Abstract
The polycomb protein Bmi-1 represses the INK4a locus, which encodes the tumor suppressors p16 and p14ARF. Here we report that Bmi-1 is downregulated when WI-38 human fibroblasts undergo replicative senescence, but not quiescence, and extends replicative life span when overexpressed. Life span extension by Bmi-1 required the pRb, but not p53, tumor suppressor protein. Deletion analysis showed that the RING finger and helix-turn-helix domains of Bmi-1 were required for life span extension and suppression of p16. Furthermore, a RING finger deletion mutant exhibited dominant negative activity, inducing p16 and premature senescence. Interestingly, presenescent cultures of some, but not all, human fibroblasts contained growth-arrested cells expressing high levels of p16 and apparently arrested by a p53- and telomere-independent mechanism. Bmi-1 selectively extended the life span of these cultures. Low O2 concentrations had no effect on p16 levels or life span extension by Bmi-1 but reduced expression of the p53 target, p21. We propose that some human fibroblast strains are more sensitive to stress-induced senescence and have both p16-dependent and p53/telomere-dependent pathways of senescence. Our data suggest that Bmi-1 extends the replicative life span of human fibroblasts by suppressing the p16-dependent senescence pathway.
Normal human fibroblasts irreversibly arrest growth after a finite number of divisions owing to a process termed replicative senescence (8-10, 17). It is generally accepted that human fibroblasts senesce because they eventually acquire one or more short, dysfunctional telomeres (14, 30, 34, 38). Telomere shortening is an inevitable consequence of the biochemistry of DNA replication and lack of telomerase expression by most somatic cells. Indeed, ectopic expression of telomerase confers an indefinite replicative life span (replicative immortality) on a variety of normal human cells without inducing significant genomic instability (5, 40, 63). However, it is now clear that many stimuli, including DNA damage and oxidative stress, cause cells to arrest growth with a senescent phenotype, independent of telomere length or structure (16, 31, 34, 35, 53, 59, 67).
Bmi-1 was identified as a c-myc-cooperating oncogene in murine B- and T-cell lymphomagenesis (25, 62). Bmi-1 is a transcriptional repressor belonging to the Polycomb group gene family (61). Polycomb group proteins, and the counteracting Trithorax group proteins, are crucial for maintaining proper gene expression patterns during development (44). A critical target of Bmi-1 is the INK4a locus, which encodes the p16 and p19ARF (p14ARF in humans) tumor suppressor proteins (28). Overexpression of Bmi-1 extends the replicative life spans of mouse and human fibroblasts (28), possibly by repressing p16 and p19/p14ARF. Studies using mouse embryo fibroblasts (MEFs) harboring specific deletions in the INK4a locus indicate that p19ARF, but not p16, is required for replicative senescence of mouse cells (36, 56). However, in contrast to human cells, mouse cells have long telomeres and express telomerase (59, 67). Thus, there are important differences between mouse and human cells. We therefore sought to determine the role of Bmi-1 in the replicative senescence of human fibroblasts.
Human cells require the p53 and pRb tumor suppressor proteins in order to senesce. Hence, the replicative life span of human cells is significantly extended when p53 and/or pRb function is suppressed by simian virus 40 (SV40) T antigen, human papilloma virus (HPV) E6 or E7 proteins, dominant negative p53 mutants, or antisense oligonucleotides (7, 17, 21, 57). Dysfunctional telomeres are thought to resemble damaged DNA and hence elicit a p53-dependent damage response (12, 49). Consistent with this idea, as human fibroblasts senesce, p53 is posttranslationally modified (3, 26, 64), and the p21 gene, a key p53 target and growth inhibitor, is highly expressed (26, 41). Some senescent cells also express high levels of p16, which inhibits the inactivating phosphorylation of pRb (1, 22, 35, 42, 52, 53, 68).
p16 is induced by a variety of stimuli (45), including DNA damage (47, 55), reactive oxidants (11), and some chemotherapeutic drugs (52). In these cases, p16 mediates a telome-re-independent senescence response, which has been termed premature or accelerated senescence. Although telome-rase appears to be sufficient to immortalize human fibroblasts, telomerase cannot immortalize human mammary epithelial cells and keratinocytes unless p16 has been inactivated (typically by methylation) (35, 46). It is generally thought that p21 establishes the senescence arrest, while p16 maintains the arrest (1, 60). However, the precise relationship between replicative life span, status of the p53 and pRb pathways, and p16 is not yet clear, especially for human fibroblasts.
It was recently shown that Bmi-1 activates telomerase in human mammary epithelial cells but not fibroblasts (18). Thus, the mechanism by which Bmi-1 extends the replicative life span of human fibroblasts remains obscure. Downregulation of p16 is an attractive candidate, but there may be other targets of Bmi-1, as important as or more important than p16, that mediate its effect on life span. Moreover, it is not known whether endogenous Bmi-1 expression changes when human cells senesce, as is the case for several growth-regulatory genes (10, 15, 23, 54, 58), nor whether Bmi-1 is responsible for upregulating p16 in senescent cells. To understand the roles of Bmi-1 and p16 in human fibroblast senescence, we examined their expression in presenescent and senescent cells and the effects of wild-type and mutant Bmi-1 proteins on the replicative life span of several human fibroblast lines. We show that the Bmi-1 is downregulated in a senescence-specific manner and that pRb, but not p53, is required for its effects on life span. We also show that highly p16-expressing cells are detectable in presenescent cultures of some, but not all, human fibroblast strains. The ability of Bmi-1 to extend replicative life span is robust only in strains with relatively few high p16-expressing cells (when presenescent) and is not altered by culturing cells in a low concentration (3%) of oxygen.
MATERIALS AND METHODS
Cells and cell culture.
WI-38 human fetal lung fibroblasts were obtained as described elsewhere (19). 82-6 (skin) and BJ (foreskin, also called HCA2) fibroblasts were from J. Oshima (University of Washington, Seattle) and J. Smith (University of Texas, San Antonio), respectively. Cells were cultured and made senescent by serial passage, as described elsewhere (19). Cells were given [3H]thymidine for 3 days, processed for autoradiography to determine the percent radiolabeled nuclei (%LN), and stained for senescence-associated β-galactosidase (SA-β-Gal), as described elsewhere (19). Cultures with >70% LN were considered presenescent, and those with <5% LN and >80% SA-β-Gal-positive cells were considered senescent. Three percent oxygen (O2) was obtained by increasing N2 while maintaining 10% CO2, using an O2 regulator (Reming Bioinstruments, Redfield, N.Y.). Cell death was measured by staining adherent cells with Mitocapture (BioVision Inc., Mountain View, Calif.) to assess mitochondrial membrane permeability (29).
Vectors and retroviruses.
Retroviruses carrying E6 or E7 were provided by D. Galloway (Fred Hutchinson Cancer Center, Seattle, Wash.). Wild-type and mutant Bmi-1 proteins were expressed by using pBabe and pMSCV-hygro (pM0) retroviral vectors, as described elsewhere (16). Infection efficiencies were always >70%.
Northern analysis.
Total cellular RNA (15 to 20 μg), purified with a commercial kit (Promega, Madison, Wis.), was analyzed by Northern blotting as described elsewhere (15). The blot was hybridized to 32P-labeled cDNA probes, stripped, and rehybridized to a control probe corresponding to Gi2α mRNA, which does not change during senescence (54).
Western analysis.
Cells were washed with phosphate-buffered saline (PBS), lysed in sample buffer (37) lacking β-mercaptoethanol, and analyzed immediately or frozen at −80°C. Prior to analysis, β-mercaptoethanol was added and samples were heated at 95°C for 5 min. Proteins were separated in 10% or 4 to 15% denaturing polyacrylamide gels and transferred to polyvinylidene difluoride membranes, and the membranes were blocked, incubated with primary and secondary antibodies, and washed, as described elsewhere (16, 18, 27). Secondary antibodies were detected by chemiluminescence (ECL Plus; Amersham-Pharmacia). Antibodies recognizing p53 (Ab6; DO-1) and α-tubulin (Ab1) were from Calbiochem. p21 (6B6), p16 (Ab-1; DCS-50.1), and Ki67 (NCL-Ki67p) antibodies were from Pharmingen, NeoMarkers, and Novocastra Laboratories, respectively. Anti-Bmi-1 (F6) was described elsewhere (2). Signals were quantified by densitometry.
Immunofluorescence.
Cells were cultured in four-well slide chambers, fixed with 3.7% formaldehyde in PBS, washed with PBS, and permeabilized with 0.5% Triton X-100 in PBS. Slides were blocked with 10% nonfat milk in PBS, incubated with primary and secondary antibodies in blocking solution for 1 h each, mounted in VectaShield containing DAPI (4′,6′-diamidino-2-phenylindole; Vector Laboratories), and viewed by epifluorescence. For p16 staining, H1299 (32) and HeLa cells served as negative and positive controls, respectively. For SA-β-Gal/p16 costaining, cells on six-well plates were stained for SA-β-Gal (19) at pH 5.5 to increase sensitivity, washed, permeabilized, and stained as described above, except that incubation with primary antibody was done for 16 h at 4°C.
Telomerase and telomere length assays.
Telomerase activity was assessed by using a telomere repeat amplification protocol (33) kit (Intergen Co., Purchase, N.Y.) as instructed by the supplier. Telomere lengths were analyzed as described elsewhere (5, 24). Briefly, 2 μg of genomic DNA was digested with HinfI and RsaI and analyzed by Southern blotting with a 32P-labeled (TTAGGG)3 probe. Signals were analyzed with a PhosphorImager (Molecular Dynamics). Mean telomere lengths were determined from the signals, as described elsewhere (24).
RESULTS
Bmi-1 levels decline in senescent. but not quiescent, cells.
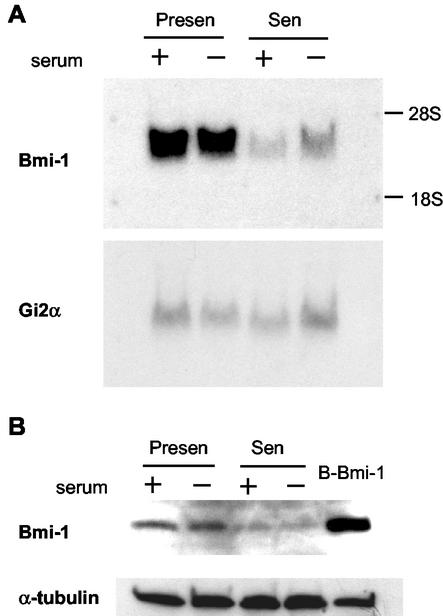
We examined Bmi-1 expression during the replicative senescence of WI-38 human fibroblasts. We analyzed by Northern blotting RNA from presenescent cells, proliferating in 10% serum or made quiescent by low-level (0.2%) serum, and senescent cells similarly cultured in 10% or low-level serum. In these experiments, the %LN of senescent cultures was <1%. Compared to a control (Gi2α) mRNA (54), Bmi-1 mRNA was highly expressed by presenescent, but not senescent, cells (Fig. 1A). Western analysis showed that Bmi-1 protein was likewise abundant in presenescent, but not senescent, cells (Fig. 1B). Importantly, the decline in Bmi-1 levels was independent of growth state per se. Bmi-1 levels did not decline in quiescent cells, suggesting specific downregulation upon senescence (Fig. 1). Similar results were obtained with TIG-3 fibroblasts (not shown). The senescence-associated decline in Bmi-1 levels was most obvious when cultures were maintained for >2 weeks after the %LN reached 5% (not shown). These data suggest that downregulation of endogenous Bmi-1 expression contributes to the senescence of human fibroblasts.
FIG. 1.
Bmi-1 is downregulated in senescent, but not quiescent, cells. Presenescent (Presen) or senescent (Sen) WI-38 cells in 10% serum (+), or cultured in 0.2% serum (−) for 3 days to render presenescent cells quiescent, were harvested for isolation of RNA or protein and analyzed as described in Materials and Methods. Senescent cells were cultured for 2 months after the labeling index reached 5% and had a labeling index of <1% when harvested. (A) Northern analysis for Bmi-1 and Gi2α (control) mRNA. Three independent experiments yielded similar results. (B) Western analysis for Bmi-1 and α-tubulin (control). Presenescent Bmi-1-overexpressing cells (B-Bmi-1) served as a positive control. Four independent experiments showed similar results.
Senescence of Bmi-1-overexpressing fibroblasts.
Bmi-1 overexpression extends the replicative life span of near-senescent human fibroblasts, accompanied by downregulation of p16 (28). p16 downregulation suppresses pRb function, which in turn can cause a crisis-like state characterized by massive cell death, as seen in human fibroblasts that express the pRb-inactivating protein E7 (6). We therefore cultured Bmi-1-overexpressing WI-38 cells to senescence and characterized their phenotype. Bmi-1 was overexpressed by using a retrovirus (pBabe-Bmi-1). Cells infected with an insertless virus (B0) served as a control.
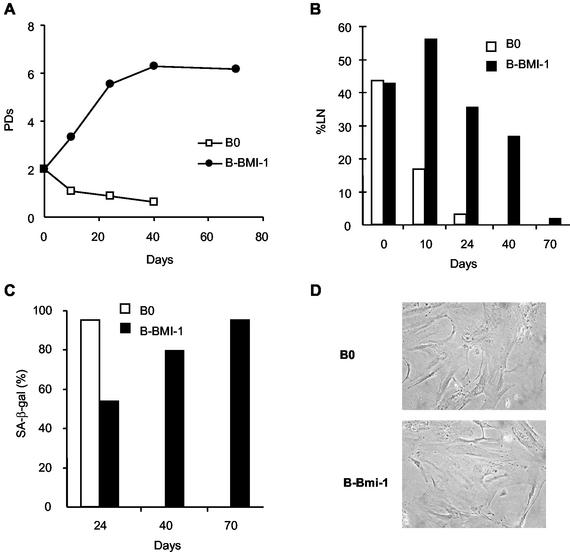
We infected near-senescent cells with pBabe-Bmi-1. Replicative life span increased by ∼4 population doublings (PD), relative to control cells (Fig. 2A). In addition, the %LN increased transiently after infection but eventually declined as the cells senesced (Fig. 2B). Consistent with these results, Bmi-1 transiently lowered the fraction of cells expressing SA-β-Gal, a marker of the senescent phenotype (19) (Fig. 2C). Eventually, however, SA-β-Gal-positive cells reached control levels as the culture senesced. The morphology of senescent Bmi-1-overexpressing cells closely resembled that of control senescent cells (Fig. 2D). Nonetheless, senescent Bmi-1-overexpressing cells continued to overexpress Bmi-1 (data not shown), as expected from studies using retroviruses to express proteins throughout the human fibroblast life span (16, 28). Thus, Bmi-1 drives cell division beyond the normal senescence checkpoint but does not alter the senescence morphology, growth arrest, absence of cell death, or SA-β-Gal expression.
FIG. 2.
Bmi-1 overexpression extends replicative life span but does not prevent senescence. Midpassage WI-38 cells (PD 34) were infected with retroviruses carrying no insert (B0) or Bmi-1 (B-Bmi-1), selected in puromycin, and passaged until senescence. (A) Cell number was determined on the indicated days after selection, and the number of PD completed was calculated. (B) DNA synthesis (%LN) during a 3-day interval was measured as described in Materials and Methods. (C) Cells were stained for SA-β-Gal, as described elsewhere (19). (D) Cells were photographed at the end of their replicative life span, 40 days after selection for control cells and 70 days after selection for Bmi-1-overexpressing cells (magnification, ×200).
Bmi-1 domains important for replicative life span extension.
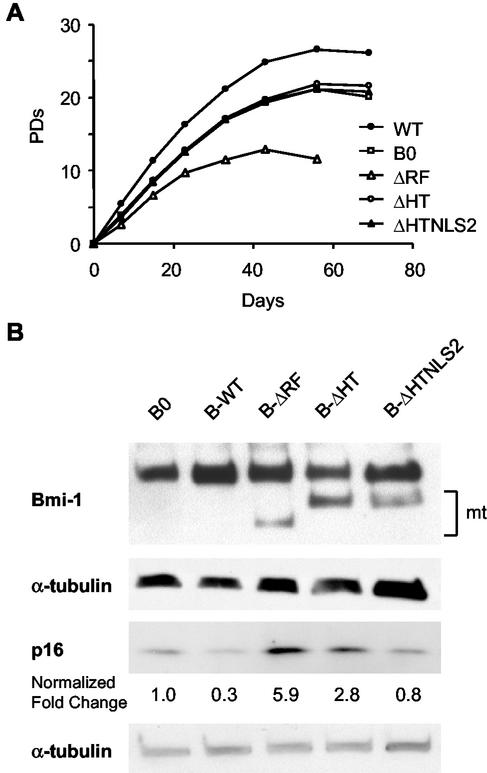
Bmi-1 has an N-terminal RING finger (RF), a central helix-turn-helix-turn-helix-turn (HT), and a C-terminal PEST-like domain (62). The C terminus also contains an essential nuclear localization signal (NLS2) (13, 18). To identify the domain(s) important for replicative life span extension, we overexpressed Bmi-1 deletion mutants lacking either the RF (ΔRF), HT (ΔHT), or HT plus NLS2 (ΔHTNLS2) domains in presenescent WI-38 cells and measured the replicative life span. Both the RING and HT domains were required for life span extension (Fig. 3A). Interestingly, although p16 levels are reported to be low in presenescent cells, wild-type Bmi-1 overexpression significantly promoted growth, even at an early passage (Fig. 3A). Overall, Bmi-1 overexpression extended replicative life span by ∼5 PD, similar to the extension seen in cells infected near senescence (Fig. 2A). Western analysis confirmed that wild-type and mutant Bmi-1 were expressed as expected (Fig. 3B).
FIG. 3.
Bmi-1 domains required for life span extension and p16 repression. (A) Presenescent WI-38 cells (PD 24) were infected with control (B0), wild-type (B-WT), or mutant (ΔRF, ΔHT, or ΔHTNLS2) Bmi-1-expressing retroviruses, as described in the text. After selection, cells were serially passaged, and cell number and number of PD completed were determined. (B) Proteins prepared from cells infected with the indicated retroviruses were analyzed by Western blotting for Bmi-1, p16, and α-tubulin (control). mt, mutant Bmi-1 proteins. Signal intensities were quantified by densitometry using multiple exposures to obtain signals in the linear range. The number beneath each band in the p16 blot is the signal intensity normalized to intensity of the α-tubulin signal, with the value for control cells (B0) set at 1.
Compared to controls, cells expressing Bmi-1 lacking the RF domain (ΔRF) senesced more rapidly (Fig. 3A). MEFs lacking Bmi-1 senesce more rapidly due to accelerated accumulation of p16 and p19ARF (28). We therefore asked whether ΔRF Bmi-1 accelerated p16 or p14ARF accumulation. p16 was strongly induced (∼5-fold) by ΔRF Bmi-1 and to a lesser extent (∼2-fold) by ΔHT Bmi-1 (Fig. 3B). The ΔHTNLS2 mutant did not alter p16 expression, consistent with failure to localize to the nucleus (18). Due to the extremely low abundance of p14ARF in human fibroblasts, its levels were not measured (not shown). Taken together, the results suggest that the RF deletion mutant accelerates senescence by upregulating p16 in presenescent human fibroblasts and thus acts as a dominant negative mutant.
Role of p53 and pRb in life span extension by Bmi-1.
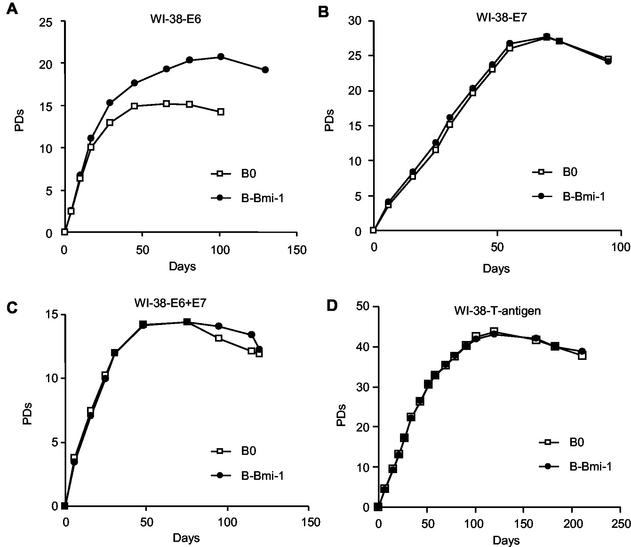
Because elimination of either the p53 or pRb tumor suppressor pathway extends replicative life span, downregulation of the INK4a locus by Bmi-1 can potentially regulate both pathways. We therefore asked which pathway is critical for control of replicative life span by Bmi-1. We overexpressed wild-type Bmi-1 in WI-38 fibroblasts that also express HPV E6 (p53 eliminated), HPV E7 (pRb eliminated), HPV E6 and E7, or SV40 large T antigen (both p53 and pRb eliminated). Bmi-1 overexpression extended the replicative life span of E6-expressing cells by 5.7 (standard error [SE], 0.7; n = 4) PD (Fig. 4A), same extent to which it did in control cells 5.7 (SE, 1.0; n = 4) PD (Fig. 2A and 3A). By contrast, Bmi-1 had no significant effect on the replicative life span of cells expressing E7, E6 and E7, or SV40 large T antigen (Fig. 4B to D). These data suggest that abolition of the pRb, but not p53, pathway is required for Bmi-1 to extend the replicative life span of human fibroblasts.
FIG. 4.
Bmi-1 overexpression extends the replicative life span of E6-expressing but not E7-, E6+E7-, or T-antigen-expressing cells. (A) Presenescent WI-38 cells expressing E6 were infected with control (B0) or Bmi-1 (B-Bmi-1)-expressing retroviruses, selected, and passaged until the end of the replicative life span, and cell number and number of PD completed were determined. (B) Presenescent WI-38 cells expressing E7 were similarly infected with B0 and B-Bmi-1 retroviruses and analyzed. (C) Early-passage E6-expressing cells were superinfected with an E7-expressing retrovirus and then further infected with B0 and B-Bmi-1 retroviruses and analyzed. (D) Presenescent WI-38 cells expressing SV40 large T antigen were infected with B0 and B-Bmi-1 retroviruses and analyzed.
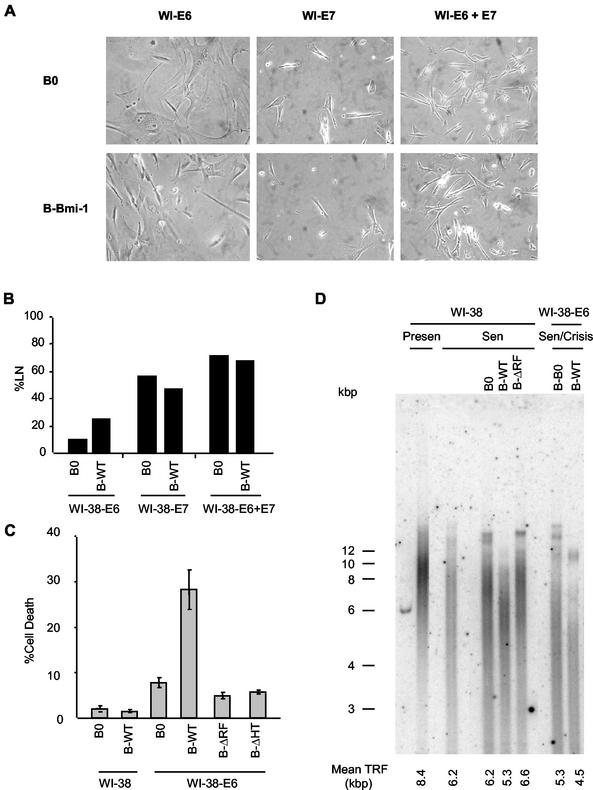
Bmi-1 overexpression causes a crisis-like state in p53-deficient fibroblasts.
Loss of p53 function extends the replicative life span of human fibroblasts, but E6-expressing cells eventually senesce (20, 60). By contrast, loss of both p53 and pRb function causes crisis at the end of the life span. Cultures in crisis have heterogeneous morphologies and growth states (growing, apoptotic, and senescent cells), and a high %LN with little or no net increase in cell number (6, 57, 66). Cultures expressing both E6 and Bmi-1 had a mixture of senescent, growing, and dying cells at the end of their life span (Fig. 5A), with an elevated %LN compared to that of cells expressing no protein, Bmi-1, or E6 alone (Fig. 2B and 5B). These cultures also had more apoptotic cells (Fig. 5C). The Bmi-1 ΔRF and HT mutants had no effect on cell death in E6-expressing cells (Fig. 5C). Together, these data suggest that overexpression of Bmi-1 induces a crisis-like state as p53-deficient cells reach the end of their replicative life span, consistent with Bmi-1 acting primarily by abolishing the pRb, but not p53, pathway.
FIG. 5.
Overexpression of Bmi-1 causes a crisis-like state in E6-expressing cells. (A) Morphology of WI-38 cells expressing E6, E7, or E6+E7, superinfected with control (B0) or Bmi-1-expressing viruses, at the end of the replicative life span. Cells were photographed under phase-contrast microscopy (magnification, ×200). (B) Labeling index (%LN) of the indicated cells was determined at the end of the replicative life span. (C) The indicated cultures at the end of the replicative life span were assessed for percentage of cells undergoing death with a Mitocapture kit, as described elsewhere (29). (D) DNA was isolated from WI-38 cells and WI-38 cells expressing E6, superinfected with control (B0) virus or viruses expressing wild-type Bmi-1 (WT) or the Bmi-1 RING deletion mutant (ΔRF), when cells were presenescent (Presen) or at the end of their replicative life span (Sen or Sen/Crisis). The DNA was analyzed for telomeric signals, as described in Materials and Methods. The mean TRF length was calculated as described elsewhere (24).
Effect of Bmi-1 on telomerase and telomere length.
It was recently reported that Bmi-1 induces telomerase in human mammary epithelial cells but not fibroblasts (18). Similarly, Bmi-1 failed to induce telomerase in E6-expressing fibroblasts, even at the end of their replicative life span, when the cultures entered crisis (data not shown).
It was recently suggested that stressful culture conditions can induce p16, and subsequent premature senescence, in mammary epithelial cells, keratinocytes, and some fibroblasts (45, 46, 59). Such cultures senesce with longer telomeres than those arrested by the p53-mediated telomere-dependent pathway. By downregulating p16, Bmi-1 might inhibit senescence due to stress, but not telomere dysfunction, and Bmi-1-overexpressing cells should senesce with average telomere lengths shorter than those of controls. Alternatively, Bmi-1 may slow telomere erosion, causing senescence with telomere lengths similar to those of controls. To distinguish between these possibilities, we measured the length of terminal restriction fragments (TRFs) of presenescent and senescent Bmi-1-overexpressing cells. Control presenescent cells had an average TRF of 8.4 kb, which shortened to 6.2 kb at senescence (Fig. 5D). Cells overexpressing Bmi-1 senesced with yet shorter TRFs of 5.3 kb (Fig. 5D). Cells expressing the ΔRF mutant, by contrast, senesced with TRFs that were slightly longer than those of control and wild-type Bmi-1-expressing cells. Similar results were obtained in E6-expressing cells. These cells senesced with shorter TRFs (5.3 kb) than control cells, but Bmi-1 caused further erosion to 4.5 kb. These results suggest that Bmi-1 can rescue cells from stress-induced senescence, driving additional cell division and telomere attrition, until cells eventually senesce by the p53/telomere-dependent mechanism.
p16 expression in presenescent cultures.
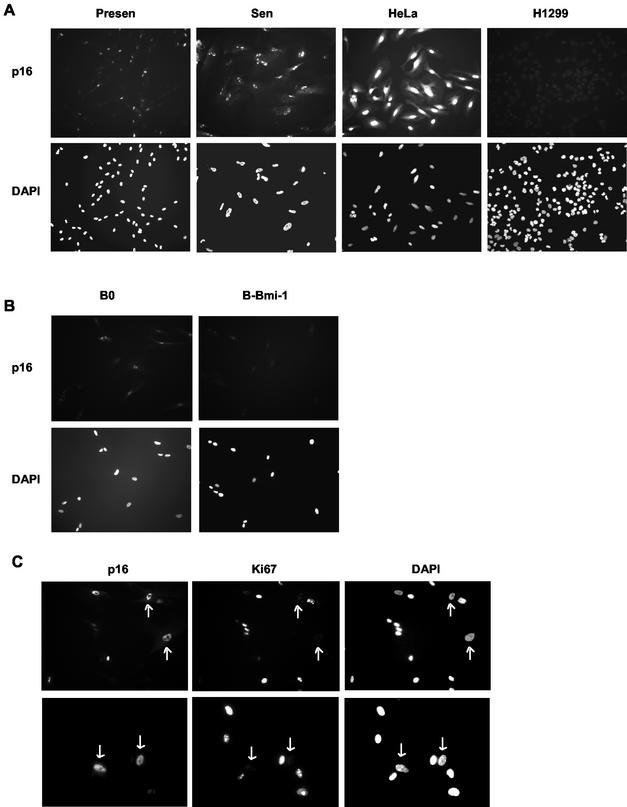
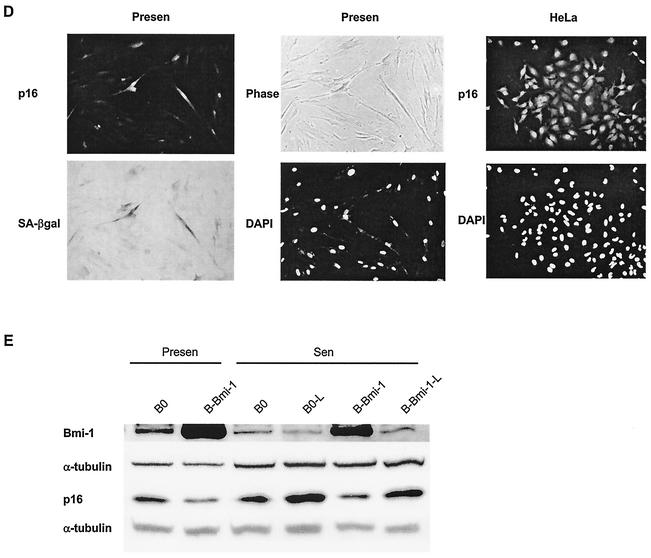
Early-passage fibroblasts have been shown to express low levels of p16, even when telomeres are relatively long (1, 20, 22). This could be due to a small fraction of high-p16-expressing cells in presenescent cultures, which senesce prematurely due to stress. Immunostaining showed that early-passage WI-38 (PD 24) cultures contained a significant fraction (20.5% ± 0.92% [SE]; n = 4) of cells that express a high level of p16, with p16 being essentially undetectable in the remaining cells (Fig. 6A; Table 1). Most cells in Bmi-1-overexpressing cultures (94.4% ± 0.75%; n = 4) did not express p16 (Fig. 6B). Moreover, most p16-positive cells in early-passage cultures failed to express the proliferation marker Ki67 (∼90%) (51) but expressed the senescence marker SA-β-Gal (>80%) (Fig. 6C and D; Table 1). Thus, even early-passage WI-38 cultures contained some cells with high p16, which Bmi-1 overexpression decreased. It is possible that these high-p16-expressing cells have one or more dysfunctional telomeres. However, phenotypic and telomere length analyses of senescent Bmi-1-overexpressing cultures are consistent with these cells having senesced prematurely, likely due to stress.
FIG. 6.
High-p16-expressing cells in presenescent WI-38 cultures and effect of Bmi-1 overexpression. (A) Presenescent cells at PD 24 (Presen) and senescent (Sen) cells were immunostained for p16 as described in Materials and Methods and photographed (magnification, ×100) at the same exposure for each set. Nuclei were visualized by DAPI staining. HeLa and H1299 cells served as positive and negative controls for p16 expression, respectively. (B) Presenescent WI-38 cells at PD 24 were infected with control (B0) or Bmi-1-expressing retroviruses, stained for p16 and DAPI after selection and photographed (magnification, ×200). (C) Presenescent WI-38 cells at PD 24 were immunostained for p16 and Ki67 as described in Materials and Methods and photographed (magnifications, ×300 [top] and ×400 [bottom]). The arrows show examples of p16-positive cells that are Ki67 negative. (D) Presenescent cells at PD 29 were stained for SA-β-Gal activity and subsequently immunostained for p16 as described in Materials and Methods. SA-β-Gal staining was photographed under phase-contrast and bright field (SA-β-Gal) microscopy. HeLa cells served as a positive control for p16 staining. Magnification, ×200. (E) Presenescent cells (PD 24, Presen) were infected with retroviruses carrying no insert (B0) or Bmi-1 (B-Bmi-1), selected, and passaged until senescence (Sen; %LN < 10%). Cells were further cultured for 2 months (B0-L and B-Bmi-1-L; %LN < 1%). Cell lysates were analyzed by Western blotting for Bmi-1, p16, and α-tubulin (control) proteins.
TABLE 1.
Expression of p16 and either Ki67 or SA-β-Gal in presenescent WI-38 fibroblastsa
| Protein | % Positive cellsb |
|---|---|
| p16 | 19.8 ± 0.55 |
| Ki67 | 61.8 ± 1.81 |
| p16 + Ki67 | 2.2 ± 0.26 |
| p16 | 20.2 ± 0.60 |
| SA-β-Gal | 20.6 ± 0.46 |
| p16 + SA-β-Gal | 16.4 ± 0.48 |
Exponentially growing presenescent WI-38 cells were stained for p16, Ki67, and/or SA-β-Gal as described in Materials and Methods. The total number of cells was determined by counting DAPI-stained nuclei or intact cells by using fluorescence or phase-contrast microscopy, respectively. SA-β-Gal-stained cells were counted under bright-field microscopy.
Values are means and SE of four independent experiments. For each experiment, at least 300 cells were scored.
Western analysis confirmed that Bmi-1 overexpression rapidly decreased p16 levels in presenescent WI-38 cultures; moreover, p16 levels increased modestly when these cultures senesced (Fig. 6E). However, p16 was highest in normal senescent cultures after they were maintained for >2 months (%LN < 1%) (Fig. 6E). p16 also increased when Bmi-1-overexpressing cells were maintained for 2 months after senescence (Fig. 6E), but at this time retrovirally expressed Bmi-1 had declined, probably due to silencing of the retroviral promoter. Importantly, p16 and Bmi-1 expression levels were always inversely correlated, supporting the idea that downregulation of Bmi-1 during replicative senescence contributes to the upregulation of p16.
Bmi-1 extends replicative life span in low concentrations of O2.
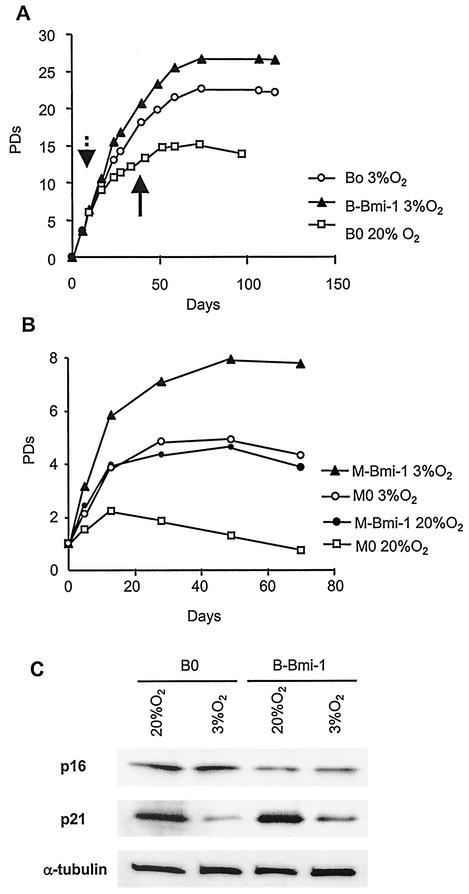
One candidate for inducing p16 and the stress-induced senescence of cultured cells is O2 concentration, which typically is 29% (atmospheric) and significantly higher than the level in most tissues. Indeed, low O2 concentrations (2 to 5%) are known to promote the growth and extend the replicative life span of human cells (43, 48). Thus, reduced O2 might suppress p16 in presenescent WI-38 cultures and abolish the ability of Bmi-1 to extend their replicative life span. To test this possibility, we grew presenescent cells (PD 24) in 3% O2 for 2 weeks, infected them with control (B0) or Bmi-1-expressing (B-Bmi-1) retroviruses in 3% O2, and continued growth in 3% O2 until senescence. Half the B0 culture was also grown in 20% O2. As expected, 3% O2 extended life span by 7.6 PD (Fig. 7A, compare B0 in 3% versus 20% O2). Unexpectedly, Bmi-1 further extended the life span of cells in 3% O2 (Fig. 7A) by >4 PD, similar to its effect in 20% O2 (Fig. 3A). To confirm this finding, we cultured B0 cells in 20% O2 until near-senescence and then superinfected them with murine stem cell virus-based retroviruses carrying no insert (control, M0) or Bmi-1 (M-Bmi-1). After selection, cells growth was monitored in 3 and 20% O2. Bmi-1 promoted growth in 3% O2 (Fig. 7B), similar to its effect in 20% O2 (Fig. 2). Thus, low O2 concentration did not abolish the ability of Bmi-1 to extend replicative life span in WI-38 cells.
FIG. 7.
Bmi-1 overexpression extends replicative life span in low O2 concentrations. (A) Presenescent WI-38 cells (PD 24) were cultured for 2 weeks in 3% O2, infected with control (B0) or Bmi-1 (B-Bmi1)-expressing retrovirus, and selected in 3% O2. Half the control culture (B0) was moved to 20% O2 on the day indicated by the dashed arrow. All cells were passaged until senescence. The solid arrow indicates the day of superinfection (B). (B) A control culture (B0) was superinfected with murine stem cell retroviruses carrying no insert (M0) or Bmi-1 (M-Bmi-1) in either in 3 or 20% O2. Cells were selected in hygromycin and passaged until senescence. (C) Presenescent cells (PD 24) were cultured in 3 or 20% O2 for 2 weeks and infected with retroviruses carrying no insert (B0) or Bmi-1 (B-Bmi-1) in either 3 or 20% O2. Cells were selected and passaged for 1 week. p16, p21, and α-tubulin (control) expression was determined by Western analysis. Three independent experiments showed similar results.
Low O2 concentration affects p21, but not p16, expression.
To determine whether reduced O2 concentration alters p16 expression and understand why it extends replicative life span, we measured p16 and p21 levels in WI-38 cells grown in 20 or 3% O2. p21 levels were greatly reduced when cells were grown in 3% O2 (Fig. 7C). By contrast, p16 levels were unchanged (Fig. 7C). In addition, Bmi-1 overexpression reduced p16 levels, regardless of the O2 concentration, but had no effect on p21 (Fig. 7C). These data suggest that the p53- and telomere-dependent pathway, and not the pRb pathway, contributes to life span extension by low-O2 culture conditions.
Effects of p16 and Bmi-1 in other fibroblast strains.
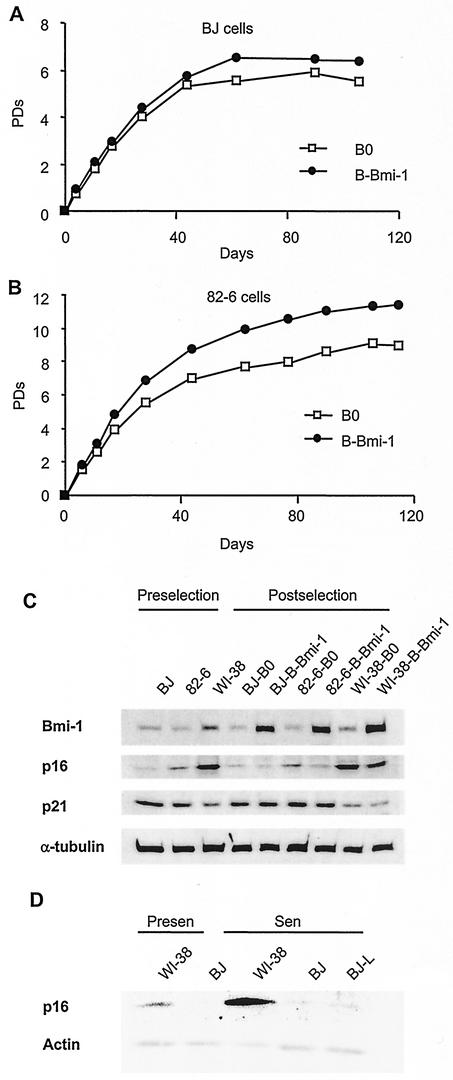
If Bmi-1 extends the replicative life span of WI-38 cells by downregulating p16 in presenescent cultures, it may not affect fibroblasts that do not have stress-induced senescent cells at an early passage. To examine this possibility, we overexpressed Bmi-1 in two additional human fibroblast strains. BJ and 82-6 cells senesce after 80 to 90 and 40 to 50 PD, respectively. These strains were passaged until the cultures reached ∼60% LN and then were infected with B0 or B-Bmi-1 retroviruses. The %LN, determined 4 days after selection, showed no increase in the case of BJ (B0%LN = 57%, versus 58% for B-Bmi-1) and a slight increase in the case of 82-6 (B0%LN = 61%, versus 66% for B-Bmi-1). Consistent with these data, Bmi-1 overexpression did not significantly extend the replicative life span of BJ cells (Fig. 8A) but slightly extended the life span of 82-6 (∼2 PD) (Fig. 8B). There was no significant difference in the levels of retrovirally expressed Bmi-1 in BJ, 82-6, and WI-38 cells (Fig. 8C). Thus, in contrast to WI-38, Bmi-1 had little (82-6) or no (BJ) effect on the replicative life span of other fibroblast strains.
FIG. 8.
p16 inducibility determines life span extension potential by Bmi-1 overexpression. (A) BJ cells (%LN ≈ 60%) were infected with retroviruses carrying no insert (B0) or Bmi-1 (B-Bmi-1) and passaged until senescence, and life span was determined as described in Materials and Methods. (B) 82-6 fibroblasts (%LN ≈ 60%) were similarly infected and analyzed. (C) Protein extracts were prepared from BJ and 82-6 (%LN ≈ 60%) and WI-38 (%LN ≈ 70%) cells prior to infection (Preselection) and after infection with B0 or B-Bmi-1 retroviruses and selection (Postselection). Extracts were analyzed by Western blotting for Bmi-1, p16, p21, and α-tubulin (control) proteins. The p16 blot was exposed for a longer interval to detect the signal in BJ cells. (D) Presenescent (%LN > 70%) and senescent (%LN < 1%) WI-38 and BJ cultures were analyzed by Western blotting for p16 and actin (control). Extracts from senescent BJ cells cultured for an additional 2 months after senescence (BJ-L, %LN < 1%) were also analyzed, with similar results.
Because Bmi-1 appeared to act primarily through p16 in WI-38 cells, we examined p16 levels in BJ and 82-6 cells by Western analysis. Early-passage (∼70% LN) WI-38 cells expressed p16 at a relatively low level, compared to senescent WI-38, but mid-passage BJ and 82-6 cells (∼60% LN) expressed even less p16 (Fig. 8C). Interestingly, compared to WI-38, BJ and 82-6 cells expressed somewhat higher levels of p21, which were not affected by Bmi-1. 82-6 cells expressed slightly more p16 than BJ cells, and Bmi-1 decreased p16 levels in these cells. By contrast, p16 was almost undetectable in BJ cells and was unaffected by Bmi-1. Even fully senescent BJ cultures expressed barely detectable levels of p16 (Fig. 8D). Consistent with the Western data, we could not detect p16 by immunofluorescence in presenescent or senescent BJ (not shown).
Taken together, our data suggest that life span extension mediated by Bmi-1 depends on the ability to repress p16 and correlates with the level of p16 expressed by presenescent cultures. Moreover, human fibroblasts differ in their propensity to induce p16 at an early passage and thus in the malleability of their replicative life span.
DISCUSSION
The p53 and pRb tumor suppressors are critical for the senescence growth arrest of human cells, which constitutes an important barrier to cellular immortalization and hence neoplastic transformation. p53 activates target genes that, in turn, induce apoptosis or arrested growth (26, 27). Because senescent fibroblasts arrest growth without signs of apoptosis (17, 26), p53 very likely acts primarily to establish and/or maintain the senescence growth arrest (17, 26). p21 is thought to be a major effector of p53 activity in senescent cells (20, 60), which, in turn, is thought to be triggered by critical telomere loss (12, 26, 34, 49, 64, 67).
p16, acting through the pRb pathway, is also thought to be important for the senescence response (1, 22, 28, 42, 52, 53, 60). p16 is induced by certain oncogenes (53, 68) and other damage or stress signals (11, 45, 47, 52) and is required for the telomere-independent senescence of some human epithelial cells (35, 46). However, p16 has been reported to rise and inhibit CDK activity only after human fibroblasts have completely arrested due to replicative senescence (60). Indeed, p16 levels were slightly elevated in near-senescent, compared to presenescent, WI-38 cells and rose substantially only after senescent cells were maintained for several weeks. Despite relatively low p16 levels prior to senescence, Bmi-1 extended the replicative life span of WI-38. However, we found that early-passage cultures had a significant number of high-p16-expressing cells, which appeared to be senescent. Bmi-1 selectively reduced the proportion of these cells. We used retroviruses, which do not infect nondividing cells, to overexpress Bmi-1. Thus, Bmi-1 could not act by repressing p16 directly in the prematurely senescent cells. Rather, it is likely that Bmi-1 prevents new induction of p16 and thus inhibits new cells from senescing prematurely, which we propose is caused by extrinsic stress.
Deletion analysis of Bmi-1 suggested that the RF and HT domains of Bmi-1 were required for life span extension of WI-38 fibroblasts. Interestingly, the ΔRF Bmi-1 mutant accelerated replicative senescence and p16 accumulation. Since RF mutants of Bmi-1 can dimerize with wild-type Bmi-1 (50) and wild-type Bmi-1 downregulates p16 expression, we suggest that the ΔRF mutant upregulates p16 by inhibiting wild-type Bmi-1 function, thus acting as a dominant negative mutant. The precise mechanism by which p16 and senescence are induced by the ΔRF mutant remains to be investigated.
We suggest that cells prematurely undergo senescence in cultures such as WI-38 independent of telomere length and function. Indeed, senescent Bmi-1-overexpressing WI-38 cells had shorter telomeres than senescent control cells, consistent with Bmi-1 driving additional cell division and telomere erosion. In addition, Bmi-1 did not extend the life span of cells expressing E7, E6+E7, or T antigen, which is expected if Bmi-1 does not act by retarding telomere erosion. These studies also show that Bmi-1 extends life span by abolishing the pRb, not the p53, pathway. p53 is thought to mediate the senescence response to telomere dysfunction (3, 6, 12, 26, 49, 67), although a p53-dependent but telomere-independent senescence pathway was recently identified in human keratinocytes (46). Finally, early-passage BJ cells were devoid of prematurely senescent (high-p16-expressing) cells, and Bmi-1 had no effect on their replicative life span.
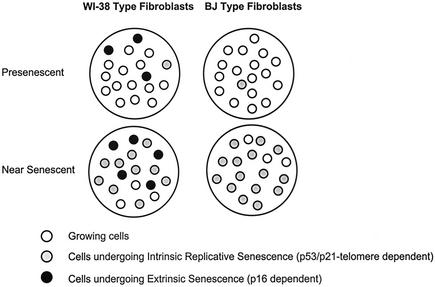
We propose two means by which human fibroblasts senesce (Fig. 9). In cultures such as WI-38 (Fig. 9, left), prematurely senescent cells may continually arise because the cells are sensitive to environmental stress. This senescence, which we term “extrinsic senescence,” is likely caused by stochastic, as-yet-unidentified, cellular or extracellular events and is mediated by p16. Extrinsic senescence is independent of telomeres, unlike replicative senescence, which is intrinsically determined by telomere function. The remaining cells in such cultures eventually undergo p53 telomere-dependent replicative senescence. p16 levels continue to rise in these cultures because replicatively senescent cells remain sensitive to stress caused by extrinsic factors. Bmi-1 suppresses extrinsic senescence by repressing p16 and thus extends the replicative capacity of the culture, but telomere erosion causes Bmi-1-overexpressing cells to eventually senesce. Consistent with this model, cells expressing a dominant negative Bmi-1 mutant senesced more rapidly, with high p16 expression, than control cultures.
FIG. 9.
Model of senescence mechanisms in human fibroblasts. Presenescent fibroblast cultures that resemble WI-38 constantly produce senescent cells by the extrinsic (p16-dependent, telomere-independent) mechanism (left). Presenescent cultures that resemble BJ fibroblasts produce few senescent cells via this extrinsic mechanism (right). As the cultures proliferate and near the end of their replicative life span, cells undergoing senescence by the p53- and telomere-dependent mechanism (intrinsic or replicative senescence) increase in both types of cultures. However, senescent cells produced by the extrinsic senescence mechanism are present only in WI-38-type cultures.
Cultures such as BJ (Fig. 9, right) may be resistant to stress induced by extrinsic factors or incapable of expressing high levels of p16 in response to such stress. Hence, p16 levels remain low and the cells undergo intrinsic replicative senescence owing to telomere erosion. Consistent with this idea, BJ cells senesce with shorter telomeres (4.7 kb) than those of senescent IMR-90 (30) or WI-38 cells (6 to 7 kb). Moreover, Bmi-1-overexpressing WI-38 cells senesced with telomere lengths similar to those of senescent BJ cells. Finally, we find that BJ cells maintain a high labeling index (%LN > 90%) after ectopic expression of telomerase, where telomerase-expressing WI-38 cells continually produce unlabeled senescent (SA-β-Gal-positive) cells (%LN = 50 to 70%) (not shown), suggesting that extrinsic senescence depends on the fibroblast strain and is independent of telomeres. Based on these data and published reports on the replicative life span of fibroblasts of different origins (39), we speculate that lung-derived fibroblasts senesce by extrinsic and intrinsic mechanisms, which depend on p16 and telomeres, respectively (Fig. 9, left), while most skin-derived fibroblasts undergo primarily intrinsic replicative senescence by the telomere-dependent mechanism (Fig. 9, right).
What type of stress induces p16 in fibroblasts? Atmospheric O2 (20%), in which most cells are cultured, is much higher than O2 concentrations in vivo, which can vary from 2 to 10% (4). Reduced O2 has long been known to promote the growth and extend the replicative life span of cultured human cells (43, 48). Therefore, we tested the idea that reduced (3%) O2 might prevent extrinsic senescence and p16 upregulation in cultures such as WI-38. However, levels of p21, not p16, declined in 3% O2. Consistent with Bmi-1 acting through p16, not p21, Bmi-1 extended the life span of WI-38 cells regardless of the O2 level. Because p21 is a p53 target gene and p21 expression declined in 3% O2, low O2 concentrations may extend replicative life span exclusively through the p53- and telomere-dependent senescence mechanism. Indeed, 40% O2 shortens replicative life span and accelerates telomere shortening (65). Although O2 levels did not affect p16 expression, BJ fibroblasts were recently shown to have relatively high antioxidant activity and low levels of protein carbonyls, peroxides, and lipofuscin, compared to WI-38 and MRC-5 fibroblasts (39). It is possible that these secondary metabolites can regulate p16 and hence induce extrinsic senescence. Whatever the case, our findings suggest that not only MEFs but also some strains of human fibroblasts are sensitive to stress in culture and presumably in vivo. Bmi-1 overcomes this p16-dependent, telomere-independent extrinsic senescence. It has been speculated that cellular senescence (whether extrinsic or intrinsic) can compromise the regenerative capacity of tissues (8, 9, 19). Bmi-1 may help maintain the regenerative capacity of these tissues.
Acknowledgments
This work was supported by grants from the National Institutes of Health (AG09909 to J.C. and AG16851 to G.P.D.) and startup funds from the Cancer Center, New England Medical Center, Boston, Mass. (G.P.D.).
We thank Miguel Rubio for providing near-senescent 82-6 cells.
REFERENCES
- 1.Alcorta, D. A., Y. Xiong, D. Phelps, G. Hannon, D. Beach, and J. C. Barrett. 1996. Involvement of the cyclin-dependent kinase inhibitor p16 (INK4a) in replicative senescence of normal human fibroblasts. Proc. Natl. Acad. Sci. USA 93:13742-13747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alkema, M. J., M. Bronk, E. Verhoeven, A. P. Otte, L. J. van't Veer, A. Berns, and M. van Lohuizen. 1997. Identification of Bmi1-interacting proteins as constituents of a multimeric mammalian Polycomb complex. Genes Dev. 11:226-240. [DOI] [PubMed] [Google Scholar]
- 3.Atadja, P., H. Wong, I. Garkavtsev, C. Veillette, and K. Riabowol. 1995. Increased activity of p53 in senescing fibroblasts. Proc. Natl. Acad. Sci. USA 92:8348-8352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balin, A. K., A. J. Fisher, and D. M. Carter. 1984. Oxygen modulates growth of human cells at physiological partial pressures. J. Exp. Med. 160:152-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bodnar, A. G., M. Ouellette, M. Frolkis, S. E. Holt, C. P. Chiu, G. B. Morin, C. B. Harley, J. W. Shay, S. Lichtsteiner, and W. E. Wright. 1998. Extension of life span by introduction of telomerase into normal human cells. Science 279:349-352. [DOI] [PubMed] [Google Scholar]
- 6.Bond, J. A., M. F. Haughton, J. M. Rowson, P. J. Smith, V. Gire, D. Wynford-Thomas, and F. S. Wyllie. 1999. Control of replicative life span in human cells: barriers to clonal expansion intermediate between M1 senescence and M2 crisis. Mol. Cell. Biol. 19:3103-3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bond, J. A., F. S. Wyllie, and D. Wynford-Thomas. 1994. Escape from senescence in human diploid fibroblasts induced directly by mutant p53. Oncogene 9:1885-1889. [PubMed] [Google Scholar]
- 8.Campisi, J. 1996. Replicative senescence: an old lives' tale? Cell 84:497-500. [DOI] [PubMed] [Google Scholar]
- 9.Campisi, J. 1997. The biology of replicative senescence. Eur. J. Cancer 33:703-709. [DOI] [PubMed] [Google Scholar]
- 10.Campisi, J. 2001. Cellular senescence as a tumor-suppressor mechanism. Trends Cell Biol. 11:27-31. [DOI] [PubMed] [Google Scholar]
- 11.Chen, Q. M. 2000. Replicative senescence and oxidant-induced premature senescence. Beyond the control of cell cycle checkpoints. Ann. N. Y. Acad. Sci. 908:111-125. [DOI] [PubMed] [Google Scholar]
- 12.Chin, L., S. E. Artandi, Q. Shen, A. Tam, S. L. Lee, G. J. Gottlieb, C. W. Greider, and R. A. DePinho. 1999. p53 deficiency rescues the adverse effects of telomere loss and cooperates with telomere dysfunction to accelerate carcinogenesis. Cell 97:527-538. [DOI] [PubMed] [Google Scholar]
- 13.Cohen, K. J., J. S. Hanna, J. E. Prescott, and C. V. Dang. 1996. Transformation by the Bmi-1 oncoprotein correlates with its subnuclear localization but not its transcriptional suppression activity. Mol. Cell. Biol. 16:5527-5535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Lange, T. 2001. Telomere capping—one strand fits all. Science 292:1075-1076. [DOI] [PubMed] [Google Scholar]
- 15.Dimri, G. P., E. Hara, and J. Campisi. 1994. Regulation of two E2F-related genes in presenescent and senescent human fibroblasts. J. Biol. Chem. 269:16180-16186. [PubMed] [Google Scholar]
- 16.Dimri, G. P., K. Itahana, M. Acosta, and J. Campisi. 2000. Regulation of a senescence checkpoint response by the E2F1 transcription factor and p14(ARF) tumor suppressor. Mol. Cell. Biol. 20:273-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dimri, G. P., and J. Campisi. 1994. Molecular and cell biology of replicative senescence. Cold Spring Harbor Symp. Quant. Biol. 59:67-73. [DOI] [PubMed]
- 18.Dimri, G. P., J. L. Martinez, J. J. Jacobs, P. Keblusek, K. Itahana, M. von Lohuizen, J. Campisi, D. E. Wazer, and V. Band. 2002. The Bmi-1 oncogene induces telomerase activity and immortalizes human mammary epithelial cells. Cancer Res. 62:4736-4745. [PubMed] [Google Scholar]
- 19.Dimri, G. P., X. Lee, G. Basile, M. Acosta, G. Scott, C. Roskelley, E. E. Medrano, M. Linskens, I. Rubelj, O. M. Pereira-Smith, M. Peacocke, and J. Campisi. 1995. A novel biomarker identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl. Acad. Sci. USA 92:9363-9367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dulic, V., G. E. Beney, G. Frebourg, L. F. Drullinger, and G. H. Stein. 2000. Uncoupling between phenotypic senescence and cell cycle arrest in aging p21-deficient fibroblasts. Mol. Cell. Biol. 20:6741-6754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hara, E., H. Tsurui, A. Shinozaki, S. Nakada, and K. Oda. 1991. Cooperative effect of antisense-Rb and antisense-p53 oligomers on the extension of life span in human diploid fibroblasts, TIG-1. Biochem. Biophys. Res. Commun. 179:528-534. [DOI] [PubMed] [Google Scholar]
- 22.Hara, E., R. Smith, D. Parry, H. Tahara, S. Stone, and G. Peters. 1996. Regulation of p16CDKN2 expression and its implications for cell immortalization and senescence. Mol. Cell. Biol. 16:859-867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hara, E., T. Yamaguchi, H. Nojima, T. Ide, J. Campisi, H. Okayama, and K. Oda. 1994. Id-related genes encoding helix-loop-helix proteins are required for G1 progression and are repressed in senescent human fibroblasts. J. Biol. Chem. 269:2139-2145. [PubMed] [Google Scholar]
- 24.Harley, C. B., A. B. Futcher, and C. W. Greider. 1990. Telomeres shorten during ageing of human fibroblasts. Nature 345:458-460. [DOI] [PubMed] [Google Scholar]
- 25.Haupt, Y., W. S. Alexander, G. Barri, S. P. Klinken, and J. M. Adams. 1991. Novel zinc finger gene implicated as myc collaborator by retrovirally accelerated lymphomagenesis in E mu-myc transgenic mice. Cell 65:753-763. [DOI] [PubMed] [Google Scholar]
- 26.Itahana, K., G. Dimri, and J. Campisi. 2001. Regulation of cellular senescence by p53. Eur. J. Biochem. 268:2784-2791. [DOI] [PubMed] [Google Scholar]
- 27.Itahana, K., G. P. Dimri, E. Hara, Y. Itahana, Y. Zou, P. Y. Desprez, and J. Campisi. 2002. A role for p53 in maintaining and establishing the quiescence growth arrest in human cells. J. Biol. Chem. 277:18206-18214. [DOI] [PubMed] [Google Scholar]
- 28.Jacobs, J. J., K. Kieboom, S. Marino, R. A. DePinho, and M. van Lohuizen. 1999. The oncogene and Polycomb-group gene bmi-1 regulates cell proliferation and senescence through the INK4a locus. Nature 397:164-168. [DOI] [PubMed] [Google Scholar]
- 29.Kaminker, P. G., S. H. Kim, R. D. Taylor, Y. Zebarjadian, W. D. Funk, G. B. Morin, P. Yaswen, and J. Campisi. 2001. TANK2, a new TRF1-associated poly(ADP-ribose) polymerase, causes rapid induction of cell death upon overexpression. J. Biol. Chem. 276:35891-35899. [DOI] [PubMed] [Google Scholar]
- 30.Karlseder, J., A. Smogorzewska, and T. de Lange. 2002. Senescence induced by altered telomere state, not telomere loss. Science 295:2446-2449. [DOI] [PubMed] [Google Scholar]
- 31.Kato, D., K. Miyazawa, M. Ruas, M. Starborg, I. Wada, T. Oka, T. Sakai, G. Peters, and E. Hara. 1998. Features of replicative senescence induced by addition of antennapedia-p16INK4A fusion protein to human diploid fibroblasts. FEBS Lett. 427:203-208. [DOI] [PubMed] [Google Scholar]
- 32.Kawabe, S., J. A. Roth, D. R. Wilson, and R. E. Meyn. 2000. Adenovirus-mediated p16INK4a gene expression radiosensitizes non-small cell lung cancer cells in a p53-dependent manner. Oncogene 19:5359-5366. [DOI] [PubMed] [Google Scholar]
- 33.Kim, N. W., M. A. Piatyszek, K. R. Prowse, C. B. Harley, M. D. West, P. L. Ho, G. M. Coviello, W. E. Wright, S. L. Weinrich, and J. W. Shay. 1994. Specific association of human telomerase activity with immortal cells and cancer. Science 266:2011-2015. [DOI] [PubMed] [Google Scholar]
- 34.Kim, S. H., P. Kaminker, and J. Campisi. 2002. Telomeres, aging and cancer: in search of a happy ending. Oncogene 21:503-511. [DOI] [PubMed] [Google Scholar]
- 35.Kiyono, T., S. A. Foster, J. I. Koop, J. K. McDougall, D. A. Galloway, and A. J. Klingelhutz. 1998. Both Rb/p16INK4a inactivation and telomerase activity are required to immortalize human epithelial cells. Nature 396:84-88. [DOI] [PubMed] [Google Scholar]
- 36.Krimpenfort, P., K. C. Quon, W. J. Mooi, A. Loonstra, and A. Berns. 2001. Loss of p16INK4a confers susceptibility to metastatic melanoma in mice. Nature 413:83-86. [DOI] [PubMed] [Google Scholar]
- 37.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 38.Levy, M. Z., R. C. Allsopp, A. B. Futcher, C. W. Greider, and C. B. Harley. 1992. Telomere end-replication problem and cell aging. J. Mol. Biol. 225:951-960. [DOI] [PubMed] [Google Scholar]
- 39.Lorenz, M., G. Saretzki, N. Sitte, S. Metzkow, and T. von Zglinicki. 2001. BJ fibroblasts display high antioxidant capacity and slow telomere shortening independent of hTERT transfection. Free Radic. Biol. Med. 31:824-831. [DOI] [PubMed] [Google Scholar]
- 40.Morales, C. P., S. E. Holt, M. Ouellette, K. J. Kaur, Y. Yan, K. S. Wilson, M. A. White, W. E. Wright, and J. W. Shay. 1999. Absence of cancer-associated changes in human fibroblasts immortalized with telomerase. Nat. Genet. 21:115-118. [DOI] [PubMed] [Google Scholar]
- 41.Noda, A., Y. Ning, S. F. Venable, O. M. Pereira-Smith, and J. R. Smith. 1994. Cloning of senescent cell-derived inhibitors of DNA synthesis using an expression screen. Exp. Cell Res. 211:90-98. [DOI] [PubMed] [Google Scholar]
- 42.Ohtani, N., Z. Zebedee, T. J. Huot, J. A. Stinson, M. Sugimoto, Y. Ohashi, A. D. Sharrocks, G. Peters, and E. Hara. 2001. Opposing effects of Ets and Id proteins on p16INK4a expression during cellular senescence. Nature 409:1067-1070. [DOI] [PubMed] [Google Scholar]
- 43.Packer, L., and K. Fuehr. 1977. Low O2 concentration extends the life span of cultured human diploid cells. Nature 267:423-425. [DOI] [PubMed] [Google Scholar]
- 44.Pirrotta, V. 1998. Polycombing the genome: PcG, trxG, and chromatin silencing. Cell 93:333-336. [DOI] [PubMed] [Google Scholar]
- 45.Ramirez, R. D., C. P. Morales, B. S. Herbert, J. M. Rohde, C. Passons, J. W. Shay, and W. E. Wright. 2001. Putative telomere-independent mechanisms of replicative aging reflect inadequate growth conditions. Genes Dev. 15:398-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rheinwald, J. G., W. C. Hahn, M. R. Ramsey, J. Y. Yu, Z. Guo, H. Tsao, M. De Luca, C. Catricala, and K. M. O'Toole. 2002. A two-stage, p16(INK4A)- and p53-dependent keratinocyte senescence mechanism that limits replicative potential independent of telomere status. Mol. Cell. Biol. 22:5157-5172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Robles, S. J., and G. R. Adami. 1998. Agents that cause DNA double strand breaks lead to p16INK4a enrichment and the premature senescence of normal fibroblasts. Oncogene 16:1113-1123. [DOI] [PubMed] [Google Scholar]
- 48.Saito, H., A. T. Hammond, and R. E. Moses. 1995. The effect of low O2 tension on the in vitro-replicative life span of human diploid fibroblast cells and their transformed derivatives. Exp. Cell Res. 217:272-279. [DOI] [PubMed] [Google Scholar]
- 49.Saretzki, G., N. Sitte, U. Merkel, R. E. Wurm, and T. von Zglinicki. 1999. Telomere shortening triggers a p53-dependent cell cycle arrest via accumulation of G-rich single stranded DNA fragments. Oncogene 18:5148-5158. [DOI] [PubMed] [Google Scholar]
- 50.Satijn, D. P., and A. P. Otte. 1999. RING1 interacts with multiple Polycomb-group proteins and displays tumorigenic activity. Mol. Cell. Biol. 19:57-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schluter, C., M. Duchrow, C. Wohlenberg, M. H. Becker, G. Key, H. D. Flad, and J. Gerdes. 1993. The cell proliferation-associated antigen of antibody Ki-67: a very large, ubiquitous nuclear protein with numerous repeated elements, representing a new kind of cell cycle-maintaining proteins. J. Cell Biol. 123:513-522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schmitt, C. A., J. S. Fridman, M. Yang, S. Lee, E. Baranov, R. M. Hoffman, and S. W. Lowe. 2002. A senescence program controlled by p53 and p16INK4a contributes to the outcome of cancer therapy. Cell 109:335-346. [DOI] [PubMed] [Google Scholar]
- 53.Serrano, M., A. W. Lin, M. E. McCurrach, D. Beach, and S. W. Lowe. 1997. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 88:593-602. [DOI] [PubMed] [Google Scholar]
- 54.Seshadri, T., J. A. Uzman, J. Oshima, and J. Campisi. 1993. Identification of a transcript that is down-regulated in senescent human fibroblasts. Cloning, sequence analysis, and regulation of the human L7 ribosomal protein gene. J. Biol. Chem. 268:18474-18480. [PubMed] [Google Scholar]
- 55.Shapiro, G. I., C. D. Edwards, M. E. Ewen, and B. J. Rollins. 1998. p16INK4A participates in a G1 arrest checkpoint in response to DNA damage. Mol. Cell. Biol. 18:378-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sharpless, N. E., N. Bardeesy, K. H. Lee, D. Carrasco, D. H. Castrillon, A. J. Aguirre, E. A. Wu, J. W. Horner, and R. A. DePinho. 2001. Loss of p16INK4a with retention of p19Arf predisposes mice to tumorigenesis. Nature 413:86-91. [DOI] [PubMed] [Google Scholar]
- 57.Shay, J. W., O. M. Pereira-Smith, and W. E. Wright. 1991. A role for both RB and p53 in the regulation of human cellular senescence. Exp. Cell Res. 196:33-39. [DOI] [PubMed] [Google Scholar]
- 58.Shelton, D. N., E. Chang, P. S. Whittier, D. Choi, and W. D. Funk. 1999. Microarray analysis of replicative senescence. Curr. Biol. 9:939-945. [DOI] [PubMed] [Google Scholar]
- 59.Sherr, C. J., and R. A. DePinho. 2000. Cellular senescence: mitotic clock or culture shock? Cell 102:407-410. [DOI] [PubMed] [Google Scholar]
- 60.Stein, G. H., L. F. Drullinger, A. Soulard, and V. Dulic. 1999. Differential roles for cyclin-dependent kinase inhibitors p21 and p16 in the mechanisms of senescence and differentiation in human fibroblasts. Mol. Cell. Biol. 19:2109-2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van der Lugt, N. M., J. Domen, K. Linders, M. van Roon, E. Robanus-Maandag, H. te Riele, M. van der Valk, J. Deschamps, M. Sofroniew, M. van Lohuizen, and A. Berns. 1994. Posterior transformation, neurological abnormalities, and severe hematopoietic defects in mice with a targeted deletion of the bmi-1 proto-oncogene. Genes Dev. 8:757-769. [DOI] [PubMed] [Google Scholar]
- 62.van Lohuizen, M., S. Verbeek, B. Scheijen, E. Wientjens, H. van der Gulden, and A. Berns. 1991. Identification of cooperating oncogenes in E mu-myc transgenic mice by provirus tagging. Cell 65:737-752. [DOI] [PubMed] [Google Scholar]
- 63.Vaziri, H., J. A. Squire, T. K. Pandita, G. Bradley, R. M. Kuba, H. Zhang, S. Gulyas, R. P. Hill, G. P. Nolan, and S. Benchimol. 1999. Analysis of genomic integrity and p53-dependent G1 checkpoint in telomerase-induced extended-life-span human fibroblasts. Mol. Cell. Biol. 19:2373-2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vaziri, H., M. D. West, R. C. Allsopp, T. S. Davison, Y. S. Wu, C. H. Arrowsmith, G. G. Poirier, and S. Benchimol. 1997. ATM-dependent telomere loss in aging human diploid fibroblasts and DNA damage lead to the post-translational activation of p53 protein involving poly(ADP-ribose) polymerase. EMBO J. 16:6018-6033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.von Zglinicki, T., G. Saretzki, W. Docke, and C. Lotze. 1995. Mild hyperoxia shortens telomeres and inhibits proliferation of fibroblasts: a model for senescence? Exp. Cell Res. 220:186-193. [DOI] [PubMed] [Google Scholar]
- 66.Wright, W. E., O. M. Pereira-Smith, and J. W. Shay. 1989. Reversible cellular senescence: implications for immortalization of normal human diploid fibroblasts. Mol. Cell. Biol. 9:3088-3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wright, W. E., and J. W. Shay. 2000. Telomere dynamics in cancer progression and prevention: fundamental differences in human and mouse telomere biology. Nat. Med. 6:849-851. [DOI] [PubMed] [Google Scholar]
- 68.Zhu, J., D. Woods, M. McMahon, and J. M. Bishop. 1998. Senescence of human fibroblast induced by oncogenic Raf. Genes Dev. 12:2997-3007. [DOI] [PMC free article] [PubMed] [Google Scholar]