxic cells (Fig. 4C).
Amounts of CDK2 protein were unaffected by either oxygen concentration or HIF-1α expression (Fig. 4D). As CDK2 is known to associate with cyclin A and cyclin E during cell cycle progression, we also examined cyclin A- and cyclin E-associated in vitro kinase activities. The cyclin A-associated kinase activity did not change (Fig. 4C); however, under hypoxia, cells lacking HIF-1α displayed a significant increase in cyclin E-related kinase activity compared to wild-type cells (Fig. 4C). In addition, cyclin E, but not cyclin A, expression was increased during hypoxia in HIF-1α null cells (Fig. 4D).
HIF-1α is required for hypoxic induction of p27 and p21 transcription.
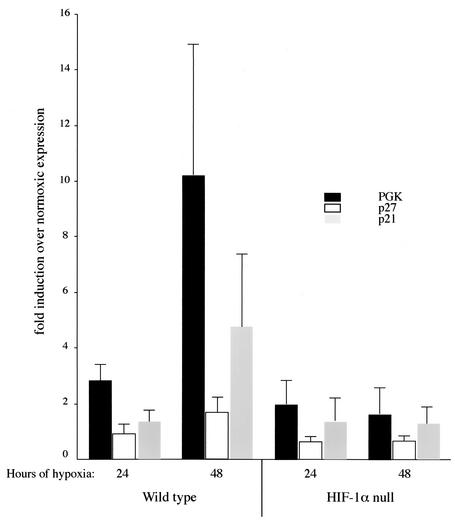
Our present data imply that hypoxic induction of p27 via HIF-1α activation is involved in cell cycle arrest. To determine whether this occurs due to transcriptional up-regulation, we examined whether mEFs and isolated B cells could respond to hypoxic conditions with changes in the transcription of genes known to be regulated by HIF-1, i.e., PGK (6, 26). As shown in Fig. 5, PGK expression is up-regulated by hypoxia in B cells. This indicates that the standard HIF-1α pathways of hypoxia-induced gene regulation are present in these cells.
FIG. 5.
Hypoxia-induced p21 and p27Kip1 transcriptional activation is dependent on HIF-1α. Real-time RT-PCR was performed to determine the relative amounts of the target transcription messages in B cells. The data are the means ± standard deviations (n = 3) of the ratio of message expressed under hypoxia compared to that under normoxia after normaliz against the rRNA as an internal control. Note that PGK expression was induced significantly by hypoxic treatment in wild-type cells, but not in HIF-1α null cells. In B cells from wild-type mice, the expression of both p21 and p27 was increased following 48 h of hypoxia, whereas induction in HIF-1α-deficient B cells was completely abolished. ANOVA tests of significance: null cells differ from the wild type at P <0.05 for all three genes.
To determine whether HIF-1α regulated transcription of either of the CKIs, we also assayed for their expression at the mRNA level. We found that both p27 and p21 are up-regulated by hypoxia and that this occurs in a HIF-1α-dependent fashion, albeit less robustly than is the case for PGK. This indicates that HIF-1 is affecting the transcription of two critical CKIs via alterations of hypoxia-induced changes in message levels. Clearly, since only p27, and not p21, shows evidence of this HIF-1α-dependent up-regulation at the protein level, there are likely other aspects of hypoxia-induced regulation of these CKIs. However, this finding demonstrates that loss of HIF-1α alters transcriptional regulation of these messages, which in turn provides a mechanism to explain the loss of hypoxia-induced growth arrest in the absence of HIF-1α.
DISCUSSION
Cell growth arrest is essential for maintenance of viability, particularly under environmental conditions which prevent normal operation of the cell division machinery. Decreased microenvironmental oxygen levels are potent signals for growth arrest, and this is likely a critical aspect of both normal cellular development and the expansion of tumors. In previous work, a number of links between cell cycle components and hypoxically induced growth arrest have been established (1, 4, 5, 10, 17, 21, 29). These include demonstrations that CKIs, such as p27, p21, and p57, are up-regulated by hypoxia and that Rb is hypophosphorylated under hypoxic conditions and work showing diverse links between the tumor suppressor p53 and the transcription factor HIF-1α (2-4, 24, 28, 37).
HIF-1 regulates the transcription of a diverse group of genes, including angiogenic factors, metabolic enzymes, and genes of unknown function (4, 5, 26, 32); however, the role of HIF-1 in regulating genes involved in cell cycle arrest has been unclear. Work by Gardner et al. (10) argued that p27 was a major determinant of hypoxically induced growth arrest, albeit in a HIF-1α-independent manner. Other recent work by Green and colleagues (12) showed that although up-regulated by hypoxia, the CKIs p27 and p21 did not affect hypoxic growth arrest in immortalized fibroblasts. Previous work from our laboratory has shown that loss of HIF-1α in transformed fibroblasts slows tumor growth (27) and inhibits cell proliferation under hypoxic conditions (31). In addition, loss of HIF-1 lessens the ability of cells to produce ATP under hypoxia (31), likely due to its role in controlling the glycolytic pathway; this in turn would argue that cells lacking HIF-1 should be retarded in cell cycle progression from the standpoint of energy metabolism alone. Thus, our finding that HIF-1 promotes cell cycle arrest in two diverse primary cell types is surprising, in particular since it is correlated with a HIF-1α-dependent induction in p21 and p27 and a decrease in CDK2 activity and Rb hypophosphorylation.
As mentioned above, work by Gardner et al. (10) showed that p27 levels increase during hypoxia in mEFs in what they argue is a HIF-1α-independent manner. This observation was based on two lines of evidence: that induction of HIF-1 did not cause growth arrest in normoxic conditions and that HIF-1α null embryonic stem (ES) cells showed no alterations in G1 arrest under hypoxic conditions. The first observation, that induced HIF-1 expression does not cause growth arrest, is perhaps best explained in light of our data by the necessity for a cofactor(s) induced by hypoxia to accompany HIF-1 expression in order for growth arrest to occur. Another possible explanation is that induction of HIF-1α by overexpression under normoxic conditions or by hypoxia mimetics, such as cobalt, does not fully duplicate the hypoxic environment necessary for full activation of HIF-1α; this has indeed been found by a number of groups (18, 22).
The second observation, that cultured ES cells do not show changes in growth arrest in the absence of HIF-1α (5), may be due to the observed differences in growth regulation of these cells compared to other cell types; they could also be due to clonal differences in the HIF-1α null ES cells themselves. In either case, our data clearly indicate that both nullizygous mEFs created by exogenous expression of Cre recombinase and nullizygous splenic B cells isolated directly from animals are defective in growth arrest in a clearly HIF-1α-dependent manner.
Our finding that p53 did not contribute directly to hypoxia-induced growth arrest is similar to other recent findings (10, 11). Interestingly, however, we did find that there was an interaction between p53 and HIF-1α in cell cycle regulation. B cells which have lost HIF-1α alone demonstrate a large increase in progression to S phase during hypoxia, whereas B cells that are nullizygous for HIF-1α and p53 together show no change in cell cycle profiles for cells in hypoxia compared to those in normoxia. Thus, the increased progression into S phase in B cells caused by loss of HIF-1α is lost following loss of p53.
This is a difficult finding to interpret but may be due to a role for p53 in regulating HIF-1α stability, as described by Ravi et al. (24). In that study, it was found that loss of p53 enhanced HIF-1α stability; if this phenomenon is extended to HIF-2α, it may be that loss of p53 results in an increased stability of HIF-2α as well, and thus an increased level of cell cycle arrest. This phenomenon, of p53 control of HIF-1α levels, may also explain why we have found increased levels of cell cycle arrest in HIF-1α wild-type, p53 null B cells. In that case, in the absence of p53 but the presence of HIF-1α, the percentage of cells in S phase drops from 30 to 14 compared to cells in which both genes are intact. Interestingly, though, and perhaps in common with Gardner et al. (10), we see no such effect in HIF-1α wild-type, p53 null mEFs.
We find that CDK2 kinase activity is completely inhibited in hypoxic cells, as has been described previously (10, 12). Regulation of this activity is known to be mediated by multiple mechanisms, including binding of cyclins by the kinase, interaction with CKIs, and phosphorylation and dephosphorylation at threonine/tyrosine residues of the protein. Our current data, showing an inverse correlation between p27 expression and CDK2 activity in wild-type cells, confirm the finding of Gardner et al. (10), on the effects of hypoxia on kinase activity acting through p27. Our finding that p27 elevation during hypoxia is HIF-1α dependent extend that observation and indicate that HIF-1 is the primary determinant of hypoxic alteration of cell cycle control.
This finding is in contrast to much other recent work, including our own, indicating that loss of HIF-1α results in slower growth of tumors and transformed cell lines (27, 31). The most likely explanation for this discrepancy is the striking differences between cell cycle control in immortalized and primary cell types. Such differences have been described extensively in regards to other growth inhibitory conditions, including hypoxia (10-12). That HIF-1α plays such different roles in primary and transformed cells was unexpected, however, and is likely an indicator that the hypoxic response and HIF-1-dependent growth arrest are two of the major hurdles that transformed cells must overcome in the process of tumorigenesis. There is currently no evidence for deletion of HIF-1 in tumors, indicating that modulation, rather than deletion, of the hypoxic response is selected for during tumorigenesis. This is consistent, in fact, with its role as a positive factor in the growth of transformed cells in vivo (27).
In the work presented here, we have shown that in two primary differentiated cell types, HIF-1α is the sole determinant of hypoxia-induced growth arrest and is correlated with alterations in p27 expression, p27 and p21 transcription, and Rb phosphorylation. These findings indicate that HIF-1 regulates cell fate during hypoxia to a surprising degree and should allow further determination of how altering HIF-1 levels may affect disease states that are marked by oxygen deprivation and ischemia.
REFERENCES
- 1.Amellem, O., T. Stokke, J. A. Sandvik, and E. O. Pettersen. 1996. The retinoblastoma gene product is reversibly dephosphorylated and bound in the nucleus in S and G2 phases during hypoxic stress. Exp. Cell Res. 227:106-115. [DOI] [PubMed] [Google Scholar]
- 2.An, W. G., M. Kanekal, M. C. Simon, E. Maltepe, M. V. Blagosklonny, and L. M. Neckers. 1998. Stabilization of wild-type p53 by hypoxia-inducible factor 1alpha. Nature 392:405-408. [DOI] [PubMed] [Google Scholar]
- 3.Blagosklonny, M. V., W. G. An, L. Y. Romanova, J. Trepel, T. Fojo, and L. Neckers. 1998. p53 inhibits hypoxia-inducible factor-stimulated transcription. J. Biol. Chem. 273:11995-11998. [DOI] [PubMed] [Google Scholar]
- 4.Blagosklonny, M. V. 2001. Hypoxia-inducible factor: Achilles' heel of antiangiogenic cancer therapy. Int. J. Oncol. 19:257-262. [DOI] [PubMed] [Google Scholar]
- 5.Carmeliet, P., Y. Dor, J. M. Herbert, D. Fukumura, K. Brusselmans, M. Dewerchin, M. Neeman, F. Bono, R. Abramovitch, P. Maxwell, C. J. Koch, P. Ratcliffe, L. Moons, R. K. Jain, D. Collen, E. Keshert, and E. Keshet. 1998. Role of HIF-1alpha in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature 394:485-490. [DOI] [PubMed] [Google Scholar]
- 6.Chen, C., N. Pore, A. Behrooz, F. Ismail-Beigi, and A. Maity. 2001. Regulation of glut1 mRNA by hypoxia-inducible factor-1. Interaction between H-ras and hypoxia. J. Biol. Chem. 276:9519-9525. [DOI] [PubMed] [Google Scholar]
- 7.Cockman, M. E., N. Masson, D. R. Mole, P. Jaakkola, G. W. Chang, S. C. Clifford, E. R. Maher, C. W. Pugh, P. J. Ratcliffe, and P. H. Maxwell. 2000. Hypoxia inducible factor-alpha binding and ubiquitylation by the von Hippel-Lindau tumor suppressor protein. J. Biol. Chem. 275:25733-25741. [DOI] [PubMed] [Google Scholar]
- 8.de Alboran, I. M., R. C. O'Hagan, F. Gartner, B. Malynn, L. Davidson, R. Rickert, K. Rajewsky, R. A. DePinho, and F. W. Alt. 2001. Analysis of C-MYC function in normal cells via conditional gene-targeted mutation. Immunity 14:45-55. [DOI] [PubMed] [Google Scholar]
- 9.Forsythe, J. A., B. H. Jiang, N. V. Iyer, F. Agani, S. W. Leung, R. D. Koos, and G. L. Semenza. 1996. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol. Cell. Biol. 16:4604-4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gardner, L. B., Q. Li, M. S. Park, W. M. Flanagan, G. L. Semenza, and C. V. Dang. 2001. Hypoxia inhibits G1/S transition through regulation of p27 expression. J. Biol. Chem. 276:7919-7926. [DOI] [PubMed] [Google Scholar]
- 11.Graeber, T. G., J. F. Peterson, M. Tsai, K. Monica, A. J. Fornace, Jr., and A. J. Giaccia. 1994. Hypoxia induces accumulation of p53 protein, but activation of a G1-phase checkpoint by low-oxygen conditions is independent of p53 status. Mol. Cell. Biol. 14:6264-6277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Green, S. L., R. A. Freiberg, and A. J. Giaccia. 2001. p21Cip1 and p27Kip1 regulate cell cycle reentry after hypoxic stress but are not necessary for hypoxia-induced arrest. Mol. Cell. Biol. 21:1196-1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hammond, E. M., N. C. Denko, M. J. Dorie, R. T. Abraham, and A. J. Giaccia. 2002. Hypoxia links ATR and p53 through replication arrest. Mol. Cell. Biol. 22:1834-1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iyer, N. V., K. L. Kotch, F. Agani, S. W. Leung, E. Laughner, R. H. Wenger, M. Gassmann, J. D. Gearhart, A. M. Lawler, A. Y. Yu, and G. L. Semenza. 1998. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1 alpha. Genes Dev. 12:149-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang, B. H., E. Rue, G. L. Wang, R. Roe, and G. L. Semenza. 1996. Dimerization, DNA binding, and transactivation properties of hypoxia-inducible factor 1. J. Biol. Chem. 271:17771-17778. [DOI] [PubMed] [Google Scholar]
- 16.Krtolica, A., N. A. Krucher, and J. W. Ludlow. 1998. Hypoxia-induced pRB hypophosphorylation results from downregulation of CDK and upregulation of PP1 activities. Oncogene 17:2295-2304. [DOI] [PubMed] [Google Scholar]
- 17.Krtolica, A., N. A. Krucher, and J. W. Ludlow. 1999. Molecular analysis of selected cell cycle regulatory proteins during aerobic and hypoxic maintenance of human ovarian carcinoma cells. Br. J. Cancer 80:1875-1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lando, D., D. J. Peet, D. A. Whelan, J. J. Gorman, and M. L. Whitelaw. 2002. Asparagine hydroxylation of the HIF transactivation domain a hypoxic switch. Science 295:858-861. [DOI] [PubMed] [Google Scholar]
- 19.Loffler, M. 1989. The biosynthetic pathway of pyrimidine (deoxy)nucleotides: a sensor of oxygen tension necessary for maintaining cell proliferation? Exp. Cell Res. 182:673-680. [DOI] [PubMed] [Google Scholar]
- 20.Loonstra, A., M. Vooijs, H. B. Beverloo, B. A. Allak, E. van Drunen, R. Kanaar, A. Berns, and J. Jonkers. 2001. Growth inhibition and DNA damage induced by Cre recombinase in mammalian cells. Proc. Natl. Acad. Sci. USA 98:9209-9214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ludlow, J. W., R. Howell, and H. C. Smith. 1993. Hypoxic stress induces reversible hypophosphorylation of pRB and reduction in cyclin A abundance independent of cell cycle progression. Oncogene 8:331-339. [PubMed] [Google Scholar]
- 22.Masson, N., C. Willam, P. H. Maxwell, C. W. Pugh, and P. J. Ratcliffe. 2001. Independent function of two destruction domains in hypoxia-inducible factor-alpha chains activated by prolyl hydroxylation. EMBO J. 20:5197-5206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maxwell, P. H., M. Wiesener, G. W. Chang, S. C. Clifford, E. C. Vaux, M. E. Cockman, C. C. Wykoff, C. W. Pugh, E. R. Maher, and P. J. Ratcliffe. 1999. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature 399:271-275. [DOI] [PubMed] [Google Scholar]
- 24.Ravi, R., B. Mookerjee, Z. M. Bhujwalla, C. H. Sutter, D. Artemov, Q. Zeng, L. E. Dillehay, A. Madan, G. L. Semenza, and A. Bedi. 2000. Regulation of tumor angiogenesis by p53-induced degradation of hypoxia-inducible factor 1alpha. Genes Dev. 14:34-44. [PMC free article] [PubMed] [Google Scholar]
- 25.Rickert, R. C., J. Rose, and K. Rajewsky. 1997. B lymphocyte-specific, Cre-mediated mutagenesis in mice. Nucleic Acids Res. 25:1317-1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ryan, H. E., J. Lo, and R. S. Johnson. 1998. HIF-1 alpha is required for solid tumor formation and embryonic vascularization. EMBO J. 17:3005-3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ryan, H. E., M. Poloni, W. McNulty, D. Elson, M. Gassmann, J. M. Arbeit, and R. S. Johnson. 2000. Hypoxia-inducible factor-1alpha is a positive factor in solid tumor growth. Cancer Res. 60:4010-4015. [PubMed] [Google Scholar]
- 28.Salnikow, K., M. Costa, W. D. Figg, and M. V. Blagosklonny. 2000. Hyperinducibility of hypoxia-responsive genes without p53/p21-dependent checkpoint in aggressive prostate cancer. Cancer Res. 60:5630-5634. [PubMed] [Google Scholar]
- 29.Schipani, E., H. Ryan, S. Didrickson, T. Kobayashi, M. Knight, and R. S. Johnson. 2001. Hypoxia in cartilage: HIF-1alpha is essential for chondrocyte growth arrest and survival. Genes Dev. 15:2865-2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmaltz, C., P. H. Hardenbergh, A. Wells, and D. E. Fisher. 1998. Regulation of proliferation-survival decisions during tumor cell hypoxia. Mol. Cell. Biol. 18:2845-2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seagroves, T. N., H. Ryan, H. Lu, B. G. Wouters, M. Knapp, P. Thibault, K. Laderoute, and R. S. Johnson. 2001. Transcription factor HIF-1 is a necessary mediator of the Pasteur effect in mammalian cells. Mol. Cell. Biol. 21:3436-3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Semenza, G. L., P. H. Roth, H. M. Fang, and G. L. Wang. 1994. Transcriptional regulation of genes encoding glycolytic enzymes by hypoxia-inducible factor 1. J. Biol. Chem. 269:23757-23763. [PubMed] [Google Scholar]
- 33.Sherr, C. J., and R. A. DePinho. 2000. Cellular senescence: mitotic clock or culture shock? Cell 102:407-410. [DOI] [PubMed] [Google Scholar]
- 34.Shimizu, S., Y. Eguchi, H. Kosaka, W. Kamiike, H. Matsuda, and Y. Tsujimoto. 1995. Prevention of hypoxia-induced cell death by Bcl-2 and Bcl-xL. Nature 374:811-813. [DOI] [PubMed] [Google Scholar]
- 35.Shweiki, D., A. Itin, D. Soffer, and E. Keshet. 1992. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature 359:843-845. [DOI] [PubMed] [Google Scholar]
- 36.Solvason, N., W. W. Wu, N. Kabra, X. Wu, E. Lees, and M. C. Howard. 1996. Induction of cell cycle regulatory proteins in anti-immunoglobulin-stimulated mature B lymphocytes. J. Exp. Med. 184:407-417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suzuki, H., A. Tomida, and T. Tsuruo. 2001. Dephosphorylated hypoxia-inducible factor 1alpha as a mediator of p53-dependent apoptosis during hypoxia. Oncogene 20:5779-5788. [DOI] [PubMed] [Google Scholar]
- 38.Tanimoto, K., Y. Makino, T. Pereira, and L. Poellinger. 2000. Mechanism of regulation of the hypoxia-inducible factor-1 alpha by the von Hippel-Lindau tumor suppressor protein. EMBO J. 19:4298-4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thelander, L., A. Graslund, and M. Thelander. 1983. Continual presence of oxygen and iron required for mammalian ribonucleotide reduction: possible regulation mechanism. Biochem. Biophys. Res. Commun. 110:859-865. [DOI] [PubMed] [Google Scholar]
- 40.Wang, G. L., B. H. Jiang, E. A. Rue, and G. L. Semenza. 1995. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc. Natl. Acad. Sci. USA 92:5510-5514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang, G. L., and G. L. Semenza. 1993. General involvement of hypoxia-inducible factor 1 in transcriptional response to hypoxia. Proc. Natl. Acad. Sci. USA 90:4304-4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang, G. L., and G. L. Semenza. 1995. Purification and characterization of hypoxia-inducible factor 1. J. Biol. Chem. 270:1230-1237. [DOI] [PubMed] [Google Scholar]