Abstract
Abasic (AP) sites represent one of the most frequently formed lesions in DNA. Here, we examine the consequences of the stalling of RNA polymerase II at AP sites in DNA in Saccharomyces cerevisiae. A severe inhibition of transcription occurs in strains that are defective in the removal of AP sites and that also lack the RAD26 gene, a homolog of the human Cockayne syndrome group B (CSB) gene, and, importantly, a dramatic rise in mutagenesis is incurred in such strains. From the various observations presented here, we infer that the stalling of transcription at AP sites is highly mutagenic.
Cellular DNA is subjected to damage inflicted by external environmental agents such as UV light from the sun and chemical pollutants, by endogenous factors such as reactive oxygen species, and by spontaneous decay. Such damage to DNA is removed by nucleotide excision repair (NER) or base excision repair (BER) processes. However, lesions that escape repair can be a block to replication and transcription. In contrast to the information available for the pathways controlling the replication of damaged DNA (19), little is known about the processes that promote transcription through DNA lesions in eukaryotes, and also, meager information is available about the consequences of the stalling of transcription at DNA lesion sites. Here we examine the effects of abasic (AP) sites on RNA polymerase II (Pol II) transcription in Saccharomyces cerevisiae and determine if the stalling of transcription at AP sites has mutagenic consequences. AP sites are formed in DNA because of spontaneous hydrolysis of the N-glycosylic bond and as intermediates in BER processes by the action of DNA glycosylases following the release of damaged bases (23).
RAD26 is the S. cerevisiae counterpart of the human CSB gene. Mutations in CSB cause Cockayne syndrome (CS), which is characterized by severe growth retardation, progressive neurological dysfunction, mental retardation, and early death (12). Also, mutations in the CSB and RAD26 genes confer a defect in preferential repair of UV lesions from the transcribed strand (21, 22), a phenomenon known as transcription-coupled repair (TCR) (11). The proteins encoded by the CSB and RAD26 genes both possess a DNA-dependent ATPase activity (3, 17). Although the mechanism of TCR in yeast or humans is not known and there is no evidence that CSB disrupts the ternary complex of Pol II stalled at a lesion site (4, 15), studies with the Escherichia coli Mfd protein, which functions in TCR by displacing the stalled RNA polymerase from the thymine dimer in an ATP hydrolysis-dependent manner and which subsequently recruits the excision nuclease (18), have suggested that Rad26 (CSB) may act in an analogous manner in TCR of UV-induced lesions. Also, a role for CSB in transcription elongation has been indicated by in vitro studies (16) and genetic studies of S. cerevisiae have provided evidence consistent with such a role for the Rad26 protein in vivo (10).
In S. cerevisiae, two class II AP endonucleases, encoded by the APN1 and APN2 genes, function in the removal of AP sites and NER comprises the third competing pathway for AP site removal (8, 20). Here we show that AP sites that accumulate in DNA in the absence of APN1, APN2, and NER block transcription and that this transcriptional block becomes more pronounced in the absence of RAD26. We also found that mutations occur at a very high rate in the rad26Δ mutant strain lacking the various pathways for the removal of AP sites. From these observations, we infer that the stalling of transcription at AP sites is extremely mutagenic.
MATERIALS AND METHODS
Strains and media.
All of the deletion strains used in this study were derived from wild-type strain EMY74.7 (MATa his3-Δ1 leu2-3,112 trp1Δ ura3-52). The generation of deletions of the APN1, APN2, RAD14, REV3 (8, 20), RAD26 (10), and POL32 (5) genes by the one-step gene disruption method has been described previously.
MMS treatment.
Cells were grown overnight in YPD (yeast extract-peptone-dextrose) medium for the determination of survival after treatment with methyl methanesulfonate (MMS). Cells were washed with distilled water and resuspended in 0.05 mM KPO4 (pH 7.0) at a density of 3 × 108/ml. After treatment of aliquots of cells with MMS at 30°C for 20 min with vigorous shaking, MMS was neutralized with 10% Na thiosulfate. Appropriate dilutions were plated on YPD for viability determinations and on synthetic complete medium lacking arginine but containing canavanine for the identification of canavanine-resistant colonies.
Transcription analysis.
For examination of GAL7 and GAL10 transcription, cells grown at 30°C to log phase in YPL (1% yeast extract, 2% peptone, 3.7% lactate) medium were diluted to an optical density at 600 nm of 0.5 in YPL medium containing 2% galactose and 0.25% MMS. Samples were taken at the indicated time points after being transferred to galactose- and MMS-containing medium, at which time they were pelleted and frozen quickly in crushed dry ice. Frozen cells were maintained at −80°C until RNA isolation. Total RNA was isolated by the hot phenol method (10), and quantitation of mRNA levels was performed in a PhosphorImager with ImageQuant software.
Determination of spontaneous CAN1S-to-can1r mutation rates.
For each strain, 11 independent cultures, each starting from ∼10 canavanine-sensitive cells, were grown in 0.5 ml of YPD medium. Rates of forward mutations at the CAN1 locus were determined from the number of canavanine-resistant colonies by the method of the median (9). Three experiments were performed with each strain.
RESULTS
Inactivation of RAD26 enhances the MMS sensitivity of mutants defective in the removal of AP sites.
RAD26 promotes TCR of UV lesions in conjunction with the NER proteins. Hence, introduction of the rad26Δ mutation into any of the NER-defective mutants, such as the rad14Δ mutant strain, causes no further increase in the UV sensitivity of NER mutants. To determine if Rad26 functions in conjunction with Apn1, Apn2, or NER for the removal of AP sites from the transcribed strand, we treated yeast cells with the alkylating agent MMS. MMS alkylates the bases in DNA, in particular, adenine at the N3 position, forming 3-methyladenine, and guanine at the N7 position, forming 7-methylguanine. In yeast, an N-methylpurine DNA glycosylase encoded by the MAG1 gene removes these and various other damaged bases (1). The resulting AP site is then removed by the Apn1 or Apn2 endonucleases or by NER (8, 20).
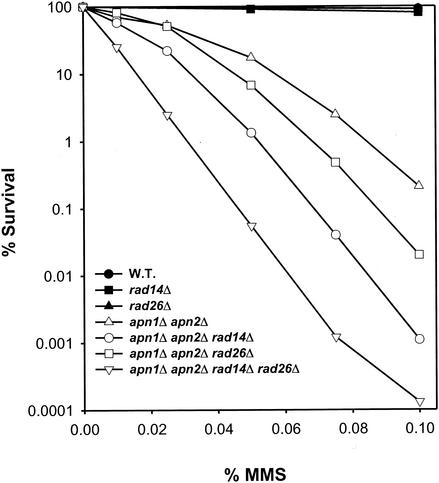
MMS sensitivity is greatly enhanced in the apn1Δ apn2Δ double mutant strain, and a further increase in MMS sensitivity occurs upon the introduction of the rad14Δ mutation into this strain (Fig. 1; see also reference 20). Interestingly, even though the rad26Δ mutation confers no perceptible increase in MMS sensitivity at the concentrations used for these experiments, introduction of the rad26Δ mutation into the apn1Δ apn2Δ or apn1Δ apn2Δ rad14Δ mutant strain enhances the MMS sensitivity of these strains (Fig. 1). These observations suggested that Rad26 promotes survival of cells harboring AP sites in their DNA by a mechanism that acts independently of Apn1, Apn2, or NER.
FIG. 1.
Effect of the rad26Δ mutation on survival of MMS-treated yeast cells lacking the APN1 and APN2 genes or, in addition, also lacking the RAD14 gene. Cells were treated with the indicated amounts of MMS (percentages, vol/vol) for 20 min at 30°C, following which MMS was inactivated with sodium thiosulfate and appropriate dilutions of cells were plated on YPD for viability determinations. The survival curves represent an average of three experiments for each strain. W.T., wild type.
Inhibition of transcription in MMS-treated rad26Δ mutant cells lacking the APN1, APN2, and RAD14 genes for the removal of AP sites.
The independence of Rad26 function from the roles of Apn1, Apn2, and NER in the removal of AP sites raised the possibility that Rad26 might enable Pol II to transcribe through AP sites. In that case, although we might expect transcription to be reduced in the apn1Δ apn2Δ and apn1Δ apn2Δ rad14Δ mutant strains because of the persistence of AP sites in DNA, transcription should be more severely curtailed in a strain lacking the various pathways for the removal of AP sites and also lacking RAD26 because, in such a strain, Pol II will continue to stall at AP sites that would persist in DNA.
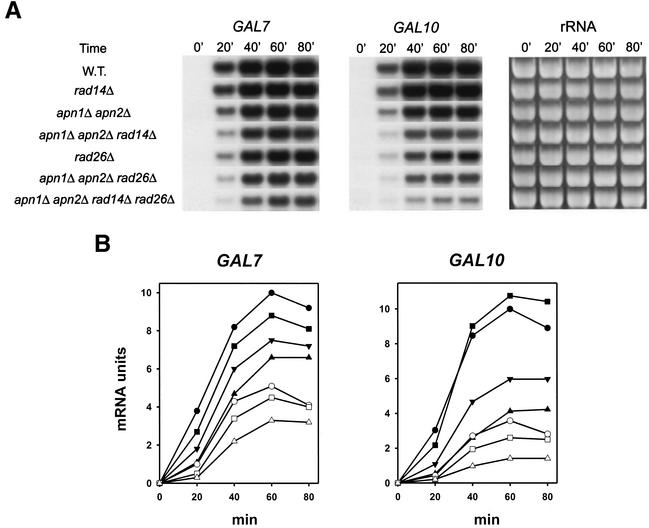
To investigate the role of RAD26 in promoting transcription through AP sites, we examined the synthesis of galactose-inducible GAL7 and GAL10 mRNAs in the wild-type and rad14Δ, apn1Δ apn2Δ, apn1Δ apn2Δ rad14Δ, rad26Δ, apn1Δ apn2Δ rad26Δ, and apn1Δ apn2Δ rad14Δ rad26Δ mutant strains treated with MMS. As shown in Fig. 2, transcription of both GAL genes was reduced in the apn1Δ apn2Δ strain and a further reduction in transcription occurred in the apn1Δ apn2Δ rad14Δ strain. Transcription was also reduced in the rad26Δ strain. Transcription in the apn1Δ apn2Δ rad26Δ strain, however, was reduced to a greater degree than in the apn1Δ apn2Δ or rad26Δ strain, and a more severe inhibition of transcription occurred in the apn1Δ apn2Δ rad14Δ rad26Δ strain than in the apn1Δ apn2Δ rad14Δ or rad26Δ strain. The increase in the inhibition of transcription that occurs in the apn1Δ apn2Δ rad14Δ mutant strain upon the inactivation of RAD26 suggests a role for Rad26 in promoting Pol II transcription through AP sites. If Rad26 had simply acted by displacing Pol II from AP sites and by subsequently promoting the recruitment of the repair machinery, akin to the role of E. coli Mfd in the TCR of thymine dimers, we would have expected transcription inhibition in the apn1Δ apn2Δ rad14Δ rad26Δ strain to be no greater than in the apn1Δ apn2Δ rad14Δ strain. That is because transcription would have continued to stall at AP sites that remain in DNA in the absence of all three pathways for their removal, regardless of whether RAD26 is present or not.
FIG. 2.
Transcription of GAL7 and GAL10 genes in MMS-treated wild-type (W.T.) and mutant strains. Total RNAs from cells grown in YPL medium containing galactose and MMS were subjected to Northern analyses. (A) Transcript levels of GAL7 (left) and GAL10 (middle) genes. The ethidium bromide-stained gel (right) indicates the levels of RNAs loaded. mRNA levels were examined at the indicated times after transfer of cells to galactose- and MMS-containing medium. (B) Quantitation of GAL7 and GAL10 mRNA levels. mRNA units at each time point are relative to the highest mRNA level in the wild-type strain. Symbols: •, wild type; ▪, rad14Δ mutant; ▴, rad26Δ mutant; ▾, apn1Δ apn2Δ mutant; ○, apn1Δ apn2Δ rad14Δ mutant; □, apn1Δ apn2Δ rad26Δ mutant; ▵, apn1Δ apn2Δ rad14Δ rad26Δ mutant.
Highly enhanced mutagenesis in MMS-treated rad26Δ mutant cells lacking the APN1, APN2, and RAD14 genes.
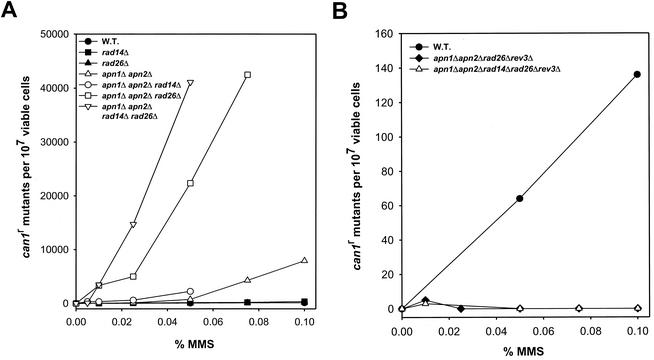
The reduction in the efficiency of transcription in the apn1Δ apn2Δ rad26Δ strain and the further inhibition of transcription in the apn1Δ apn2Δ rad14Δ rad26Δ strain implies that in these mutant strains, transcription stalls at AP sites that accumulate in DNA in the absence of the various pathways for their removal. This observation has presented the opportunity to determine if such transcriptional stalling has mutagenic consequences. To examine this, cells were treated with MMS and the frequency of MMS-induced CAN1S-to-can1r forward mutations was determined in various mutant strains. As we have shown previously (8, 20), and show here in Fig. 3A for comparison, the frequency of MMS-induced can1r mutations is elevated in the apn1Δ apn2Δ strain and a further increase in the can1r mutation frequency occurs in the apn1Δ apn2Δ rad14Δ strain. Since AP sites are noncoding lesions, translesion synthesis through them is highly mutagenic and in yeast, REV3-encoded DNA polymerase ζ is indispensable for the mutagenic bypass of these lesions (8).
FIG. 3.
MMS-induced can1r mutations in various mutant strains. Cells were treated with MMS at the indicated concentrations for 20 min at 30°C, and following inactivation of the MMS, cells were plated on YPD medium for viability determination and on synthetic complete medium lacking arginine and containing canavanine for determination of the can1r mutation frequency. Each curve represents the average of two or three experiments. (A) Elevated mutability in rad26Δ mutant strains lacking the APN1, APN2, and RAD14 genes required for the removal of AP sites. (B) Requirement of REV3 for mutagenesis in the apn1Δ apn2Δ rad26Δ and apn1Δ apn2Δ rad14Δ rad26Δ mutant strains. W.T., wild type.
Even though transcription is reduced in MMS-treated rad26Δ cells, MMS induces no further increase in mutagenesis in the rad26Δ strain beyond that seen in the wild-type strain (Fig. 3A). This suggests that transcriptional stalling at MMS-induced DNA lesions, such as 3-methyladenine and 7-methylguanine, that would abound in this genetic background, is not particularly mutagenic. In striking contrast, a very large increase in the frequency of MMS-induced can1r mutations occurs in the apn1Δ apn2Δ rad26Δ strain over that in the apn1Δ apn2Δ mutant strain. For example, in comparison to the frequency of ∼700 can1r mutations per 107 cells in the apn1Δ apn2Δ strain treated with 0.05% MMS, the frequency rises to over 20,000 can1r mutations per 107 cells in the similarly treated apn1Δ apn2Δ rad26Δ strain (Fig. 3A). To ascertain that the greatly enhanced mutagenesis in the apn1Δ apn2Δ rad26Δ strain was, in fact, due to the rad26Δ mutation, we introduced the wild-type RAD26 gene carried on a low-copy-number CEN/ARS plasmid into this strain and showed that the frequency of MMS-induced can1r mutations was now reduced to the level seen in the apn1Δ apn2Δ strain (data not shown). A large increase in the frequency of can1r mutations was also observed in the apn1Δ apn2Δ rad14Δ rad26Δ strain, compared to that in the apn1Δ apn2Δ rad14Δ strain. For example, the frequencies of can1r mutations in the apn1Δ apn2Δ rad14Δ mutant strain treated with 0.025 and 0.05% MMS were ∼300 and 2,800/107 cells, respectively, whereas the corresponding frequencies in the apn1Δ apn2Δ rad14Δ rad26Δ strain were ∼15,000 and 41,000 can1r mutants per 107 cells, respectively (Fig. 3A). From these observations, we infer that AP sites that remain in DNA in the absence of Apn1, Apn2, and NER become highly mutagenic in cells lacking a functional RAD26 gene.
Elevated spontaneous mutability in rad26Δ mutant strains lacking the pathways for AP lesion removal.
AP sites arise in DNA as a result of spontaneous hydrolysis of the glycosylic bond or as an intermediate in BER. Although, in the absence of MMS treatment, we have seen no evidence of a decrease in the transcription of the GAL7 and GAL10 genes in the apn1Δ apn2Δ rad26Δ or apn1Δ apn2Δ rad14Δ rad26Δ strain compared with that in the rad26Δ strain (data not shown), we reasoned that, despite the infrequent occurrence of AP sites in untreated cells, it may still be possible to determine the effects of the stalling of transcription at these sites on spontaneous mutability.
Table 1 presents the rates of spontaneously occurring can1r mutations in the wild-type strain and in various mutant yeast strains. Compared to that of the wild type, mutation rates increase about twofold in the apn1Δ apn2Δ strain but show no increase in the rad26Δ strain. In striking contrast, can1r mutations occur in the apn1Δ apn2Δ rad26Δ strain at a rate that is >40-fold higher than the rate in the wild-type strain. That the large increase in the mutation rate in the apn1Δ apn2Δ rad26Δ strain was due to the rad26Δ mutation was verified by observing that the introduction of the wild-type RAD26 gene carried on a low-copy-number yeast plasmid into the apn1Δ apn2Δ rad26Δ strain lowered the mutation rate very substantially. Mutation rates were also higher in the apn1Δ apn2Δ rad14Δ rad26Δ strain than in the apn1Δ apn2Δ rad14Δ strain.
TABLE 1.
Rates of spontaneous CAN1S to can1r mutations in various mutant yeast strains
| Strain | can1r mutation rate (10−7) | Fold increase |
|---|---|---|
| Wild type | 4.4 ± 0.8 | 1.0 |
| apn1Δ apn2Δ | 10.2 ± 1.7 | 2.3 |
| rad14Δ | 15.0 ± 3.4 | 3.4 |
| apn1Δ apn2Δ rad14Δ | 32.8 ± 6.1 | 7.4 |
| rad26Δ | 3.3 ± 0.5 | 0.74 |
| apn1Δ apn2Δ rad26Δ | 185.5 ± 58.6 | 42.0 |
| apn1Δ apn2Δ rad26Δ(pRAD26)a | 25.4 ± 4.0 | 5.7 |
| apn1Δ apn2Δ rad14Δ rad26Δ | 367.6 ± 70.0 | 83.0 |
| rev3Δ | 3.3 ± 0.7 | 0.75 |
| apn1Δ apn2Δ rev3Δ | 7.5 ± 2.0 | 1.7 |
| apn1Δ apn2Δ rad14Δ rev3Δ | 9.0 | 2.0 |
| apn1Δ apn2Δ rad26Δ rev3Δ | 5.0 ± 0.7 | 1.1 |
| apn1Δ apn2Δ rad14Δ rad26Δ rev3Δ | 19.6 ± 4.9 | 4.4 |
| pol32Δ | 12.6 ± 0.6 | 2.9 |
| apn1Δ apn2Δ pol32Δ | 14.9 ± 2.8 | 3.4 |
| apn1Δ apn2Δ rad26Δ pol32Δ | 746.5 ± 78.8 | 168.5 |
| apn1Δ apn2Δ rad26Δ pol32Δ rev3Δ | 20.8 ± 8.6 | 4.7 |
apn1Δ apn2Δ rad26Δ(pRAD26) denotes the presence of the wild-type RAD26 gene carried on a plasmid in the apn1Δ apn2Δ rad26Δ mutant strain.
To determine the spectrum of spontaneous can1r mutations generated in the apn1Δ apn2Δ rad26Δ strain, we amplified the can1 gene from 30 independent, spontaneously arising canavanine-resistant colonies by PCR and sequenced the entire gene. The changes in the apn1Δ apn2Δ rad26Δ strainexhibited a prevalence of C · G-to-T · A transitions (56% of all base changes), where the original cytosine was present in the transcribed strand. The C · G-to-T · A mutations could have been formed by the incorporation of adenine opposite an AP site originating from cytosine, most likely because of its deamination to uracil, followed by the removal of uracil by a DNA glycosylase. The other base changes that we observed could be accounted for by the insertion of nucleotides other than A opposite the AP site resulting from the deamination of cytosine present in the transcribed strand or from the insertion of an A or a C opposite the AP site resulting from the spontaneous loss of G or A from the transcribed strand.
Genetic control of elevated mutagenesis in rad26Δ mutant strains lacking the pathways for AP lesion removal.
Mutagenesis resulting from the replication of DNA containing AP sites is dependent upon REV3-encoded DNA polymerase ζ (5, 8). We found that the highly elevated mutagenesis that occurs in the apn1Δ apn2Δ rad26Δ and apn1Δ apn2Δ rad14Δ rad26Δ strains also requires polymerase ζ, since the introduction of the rev3Δ mutation into these strains led to a large reduction in the frequency of MMS-induced can1r mutations (Fig. 3B). The rates of spontaneous can1r mutations also declined dramatically in these mutant strains in the absence of REV3 (Table 1).
Although AP site-induced mutagenesis resulting from replicative bypass or transcriptional stalling requires polymerase ζ, the two processes differ in their requirements for the Pol32 subunit of DNA polymerase δ. Yeast polymerase δ is composed of three subunits of 125, 58, and 55 kDa, which are encoded by the POL3, POL31, and POL32 genes, respectively (2). By contrast to POL3 and POL31, which are essential for viability, POL32 is not essential (2); inactivation of POL32, however, causes a deficiency in damage-induced mutagenesis. The pol32Δ mutation is UV sensitive and deficient in UV mutagenesis (7), and MMS-induced mutagenesis that would result from the replication of DNA containing AP lesions is not observed in the apn1Δ apn2Δ pol32Δ strain (5). We have previously suggested that the requirement of the Pol32 subunit for mutagenic translesion synthesis during replication obtains from its role in connecting polymerase δ bound to the nondamaged DNA strand with polymerase ζ bound to the damaged strand (5). As shown in Table 1, the rate of spontaneous can1r mutations in the apn1Δ apn2Δ pol32Δ strain was about the same as in the pol32Δ strain, ∼0.14 × 10−5; however, and in striking contrast, the rate of spontaneous can1r mutations rose to 7.5 × 10−5 in the apn1Δ apn2Δ rad26Δ pol32Δ strain, compared to the rate of 1.85 × 10−5 in the apn1Δ apn2Δ rad26Δ strain. Similarly, MMS-induced mutations also occur in the apn1Δ apn2Δ rad26Δ pol32Δ strain. For example, in the apn1Δ apn2Δ pol32Δ and apn1Δ apn2Δ rad26Δ pol32Δ strains treated with 0.001% MMS, the frequencies of induced can1r mutations were ∼9 × 10−7 and 450 × 10−7, respectively. Thus, by contrast to the requirement of POL32 for mutagenic translesion synthesis through AP sites occurring during replication, this subunit is dispensable for mutagenesis resulting from the stalling of transcription at AP sites. The spontaneous and MMS-induced mutagenesis occurring in the apn1Δ apn2Δ rad26Δ pol32Δ strain is polymerase ζ dependent (Table 1 and data not shown).
DISCUSSION
Rad26 functions independently of BER and NER in promoting transcription through AP sites.
For the repair of UV lesions, RAD26 functions together with the components of NER, and hence, the introduction of the rad26Δ mutation into any of the NER-defective mutants, such as, for example, the rad14Δ mutant, causes no further increase in UV sensitivity. Here we show that introduction of the rad26Δ mutation into the apn1Δ apn2Δ and apn1Δ apn2Δ rad14Δ strains, which lack both the AP endonucleases or, in addition, also lack the NER system for the removal of AP sites, enhances the MMS sensitivity of these strains. These observations suggested that RAD26 promotes survival of cells harboring AP sites by a mechanism that acts independently of the BER and NER processes.
Because Rad26 promotes TCR of UV-induced DNA lesions in conjunction with the NER proteins, transcription is greatly inhibited in UV-irradiated, NER-defective mutants and the inhibition of transcription that occurs in the NER-defective mutants far exceeds that seen in the rad26Δ strain (13). Although the mechanism of Rad26 action in TCR is not known, it presumably functions in a manner analogous to that of the Mfd protein in E. coli. In that case, we would expect transcription to remain inhibited in the absence of any of the essential NER proteins, as is observed. That will occur because in spite of the displacement of Pol II from UV lesions, transcription will continue to stall because of the persistence of lesions in DNA. Here we show that after MMS treatment, the levels of GAL7 and GAL10 transcripts are reduced in apn1Δ apn2Δ cells and a further reduction occurs in apn1Δ apn2Δ rad14Δ cells. Transcription is also reduced in rad26Δ cells; however, and importantly, the apn1Δ apn2Δ rad26Δ strain displays a much greater reduction in transcription than that seen in the apn1Δ apn2Δ or rad26Δ strain and a more severe inhibition of transcription occurs in the apn1Δ apn2Δ rad14Δ rad26Δ strain than in the apn1Δ apn2Δ rad14Δ or rad26Δ strain. The increase in transcriptional inhibition that occurs in the absence of RAD26 in the apn1Δ apn2Δ rad14Δ strain, which lacks all of the major pathways for AP site removal, suggests that Rad26 enables Pol II to transcribe through AP sites and that, in this role, it acts independently of the Apn1, Apn2, and NER proteins.
Stalling of transcription at AP sites is highly mutagenic.
The severe inhibition of transcription that we observed in the MMS-treated apn1Δ apn2Δ rad26Δ and apn1Δ apn2Δ rad14Δ rad26Δ strains, which must result from the stalling of Pol II at AP sites, has enabled us to determine the mutagenic consequences of such stalling. We show here that the frequency of MMS-induced can1r mutations is highly elevated in the apn1Δ apn2Δ and apn1Δ apn2Δ rad14Δ strains which lack the RAD26 gene, and the rate of spontaneous can1r mutations is also much higher in the apn1Δ apn2Δ rad26Δ and apn1Δ apn2Δ rad14Δ rad26Δ strains than in the apn1Δ apn2Δ and apn1Δ apn2Δ rad14Δ strains, respectively.
Polymerase ζ is indispensable for the mutagenic bypass of AP sites during replication (5, 8). Here we show that mutagenesis in the apn1Δ apn2Δ rad26Δ or apn1Δ apn2Δ rad14Δ rad26Δ mutant strain is also dependent upon polymerase ζ. However, by contrast to the requirement of the Pol32 subunit of polymerase δ for mutagenic translesion synthesis through AP sites during replication (5), we found that enhanced mutability in the apn1Δ apn2Δ rad26Δ strain does not require POL32. Thus, although mutagenic translesion synthesis through AP sites during both replication and transcription requires polymerase ζ, the two processes differ in their requirements for POL32. The requirement of POL32 for mutagenic translesion synthesis during replication may obtain from its role in connecting polymerase δ bound to the nondamaged strand to polymerase ζ bound to the damaged strand, as we have suggested before (5).
The mutagenic bypass of AP sites during replication involves the sequential action of two DNA polymerases, in which one polymerase inserts the nucleotide opposite the lesion and polymerase ζ then extends from the inserted nucleotide (5). For the insertion reaction, polymerase δ will be a key player because of its proximity to the lesion site and also because of its proficiency at inserting an A opposite an AP site, which is the nucleotide inserted most often, as was inferred from the analyses of can1r mutations arising in MMS-treated apn1Δ apn2Δ cells (5). The nucleotides G, C, and T are also incorporated, but much less frequently, and polymerase η and Rev1 have been suggested to contribute to the insertion step but in a much less prominent manner than polymerase δ (5).
Similar to the can1r mutations arising from translesion synthesis through AP sites during replication, spontaneous can1r mutations in the apn1Δ apn2Δ rad26Δ mutant strain also occur predominantly by the incorporation of an A opposite the AP site and much less frequently by the incorporation of nucleotides other than A. This suggests that following the stalling of Pol II at an AP site, mutagenic translesion synthesis opposite this lesion also occurs by the sequential action of two different DNA polymerases, in which polymerase ζ extends from the A nucleotide inserted by polymerase δ or from the other nucleotides inserted by polymerase η or Rev1.
A model of hypermutability resulting from the stalling of transcription at AP sites.
All of our observations—the much greater inhibition of transcription in the apn1Δ apn2Δ and apn1Δ apn2Δ rad14Δ strains in the absence of RAD26 than in its presence, the much higher mutagenesis in the apn1Δ apn2Δ rad26Δ and apn1Δ apn2Δ rad14Δ rad26Δ strains than in the apn1Δ apn2Δ and apn1Δ apn2Δ rad14Δ strains, respectively, and our inference that most of the base changes occurring among spontaneous can1r mutants obtained from the apn1Δ apn2Δ rad26Δ strain can be accounted for by the incorporation of a nucleotide opposite AP sites present in the transcribed strand—indicate that an AP site in the transcribed strand presents a block to Pol II but that this block can be overcome by a process involving the Rad26 protein. Thus, in the absence of the pathways for the removal of AP lesions and the Rad26 protein, transcription stalls at AP sites and this stalling at AP sites is highly mutagenic.
We suggest that in a Pol II ternary complex stalled at an AP site, the nontranscribed DNA strand becomes subject to nicking by single-stranded DNA endonucleases and subsequent exonucleolytic degradation creates a gap that spans the AP site present in the transcribed strand. Thereafter, repair synthesis by the sequential action of two DNA polymerases, in which one polymerase, such as polymerase δ, polymerase η, or Rev1, inserts the nucleotide opposite the AP site and polymerase ζ then extends from the inserted base, completes the repair process (5).
During DNA replication, the presence of a closely aligned sister duplex provides an error-free means, such as recombination (14) or a copy choice type of DNA synthesis (6), by which to bypass lesions. The very high mutagenicity of transcriptional stalling at AP sites, compared to that resulting from the replicative bypass of this lesion, may arise from the relative lack of such alternate error-free lesion bypass pathways during transcription.
Acknowledgments
This work was supported by National Institutes of Health grants CA35035 and CA41261. Sequencing of can1r mutations was done in the Molecular Biology Core Laboratory, which is supported by NIEHS Center grant P30-ES06676.
REFERENCES
- 1.Chen, J., B. Derfler, and L. Samson. 1990. Saccharomyces cerevisiae 3-methyladenine DNA glycosylase has homology to the AlkA glycosylase of E. coli and is induced in response to DNA alkylation damage. EMBO J. 9:4569-4575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gerik, K. J., X. Li, A. Pautz, and P. M. J. Burgers. 1998. Characterization of the two small subunits of Saccharomyces cerevisiae DNA polymerase δ. J. Biol. Chem. 273:19747-19755. [DOI] [PubMed] [Google Scholar]
- 3.Guzder, S. N., Y. Habraken, P. Sung, L. Prakash, and S. Prakash. 1996. RAD26, the yeast homolog of human Cockayne's syndrome group B gene, encodes a DNA dependent ATPase. J. Biol. Chem. 271:18314-18317. [DOI] [PubMed] [Google Scholar]
- 4.Hara, R., C. P. Selby, M. Liu, D. H. Price, and A. Sancar. 1999. Human transcription release factor 2 dissociates RNA polymerases I and II stalled at a cyclobutane thymine dimer. J. Biol. Chem. 274:24779-24786. [DOI] [PubMed] [Google Scholar]
- 5.Haracska, L., I. Unk, R. E. Johnson, E. Johansson, P. M. J. Burgers, S. Prakash, and L. Prakash. 2001. Roles of yeast DNA polymerases δ and ζ and of Rev1 in the bypass of abasic sites. Genes Dev. 15:945-954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Higgins, N. P., K. Kato, and B. Strauss. 1976. A model for replication repair in mammalian cells. J. Mol. Biol. 101:417-425. [DOI] [PubMed] [Google Scholar]
- 7.Huang, M.-E., A. de Calignon, A. Nicolas, and F. Galibert. 2000. POL32, a subunit of the Saccharomyces cerevisiae DNA polymerase δ, defines a link between DNA replication and the mutagenic bypass repair pathway. Curr. Genet. 38:178-187. [DOI] [PubMed] [Google Scholar]
- 8.Johnson, R. E., C. A. Torres-Ramos, T. Izumi, S. Mitra, S. Prakash, and L. Prakash. 1998. Identification of APN2, the Saccharomyces cerevisiae homolog of the major human AP endonuclease HAP1, and its role in the repair of abasic sites. Genes Dev. 12:3137-3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lea, D. E., and C. A. Coulson. 1949. The distribution of the numbers of mutants in bacterial populations. J. Genet. 49:264-285. [DOI] [PubMed] [Google Scholar]
- 10.Lee, S.-K., S.-L. Yu, L. Prakash, and S. Prakash. 2001. Requirement for yeast RAD26, a homolog of the human CSB gene, in elongation by RNA polymerase II. Mol. Cell. Biol. 21:8651-8656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mellon, I., G. Spivak, and P. C. Hanawalt. 1987. Selective removal of transcription-blocking DNA damage from the transcribed strand of the mammalian DHFR gene. Cell 51:241-249. [DOI] [PubMed] [Google Scholar]
- 12.Nance, M. A., and S. A. Berry. 1992. Cockayne syndrome: review of 140 cases. Am. J. Med. Genet. 42:68-84. [DOI] [PubMed] [Google Scholar]
- 13.Reagan, M. S., and E. C. Friedberg. 1997. Recovery of RNA polymerase II synthesis following DNA damage in mutants of Saccharomyces cerevisiae defective in nucleotide excision repair. Nucleic Acids Res. 25:4257-4263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rupp, W. D., C. E. I. Wilde, D. L. Reno, and P. Howard-Flanders. 1971. Exchanges between DNA strands in ultraviolet-irradiated Escherichia coli. J. Mol. Biol. 61:25-44. [DOI] [PubMed] [Google Scholar]
- 15.Selby, C. P., R. Drapkin, D. Reinberg, and A. Sancar. 1997. RNA polymerase II stalled at a thymine dimer: footprint and effect on excision repair. Nucleic Acids Res. 25:787-793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Selby, C. P., and A. Sancar. 1997. Cockayne syndrome group B protein enhances elongation by RNA polymerase II. Proc. Natl. Acad. Sci. USA 94:11205-11209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Selby, C. P., and A. Sancar. 1997. Human transcription-repair coupling factor CSB/ERCC6 is a DNA-stimulated ATPase but is not a helicase and does not disrupt the ternary transcription complex of stalled RNA polymerase II. J. Biol. Chem. 272:1885-1890. [DOI] [PubMed] [Google Scholar]
- 18.Selby, C. P., and A. Sancar. 1993. Molecular mechanism of transcription-repair coupling. Science 260:53-58. [DOI] [PubMed] [Google Scholar]
- 19.Torres-Ramos, C., S. Prakash, and L. Prakash. 2002. Requirement of RAD5 and MMS2 for postreplication repair of UV-damaged DNA in Saccharomyces cerevisiae. Mol. Cell. Biol. 22:2419-2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Torres-Ramos, C. A., R. E. Johnson, L. Prakash, and S. Prakash. 2000. Evidence for the involvement of nucleotide excision repair in the removal of abasic sites in yeast. Mol. Cell. Biol. 20:3522-3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Gool, A. J., R. Verhage, S. M. A. Swagemakers, P. van de Putte, J. Brouwer, C. Troelstra, D. Bootsma, and J. H. J. Hoeijmakers. 1994. RAD26, the functional S. cerevisiae homolog of the Cockayne syndrome B gene ERCC6. EMBO J. 13:5361-5369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Hoffen, A., A. T. Natarajan, L. V. Mayne, A. A. van Zeeland, L. H. F. Mullenders, and J. Venema. 1993. Deficient repair of the transcribed strand of active genes in Cockayne's syndrome cells. Nucleic Acids Res. 21:5890-5895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wallace, S. 1997. Oxidative damage to DNA and its repair, p. 49-90. In J. G. Scandalios (ed.), Oxidative stress defenses. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.