Abstract
p38 mitogen-activated protein (MAP) kinases play an important role in the regulation of cellular responses to all kinds of stresses. The most abundant and broadly expressed p38 MAP kinase is p38α, which can also control the proliferation, differentiation, and survival of several cell types. Here we show that the absence of p38α correlates with the up-regulation of one of its upstream activators, the MAP kinase kinase MKK6, in p38α−/− knockout mice and in cultured cells derived from them. In contrast, the expression levels of the p38 activators MKK3 and MKK4 are not affected in p38α-deficient cells. The increase in MKK6 protein concentration correlates with increased amounts of MKK6 mRNA in the p38α−/− cells. Pharmacological inhibition of p38α also up-regulates MKK6 mRNA levels in HEK293 cells. Conversely, reintroduction of p38α into p38α−/− cells reduces the levels of MKK6 protein and mRNA to the normal levels found in wild-type cells. Moreover, we show that the MKK6 mRNA is more stable in p38α−/− cells and that the 3′untranslated region of this mRNA can differentially regulate the stability of the lacZ reporter gene in a p38α-dependent manner. Our data indicate that p38α can negatively regulate the stability of the MKK6 mRNA and thus control the steady-state concentration of one of its upstream activators.
Mitogen-activated protein kinase (MAPK) signal transduction pathways have been implicated in the regulation of numerous cellular processes including cell growth, differentiation, and survival. Some mammalian MAPKs, for example the extracellular signal-regulated kinases (ERK1 and ERK2), are activated mainly by mitogens, while others, like the c-Jun N-terminal kinase (JNK) and p38 pathways, are activated by many environmental and genotoxic stresses.
The p38 MAPK pathway is strongly activated by proinflammatory cytokines, such as interleukin-1 (IL-1) and tumor necrosis factor alpha (32, 46, 60). Four vertebrate p38 MAPK family members have been cloned and named p38α, p38β, p38γ, and p38δ (24, 30, 40, 41, 45, 51). They are 60 to 70% identical in their amino acid sequence but differ in their expression pattern and sensitivity to chemical inhibitors such as SB203580 (reviewed in references 42 and 56). Like other MAPKs, p38s are activated by dual phosphorylation on the Thr-X-Tyr motif of the activation loop. The MAPK kinases MKK6 (17, 30), MKK3 (24, 34), and MKK4 (20, 21, 48) are all able to phosphorylate and activate p38 MAPKs, but only MKK6 can activate all of the p38 isoforms, albeit with different efficiencies (2). Different MKK6 isoforms have been cloned, and the pattern of expression appears to be tissue specific (15, 33, 58). A MKK-independent mechanism for the activation of p38α has also been recently proposed, based on the ability of the adapter protein TAB-1 to bind to and stimulate autophosphorylation of p38α (29). Gene-targeted inactivation of the mouse p38α gene results in embryonic death, mainly due to a defect in placental organogenesis (1, 54, 63), although a role for p38α in embryonic erythropoiesis has also been proposed (39, 63).
Changes in the cellular concentration of p38 MAPKs and their MKK activators have rarely been described, suggesting that these proteins may be constitutively expressed and mainly regulated by cycles of phosphorylation and dephosphorylation. One exception is p38γ/SAPK3, which strongly accumulates on myocyte differentiation, probably due to increased transcription, although this possibility was not formally investigated (16).
The substrates of p38 MAPKs include many transcription factors (reviewed in reference 14), which are involved in the transcriptional regulation of several genes including those encoding cell surface receptors, other transcription factors, and cytokines (42, 56). In particular, the role of p38 MAPKs in the regulation of genes activated during inflammation has been extensively investigated. For example, p38 MAPK activation can induce the accumulation of mRNAs encoding IL-1, tumor necrosis factor alpha, and IL-6 (28, 35, 53, 68) as well as cyclooxygenase-2 (23, 44) via either stimulation of transcription (71), increased mRNA stability (50), or both (44, 68).
The importance of mRNA turnover for the control of gene expression has been known for a long time (reviewed in references 5, 13, and 59). Genes known to be controlled by this mechanism include the proto-oncogenes c-fos and c-myc (11, 69) and the cell cycle regulatory genes encoding cyclin B1, cyclin A, and p21 (65, 66). Despite the key role of this process in the regulation of gene expression, the underlying molecular mechanisms have only recently started to be elucidated. The turnover of labile mRNAs is usually controlled through the poly(A) tail as well as AU-rich elements (AREs), which are generally located in the 3′-untranslated region (UTR) (62, 64). AREs also influence mRNA translation and nuclear export (4, 13). Several ARE-binding proteins have been cloned (11, 12, 70); some of them, such as HuD and HuR, can enhance mRNA stability (25, 66), whereas others, including AUF1 and tristetraprolin (TTP), stimulate mRNA degradation (7, 43). The stability of mRNAs can be regulated by extracellular stimuli, and the involvement of MAPKs, in particular the p38 MAPK pathway, has been proposed in different cellular systems (38, 44, 47, 61). For example, the p38 pathway can modulate both the expression and posttranslational modification of TTP and can also cooperate with HuR in the stabilization of the IL-3 transcript (50, 52).
Here we show that cells lacking p38α contain increased levels of its upstream activator MKK6. The increase in MKK6 concentration correlates with increased stability of the MKK6 mRNA in p38α−/− cells, which appears to be regulated by the MKK6 3′-UTR. Both MKK6 protein and mRNA levels are decreased to normal on reintroduction of p38α. Thus, p38α can directly control the concentration of one of its upstream activators, MKK6, via regulation of the stability of its mRNA.
MATERIALS AND METHODS
Cell lines.
The generation of p38α knockout mice and the establishment of the cardiomyocytic cell lines have been previously described (1). The cardiomyocytic cell lines were grown on collagen-coated tissue culture plates (Falcon) containing Dulbecco minimal essential medium plus 10% fetal calf serum (Gibco-BRL), 2 mM glutamine (Gibco-BRL), 100 U of penicillin per ml and 100 mg of streptomycin per ml (Gibco-BRL), 10 U of gamma interferon (Sigma), and 0.2 ng of cardiotrophin per ml (R&D Systems) at 33°C and 5% CO2.
Mouse embryo fibroblasts (MEFs) were derived from E11.5 and E12.5 embryos obtained by crossing heterozygous p38α+/− mice. After removal of the head (for genotyping by immunoblotting) and rinsing in phosphate-buffered saline (PBS), the embryos were disrupted in 1 ml of trypsin-EDTA (0,01% trypsin, 0.1 mM EDTA [Gibco-BRL]) by several passages through a syringe and then incubated for 1 h at 37°C in 5% CO2. Cells from a single embryo were then plated in tissue culture dishes (15 cm in diameter) in Dulbecco minimal essential medium supplemented with 10% Fetal calf serum, 2 mM glutamine, 100 U of penicillin per ml, and 100 mg of streptomycin per ml. When the plates were confluent (about 48 h), the cells were frozen. Early-passage cells (between P2 and P4) were used for the experiments.
The 293GP/BRS and HEK293 cells were routinely grown in the same medium as MEFs (see above) at 37°C in 5% CO2. Stably transfected and retrovirally transduced cell lines were grown in medium supplemented with 100 μg of hygromycin (Sigma) per ml.
Transfection, retroviral infection, and cell stimulation.
Wild-type and p38α−/− cardiomyocytes were plated in 6-cm dishes and 24 h later were cotransfected with 7.2 μg of pEFmlink-p38α or pEFmlink and 0.8 μg of pHygro (containing the gene for hygromicin resistance) using the FuGENE6 reagent (Roche). The medium was removed 16 h posttransfection, and the cells were washed and selected with 100 μg of hygromycin per ml. Single clones as well as pools were screened for the expression of p38α.
Retroviral infection was performed as reported elsewhere (36). Briefly, the viruses were generated by cotransfection of the plasmidic form of the retroviral vectors (MSCV-hygro plasmid, 25 μg) and the packaging construct (Φ-Eco, 5 μg) in the 293 GP/BRS cell line by the calcium phosphate precipitation technique. The following day, the precipitates were removed and fresh medium was added. This medium, containing the viral particles, was recovered 24 h later, centrifuged at 3,000 x g for 10 min, and filtered through a 0.45-μm-pore size filter (Millipore). Subconfluent cultures of cardiomyocytes were infected by adding to each plate 3 ml of medium containing the viral particles and 4 μg of polybrene (Sigma) per ml. A second round of infection was performed 12 h later. The medium was removed 30 h later, and the cells were selected for 15 days in the presence of 100 μg of hygromycin per ml.
HEK293 cells were transiently transfected by the calcium phosphate precipitation technique using 0.5 μg of pEGFP, 5 μg of the reporter plasmid MCS-lacZ-MKK6 3′UTR, and 10 μg of either pEFmlink-MKK6-DD (2), expressing a constitutive active MKK6 mutant, or the empty vector. Cells were collected 48 h after removal of the precipitates and used to either extract RNA or prepare protein extracts.
For UV treatment, subconfluent cultures were treated with UV (2) and then incubated for 30 min before preparation of cell lysates. Subconfluent cultures were also used for cycloheximide (25 μg/ml) (Sigma), and actinomycin D (5 μg/ml) (Sigma) treatments. The p38 MAPK inhibitor SB203580 (Calbiochem) was used at 10 μM.
DNA expression constructs.
pEFmlink-p38α was constructed by cloning human p38α cDNA (provided by Philip Cohen, Medical Research Council, Dundee, Scotland) derived from the pGEX-p38α plasmid (2) into the BamHI site of the pEFmlink expression vector. To obtain MSCV-p38α, the same BamHI fragment was filled in with Klenow DNA polymerase and cloned into HpaI-digested MSCV-Hygro. The MSCV-p38α D168A mutant was obtained by site-directed mutagenesis using the oligonucleotide 5′-AGCCAGTCCAAAAGCCAGAATCTTCAG-3′ (the mutated codon is underlined) and the QuickChange kit (Stratagene).
The MKK6-3′UTR was cloned by reverse transcription PCR (RT-PCR) using RNA purified from cardiomyocytes (3 μg) and the SuperScript first-strand synthesis system (Invitrogen). Restriction sites for PstI and HindIII were inserted at the 5′ and 3′ end, respectively, using oligonucleotides 5′-CCTGCAGAACTGATACTTGGGGACTAA-3′ and 5′-GTTCGAAGCCCTATAAATACTGCCGA-3′. The PCR product was cloned into pCMV-Script (Stratagene). pCMV-MKK6-3′UTR was digested with PstI to insert the lacZ open reading frame derived from pMC1871 (Promega). Finally, the lacZ-MKK6-3′UTR cassette was obtained by HindIII digestion, filled in with Klenow DNA polymerase, and cloned into the HpaI site of MSCV-Hygro.
Cell extracts, immunoblotting, and antibodies.
Cell extracts were prepared as reported previously (2). Briefly, cells were washed in cold PBS and lysed in IP buffer (50 mM Tris-HCl [pH 7.5], 150 mM NaCl, 1% NP-40, 5 mM EDTA, 5 mM EGTA, 20 mM NaF, 0.1 mM sodium orthovanadate, and Complete Mini protease inhibitor cocktail [Roche]). After 10 min of incubation on ice, lysates were centrifuged for 20 min at 10,000 × g. Mouse tissues were homogenized in 5 volumes of IP buffer, incubated on ice for 10 min, sonicated three times for 10 s each, and centrifuged for 15 min at 10,000 × g.
For immunoblotting, 30 μg of total protein was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose (BA85; Scleicher & Schuell) by using a semidry blotting apparatus. The membranes were blocked in 4% nonfat dry milk in TTBS (2) for at least 1 h at room temperature. Primary antibodies were incubated in TTBS containing 1% milk (except for the phospho-MKK6 antibody, which was incubated in 3% bovine serum albumin) overnight at 4°C. Binding of the primary antibodies was detected with horseradish peroxidase-coupled secondary antibodies (Dako) followed by enhanced chemiluminescence detection (Amersham Pharmacia Biotech.).
The following antibodies were used: p38α (C20), MKK3 (I20) and cyclin D1 (C-20) (Santa Cruz), phospho-p38 and phospho-MKK6/MKK3 (Cell Signaling Technology), antitubulin (Sigma), and MKK6 rabbit antiserum (2).
Northern blotting and RT-PCR.
RNA was prepared from actively growing cells by using the TRIzol reagent (Life Technologies) as specified by the manufacturer. Total RNA (40 μg for cardiomyocytes and 30 to 50 μg for HEK293 cells) was separated by electrophoresis on a 1.2% agarose gel containing 0.04 M morpholinepropanesulfonic acid (MOPS) and 0.06 M formaldehyde, transferred onto a nylon membrane (GeneScreen Plus; Dupont), and cross-linked by UV treatment (Stratalinker apparatus). The membrane was hybridized in ULTRAHyb solution (Ambion), using the murine full-length MKK6 ORF or a fragment of the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) ORF (nucleotides 134 to 600) as probes. After several washes, the bands were visualized by autoradiography and quantified in a Fuji FLA2000 phosphorimaging device. Each experiment was performed at least twice.
For the RT-PCR, after DNase I treatment (Life Technology), 3 μg of total RNA was retrotranscribed using the Superscript kit (Life Technology). PCR was performed using the following oligonucleotides: lacZ (5′-GATTGGTGGCGACGACTCCTG-3′) and MKK6-3′UTR (5′-GTTCGAAGCCCTATAAATACTGCCGA-3′) to detect the hybrid lacZ-MKK6-3′UTR, and 5′-GAGGGGCCATCCACAGTCTTC-3 and 5′-CAGTATGACTCCACTCACGGC-3′ for GAPDH. The PCR products were separated on 1.2% agarose gels.
Nuclear run-on assay.
The run-on assay was performed as reported previously (49). Briefly, nuclei were collected from exponentially growing cardiomyocytic cells lysed in a buffer containing 10 mM Tris (pH 7.4), 10 mM NaCl, 3 mM MgCl2 and 0.5% NP-40 and pelleted onto a 1 M sucrose cushion. The pellet was resuspended in storage buffer containing 50 mM Tris (pH 8.3), 40% glycerol, 5 mM MgCl2 and 0.1 mM EDTA. About 4 × 107 nuclei (200 μl) were incubated in 10 mM Tris (pH 8.0)-5 mM MgCl2-0.3 mM KCl-1 mM each ATP, CTP, and GTP-2.5 mM dithiothreitol-250 μCi [α-32P]UTP at 30°C for 40 min. After DNase I digestion, the RNA was purified by phenol-chloroform extraction and precipitated in ethanol. Linearized DNA (5 μg) obtained from either the pGEM2 vector alone or pGEM2 containing the MKK6-5′UTR or GAPDH was denatured and dot blotted onto a membrane (GeneScreen). Membranes were hybridized at 42°C in ULTRAHyb solution using 3 × 106 cpm of labeled RNA per ml. After 72 h, the membranes were washed in 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) plus 0.1% SDS followed by 2× SSC alone and then incubated for 30 min at 37°C in 2× SSC containing 300 μg of RNase A per ml. After a final wash in 0.2× SSC plus 0.1% SDS, signals were visualized by autoradiography using a PhosphorImager (Molecular Dynamics).
Preparation of transcripts and RNA-binding assays.
Plasmid pCMV-MKK6-3′UTR was lineralized with NotI and used for in vitro transcription with T7 RNA polymerase in the presence of either [α-32P]UTP for preparing radiolabeled transcripts, or biotin-11-CTP (Sigma) for preparing biotinylated transcripts. Radiolabeled transcripts were resuspended in water, and biotinylated transcripts were resuspended in 2× TENT buffer (20 mM Tris-HCl [pH 8.0], 2 mM EDTA [pH 8.0], 500 mM NaCl, 1% [vol/vol] Triton X-100).
For electrophoretic mobility shift and supershift assays, reaction mixtures (10 μl) containing 1 μg of tRNA, 2 to 10 fmol of RNA, and 10 μg of cytoplasmic lysate prepared as described previously (66) were incubated in reaction buffer (15 mM HEPES [pH 7.9], 10 mM KCl, 10% glycerol, 0.2 mM dithiothreitol, 5 mM MgCl2) for 30 min at 25°C and digested with RNase T1 (100 U/reaction) for 15 min at 37°C. For supershift analysis, antibodies (1 μg) recognizing HuR (Molecular Probes, Eugene, Oreg.), AUF1 (Phoenix Pharmaceuticals, Inc., Belmont, Calif.), or TTP (Santa Cruz), were added to lysates for 1 h on ice before the addition of radiolabeled RNA. Complexes were resolved by electrophoresis through native gels (7% acrylamide in 0.25 M Tris-borate-EDTA buffer) without loading buffer (160 V for 2 h at 4°C); the gels were dried, and radioactivity was visualized with a PhosphorImager.
For pull-down Western assays, 0.2 μg of biotin-labeled MKK6 RNA and 80 μg of cytoplasmic lysates prepared from either wild-type or p38α−/− cells were incubated in 1× TENT buffer for 30 min at room temperature. Then 10 μl of magnetic streptavidin-coated Dynabeads (Dynal) that had been prewashed twice in a solution of 0.1 M NaOH and 0.05 M NaCl and once in a solution of 0.1 M NaCl and resuspended in 1× TENT was added to the binding-reaction mixture and incubated at room temperature for an additional 30 min. Protein-RNA complexes were “pulled down” on a magnetic base, washed twice with ice-cold PBS, boiled in protein-loading buffer, and electrophoresed through 12% polyacrylamide-SDS gels alongside aliquots of cytoplasmic lysate (10 μg each). Following transfer, RNA-binding proteins were visualized using antibodies that recognize HuR (Santa Cruz); hybridizations using an anti-β-actin antibody (ABCAM, Cambridge, England) were routinely conducted to control for the evenness of loading and transfer of samples, as well as to assess the quality of sample preparation.
β-Galactosidase assay.
Actively growing cells were washed in cold PBS and resuspended in lysis buffer containing 0.25 M Tris-HCl (pH 7.8) and 0.5 % NP-40. After incubation on ice for 10 min, the cell extracts were centrifuged at 12,000 × g, for 10 min at 4°C. β-Galactosidase activity was determined by incubating 3 and 30 μg of total cellular protein in 1 ml of buffer Z (100 mM sodium phosphate [pH 7.5], 10 mM KCl, 1 mM MgSO4, 50 mM β-mercaptoethanol). After a 5-min incubation at 37°C, 200 μl of o-nitrophenyl-β-d-galactopyranoside (ONPG) was added. On the appearance of a pale yellow color, reactions were stopped by adding 1 M Na2CO3 (500 μl) and quantified by measuring the optical density at 420 nm (A420). β-Galactosidase activity was determined as follows: units of β-galactosidase = A420 × 1,000/(amount of protein [micrograms] × reaction time [hours]).
RESULTS
Upregulation of MKK6 in p38α-deficient cells.
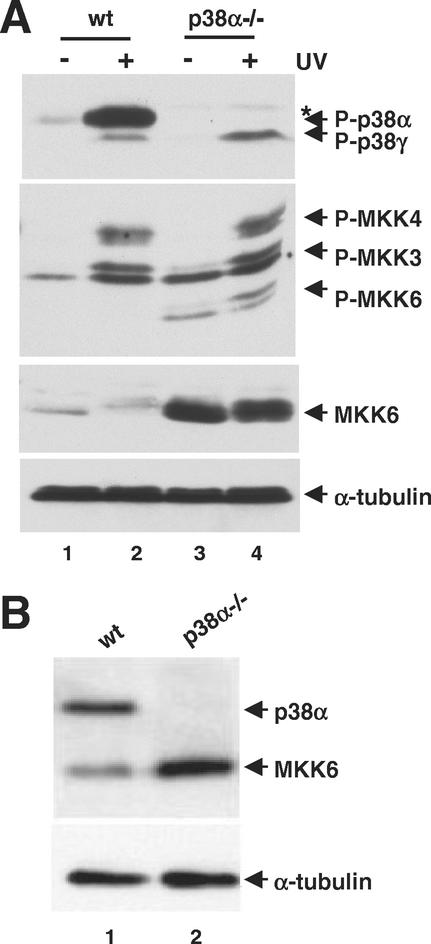
We have investigated how the absence of p38α MAPK, which is the most abundant and broadly expressed p38 isoform, affects other p38 MAPKs and their MKK activators. For this purpose, cell lines expressing cardiomyocyte-specific markers were derived from the hearts of wild-type and p38α−/− E10.5 mouse embryos (1). These cells were UV irradiated, and the activation of the p38 MAPK pathway was investigated in whole-cell extracts by immunoblotting using antibodies specific for the phosphorylated form of p38 MAPKs. In wild-type cells stimulated with UV, we detected a strong phosphorylated band (Fig. 1A, lane 2) corresponding to p38α, as it was recognized by p38α-specific antibodies (data not shown) and was absent from p38α−/− cells (Fig. 1A, lane 4). Phosphorylation of a faster-migrating p38 MAPK isoform was detected in the p38α−/− cells only on UV treatment (lane 4). This band was weaker in wild-type cells; it was identified as p38γ by using specific antibodies (data not shown). In parallel, the activation of p38 MKKs was studied by immunoblotting using antibodies that recognize the phosphorylated forms of MKK3 and MKK6. Increased levels of MKK6 phosphorylation were detected in p38α-deficient cells, either unstimulated or UV treated (Fig. 1A, lanes 3 and 4). However, MKK3 was activated to similar extents in the UV stimulated p38α−/− and wild-type cells (Fig. 1A). This antibody also cross-reacted with MKK4 (2), whose phosphorylation was unchanged in the two cell lines (Fig. 1A). We next investigated if the increased phosphorylation of MKK6 correlated with differences in MKK6 protein levels. Surprisingly, using antibodies that did not depend on phosphorylation, we detected a significant increase in the amount of MKK6 protein in the p38α−/− cells (Fig. 1A, compare lanes 1 and 2 with lanes 3 and 4), which was estimated to be about six to seven times greater than in wild-type cells (data not shown).
FIG. 1.
Upregulation of MKK6 protein levels in p38α-deficient cells. (A) Wild-type (wt) and p38α−/− cardiomyocytic cells were treated with UV or left untreated as indicated. The phosphorylation status of p38 MAPKs and p38 MKKs, as well as the levels of MKK6 and tubulin proteins in whole-cell extracts, were determined by immunoblotting using specific antibodies. The asterisk indicates a phospho-p38 band that cross-reacts with antibodies against p38β and p38δ. (B) MEFs were prepared from wild-type and p38α−/− embryos, and whole-cell extracts were analyzed by immunoblotting with p38α, MKK6, and tubulin antibodies.
To investigate whether MKK6 protein up-regulation in the absence of p38α was a cell-type-specific phenomenon, primary MEFs were prepared from p38α−/− and wild-type mouse embryos. As with the cardiomyocytic cell lines, we also detected up-regulation of the MKK6 protein levels in p38α−/− MEFs (Fig. 1B).
MKK6 up-regulation requires p38α kinase activity.
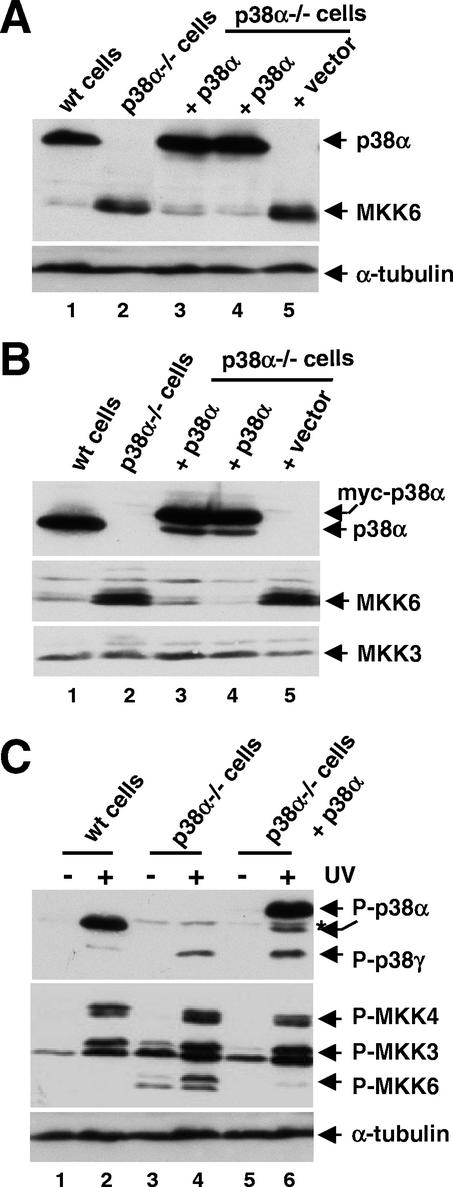
To confirm that MKK6 up-regulation was directly related to the absence of p38α, we reintroduced p38α into the p38α−/− cells and looked for a reduction in MKK6 expression by using two approaches. First, the p38α−/− cells were infected with a retroviral vector expressing the p38α cDNA and selected with hygromycin. We obtained hygromycin-resistant cells expressing p38α at a similar level to wild-type cells, which showed strong down-regulation of the MKK6 protein levels compared with the parental p38α−/− cells (Fig. 2A, lanes 3 and 4). Similar results were obtained when p38α−/− cells were stably transfected with a plasmid encoding myc-tagged p38α (Fig. 2B, lanes 3 and 4). In these experiments, we also confirmed that the expression of MKK3 was independent of the changes in MKK6 protein levels (Fig. 2B, lower panel). Moreover, the levels of MKK3 and MKK4 phosphorylation in UV-stimulated cells were also similar, in either the presence or absence of p38α (Fig. 2C). The expression and phosphorylation of p38γ were not significantly changed after p38α reintroduction in the p38α−/− cells (Fig. 2C and data not shown).
FIG. 2.
Reintroduction of p38α in p38α−/− cells down-regulates MKK6 protein levels. Ectopic p38α was expressed in p38α−/− cells either by retroviral infection (A) or by stable transfection with a myc-tagged p38α-expressing construct (B). Two clones were analyzed in each case, and the expression of p38α, MKK6, and MKK3 proteins was determined by immunoblotting of whole-cell extracts. (C) The same cell lines as in panel B were either UV treated or left untreated, and the lysates were analyzed by immunoblotting with antibodies specific for the phosphorylated forms of p38 MAPKs and p38 MKKs. Note thedifferent electrophoretic mobility of endogenous and myc-tagged p38α (lanes 2 and 6, respectively). The asterisk indicates a phospho-p38 band that cross-reacts with antibodies against p38β and p38δ. wt, wild type.
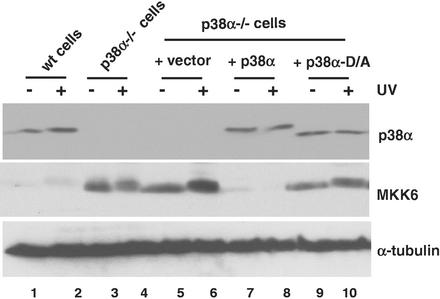
To verify if the kinase activity of p38α was required for MKK6 down-regulation, we generated a catalytically inactive p38α mutant with Asp-168 in the phosphorylation lip changed to Ala (D168A). We found that in contrast to the wild-type p38α, expression of similar levels of the D168A p38α mutant (Fig. 3, top panel) only slightly decreased the MKK6 protein levels in p38α−/− cells (Fig. 3, middle panel). We also investigated whether overexpression of p38γ in p38α−/− cells could change the expression levels of MKK6. However, we never obtained more than a modest increase (about twofold) in the amount of endogenous p38γ, which did not change MKK6 expression levels in p38α−/− cells (data not shown).
FIG. 3.
Catalytically inactive p38α does not down-regulate MKK6 protein levels in p38α−/− cells. Wild-type (wt), p38α-deficient cells and the p38α-deficient cells infected with either the retroviral vector or retroviruses expressing wild-type p38α or the kinase-dead mutant D168A were UV treated or left untreated, as indicated. The expression levels of p38α, MKK6, and tubulin in whole-cell extracts were determined by immunoblotting.
Taken together, the results suggest that the increase in the level of MKK6 in p38α−/− cells was specifically related to the absence of p38α kinase activity.
MKK6 mRNA is up-regulated in p38α−/− cells.
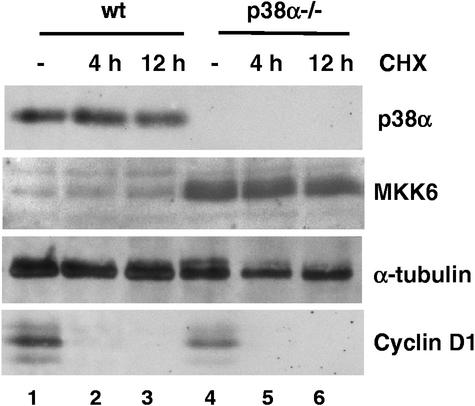
The cellular concentrations of p38 MAPKs and MKKs are thought to be relatively constant, even after activation of the signaling pathway. To study the molecular mechanism of MKK6 protein accumulation in the absence of p38α, we first investigated MKK6 protein turnover in wild-type and p38α−/− cell lines. When cells were treated with cycloheximide, an inhibitor of protein synthesis, we could detect no differences in MKK6 stability in the presence or absence of p38α (Fig. 4). Moreover, we found that both MKK6 and p38α seemed to be quite stable proteins, with a half-life greater than 24 h (data not shown). In contrast, cyclin D1 totally disappeared after 4 h of incubation with cycloheximide (Fig. 4). This suggests that the accumulation of MKK6 in p38α-deficient cells is probably not due to differences in MKK6 protein stability.
FIG. 4.
MKK6 protein stability is not affected in p38α-deficient cells. Wild-type (wt) and p38α−/− cells were incubated with cycloheximide (CHX, 25 μg/ml) for 4 and 12 h. Cell extracts were analyzed by immunoblotting for expression of p38α, MKK6, tubulin, and cyclin D1.
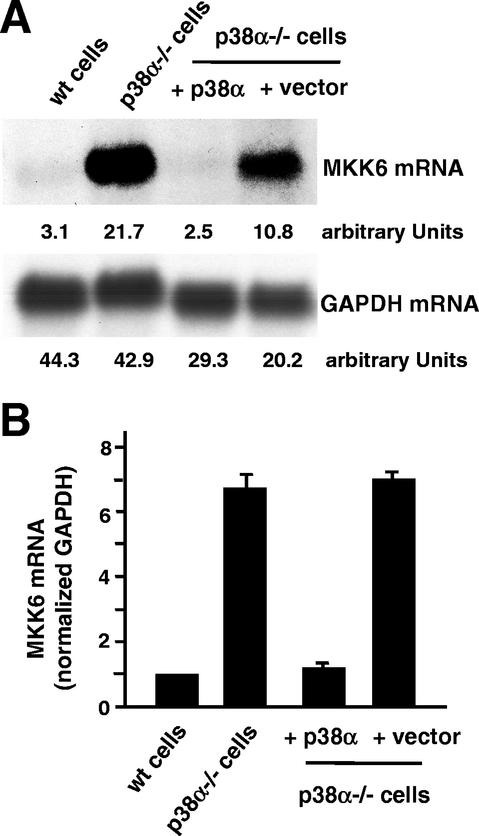
This result prompted us to investigate MKK6 mRNA levels in p38α−/− cell lines. We found that the increased levels of MKK6 protein in p38α−/− cells correlated with the up-regulation of the MKK6 mRNA, as determined by Northern blotting (Fig. 5A). When the transcript amount was standardized against endogenous GAPDH mRNA levels, we found that the MKK6 mRNA was about sixfold more abundant in the p38α−/− cells than in the wild-type cells (Fig. 5B). Interestingly, as with the MKK6 protein (Fig. 2 and 3), the MKK6 mRNA levels could also be reversed to about the wild-type concentration by reintroduction of p38α into the p38α−/− cells (Fig. 5).
FIG. 5.
Up-regulation of MKK6 mRNA in p38α-deficient cells. (A) Northern blot analysis of RNA prepared from actively growing wild-type (wt) and p38α−/− cells and from p38α−/− cells infected with either p38α-expressing retroviruses or the empty retroviral vector. The membrane was hybridized with the MKK6 (upper panel) and GAPDH (lower panel) probes. (B) PhosphorImager quantification of MKK6 mRNA levels (normalized against the GAPDH values) in the indicated cell lines. The results were compiled from six independent experiments.
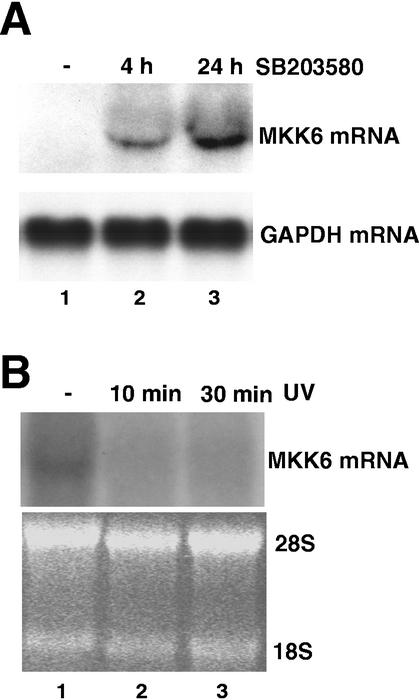
We next used a complementary approach to confirm these results in a different cellular system. We investigated whether pharmacological inhibition of p38α would result in the accumulation of MKK6 transcript in HEK293 cells. We found that treatment of HEK293 cells with the p38α/β inhibitor SB203580 (18) resulted in a striking time-dependent accumulation of the MKK6 mRNA (Fig. 6A), which also correlated with an increase in MKK6 protein levels (data not shown). Conversely, treatment of HEK293 cells with UV, a powerful activator of the p38 MAPK pathway, decreased the levels of the MKK6 transcript (Fig. 6B). These results indicated that the down-regulation of MKK6 mRNA levels by p38α kinase activity also occurs in wild-type cells.
FIG. 6.
Regulation of the MKK6 transcript levels by the p38 MAPK pathway in wild-type cells. (A) Northern blotting of total RNA (30 μg) purified from HEK293 cells that were either untreated (lane 1) or treated with the p38 MAPK inhibitor SB203580 (10 μM) for the indicated times (lanes 2 and 3). The membrane was hybridized with the MKK6 (upper panel) and GAPDH (lower panel) probes. (B) HEK293 cells were either untreated (lane 1) or treated with UV and then incubated for the indicated times (lanes 2 and 3) before purification of total RNA, which was analyzed (50 μg) by Northern blotting. The hybridization with the MKK6 probe and the ethidium bromide gel showing the rRNA as a loading control are shown in the upper and lower panels, respectively.
p38α negatively regulates MKK6 mRNA stability through its 3′UTR.
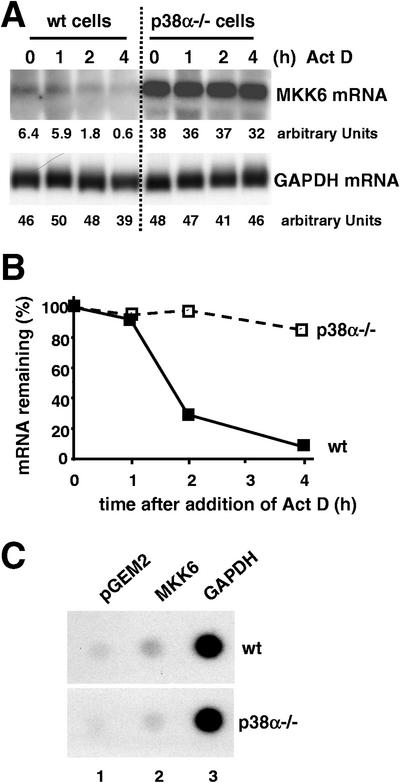
To study if the increase in MKK6 mRNA levels in p38α−/− cells occurred at the transcriptional or posttranscriptional level, we investigated the stability of MKK6 transcripts in wild-type and p38α-deficient cells treated with actinomycin D, an inhibitor of RNA polymerase II (Fig. 7A). We found that the MKK6 mRNA had a half-life of about 1.5 h in wild-type cells but was significantly more stable in p38α-deficient cells (Fig. 7B). In the same experiment, we could detect no differences in the turnover of the leukemia inhibitor factor (22) mRNA between wild-type and p38α−/− cells (data not shown).
FIG. 7.
Increased stability of MKK6 mRNA but normal MKK6 transcriptional rate in p38α-deficient cells. (A) Wild-type (wt) and p38α−/− cells were treated with actinomycin D (Act D) (5 μg/ml), and at the indicated times, RNA was extracted and analyzed by Northern blotting. A representative example of three independent experiments is shown, with the relative expression levels indicated as arbitrary PhosphorImager units. (B) Representation of the MKK6 transcript levels remaining in the cells after actinomycin D treatment. Values were normalized to GAPDH levels. (C) Logarithmically growing wild-type and p38α−/− cells were subjected to nuclear run-on analysis. The membrane was blotted with the indicated plasmid DNAs, and signals were visualized by autoradiography.
To rule out the possibility that p38α could also be regulating MKK6 mRNA turnover at the transcriptional level, we performed a nuclear run-on assay. In these experiments, we could detect no significant difference in the transcriptional rate of the MKK6 gene between wild-type and p38α-deficient cells (Fig. 7C). Thus, the MKK6 mRNA levels are posttranscriptionally regulated by p38α.
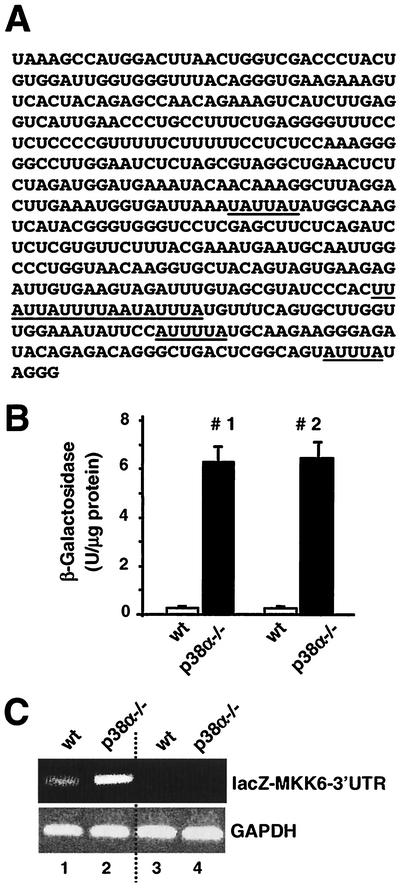
AREs, and in particular AUUUA pentamers, play a key role in the control of mRNA stability (13). Some of the proteins that regulate mRNA stability, such as TTP, can bind to AUUUA sequences and are regulated by p38 MAPKs (50). We have identified AU-rich sequences in the 3′-UTR of the mouse MKK6 transcript (Fig. 8A). To investigate their role in the differential accumulation of MKK6 mRNA in p38α-deficient cells, we fused the 3′-UTR of MKK6 downstream of the β-galactosidase-encoding lacZ cDNA in a hygromycin resistance retroviral vector. Following infection with the retrovirus, hygromycin-resistant clones were selected to test the expression (and thus, indirectly, the stability) of the hybrid mRNA in wild-type and p38α−/− cells. We measured about 10-fold-higher levels of β-galactosidase activity in the cellular lysates of p38α-deficient cells after expression of the chimeric mRNA (Fig. 8B). This also correlated with higher levels of hybrid mRNA, as determined by RT-PCR, in the p38α−/− cells than in the wild-type cells (Fig. 8C).
FIG. 8.
The MKK6 3′-UTR regulates mRNA stability. (A) Sequence of the mouse MKK6 mRNA 3′-UTR. The first three nucleotides correspond to the UAA stop codon of the MKK6 open reading frame. AUUUA sequences potentially involved in the regulation of mRNA stability are underlined. (B) The MKK6 3′-UTR region was cloned downstream of the lacZ cDNA in the MSCV retrovirus. Wild-type (wt) and p38α−/− cells were infected in duplicate with two different lacZ MKK6 3′-UTR clones (numbers 1 and 2). After selection for 15 days with hygromycin, the cells were lysed and the β-galactosidase activity in the whole-cell extracts was quantified. The data are representative of four independent experiments. (C) RNA was obtained from wild-type and p38α−/− cells infected with retrovirus expressing the lacZ MKK6 3′-UTR (lanes 1 and 2) or the retroviral vector alone (lanes 3 and 4). The amounts of the lacZ MKK6 3′-UTR (upper panel) and GAPDH (lower panel) RNAs were assessed by RT-PCR.
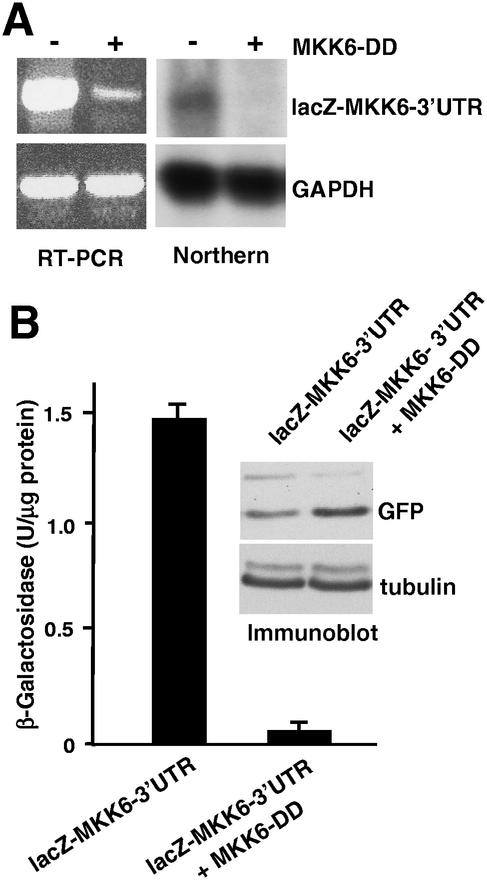
We also investigated the effect of activating the p38 MAPK pathway on the stability of the hybrid lacZ-MKK6-3′UTR mRNA by cotransfection of HEK293 cells with the constitutively active MKK6-DD mutant. We found that in cells expressing MKK6-DD, there was a significant reduction both in the amount of the lacZ-MKK6-3′UTR mRNA, as determined by either RT-PCR or Northern blotting (Fig. 9A), and in the levels of β-galactosidase activity (Fig. 9B).
FIG. 9.
Down-regulation of the MKK6 3′-UTR transcript levels by activation of the p38 MAPK pathway. (A) HEK293 cells were transiently cotransfected with plasmids carrying lacZ MKK6 3′-UTR, pEGFP, and either pEFmlink-MKK6-DD or pEFmlink alone. The amounts of the lacZ MKK6 3′-UTR (upper panel) and GAPDH RNAs were determined by both Northern blotting and RT-PCR. (B) Quantification of the β-galactosidase activity in whole-cell extracts from the same experiment. Data are normalized for the efficiency of transfection, as determined by NIH image analysis of green fluorescent protein (GFP) immunoblots (upper panel). The quantification of two independent experiments is presented.
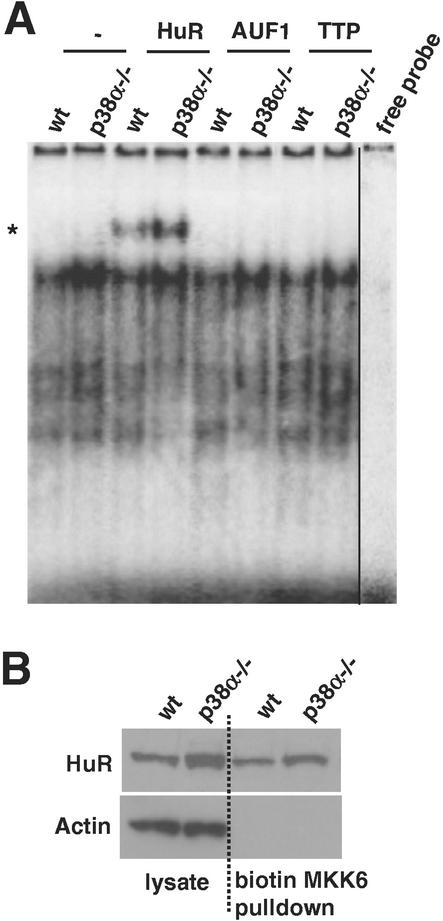
Next we sought to investigate whether the MKK6 3′-UTR was the target of ARE-binding proteins known to modulate the turnover of target mRNAs. To this end, we used two different RNA-binding assays. First, as shown in Fig. 10A, radiolabeled MKK6 RNA and proteins present in the cytoplasm of cardiomyocytes (and MEFs [data not shown]) formed abundant protein-RNA complexes exhibiting lower electrophoretic mobility on native gels. Complexes were moderately more abundant when cytoplasmic extracts from p38α−/− cells were used. That cytoplasmic HuR was bound to the MKK6 transcript in these complexes was evidenced by using anti-HuR antibodies, which were capable of supershifting a fraction of the radiolabeled bands. No such interaction was detected with antibodies that recognize AUF1 or TTP (Fig. 10A). Second, the HuR-MKK6 RNA interaction was further assessed using pull-down immunoblot assays (65): biotinylated MKK6-3′UTR transcript was used to pull down interacting proteins by using streptavidin-conjugated magnetic beads, followed by immunoblot analysis to detect HuR. In keeping with the supershift results, the level of HuR in MKK6 transcript-containing complexes was also found to be slightly elevated (approximately 1.5- to 2-fold) when lysates from p38α−/− cells were used. Pull-down immunoblot assays that were carried out either in the absence of biotinylated RNA or using biotinylated transcripts lacking AREs (such as the coding region of p21) did not pull down HuR (data not shown).
FIG. 10.
Identification of cytoplasmic RNA-binding proteins forming complexes with the MKK6 3′-UTR. (A) Cytoplasmic fractions of either wild-type (wt) or p38α−/− cells were incubated with radiolabeled MKK6 3′-UTR, and the electrophoretic mobility of the resulting complexes was visualized on native polyacrylamide gels. The presence of specific RNA-binding proteins in the complexes (*, supershift) was visualized by incubation with the indicated antibodies. “free probe” indicates a radiolabeled transcript that was not incubated with cytoplasmic extracts. (B) Cytoplasmic lysates (80 μg) prepared from either wild-type or p38α−/− cells were incubated with a biotinylated transcript corresponding to the MKK6 3′-UTR, and complexes were pulled down using streptavidin-conjugated magnetic beads. The presence of HuR was subsequently analyzed by immunoblotting. Cytoplasmic lysates (10 μg) were analyzed in parallel for determination of total cytoplasmic HuR levels. After being stripped, the membrane was reprobed with actin antibodies to assess the evenness in loading and transfer as well as to verify the absence of contaminating cytoplasmic material in the pull-down samples.
Taken together, these results show that the MKK6 3′-UTR can regulate mRNA stability in a p38α-dependent manner and can identify HuR as a potential regulator of this process.
Up-regulation of MKK6 in p38α-deficient mouse tissues.
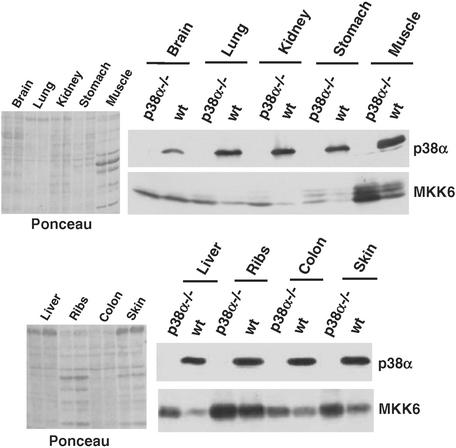
The expression of p38α is ubiquitous, while the other p38 isoforms and the p38 MAPK kinases are differentially expressed in some tissues. Therefore, we investigated the expression levels of MKK6 in tissues of p38α-deficient mice. p38α and MKK6 were expressed at different levels in the mouse tissues analyzed, with MKK6 in particular showing greater variation. Interestingly, we observed that the absence of p38α correlated with an increase in MKK6 protein levels in most tissues analyzed, although the up-regulation was more clear in muscle, kidney, and liver tissue (Fig. 11). In brain tissue, however, MKK6 protein levels were about the same in the presence and absence of p38α. These results demonstrate that p38α can down-regulate MKK6 expression not only in cultured cells but also in mice.
FIG. 11.
Up-regulation of MKK6 protein in p38α knockout mice. The indicated mouse tissues were obtained from control and p38α-deficient littermates (2 month old) generated by tetraploid fusions (1). Tissue extracts were analyzed by immunoblotting with p38α and MKK6 antibodies (right panels). Ponceau staining of the same blots is shown in the left panels. wt, wild type.
DISCUSSION
The predominant p38 MAPK in most cell types is p38α, which is also ubiquitously expressed and has been implicated in a variety of cellular responses (reviewed in references 3, 42, 55, and 56). Unexpectedly, mouse embryos lacking p38α die mainly of a defect in placental organogenesis (1, 54, 63), suggesting that this is the only function of p38α during mouse development that cannot be compensated for by other p38 MAPKs or alternative signaling pathways.
We have investigated how the absence of p38α might affect other p38 MAPKs and their activators. One striking difference that we observed is the increased levels of phosphorylated MKK6, in both UV-treated and untreated p38α−/− cells, which is due to an increase in the total amount of MKK6 protein. Importantly, we also found increased levels of MKK6 in different tissues of a p38α−/− knockout mouse, indicating that the regulation of MKK6 expression levels by p38α is not restricted to cultured cells. The reintroduction into p38α−/− cells of wild-type p38α, but not of a kinase-dead p38α mutant, led to the reduction of the MKK6 protein concentration (as well as both basal and UV-stimulated MKK6 phosphorylation) to a similar level to that in wild-type cells. It has been reported that incubation of C3H T101/2 and HL60 cells with the p38α/β inhibitor SB203580 up-regulates the basal levels of MKK6 activity and MKK6 phosphorylation, respectively, although in these cases the mechanisms of MKK6 up-regulation were not investigated (37, 67).
The expression of several genes is known to be regulated by p38 MAPKs at both the transcriptional and posttranscriptional levels (56). Many transcription factors are regulated by p38 MAPK phosphorylation (10), but several proteins, including cyclin D1 (9) and p53 (6), have also been reported to be stabilized by direct p38α (or p38β) phosphorylation. We could not detect changes in MKK6 protein turnover in p38α−/− cells; however, we found that the absence of p38α resulted in increased levels of MKK6 mRNA. Consistently, we also observed that HEK293 cells treated with SB203580 accumulated MKK6 transcripts. It is also becoming clear that the regulation of mRNA turnover may be as important as transcriptional regulation in controlling gene expression by p38 MAPKs (19, 50, 68). We found that in the absence of p38α, the transcriptional rate of the MKK6 gene was not changed whereas its transcript was more stable, suggesting that p38α down-regulates MKK6 expression through destabilization of its mRNA.
The binding of stabilizing or destabilizing proteins to AREs located in the 3′-UTR of mRNAs is one of the mechanisms known to regulate mRNA stability (13). The MKK6 3′-UTR contains several putative AREs that, when fused to the lacZ cDNA, can significantly stabilize the fusion construct in p38α−/− cells. Conversely, activation of the p38 MAPK pathway can destabilize the lacZ MKK6 3′-UTR mRNA in transiently transfected cells. Taken together, our results indicate that the MKK6 3′-UTR plays an important role in the regulation of MKK6 mRNA turnover by p38α.
How could p38α regulate MKK6 mRNA turnover? Since mRNA-stabilizing and -destabilizing factors appear to bind the same elements (13, 57), it is in principle possible that p38α either activates an mRNA-destabilizing protein or negatively regulates an mRNA-stabilizing factor or both. The regulation of these proteins by p38α could occur at the level of protein accumulation, localization, and/or RNA-binding activity. Examples of all of these possibilities have been previously shown, although these studies have always been performed after stress stimulation and it has typically been found that the stabilities of the mRNAs investigated were enhanced (52, 68). Here, we investigated whether the MKK6 3′-UTR was a target of the RNA-binding proteins whose roles in modulating mRNA turnover are best established: HuR, AUF1, and TTP (8, 27, 50, 57, 66). We show that cytoplasmic HuR associates with the 3′-UTR of MKK6 in cells with different p38α status. Given the observation that HuR abundance and HuR complexing with the MKK6 3′-UTR transcripts were moderately higher in p38α−/− cells, as determined by supershift assay and in biotin pull-down immunoblots, we postulate that the elevated presence of HuR may contribute to the heightened stability of MKK6 mRNA in p38α−/− cells. Nevertheless, HuR is unlikely to be the only player, given the small p38α-dependent differences in levels and binding activity. Moreover, while HuR is present in virtually all tissue types, our observation that MKK6 is not up-regulated in some p38α−/− organs suggests that other factors involved in regulating MKK6 mRNA turnover may not be ubiquitously expressed. The relative abundance of cytoplasmic AUF1 was unchanged in cells with different p38α status, and AUF1 did not appear to associate with the MKK6 3′-UTR in our binding assays; TTP could not be detected in cytoplasmic lysates. Further work is necessary to establish the potential influence of HuR on the p38α-dependent differences in MKK6 mRNA stability, as well as to identify additional regulatory proteins.
To our knowledge, this is the first report showing negative feedback regulation of the expression of an MKK (MKK6) by its MAPK substrate (p38α). Moreover, we provide evidence for a stress-independent role of the p38 MAPK pathway in the down-regulation of mRNA stability. In contrast to previous reports that typically associated the activation of this pathway with mRNA stabilization in stressed cells, the MKK6 transcript is an example of p38α-destabilized mRNA. Our results also indicate that the p38α kinase activity present in normally proliferating cells is specifically required for this effect, since the kinase-dead p38α mutant is unable to down-regulate MKK6 expression. Thus, the basal, low-level activity of p38α in non stressed cells can regulate the expression of specific genes, which may be related to either cell proliferation or some housekeeping function of p38α.
It is unclear why the concentration of MKK6 should be maintained low in normal cells and why among the three MKKs that can activate p38α only MKK6 is controlled by what probably is its main substrate. It is important to keep in mind that although MKK6 can activate all of the p38 MAPKs, lower MKK6 concentrations preferentially activate p38α (2). We found that in mice, the amount of MKK6 protein is quite different from tissue to tissue. It is therefore possible that the regulation of MKK6 levels by p38α reflects the need to keep a particular ratio between the two proteins, ensuring the appropriate cellular response to a particular signal (via p38α) and avoiding the possible activation of other p38 MAPKs, which may have different functions. It is of note that in our cell lines the increased amount of MKK6 protein also correlates with increased basal MKK6 phosphorylation, which has rarely been described. Similarly, the muscle-specific deletion of the insulin like growth factor 1 receptor results in the increase of both the level of p38α protein and its basal phosphorylation (26). This result, together with our observations reported here, suggests that in nonstimulated cells there is a correlation between the phosphorylation level of MAPK pathway components and their steady-state protein concentrations.
The difference in the susceptibility to activation of p38 MAPKs depending on the MKK6 concentration observed in vitro (2) has also been verified under physiological conditions. For example, both p38α and p38δ are highly expressed in primary macrophages but lipopolysaccharide stimulation can activate p38α to a greater extent whereas endothelial cells also express comparable levels of p38α and p38β but IL-1β preferentially activates p38α (31). Although in these cases the activity of MKK6 (and other p38 MKKs) was not investigated, the results are consistent with the idea that high levels of MKK6 may be important for the full activation of p38 MAPKs other than p38α.
Our work suggests that an appropriate ratio of MKK6 to its main substrate, p38α, is important for the specificity of signaling by p38 MAPKs. Thus, the regulation of MKK6 expression by p38α may contribute to ensuring the precise cellular response to extracellular signals. Further work on the mechanisms involved should also help to elucidate the factors implicated in the regulation of mRNA turnover by p38α in nonstressed cells.
Acknowledgments
We thank Juan Valcarcel for many useful suggestions and for critical reading of the manuscript. We are also grateful to Almudena Porras and Margaret Jones for their help during the early stages of this work and to Stefania Castagnetti for technical advice.
REFERENCES
- 1.Adams, R. H., A. Porras, G. Alonso, M. Jones, K. Vintersten, S. Panelli, A. Valladares, L. Perez, R. Klein, and A. R. Nebreda. 2000. Essential role of p38alpha MAP kinase in placental but not embryonic cardiovascular development. Mol. Cell 6:109-116. [PubMed] [Google Scholar]
- 2.Alonso, G., C. Ambrosino, M. Jones, and A. R. Nebreda. 2000. Differential activation of p38 mitogen-activated protein kinase isoforms depending on signal strength. J. Biol. Chem. 275:40641-40648. [DOI] [PubMed] [Google Scholar]
- 3.Ambrosino, C., and A. R. Nebreda. 2001. Cell cycle regulation by p38 MAP kinases. Biol. Cell 93:47-51. [DOI] [PubMed] [Google Scholar]
- 4.Antic, D., N. Lu, and J. D. Keene. 1999. ELAV tumor antigen, Hel-N1, increases translation of neurofilament M mRNA and induces formation of neurites in human teratocarcinoma cells. Genes Dev. 13:449-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Atwater, J. A., R. Wisdom, and I. M. Verma. 1990. Regulated mRNA stability. Annu. Rev. Genet. 24:519-541. [DOI] [PubMed] [Google Scholar]
- 6.Bulavin, D. V., S. Saito, M. C. Hollander, K. Sakaguchi, C. W. Anderson, E. Appella, and A. J. Fornace, Jr. 1999. Phosphorylation of human p53 by p38 kinase coordinates N-terminal phosphorylation and apoptosis in response to UV radiation. EMBO J. 18:6845-6854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buzby, J. S., G. Brewer, and D. J. Nugent. 1999. Developmental regulation of RNA transcript destabilization by A + U-rich elements is AUF1-dependent. J. Biol. Chem. 274:33973-33978. [DOI] [PubMed] [Google Scholar]
- 8.Carballo, E., H. Cao, W. S. Lai, E. A. Kennington, D. Campbell, and P. J. Blackshear. 2001. Decreased sensitivity of tristetraprolin-deficient cells to p38 inhibitors suggests the involvement of tristetraprolin in the p38 signaling pathway. J. Biol. Chem. 276:42580-42587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Casanovas, O., F. Miro, J. M. Estanyol, E. Itarte, N. Agell, and O. Bachs. 2000. Osmotic stress regulates the stability of cyclin D1 in a p38SAPK2-dependent manner. J. Biol. Chem. 275:35091-35097. [DOI] [PubMed] [Google Scholar]
- 10.Chellappan, S. P. 2001. HOG on the promoter: regulation of the osmotic stress response. Science STKE 2001(93):PE1. [DOI] [PubMed] [Google Scholar]
- 11.Chen, C. Y., T. M. Chen, and A. B. Shyu. 1994. Interplay of two functionally and structurally distinct domains of the c-fos AU-rich element specifies its mRNA-destabilizing function. Mol. Cell. Biol. 14:416-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen, C. Y., R. Gherzi, S. E. Ong, E. L. Chan, R. Raijmakers, G. J. Pruijn, G. Stoecklin, C. Moroni, M. Mann, and M. Karin. 2001. AU binding proteins recruit the exosome to degrade ARE-containing mRNAs. Cell 107:451-464. [DOI] [PubMed] [Google Scholar]
- 13.Chen, C. Y., and A. B. Shyu. 1995. AU-rich elements: characterization and importance in mRNA degradation. Trends Biochem. Sci. 20:465-470. [DOI] [PubMed] [Google Scholar]
- 14.Cohen, P. 1997. The search for physiological substrates of MAP and SAP kinases in mammalian cells. Trends Cell Biol. 7:353-361. [DOI] [PubMed] [Google Scholar]
- 15.Cuenda, A., G. Alonso, N. Morrice, M. Jones, R. Meier, P. Cohen, and A. R. Nebreda. 1996. Purification and cDNA cloning of SAPKK3, the major activator of RK/p38 in stress-and cytokine-stimulated monocytes and epithelial cells. EMBO J. 15:4156-4164. [PMC free article] [PubMed] [Google Scholar]
- 16.Cuenda, A., and P. Cohen. 1999. Stress-activated protein kinase-2/p38 and a rapamycin-sensitive pathway are required for C2C12 myogenesis. J. Biol. Chem. 274:4341-4346. [DOI] [PubMed] [Google Scholar]
- 17.Cuenda, A., P. Cohen, V. Buee-Scherrer, and M. Goedert. 1997. Activation of stress-activated protein kinase-3 (SAPK3) by cytokines and cellular stresses is mediated via SAPKK3 (MKK6); comparison of the specificities of SAPK3 and SAPK2 (RK/p38). EMBO J. 16:295-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davies, S. P., H. Reddy, M. Caivano, and P. Cohen. 2000. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem. J. 351:95-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dean, J. L., M. Brook, A. R. Clark, and J. Saklatvala. 1999. p38 mitogen-activated protein kinase regulates cyclooxygenase-2 mRNA stability and transcription in lipopolysaccharide-treated human monocytes. J. Biol. Chem. 274:264-269. [DOI] [PubMed] [Google Scholar]
- 20.Derijard, B., J. Raingeaud, T. Barrett, I. H. Wu, J. Han, R. J. Ulevitch, and R. J. Davis. 1995. Independent human MAP-kinase signal transduction pathways defined by MEK and MKK isoforms. Science 267:682-685. [DOI] [PubMed] [Google Scholar]
- 21.Doza, N. Y., A. Cuenda, G. M. Thomas, P. Cohen, and A. R. Nebreda. 1995. Activation of the MAP kinase homologue RK requieres the phosphorylation of Thr-180 and Tyr-182 and both residues are phosphorylated in chemically stressed KB cells. FEBS Lett. 364:223-228. [DOI] [PubMed] [Google Scholar]
- 22.Duval, D., B. Reinhardt, C. Kedinger, and H. Boeuf. 2000. Role of suppressors of cytokine signaling (Socs) in leukemia inhibitory factor (LIF)-dependent embryonic stem cell survival. FASEB J. 14:1577-1584. [DOI] [PubMed] [Google Scholar]
- 23.Engelman, J. A., M. P. Lisanti, and P. E. Scherer. 1998. Specific inhibitors of p38 mitogen-activated protein kinase block 3T3- L1 adipogenesis. J. Biol. Chem. 273:32111-32120. [DOI] [PubMed] [Google Scholar]
- 24.Enslen, H., J. Raingeaud, and R. J. Davis. 1998. Selective activation of p38 mitogen-activated protein (MAP) kinase isoforms by the MAP kinase kinases MKK3 and MKK6. J. Biol. Chem. 273:1741-1748. [DOI] [PubMed] [Google Scholar]
- 25.Fan, X. C., and J. A. Steitz. 1998. Overexpression of HuR, a nuclear-cytoplasmic shuttling protein, increases the in vivo stability of ARE-containing mRNAs. EMBO J. 17:3448-3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fernandez, A. M., J. Dupont, R. P. Farrar, S. Lee, B. Stannard, and D. Le Roith. 2002. Muscle-specific inactivation of the IGF-I receptor induces compensatory hyperplasia in skeletal muscle. J. Clin. Investig. 109:347-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gallouzi, I. E., C. M. Brennan, M. G. Stenberg, M. S. Swanson, A. Eversole, N. Maizels, and J. A. Steitz. 2000. HuR binding to cytoplasmic mRNA is perturbed by heat shock. Proc. Natl. Acad. Sci. USA 97:3073-3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garcia, J., B. Lemercier, S. Roman-Roman, and G. Rawadi. 1998. A Mycoplasma fermentans-derived synthetic lipopeptide induces AP-1 and NF-kappaB activity and cytokine secretion in macrophages via the activation of mitogen-activated protein kinase pathways. J. Biol. Chem. 273:34391-34398. [DOI] [PubMed] [Google Scholar]
- 29.Ge, B., H. Gram, F. Di Padova, B. Huang, L. New, R. J. Ulevitch, Y. Luo, and J. Han. 2002. MAPKK-independent activation of p38 mediated by TAB1-dependent autophosphorylation of p38. Science 295:1291-1294. [DOI] [PubMed] [Google Scholar]
- 30.Goedert, M., A. Cuenda, M. Craxton, R. Jakes, and P. Cohen. 1997. Activation of the novel stress-activated protein kinase SAPK4 by cytokines and cellular stresses is mediated by SKK3 (MKK6); comparison of its substrate specificity with that of other SAP kinases. EMBO J. 16:3563-3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hale, K. K., D. Trollinger, M. Rihanek, and C. L. Manthey. 1999. Differential expression and activation of p38 mitogen-activated protein kinase alpha, beta, gamma, and delta in inflammatory cell lineages. J. Immunol. 162:4246-4252. [PubMed] [Google Scholar]
- 32.Han, J., J. D. Lee, L. Bibbs, and R. J. Ulevitch. 1994. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science 265:808-811. [DOI] [PubMed] [Google Scholar]
- 33.Han, J., J. D. Lee, Y. Jiang, Z. Li, L. Feng, and R. J. Ulevitch. 1996. Characterization of the structure and function of a novel MAP kinase kinase (MKK6). J. Biol. Chem. 271:2886-2891. [DOI] [PubMed] [Google Scholar]
- 34.Han, J., X. Wang, Y. Jiang, R. J. Ulevitch, and S. Lin. 1997. Identification and characterization of a predominant isoform of human MKK3. FEBS Lett. 403:19-22. [DOI] [PubMed] [Google Scholar]
- 35.Hashimoto, A., H. Okada, A. Jiang, M. Kurosaki, S. Greenberg, E. A. Clark, and T. Kurosaki. 1998. Involvement of guanosine triphosphatases and phospholipase C-gamma2 in extracellular signal-regulated kinase, c-Jun NH2-terminal kinase, and p38 mitogen-activated protein kinase activation by the B cell antigen receptor. J. Exp. Med. 188:1287-1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hawley, R. G., F. H. Lieu, A. Z. Fong, and T. S. Hawley. 1994. Versatile retroviral vectors for potential use in gene therapy. Gene Ther. 1:136-138. [PubMed] [Google Scholar]
- 37.Hazzalin, C. A., A. Cuenda, E. Cano, P. Cohen, and L. C. Mahadevan. 1997. Effects of the inhibition of p38/RK MAP kinase on induction of five fos and jun genes by diverse stimuli. Oncogene 15:2321-2331. [DOI] [PubMed] [Google Scholar]
- 38.Huang, S., L. New, Z. Pan, J. Han, and G. R. Nemerow. 2000. Urokinase plasminogen activator/urokinase-specific surface receptor expression and matrix invasion by breast cancer cells requires constitutive p38alpha mitogen-activated protein kinase activity. J. Biol. Chem. 275:12266-12272. [DOI] [PubMed] [Google Scholar]
- 39.Ihle, J. N. 2000. The challenges of translating knockout phenotypes into gene function. Cell 102:131-134. [DOI] [PubMed] [Google Scholar]
- 40.Jiang, Y., C. Chen, Z. Li, W. Guo, J. A. Gegner, S. Lin, and J. Han. 1996. Characterization of the structure and function of a new mitogen-activated protein kinase (p38beta). J. Biol. Chem. 271:17920-17926. [DOI] [PubMed] [Google Scholar]
- 41.Jiang, Y., H. Gram, M. Zhao, L. New, J. Gu, L. Feng, F. Di Padova, R. J. Ulevitch, and J. Han. 1997. Characterization of the structure and function of the fourth member of p38 group mitogen-activated protein kinases, p38delta. J. Biol. Chem. 272:30122-30128. [DOI] [PubMed] [Google Scholar]
- 42.Kyriakis, J. M., and J. Avruch. 2001. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol. Rev. 81:807-869. [DOI] [PubMed] [Google Scholar]
- 43.Lai, W. S., E. Carballo, J. R. Strum, E. A. Kennington, R. S. Phillips, and P. J. Blackshear. 1999. Evidence that tristetraprolin binds to AU-rich elements and promotes the deadenylation and destabilization of tumor necrosis factor alpha mRNA. Mol. Cell. Biol. 19:4311-4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lasa, M., K. R. Mahtani, A. Finch, G. Brewer, J. Saklatvala, and A. R. Clark. 2000. Regulation of cyclooxygenase 2 mRNA stability by the mitogen-activated protein kinase p38 signaling cascade. Mol. Cell. Biol. 20:4265-4274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lechner, C., M. A. Zahalka, J. F. Giot, N. P. Moller, and A. Ullrich. 1996. ERK6, a mitogen-activated protein kinase involved in C2C12 myoblast differentiation. Proc. Natl. Acad. Sci. USA 93:4355-4359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee, J. C., J. T. Laydon, P. C. McDonnell, T. F. Gallagher, S. Kumar, D. Green, D. McNulty, M. J. Blumenthal, J. R. Heys, S. W. Landvatter, et al. 1994. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature 372:739-746. [DOI] [PubMed] [Google Scholar]
- 47.Lee, N. H., and R. L. Malek. 1998. Nerve growth factor regulation of m4 muscarinic receptor mRNA stability but not gene transcription requires mitogen-activated protein kinase activity. J. Biol. Chem. 273:22317-22325. [DOI] [PubMed] [Google Scholar]
- 48.Lin, A., A. Minden, H. Marinetto, F.-X. Claret, C. Lange-Carter, F. Mercurio, G. L. Johnson, and M. Karin. 1995. Identification of a dual specificity kinase that activates the Jun kinases and p38-Mpk2. Science 268:286-290. [DOI] [PubMed] [Google Scholar]
- 49.Lin, S., W. Wang, G. M. Wilson, X. Yang, G. Brewer, N. J. Holbrook, and M. Gorospe. 2000. Down-regulation of cyclin D1 expression by prostaglandin A(2) is mediated by enhanced cyclin D1 mRNA turnover. Mol. Cell. Biol. 20:7903-7913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mahtani, K. R., M. Brook, J. L. Dean, G. Sully, J. Saklatvala, and A. R. Clark. 2001. Mitogen-activated protein kinase p38 controls the expression and posttranslational modification of tristetraprolin, a regulator of tumor necrosis factor alpha mRNA stability. Mol. Cell. Biol. 21:6461-6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mertens, S., M. Craxton, and M. Goedert. 1996. SAP kinase-3, a new member of the family of mammalian stress-activated protein kinases. FEBS Lett. 383:273-276. [DOI] [PubMed] [Google Scholar]
- 52.Ming, X. F., G. Stoecklin, M. Lu, R. Looser, and C. Moroni. 2001. Parallel and independent regulation of interleukin-3 mRNA turnover by phosphatidylinositol 3-kinase and p38 mitogen-activated protein kinase. Mol. Cell. Biol. 21:5778-5789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Miyazawa, K., A. Mori, H. Miyata, M. Akahane, Y. Ajisawa, and H. Okudaira. 1998. Regulation of interleukin-1 beta-induced interleukin-6 gene expression in human fibroblast-like synoviocytes by p38 mitogen-activated protein kinase. J. Biol. Chem 273:24832-24838. [DOI] [PubMed] [Google Scholar]
- 54.Mudgett, J. S., J. Ding, L. Guh-Siesel, N. A. Chartrain, L. Yang, S. Gopal, and M. M. Shen. 2000. Essential role for p38alpha mitogen-activated protein kinase in placental angiogenesis. Proc. Natl. Acad. Sci. USA 97:10454-10459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nebreda, A. R., and A. Porras. 2000. p38 MAP kinases: beyond the stress response. Trends Biochem. Sci. 25:257-260. [DOI] [PubMed] [Google Scholar]
- 56.Ono, K., and J. Han. 2000. The p38 signal transduction pathway: activation and function. Cell. Signal. 12:1-13. [DOI] [PubMed] [Google Scholar]
- 57.Raghavan, A., R. L. Robison, J. McNabb, C. R. Miller, D. A. Williams, and P. R. Bohjanen. 2001. HuA and tristetraprolin are induced following T cell activation and display distinct but overlapping RNA binding specificities. J. Biol. Chem. 276:47958-47965. [DOI] [PubMed] [Google Scholar]
- 58.Raingeaud, J., A. J. Whitmarsh, T. Barret, B. Derijard, and R. J. Davis. 1996. MKK3-and MKK6-regulated gene expression is mediated by the p38 mitogen-activated protein kinase signal transduction pathway. Mol. Cell. Biol. 16:1247-1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ross, J. 1997. A hypothesis to explain why translation inhibitors stabilize mRNAs in mammalian cells: mRNA stability and mitosis. Bioessays 19:527-529. [DOI] [PubMed] [Google Scholar]
- 60.Rouse, J., P. Cohen, S. Trigon, M. Morange, A. Alonso-Llamazares, D. Zamanillo, T. Hunt, and A. R. Nebreda. 1994. A novel kinase cascade triggered by stress and heat shock that stimulates MAPKAP kinase-2 and phosphorylation of the small heat shock proteins. Cell 78:1027-1037. [DOI] [PubMed] [Google Scholar]
- 61.Rutault, K., C. A. Hazzalin, and L. C. Mahadevan. 2001. Combinations of ERK and p38 MAPK inhibitors ablate tumor necrosis factor-alpha (TNF-alpha) mRNA induction. Evidence for selective destabilization of TNF-alpha transcripts. J. Biol. Chem. 276:6666-6674. [DOI] [PubMed] [Google Scholar]
- 62.Sachs, A. B. 1993. Messenger RNA degradation in eukaryotes. Cell 74:413-421. [DOI] [PubMed] [Google Scholar]
- 63.Tamura, K., T. Sudo, U. Senftleben, A. M. Dadak, R. Johnson, and M. Karin. 2000. Requirement for p38alpha in erythropoietin expression: a role for stress kinases in erythropoiesis. Cell 102:221-231. [DOI] [PubMed] [Google Scholar]
- 64.Vasudevan, S., and S. W. Peltz. 2001. Regulated ARE-mediated mRNA decay in Saccharomyces cerevisiae. Mol. Cell 7:1191-1200. [DOI] [PubMed] [Google Scholar]
- 65.Wang, W., M. C. Caldwell, S. Lin, H. Furneaux, and M. Gorospe. 2000. HuR regulates cyclin A and cyclin B1 mRNA stability during cell proliferation. EMBO J. 19:2340-2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang, W., H. Furneaux, H. Cheng, M. C. Caldwell, D. Hutter, Y. Liu, N. Holbrook, and M. Gorospe. 2000. HuR regulates p21 mRNA stabilization by UV light. Mol. Cell. Biol. 20:760-769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang, X., and G. P. Studzinski. 2001. Inhibition of p38MAP kinase potentiates the JNK/SAPK pathway and AP-1 activity in monocytic but not in macrophage or granulocytic differentiation of HL60 cells. J. Cell. Biochem. 82:68-77. [DOI] [PubMed] [Google Scholar]
- 68.Winzen, R., M. Kracht, B. Ritter, A. Wilhelm, C. Y. Chen, A. B. Shyu, M. Muller, M. Gaestel, K. Resch, and H. Holtmann. 1999. The p38 MAP kinase pathway signals for cytokine-induced mRNA stabilization via MAP kinase-activated protein kinase 2 and an AU-rich region-targeted mechanism. EMBO J. 18:4969-4980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yeilding, N. M., W. N. Procopio, M. T. Rehman, and W. M. Lee. 1998. c-myc mRNA is down-regulated during myogenic differentiation by accelerated decay that depends on translation of regulatory coding elements. J. Biol. Chem. 273:15749-15757. [DOI] [PubMed] [Google Scholar]
- 70.Zhang, W., B. J. Wagner, K. Ehrenman, A. W. Schaefer, C. T. DeMaria, D. Crater, K. DeHaven, L. Long, and G. Brewer. 1993. Purification, characterization, and cDNA cloning of an AU-rich element RNA-binding protein, AUF1. Mol. Cell. Biol. 13:7652-7665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhu, W., J. S. Downey, J. Gu, F. Di Padova, H. Gram, and J. Han. 2000. Regulation of TNF expression by multiple mitogen-activated protein kinase pathways. J. Immunol. 164:6349-6358. [DOI] [PubMed] [Google Scholar]