Abstract
The present study addresses the capacity of heregulin (HRG), a ligand of type I receptor tyrosine kinases, to transactivate the progesterone receptor (PR). For this purpose, we studied, on the one hand, an experimental model of hormonal carcinogenesis in which the synthetic progestin medroxyprogesterone acetate (MPA) induced mammary adenocarcinomas in female BALB/c mice and, on the other hand, the human breast cancer cell line T47D. HRG was able to exquisitely regulate biochemical attributes of PR in a way that mimicked PR activation by progestins. Thus, HRG treatment of primary cultures of epithelial cells of the progestin-dependent C4HD murine mammary tumor line and of T47D cells induced a decrease of protein levels of PRA and -B isoforms and the downregulation of progesterone-binding sites. HRG also promoted a significant increase in the percentage of PR localized in the nucleus in both cell types. DNA mobility shift assay revealed that HRG was able to induce PR binding to a progesterone response element (PRE) in C4HD and T47D cells. Transient transfections of C4HD and T47D cells with a plasmid containing a PRE upstream of a chloramphenicol acetyltransferase (CAT) gene demonstrated that HRG promoted a significant increase in CAT activity. In order to assess the molecular mechanisms underlying PR transactivation by HRG, we blocked ErbB-2 expression in C4HD and T47D cells by using antisense oligodeoxynucleotides to ErbB-2 mRNA, which resulted in the abolishment of HRG's capacity to induce PR binding to a PRE, as well as CAT activity in the transient-transfection assays. Although the inhibition of HRG binding to ErbB-3 by an anti-ErbB-3 monoclonal antibody suppressed HRG-induced PR activation, the abolishment of HRG binding to ErbB-4 had no effect on HRG activation of PR. To investigate the role of mitogen-activated protein kinases (MAPKs), we used the selective MEK1/MAPK inhibitor PD98059. Blockage of MAPK activation resulted in complete abrogation of HRG's capacity to induce PR binding to a PRE, as well as CAT activity. Finally, we demonstrate here for the first time that HRG-activated MAPK can phosphorylate both human and mouse PR in vitro.
Recent evidence has clearly shown the presence of cross talks between steroid hormone and growth factor (GF) signaling pathways, which were previously thought to be distinctly separate processes. Members of the steroid receptor superfamily are heavily phosphorylated proteins that, upon ligand binding, act as nuclear transcription factors (for a review, see reference 72). Particularly, progesterone receptor (PR), the focus of the present work, is phosphorylated in the absence of hormone and undergoes an increase in phosphorylation upon hormonal stimulation (19, 20, 67). Although the functional role of PR phosphorylation remains elusive, increased evidence indicates that it plays a role in the regulation of PR transcriptional activity (3, 7, 14, 22, 40, 66). On the other hand, most GFs bind to transmembrane receptors that carry an intrinsic activity of tyrosine kinase. Tyrosine residues in the intracellular domains of the type I and II receptor tyrosine kinase (RTK) families, phosphorylated as a result of ligand binding, serve as docking sites for a number of SH2 and phosphotyrosine-binding domain-containing proteins (15, 34, 42, 50), which link RTKs to several signal transduction pathways. Among these, one of the best characterized is the mitogen-activated protein kinase (MAPK) cascade (23).
Accumulated evidence has highlighted the importance of phosphorylation as one of the molecular mechanisms by which steroid hormone and GF signaling converge. First, the majority of the phosphorylation sites in PR, as well as in the other steroid receptors, contain a Ser/Thr-Pro motif, which is a core consensus sequence for proline-directed kinases such as MAPKs (reviewed in reference 72). Second, modulation of protein kinase or phosphatase activity has been shown to result in regulation of the ligand-induced transcriptional activity of PR (7, 22, 55, 64), as well as in induction of ligand-independent activation of the receptor (19, 77).
The positive role of estrogens in breast cancer development has long been acknowledged. Accumulated evidence also indicates that progestins are involved in controlling mammary tumorigenesis (9, 10, 13, 29, 35, 39, 43, 64). In addition, type I RTKs have been implicated in the induction of proliferative signaling in breast cancer (6, 52). Type I RTKs includes four members: epidermal GF receptor (EGFR/ErbB-1) (69), ErbB-2 (73), ErbB-3 (37, 53), and ErbB-4 (54). A large number of ligands for type I RTKs have been described. These include six ligands for EGFR (59) and all isoforms of Neu differentiation factor/heregulin (HRG) which bind to ErbB-3 and ErbB-4 (68). Interestingly, ErbB-2 is an orphan receptor. Despite the absence of a known ligand that binds ErbB-2 directly, this receptor participates in an extensive network of ligand-induced formation of ErbBs dimers (28).
Convergence between steroid hormones and RTK signaling pathways has a bidirectional nature in which steroid hormones activate either RTKs or their downstream signaling pathways (4, 5, 9, 39, 45, 58) and where, conversely, RTK ligands are able to modulate steroid receptor transcriptional activity (32, 33, 51, 60, 64). Steroid-independent activation of estrogen receptor (ER) by EGF and IGF-I, ligands of the type I and II RTKs, respectively, has long been described (11, 31, 32, 33). HRG, another type I RTK ligand, has also been found to transactivate ER (51). Although the capacity of RTK ligands to transactivate PR remains poorly studied, EGF is able to induce transactivation of chicken PRA (77).
We have recently proved the presence of interactions between progestins and HRG signaling pathways in an experimental model of hormonal carcinogenesis in which the synthetic progestin medroxyprogesterone acetate (MPA) induced mammary adenocarcinomas in female BALB/c mice (4, 5). Our previous studies have demonstrated the capacity of progestins to activate ErbB-2 and ErbB-3 and to induce HRG synthesis (4, 5). Therefore, the present work focuses on the assessment of the other likely scenario of HRG and progestin interaction, i.e., the capacity of HRG to transactivate PR. Our findings show that HRG was able to induce PR transactivation through a mechanism that requires both a functional ErbB-2 and MAPK activation. One of the most salient aspects of the present study is that we have demonstrated, for the first time, that HRG-activated MAPK is able to phosphorylate both human PR (hPR) and mouse PR in vitro.
MATERIALS AND METHODS
Animals and tumors.
Experiments were carried out in virgin female BALB/c mice raised at the Institute of Biology and Experimental Medicine of Buenos Aires. All animal studies were conducted in accordance with the highest standards of animal care as outlined in the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The hormone-dependent ductal tumor line C4HD originated in mice treated with 40 mg of MPA every 3 months for 1 year and was maintained by serial transplantation in animals treated with 40 mg of MPA given subcutaneously in the flank opposite to the tumor inoculum (38, 47). The C4HD tumor line is of ductal origin and expresses PR and ER (38, 47).
Cell cultures and proliferation assays.
Primary cultures of epithelial cells from C4HD tumors, growing in MPA-treated mice, were performed as previously described (21). In brief, tumors were aseptically removed, minced, and washed with DMEM-F12 (Dulbecco modified Eagle medium-Ham F12 [1:1], without phenol red, with 100 U of penicillin/ml, and with 100 μg of streptomycin/ml). The tissue was suspended in 5 ml of enzymatic solution (trypsin, 2.5 mg/ml; albumin, 5 mg/ml; collagenase type II [Gibco-BRL, Gaithersburg, Md.], 239 U/ml) in phosphate-buffered saline (PBS) and then incubated at 37°C for 20 min with continuous stirring. The liquid phase of the suspension was then removed, and the undigested tissue was incubated with fresh enzymatic solution for 20 min. Enzyme action was stopped by adding DMEM-F12 plus 5% heat-inactivated fetal calf serum (FCS; Gen S.A., Buenos Aires, Argentina). Epithelial and fibroblastic cells were separated as previously described (21). Briefly, the cell suspension was resuspended in 15 ml of DMEM-F12 plus 10% FCS and allowed to sediment for 20 min. The sedimented cells, corresponding to the epithelial enriched fraction, were resuspended again in 15 ml of DMEM-F12 plus 5% FCS and allowed to sediment for another 20 min. The upper 15 ml was discarded, and this procedure was subsequently repeated until no fibroblasts were observed in the supernatant. Cells were plated in culture flasks with DMEM-F12 plus 5% steroid-stripped FCS (ChFCS) (21) and allowed to attach for 24 to 48 h. The purity of the epithelial cultures was evaluated by cytokeratin staining. Cells were incubated in DMEM-F12 (without phenol red, with 100 U of penicillin/ml, and with 100 μg of streptomycin/ml) with 2.5% ChFCS in the presence of 10 nM MPA, 10 nM RU486, HRG at 20 ng/ml, and MPA+RU486 or HRG+RU486. In experiments in which ErbB-2 was blocked, cells were pretreated with a 2 μM concentration of antisense oligodeoxynucleotides (ASODNs) to ErbB-2 mRNA or, as control, with a 2 μM concentration of sense oligodeoxynucleotides (SODNs) for 48 h before the addition of HRG. ErbB-2 antisense (5′-GGC CGC CAG CTC CAT) and sense (5′-ATG GAG CTG GCG GCC) oligodeoxynucleotides (ODNs) correspond to the translation start region, including the initiation codon of the ErbB-2 mRNA (16). In experiments assessing the role of ErbB-3 or ErbB-4 in HRG-induced proliferation, cells were preincubated with increasing concentrations of either an ErbB-3 mouse monoclonal antibody (oncoprotein Ab-5, clone H3.105.5; Neomarkers, Freemont, Calif.) or an ErbB-4 mouse monoclonal antibody (oncoprotein Ab-3, clone H4.72.8; Neomarkers) prior to HRG treatment. As control, cells were also incubated with preimmune mouse serum. To block MAPK activation, PD98059 (10 μM, dissolved 1:2,000 in dimethyl sulfoxide) was added to cells 30 min before the 48 h of incubation with HRG. Controls were performed in order to verify that dimethyl sulfoxide (1:2,000) did not modify HRG-stimulated proliferation. After a 24-h incubation, 50% of the medium was replaced with fresh medium, and the cells were incubated for another 24 h in the presence of 0.8 μCi of [3H]thymidine (specific activity, 70 to 90 Ci/mmol; NEN/Dupont, Boston, Mass.). Cells were then trypsinized and harvested. Assays were performed in octuplicate. The differences between control and experimental groups were analyzed by analysis of variance and the Tukey t test between groups. In earlier experiments we had demonstrated that thymidine uptake correlates with the number of cells/well (21). T47D cells were obtained from the American Type Culture Collection. Cells were cultured in DMEM (without phenol red) supplemented with 0.1% ChFCS and then subjected to the treatments described above for C4HD cells. Cell proliferation assays and data analysis were performed as described for C4HD cells.
ErbB-2, ErbB-3, ErbB-4, and MAPK expression and activation.
Lysates were prepared from C4HD or T47D cells subjected to the different treatments described in each experiment. Cells were lysed in buffer containing 50 mM Tris (pH 7.4), 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 10% glycerol, 0.5% Nonidet P-40, 1 mM MgCl2, 1 mM phenylmethylsulfonyl fluoride (PMSF), 10 μg of leupeptin/ml, 5 μg of pepstatin/ml, 5 μg of aprotinin/ml, 1 mM sodium orthovanadate, 5 mM NaF, 20 mM sodium molybdate, and 5 mM sodium pyrophosphate. Lysates were centrifuged at 40,000 × g for 40 min at 4°C, and the protein content in the supernatant was determined by using a Bio-Rad kit (Richmond, Calif.). Proteins were solubilized in sample buffer (60 mM Tris-HCl [pH 6.8], 2% sodium dodecyl sulfate [SDS], 10% glycerol, 0.01% bromophenol blue) and subjected to SDS-polyacrylamide gel electrophoresis (PAGE). Proteins were electroblotted onto nitrocellulose. Membranes were blocked with PBS-0.1% Tween 20 and immunoblotted with the following antibodies: ErbB-2 rabbit polyclonal antibody Neu C-18 (Santa Cruz Biotechnology, Santa Cruz, Calif.), ErbB-3 rabbit polyclonal antibody C-17 (Santa Cruz), ErbB-4 rabbit polyclonal antibody C-18 (Santa Cruz), ErbB-4/HER-4 oncoprotein Ab-2 rabbit polyclonal antibody (Neomarkers), anti-total p42/p44 MAPK (C-14; Santa Cruz), anti-phospho p42/p44 MAPK (E-4 Santa Cruz), and antihemagglutinin (anti-HA) antibody HA.11 (Berkeley Antibody Co., Berkeley, Calif.). After a washing step, the membranes were incubated with horseradish peroxidase-conjugated secondary antibody (Amersham International). Enhanced chemiluminescence was performed according to the manufacturer's instructions (Amersham). To perform ErbB-3 and ErbB-4 tyrosine phosphorylation analysis, cell lysates (1 mg of protein) were precleared with protein A-agarose (Santa Cruz). Then, 2 to 5 μg of either ErbB-3 C-17 (Santa Cruz) or ErbB-4 Ab-2 (Neomarkers) was used in each immunoprecipitation reaction, which were rocked for 2 h at 4°C. Thereafter, the immunocomplexes were captured by adding protein A-agarose and rocked for an additional 2 h. Beads were washed three times with lysis buffer and then boiled for 10 min in sample buffer and subjected to SDS-PAGE on a 6% gel. Proteins were electroblotted onto nitrocellulose, and filters were probed with mouse monoclonal anti-P-Tyr PY-99 (Santa Cruz).
PR Western blotting.
To study PR expression at protein level, whole-cell extracts were prepared. In brief, C4HD or T47D cells were lysed by scraping at 4°C in buffer containing 50 mM Tris (pH 7.4), 7.5 mM EDTA, 10% glycerol, 0.4 M KCl, 1 mM PMSF, 10 μg of leupeptin/ml, 5 μg of pepstatin/ml, 5 μg of aprotinin/ml, 5 mM NaF, and 5 mM sodium pyrophosphate. Cell homogenates were incubated for 30 min with intermittent vortexing and centrifuged at 14,000 rpm for 30 min. Soluble proteins in clarified lysates were quantified by using a Bio-Rad (Richmond, Calif.) kit. Proteins were resolved by SDS-PAGE on 7.5% gels, electroblotted onto nitrocellulose, and immunoblotted with the PR mouse monoclonal antibody hPR Ab-7 (clone 7) (Neomarkers) or with the phospho-294-specific PR mouse monoclonal antibody Ab-12 (clone 608; Neomarkers).
Preparation of nuclear and cytosolic extracts.
C4HD and T47D cells were lysed by scraping at 4°C in buffer A, which contains 50 mM Tris (pH 7.4), 7.5 mM EDTA, 10% glycerol, 0.25 M sucrose, 0.5 mM dithiothreitol (DTT), 1 mM PMSF, 10 μg of leupeptin/ml, 5 μg of pepstatin/ml, 5 μg of aprotinin/ml, and 5 mM NaF. The homogenate was centrifuged for 10 min at 1,000 × g, and the supernatant fraction (cytosol) was then collected. The crude nuclear pellet was washed once with buffer A containing NP-40 0.01% and then resuspended in buffer A containing 0.4 M KCl and was later incubated for 30 min at 4°C with resuspension every 10 min. The suspension was centrifuged at 180,000 × g (30 min) diluted with the same volume of buffer A to reduce the salt concentration.
PR binding assay.
PR content was evaluated by using a single-saturating-dose [3H]R5020 binding assay. Briefly, triplicate 20-μl aliquots of cytosolic or nuclear extracts were incubated for 18 h at 4°C with 10 nM [3H]R5020 in the presence or absence of a 100-fold molar excess of unlabeled R5020 and of a 200-fold molar excess of cortisol to suppress nonspecific binding to plasma proteins. At the end of the incubation, samples were treated with 100 μl of a suspension of 1% charcoal- 0.1% dextran in buffer containing 20 mM Tris (pH 7.4), 1.5 mM EDTA, 0.25 mM DTT, 20 mM Na2MoO4, and 10% glycerol. A free fraction was separated by centrifugation at 3,500 rpm for 10 min and then counted in a Beckman liquid scintillation counter. Protein concentrations were determined by using a Bio-Rad kit, and results were expressed as femtomoles of protein per milligram.
EMSA.
The DNA-binding activity of PR in nuclear cell extracts of C4HD and T47D cells was analyzed by electrophoretic mobility shift assay (EMSA). Nuclear extracts were prepared from cells after incubation for 2 h at 37°C with 10 nM MPA or 20 ng of HRG/ml. In some experiments, the cells were pretreated for 48 h at 37°C with 2 μM ErbB-2 ASODNs or SODNs or preincubated for 30 to 90 min with a ErbB-3 mouse monoclonal antibody (oncoprotein Ab-5, clone H3.105.5; Neomarkers), a ErbB-4 mouse monoclonal antibody (oncoprotein Ab-3, clone H4.72.8; Neomarkers), preimmune mouse serum, or 10 μM PD98059. A 27-bp double-stranded synthetic oligonucleotide (5′-GAT CCT GTA CAG GAT GTT CTA GCT ACA-3′) containing the progesterone response element (PRE) from the rat tyrosine aminotransferase gene promoter (Santa Cruz) was used as the target DNA. The PRE oligonucleotide was end labeled with [γ-32P]ATP by T4 polynucleotide kinase to a specific activity of ca. 30,000 to 50,000 cpm/0.1 ng. Nuclear extracts containing 5 to 10 fmol of receptor (based on a steroid-binding assay) and 20 μg of nuclear protein were incubated for 20 min at room temperature in a total volume of 30 μl with 0.8 ng of the [32P]PRE. The DNA-binding reaction also consisted of 2.5 μg of poly(dI-dC) as a nonspecific competitor DNA in a binding buffer containing 10 mM Tris-HCl (pH 7.4), 50 mM NaCl, 10% glycerol, 1 mM DTT, 5 mM MgCl2, 5 μg of gelatin/μl, 1 mM PMSF, 10 μg of leupeptin/ml, 5 μg of pepstatin/ml, and 5 μg of aprotinin/ml. The specificity of the PR-PRE complexes was studied by competition with 25- and 100-fold mass excesses of unlabeled PRE oligonucleotide and with a 100-fold mass excess of a mutant PRE with two GT-CA substitutions in the PR-binding motif (5′-GAT CCT CAA CAG GAT CAT CTA GCT ACA-3′; Santa Cruz). In the supershift assays, the rabbit polyclonal anti-PR antibody C-19 (Santa Cruz) at a concentration of 4 μg/assay or, as a control, preimmune rabbit serum was used. Samples were subjected to electrophoresis on nondenaturing 5.5% acrylamide gels (30:1) in low-ionic-strength TBE buffer (10 mM Tris-borate, pH 7.5; 0.025 mM EDTA). Gels were preelectrophoresed at 10 mA for 60 min, and samples were run at 20 mA/gel at room temperature for 120 min. To prevent warming of the gels, water was recirculated through the gel apparatus. Gels were dried without fixation under vacuum and were autoradiographed by exposure to Kodak X-Omat film at −70°C.
Transient transfections.
Reporter plasmid PRE2-TATAtk-CAT contains the TATA box and flanking sequences of the tk promoter (−60/+51) cloned downstream of two copies of the TAT promoter PRE (61). C4HD and T47D cells were plated in six-well dishes in DMEM supplemented with 5% ChFCS and with 10 nM MPA for C4HD cells or in DMEM with 10% ChFCS for T47D, without antibiotics, and were cultured for 48 h prior to transfection. Transient transfections were performed for 48 h at 37°C with 4 μg per well of the PRE2-TATAtk-CAT plasmid DNA and with 4 μg of the β-galactosidase expression plasmid CMV-β-Gal (Clontech, Palo Alto, Calif.) used to correct variations in transfection efficiency since control transfections were also performed with a tk-CAT reporter lacking a PRE (61). The DOTAP liposomal transfection reagent technique (Roche Biochemicals) was used as recommended by the manufacturer. After transfection, cells were washed and cultured for 24 h in 2.5% ChFCS (C4HD) or in 0.1% ChFCS (T47D) before treatment with the different activators. Cells were then harvested, and lysates were analyzed for chloramphenicol acetyltransferase (CAT) activity. The plasmids used in experiments studying in vitro hPR phosphorylation by MAPK included Flag epitope-tagged hPRB (58), a gift of K. B. Horwitz, and HA-tagged Erk2 (p42 MAPK) expression vector (17). Transfections were performed according to the protocol described above. Cells transfected with the HA-tagged Erk2 (p42 MAPK) expression vector were treated for 10 min with HRG (20 ng/ml) to obtain activated p42 MAPK, and cells transfected with the Flag epitope-tagged hPRB were grown after transfection for 48 h in DMEM without serum (T47D cells) or supplemented with 2.5% ChFCS (C4HD cells) for 48 h to obtain transcriptionally inactive PR.
CAT assays.
Cells were washed once with PBS without calcium and magnesium, scraped into 500 μl of 1× reporter buffer (Promega, Madison, Wis.), and collected by centrifugation at 14,000 rpm for 15 min. Protein concentrations were determined by using a Bio-Rad kit. The CAT activity was determined by incubating 100 μg of protein with 0.2 μCi of [3H]chloramphenicol (20 μCi/μmol; DuPont/NEN) and with 250 μM butyryl-coenzyme A (Sigma) in 100 μl of 100 mM Tris-HCl (pH 7.5) overnight at 37°C. Acylated chloramphenicol was extracted by using a mixture of 200 μl of TMPD (2:1) and xylenes (Sigma) and counted in a scintillation counter. Values of reagent blanks were subtracted from all samples before calculation of data. To control variation in transfection efficiency, cells were cotransfected with a CMV-β-Gal reporter plasmid (Clontech). CAT results were calculated as the ratio of CAT activity per unit of β-Gal activity. Duplicate samples were analyzed for each datum point. The MPA-dependent transcriptional activity was set as 100%.
MAPK immunoprecipitation and activity assays.
C4HD and T47D cells were incubated for 10 min with HRG at 20 ng/ml or kept untreated and growing in ChFCS. Blockage of MAPK activity was accomplished by preincubating the cells for 30 min with 10 μM PD98059 before stimulation with HRG. Cell lysates were immunoprecipitated by using anti-total p42/p44 MAPK antibody (C-14; Santa Cruz). Immunoprecipitations were rocked for 2 h at 4°C, and the immunocomplexes were then captured by adding protein A-agarose, followed by rocking for an additional 2 h. Beads were washed, and an in vitro kinase assay was performed with 1.5 μg of myelin basic protein (MBP; Sigma)/μl as the substrate and 10 μCi of [γ-32P]ATP. Samples were analyzed by SDS-PAGE on 12% acrylamide gels. Gels were dried without fixation under vacuum and were autoradiographed by exposure to Kodak X-Omat film at −70°C.
MAPK activation was also studied by performing Western blot with an anti-phospho p42/p44 MAPK monoclonal antibody (E-4; Santa Cruz). Membranes were stripped and hybridized with the antibody anti-total p42/p44 MAPK (C-14; Santa Cruz).
In vitro PR phosphorylation.
To study the capacity of endogenous MAPK to phosphorylate PR, C4HD and T47D cells growing in six-well plates were treated with HRG for 10 min, pretreated with PD98059 for 30 min prior to HRG treatment, or kept untreated and growing in ChFCS. Cells were lysed and MAPK were immunoprecipitated from 4 mg of protein extracts from each cell treatment with an anti-p42/p44 MAPK antibody (C-14). Transcriptionally inactive PR was immunoprecipitated from 4 mg of protein from C4HD or T47D cells growing in 2.5% ChFCS (C4HD) or in DMEM without serum (T47D) by using the PR mouse monoclonal antibody hPR Ab-7 (clone 7; Neomarkers). To assess the capacity of transfected p42 MAPK to phosphorylate hPR, C4HD and T47D cells were transiently transfected with 4 μg of an HA-tagged p42 MAPK expression vector/well, and 48 h later the cells were treated with HRG for 10 min, pretreated with PD98059 for 30 min prior to HRG treatment, or kept untreated and growing in ChFCS. Cells were lysed, and p42 MAPK was immunoprecipitated from 4-mg protein extracts from each cell treatment with an anti-HA antibody (HA.11; Berkeley Antibody Co). On the other hand, as a source of transcriptionally inactive hPR, C4HD and T47D cells were transfected with 4 μg of the Flag-tagged hPRB expression vector/well, and 48 h later PR was immunoprecipitated from 4 mg of protein from lysates of cells growing in ChFCS by using a mouse monoclonal anti-Flag M2 antibody (Sigma). Immunoprecipitated PR was then subjected to an in vitro phosphorylation assay with MAPKs immunoprecipitated from cells subjected to each of the treatments described above. In brief, PR and p42 MAPK immunoprecipitates were washed once with PBS containing 1% NP-40, protease (1 mM PMSF, 10 μg of leupeptin/ml, 5 μg of pepstatin/ml, 5 μg of aprotinin/ml), and phosphatase (2 mM sodium orthovanadate, 5 mM sodium fluoride, 5 mM sodium pyrophosphate, 12.5 mM β-glycerophosphate) inhibitors, once again in 100 mM Tris-HCl (pH 7.5)-0.5 mM LiCl containing protease and phosphatase inhibitors, and then three times with kinase reaction buffer (12.5 mM morpholinepropanesulfonic acid [pH 7.5], 12.5 mM β-glycerophosphate, 7.5 mM MgCl2, 0.5 mM EGTA, 0.5 mM NaF, 0.5 mM sodium orthovanadate). The kinase reaction was performed by incubating MAPK immunocomplexes with equal amounts of immunoprecipitated PR as the peptide substrate in 30 μl of kinase buffer containing 3.3 mM DTT, 0.05 mM unlabeled ATP, and 10 μCi of [γ-32P]ATP for 30 min at 30°C. Samples were analyzed by SDS-PAGE on 10% acrylamide gels. The upper part of the gels, containing the PR, was dried, and phosphorylation of the PR was visualized by autoradiography. The lower part of the gel was transferred onto nitrocellulose, and an immunoblot was performed with an anti-phospho MAPK antibody. The filter was then stripped and hybridized with anti-total p42/p44 MAPK antibody. To directly assess whether MAPK could induce phosphorylation of Ser294, a cold in vitro kinase assay that was similar to the one described above except that the reaction contained 100 μM cold ATP instead of [3γ-2P]ATP was performed. At the end of the incubation, proteins were subjected to SDS-PAGE. Gels were transferred onto nitrocellulose, and in the upper part of the gels containing PR immunoblots were performed with anti-phospho Ser294 PR antibody. The lower part of the gel in which MAPK was resolved was revealed with an anti-phospho p42/p44 MAPK monoclonal antibody (E-4).
RESULTS
The progestin antagonist RU486 blocks HRG-induced proliferation of C4HD and T47D cells.
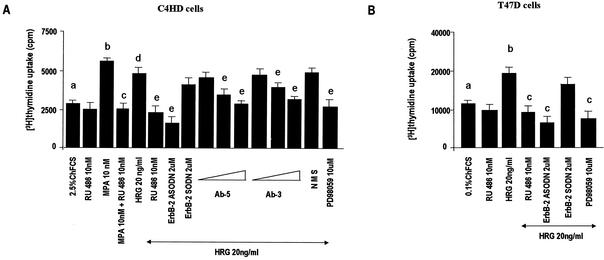
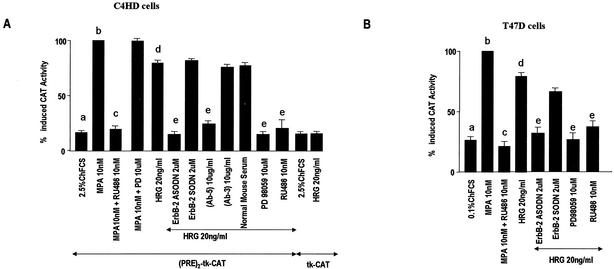
In previous research we had already found that progestins are able to modulate the HRG/ErbBs transduction pathway, sensitizing breast cancer cells to the proliferative effect of this signaling pathway (4, 5). In the present study, we explored the probable bidirectional nature of the interaction between progestins and HRG through the study of HRG capacity to transactivate the PR. We performed these studies in an experimental model of hormonal carcinogenesis in which the synthetic progestin MPA induced mammary adenocarcinomas in female BALB/c mice (38, 47). Primary cultures of the C4HD murine mammary tumor line (21) were used. As we have previously described in detail (21), C4HD epithelial cells derive from a ductal, progestin-dependent mammary tumor line (38, 47). C4HD cells require MPA administration to proliferate and express high levels of PR (21). We have recently found that HRG was able to induce a proliferative response as potent as that induced by MPA on C4HD cells (5). Here, we first investigated whether the progestin antagonist RU486 had any effect on HRG-induced proliferation. As shown in Fig. 1, the stimulatory effect of HRG was inhibited by RU486 (Fig. 1). We had also already shown that HRG proliferative effects were abolished when C4HD cells were treated with ASODNs to ErbB-2 mRNA (5) (Fig. 1). In previous studies we demonstrated that C4HD cells do not express ErbB-1/EGFR (38), exhibit high levels of ErbB-3 (4, 5), and show low ErbB-4 expression (4, 5). Here, we assessed the role of ErbB-3 and ErbB-4 in transmitting HRG proliferative signals in C4HD cells. For this purpose, we blocked HRG binding to ErbB-3 by using the mouse monoclonal ErbB-3 antibody Ab-5. The addition of the ErbB-3 antibody to HRG-treated C4HD cells resulted in a dose-dependent inhibition of HRG proliferative effects (Fig. 1). In order to investigate ErbB-4 involvement in HRG induction of C4HD cell growth, we used the same experimental approach. As shown in Fig. 1, the ErbB-4 mouse monoclonal antibody Ab-3 inhibited in a dose-dependent fashion the HRG proliferative response (Fig. 1). We then studied signal transduction pathways activated by HRG downstream from these receptors. It has been well acknowledged that HRG activates p42/p44 MAPK in breast cancer cells (44, 63). Thus, we investigated the participation of MAPK in HRG-induced proliferation of C4HD cells by preincubation with PD98059, a specific inhibitor of MEK1. As shown in Fig. 1, HRG induction of C4HD cell growth was abolished by PD98059. Similar experiments were conducted in T47D breast cancer cells, which naturally express both the A and B isoforms of PR (30). As previously reported (25), HRG stimulated T47D cell proliferation, and this effect was blocked by PD98059 (Fig. 1). Blockage of ErbB-2 expression also resulted in the suppression of HRG mitogenic activity (Fig. 1). Interestingly, we found that in T47D cells RU486 was able to abolish HRG-induced growth, as observed in C4HD cells (Fig. 1). These findings suggest that physiological actions of HRG in breast cancer cells might be mediated in part by the PR. In all of the experiments described, C4HD cells used as a control were grown in 2.5% ChFCS and T47D cells were grown in 0.1% ChFCS, which were the best conditions for detecting a strong growth stimulatory effect of HRG. No presence of progesterone was detected by radioimmunoassay in ChFCS or in the culture medium of C4HD and T47D cells growing in either ChFCS or treated with HRG. This indicates that progesterone levels are <10−13 M, a level insufficient to induce PR transactivation in mammalian cells (26).
FIG. 1.
Proliferative effects of HRG require PR and p42/p44 MAPK activation. C4HD (A) and T47D (B) cells were incubated for 48 h in medium with ChFCS supplemented, as indicated, with RU486, 10 nM MPA, MPA-RU486, HRG, HRG-RU486, HRG-ASODN or -SODN plus ErbB-2, or HRG-PD98059. C4HD cells were also treated with HRG plus increasing concentrations (1, 5, and 10 μg/ml) of either ErbB-3 monoclonal antibody (Ab-5) or ErbB-4 monoclonal antibody (Ab-3) and HRG plus preimmune mouse serum (NMS). Incorporation of [3H]thymidine was used as a measure of DNA synthesis. Data are presented as means ± the standard deviation (SD). Significance for columns b versus a, columns c versus b, columns d versus a, and columns e versus d, P < 0.001. The experiments shown are representative of a total of five for each cell type.
HRG induces downregulation of both PRA and PRB isoforms.
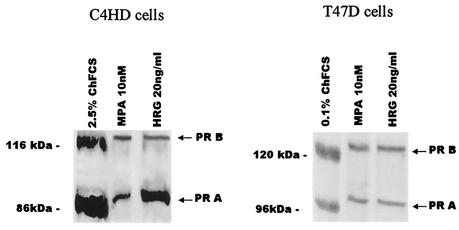
We investigated the effect of HRG on the expression at protein level of PR in C4HD and T47D cells. Both PRA and PRB isoforms were detected on C4HD cell immunoblots (Fig. 2). As previously described in the murine mammary gland (65), PRA was the predominant isoform expressed in C4HD cells (Fig. 2). After a 2-h treatment of C4HD cells, HRG induced a significant downregulation of PRB (71% ± 7%) and PRA (67% ± 6%) isoforms. MPA exerted a similar downregulation of PRB (82% ± 6%) and PRA (87% ± 7%) in C4HD cells. Comparable results were found in T47D cells, in which HRG induced a 71% ± 5% decrease in PRB and a 64% ± 4% decrease in PRA expressions (Fig. 2), an effect similar to the one exerted by MPA, which decreases PRB by expression by 78% ± 6% and PRA expression by 82% ± 7% in these cells (Fig. 2). It is well acknowledged that progestin treatment induces changes in the electrophoretic mobility of PR on SDS-gels, resulting in an upward shift of both PR isoforms (7, 14, 67, 72). This is associated with a second step of ligand-induced increase in PR phosphorylation that requires 40 to 60 min for completion (75). We found here that HRG treatment of C4HD and T47D cells induced an upward shift of both PR isoforms, an effect similar to that observed with MPA (Fig. 2). Therefore, this was our first finding suggesting that HRG might be involved in the induction of PR phosphorylation.
FIG. 2.
HRG induces downregulation of PR expression at the protein level. C4HD and T47D cells were incubated for 2 h with MPA or HRG or were left untreated and growing in ChFCS. A total of 100 μg of protein from whole-cell lysates, obtained as described in Materials and Methods, were electrophoresed and immunoblotted for PR. The experiment shown is representative of a total of four in which the standard error of the mean (SEM) was within 10%.
As previously reported for progestin treatment of breast cancer cells (14, 49, 57, 70, 71), MPA treatment of C4HD and T47D cells resulted in the downregulation of progesterone-binding sites by using a single-saturating-dose [3H]R5020 binding assay (Table 1) . Interestingly, decreased concentration of PR protein induced by HRG in both C4HD and T47D cells, also correlated with downregulation of progesterone-binding sites (Table 1).
TABLE 1.
HRG-induced downregulation of progesterone-binding sites
| Cell type | Mean amt (fmol/mg of protein) of PRa ± SEM with:
|
||
|---|---|---|---|
| ChFCS | MPA (10 nM) | HRG (20 ng/ml) | |
| C4HD | 1,424.0 ± 355.8 | 350.3 ± 62.5b | 520.0 ± 80.1b |
| T47D | 939.0 ± 298.7 | 203.2 ± 58.9b | 198.2 ± 128.6b |
PR levels were measured by a single-saturating-dose [3H]R5020-binding assay as described in Materials and Methods. Each value represents the mean of six independent determinations.
P < 0.001 compared to the respective cell line growing in ChFCS.
HRG enhances nuclear binding of the PR.
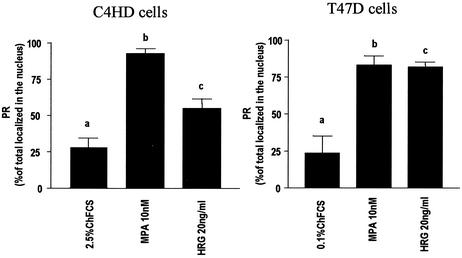
It has long been known that in the absence of hormone, PR distributes between NaCl-extractable nuclear and cytosol fractions of human breast cells, whereas hormone treatment stimulates the major amount of cellular PR to bind tightly to the nuclei (14, 22, 24, 46). Therefore, we investigated the effect of HRG on the cellular localization of PR. As shown in Fig. 3, HRG treatment of C4HD cells for 2 h resulted in a significant increase in PR nuclear localization compared to cells growing in 2.5% ChFCS, as determined by a [3H]R5020 binding assay. Treatment with MPA elicited the anticipated increased specific binding of [3H]R5020 in the nuclear fractions compared to controls (Fig. 3). Similar results were found in T47D cells in which both HRG and MPA treatment induced nuclear localization of PR (Fig. 3).
FIG. 3.
HRG promotes the nuclear localization of PR. C4HD and T47D cells were treated for 2 h at 37°C with MPA or HRG or were left untreated and growing in ChFCS. PR levels were measured in nuclear and cytoplasmic extracts as described in Materials and Methods. The total PR number, which was different for each treatment because of MPA- and HRG-induced downregulation of PR, was obtained as the sum of the sites present in the two compartments. Data are expressed as a percentage of the total PR localized in the nucleus. Values shown in the histogram are the means of eight independent experiments ± the SEM. Significance for columns b versus a and columns c versus a, P < 0.001.
HRG induces PR binding to a PRE.
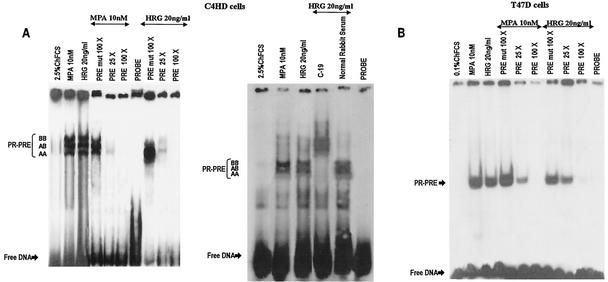
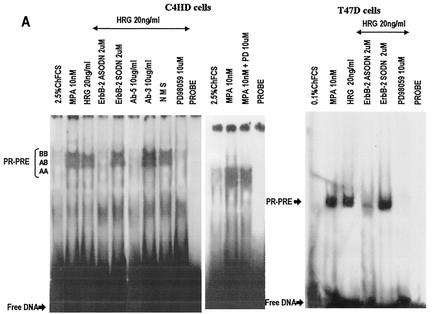
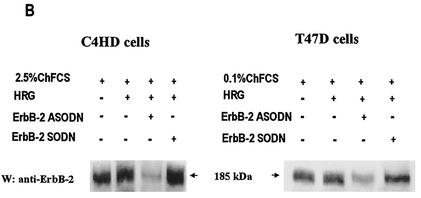
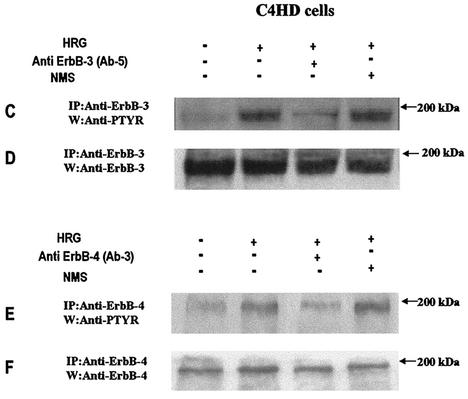
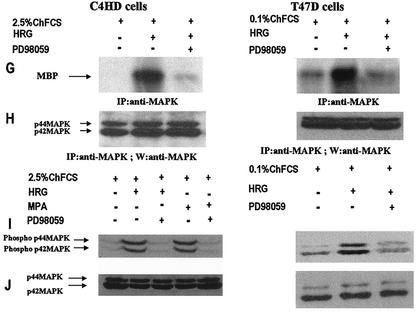
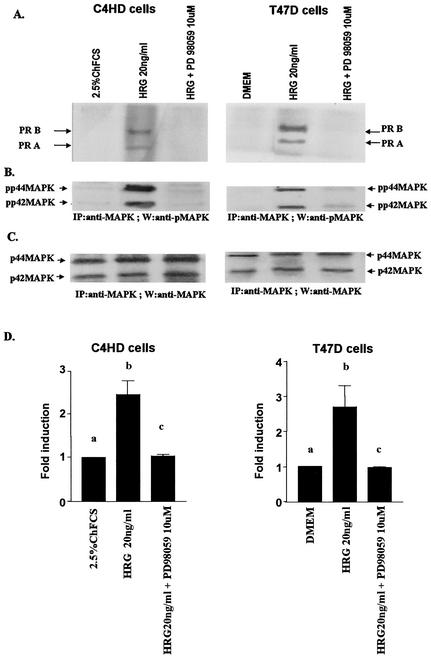
We investigated whether HRG was able to induce PR binding to a PRE, a prerequisite for PR-induced transcriptional activation. Nuclear extracts from C4HD treated with HRG for 2 h were incubated with a [32P]PRE oligonucleotide probe and analyzed for the formation of PR-DNA complexes by EMSA. The results shown in Fig. 4A demonstrate that HRG was able to promote PR binding to the PRE. The band-shifting pattern observed after HRG treatment was very similar to that seen after MPA treatment (Fig. 4A). The presence of specific PR-PRE complexes after HRG or MPA treatment was demonstrated by competition with excess unlabeled PRE oligonucleotide and by the lack of competition with a mutated PRE (Fig. 4A). The inclusion of an anti-PR (Fig. 4, right panel, antibody C-19) in the EMSA reaction supershifted the protein-DNA complex. An equivalent amount of preimmune rabbit serum, used as a control, had no effect (Fig. 4, right panel, NRS). These data clearly show the presence of PR in the DNA-protein complex. Comparable results were obtained in T47D cells in which HRG induced a specific binding of PR to the PRE (Fig. 4B). We next investigated molecular mechanisms involved in HRG action. Since we have found that ErbB-2 plays a key role in HRG-induced proliferation of C4HD cells (4, 5) (Fig. 1), we initiated the present study to assess the effect of the blockage of ErbB-2 expression on HRG-induced PR binding to a PRE. As can be seen in Fig. 5A, blockage of ErbB-2 expression by using ASODNs of ErbB-2 mRNA resulted in almost complete inhibition of HRG capacity to promote PR binding to DNA. As a model for studying ErbB-3 and ErbB-4 involvement in the mechanism of HRG induction of PR binding to a PRE, we chose C4HD cells. We did this, first of all, because they overexpress ErbB-3, which might result in enhanced sensitivity to signaling pathways involving ErbB-3 activation. Second, in present and previous studies we found that HRG treatment of C4HD cells induced tyrosine phosphorylation of ErbB-3 and ErbB-4 (4, 5) and that both receptors play a role in HRG proliferative effects (Fig. 1). Thus, HRG binding to either ErbB-3 or ErbB-4 in C4HD cells was abolished by using the respective monoclonal antibody. Whereas ErbB-3 antibody Ab-5, at 10 μg/ml, completely abolished HRG's capacity to induce PR binding to DNA, no effect was observed when HRG binding to ErbB-4 was disrupted by ErbB-4 antibody Ab-3 (Fig. 5A). We next investigated whether blockge of the MEK1-MAPK signaling pathway would inhibit HRG capacity to regulate PR binding to a PRE. As shown in Fig. 5A, treatment of C4HD cells with PD98059 resulted in complete abolishment of HRG induction of the formation of PR-PRE complexes. As a way to compare signal transduction pathways involved in ligand-dependent and -independent activation of PR, we next investigated whether blockage of the MEK1/MAPK signaling pathway affects MPA induction of PR binding to a PRE in C4HD cells. As shown in Fig. 5A, blockage of MAPK activation with PD98059 did not have any significant effect on progestin-induced PR binding to DNA. Comparable results were found in T47D cells (Fig. 5A). Figure 5B to J show the specific effect of ASODNs on ErbB-2 protein expression, the capacity of ErbB-3 and ErbB-4 monoclonal antibodies to abolish HRG-induced phosphorylation of these receptors, and the effect of PD98059 on MAPK activation. The effect of ErbB-2 ASODNs on ErbB-2 protein expression was determined by immunoblotting C4HD and T47D cell lysates (Fig. 5B). As we had previously found (4, 5), treatment of C4HD cells with 2 μM concentrations of ASODNs reduced ErbB-2 levels by 65 to 75%, whereas SODNs did not have any effect on ErbB-2 protein (Fig. 5B). Similarly, ErbB-2 ASODNs reduced ErbB-2 protein by 70 to 80%, whereas SODNs did not affect ErbB-2 expression in T47D cells (Fig. 5B). To confirm that inhibition of HRG binding to ErbB-3 or ErbB-4, by the use of the respective blocking antibody, resulted in the abrogation of HRG capacity to activate these receptors, we assessed their degree of tyrosine phosphorylation. Therefore, C4HD cells were preincubated with anti-ErB-3 (Ab-5) or ErbB-4 (Ab-3) blocking antibodies prior to HRG treatment. As seen in Fig. 5C, abolishment of HRG-ErbB-3 binding resulted in the abrogation of HRG-induced ErbB-3 phosphorylation. Similarly, blockage of HRG binding to ErbB-4 resulted in the inhibition of the HRG capacity to phosphorylate ErbB-4 (Fig. 5E). MAPK activity in C4HD and T47D cells pretreated with PD98059 was evaluated by immunoprecipitating cell lysates with an anti-p42/p44 MAPK antibody and by subjecting immunoprecipitates to an in vitro phosphorylation assay with MBP as the substrate. Activation of MAPK by HRG in both cell types was suppressed when cells were treated with 10 μM PD98059 (Fig. 5G). We also examined the effect of PD98059 on the activity of p42/p44 MAPK by using antisera specific for the dually phosphorylated, active form of this kinase. As shown in Fig. 5I, this experimental approach yielded the same results as the in vitro phosphorylation assay. Figure 5I also shows MPA-induced MAPK activation in C4HD cells and its abolishment by pretreatment of cells with PD98059.
FIG. 4.
HRG induces PR binding to a PRE. (A) In the left panel, C4HD cells were treated for 2 h at 37°C with MPA or HRG or were left untreated and growing in ChFCS. Aliquots of nuclear extracts from both cell types containing 4 fmol of PR were incubated with 1 ng of a 27-bp [32P]PRE oligonucleotide probe for 20 min at room temperature and then analyzed by EMSA. The specificity of the PR-PRE complexes is shown by competition with a 25- and 100-fold mass excess of unlabeled PRE oligonucleotide and by the lack of competition with a 100-fold mass excess of a mutant PRE (PRE mut 100 X). In the right panel, supershift analysis was performed by including either an anti-PR antibody (Ab-C19) or an equivalent amount of preimmune rabbit serum, as a control, in the EMSA reaction. (B) Aliquots of nuclear extracts from T47D cells containing 4 fmol of PR were analyzed by EMSA as described for panel A.
FIG. 5.
Blockage of ErbB-2 expression, ErbB-3 activation, or MAPK activity abolishes HRG capacity to induce PR binding to a PRE. (A) C4HD and T47D cells were treated for 2 h at 37°C with 10 nM MPA or HRG at 20 ng/ml or remained untreated and growing in ChFCS. ErbB-2 expression was blocked by pretreatment of both cell types for 48 h with 2 μM ASODN to ErbB-2 before the 2-h treatment with HRG. As a control, cells were preincubated with 2 μM SODN. MEK1/MAPK activity was abolished by preincubating the cells for 30 min with 10 μM PD98059 before treatment with HRG for 2 h. C4HD cells were also pretreated with 10 μg of ErbB-3 monoclonal antibody (Ab-5), ErbB-4 monoclonal antibody (Ab-3), or preimmune mouse serum (NMS)/ml before treatment with HRG for 2 h. Aliquots of nuclear extracts (4 fmol of PR) were incubated with a [32P]PRE oligonucleotide and subjected to an EMSA. (B) C4HD and T47D cells were incubated for 2 h with HRG at 20 ng/ml or preincubated for 48 h with 2 μM ErbB-2 ASODN or SODN and then treated for 2 h with HRG as described above. A total of 80 μg of protein from cell lysates was electrophoresed and immunoblotted for ErbB-2. Densitometric analysis of an ErbB-2 band expressed as percentages of the control value, i.e., cells growing in 20 ng of HRG/ml, yielded 32% for C4HD cells and 26% for T47D cells treated with 2 μM ASODN. No significant differences were found in the densitometric values of ErbB-2 bands between control cells and cells treated with 2 μM SODN. Autoradiograms from representative experiments of a total of three performed for each cell type are shown. (C to F) To evaluate the role of blocking antibodies on HRG-induced ErbB-3 and ErbB-4 tyrosine phosphoryation, C4HD cells were preincubated with 10 μg of either a ErbB-3 mouse monoclonal antibody (Ab-5) or a ErbB-4 mouse monoclonal antibody (Ab-3)/ml prior to HRG treatment for 10 min. As a control, cells were also incubated with preimmune normal mouse serum (NMS). ErbB-3 and ErbB-4 were immunoprecipitated as described in Materials and Methods, and immunocomplexes were subjected to SDS-PAGE (7.5% gel) and analyzed by Western blotting with an anti-P-Tyr monoclonal antibody (C and E). Identical aliquots of each immunoprecipitate were subjected to immunoblot analysis with anti-ErbB-3 (D) or ErbB-4 (F) antibodies to verify that nearly equal amounts of immunoprecipitated proteins were loaded. W, Western blot; IP, immunoprecipitation. (G) C4HD and T47D cells were incubated for 10 min with HRG at 20 ng/ml or were left untreated and growing in ChFCS. Blockage of MAPK activity was accomplished by preincubating the cells for 30 min with 10 μM PD98059 before stimulation with HRG. Cell lysates were immunoprecipitated by using an anti-p42/p44 MAPK antibody. The immunoprecipitates were assayed for MAPK activity with MBP as the substrate. (H) As loading control, identical aliquots of each immunoprecipitate assayed in panel G were subjected to immunoblot analysis with the anti-p42/p44 MAPK antibody. (I) C4HD and T47D cells were treated as described in panel G and, in addition, C4HD were treated with MPA for 10 min or were preincubated for 30 min with 10 μM PD98059 before stimulation with MPA. A total of 100 μg of protein from cell lysates was electrophoresed on 12% SDS-gels and immunoblotted with an anti-phospho p42/p44 MAPK antibody. (J) The membrane shown in panel I was stripped and hybridized with an antibody anti-total p42/p44 MAPK antibody.
HRG induces PR transcriptional activity.
To investigate HRG's capacity to transactivate PR, C4HD cells were transiently transfected with a PRE2-tk-CAT reporter plasmid and a β-galactosidase expression vector as an internal control. Treatment of C4HD cells with MPA induced activation of the PRE-CAT reporter construct that was completely inhibited by preincubation with RU486 (Fig. 6A). Blockage of MAPK activity with PD98059 did not inhibit MPA capacity to activate the PRE-CAT reporter (Fig. 6A). HRG was also able to promote strong activation of the PRE-CAT gene, which was abolished by treating C4HD cells with ASODNs to ErbB-2, by blocking HRG binding to ErbB-3 with the anti-ErbB-3 monoclonal antibody Ab-5, and by preincubating cells with PD98059 (Fig. 6A). Abolishment of HRG binding to ErbB-4 with the ErbB-4 antibody Ab-3 did not result in inhibition of HRG ability to activate the PRE reporter construct (Fig. 6A). These findings demonstrate that HRG-induced transactivation of PR requires both the presence of functional ErbB-2 and ErbB-3 and MAPK activation. HRG did not stimulate CAT transcription from the minimal thymidine kinase promoter lacking a PRE in C4HD cells (Fig. 6A). This indicates that HRG effects are mediated through the PRE. HRG also activated the PRE-CAT gene in T47D cells (Fig. 6B). This effect was abolished by treating T47D cells with ASODNs to ErbB-2 or with PD98059 (Fig. 6B). HRG-induced activation is mediated by the PR, as indicated by the finding that RU486 completely abolished HRG response in both cell types (Fig. 6). Since glucocorticoid receptor (GR) and androgen receptor (AR) bind to the same GRE/PRE sequences as PR, it is worth pointing out that C4HD cells express neither GR nor AR (48). T47D cells do not express GR (26) and, as previously reported (26), T47D maintained in our laboratory lack detectable AR.
FIG. 6.
HRG induces transcriptional activity of PR. C4HD (A) and T47D (B) cells were transiently transfected with 4 μg of a PRE2-tk-CAT reporter plasmid/well and with 4 μg of a CMV-β-Gal expression vector/well as an internal control. C4HD cells were also transfected with a tk-CAT reporter lacking a PRE. Cells were then treated with MPA, MPA-RU486, HRG, and HRG-RU486 at 37°C for 48 h for C4HD cells or for 24 h for T47D cells or were left untreated and growing in ChFCS. To study the effect of ErbB-2 expression blockage, 2 μM ErbB-2 ASODN or 2 μM SODN was added, along with HRG, during the 48-h treatment. To inhibit MAPK activation, cells were preincubated with 10 μM PD98059 before HRG treatment. C4HD cells were also pretreated with 10 μg of ErbB-3 monoclonal antibody (Ab-5) or ErbB-4 monoclonal antibody (Ab-3)/ml or preimmune mouse serum before treatment with HRG. Cells were harvested and lysed, and the CAT and β-galactosidase activities were measured as described in Materials and Methods. The results are presented as the percent activation, where 100% represents the maximal activity of PR in the presence of MPA. The data shown represent the means of six independent experiments ± the SEM. Significance for columns b versus a, columns c versus b, columns d versus a, and columns e versus d, P < 0.001.
MAPK induces PR phosphorylation.
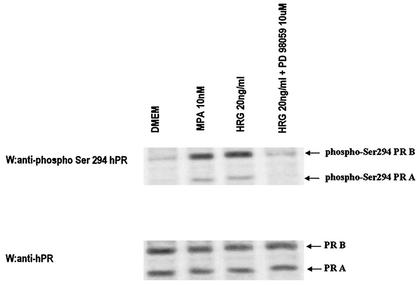
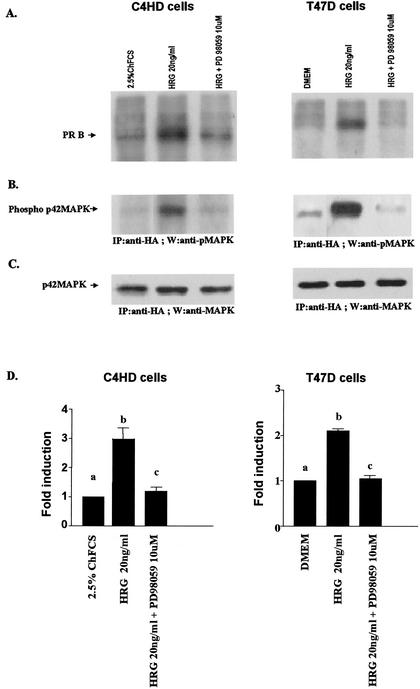
Although the functional role of PR phosphorylation remains elusive, increasing evidence indicates that phosphorylation plays a role in both ligand-dependent and -independent regulation of PR transcriptional activity (3, 7, 14, 22, 40, 66). The majority of the phosphorylation sites in PR contain a Ser/Thr-Pro motif, which is a core consensus sequence for proline-directed kinases such as MAPK. In the present study, we found that blockage of MAPK activation results in inhibition of HRG capacity to induce PR binding to a PRE, as well as CAT activity (Fig. 5 and 6). Therefore, we hypothesize that MAPK activated by HRG could mediate PR phosphorylation. The MAPK consensus site Ser294 in hPR is rapidly phosphorylated in response to progestins (75). Recent work has demonstrated that MAPK appears to be involved in phosphorylation of the Ser294 residue, which is an absolute requirement for ligand-induced PR downregulation (40). In addition, EGF, a well-characterized activator of MAPK, induced strong phosphorylation of Ser294 after 5 min and after 1 h treatment of T47D cells expressing the B isoform of PR (64). Therefore, we investigated here the effect of HRG on the phosphorylation of Ser294 in T47D cells. As shown in Fig. 7, HRG was able to induce a robust phosphorylation of Ser294 in PRA and PRB after 10 min treatment. MPA exerted the same rapid induction of PR phosphorylation on Ser294 as HRG (Fig. 7). As previously reported (14), the anti-Ser294 antibody exhibited strong preferential reactivity for PRB. Blockage of MAPK activity by preincubation with PD98059 resulted in the complete abolishment of HRG capacity to phosphorylate Ser294, indicating that MAPKs are involved in HRG-induced phosphorylation of this residue. We then investigated whether MAPK could induce PR phosphorylation in vitro. For this purpose, we immunoprecipitated p42 and p44 MAPK from protein extracts of C4HD cells treated or untreated with HRG for 10 min and from C4HD cells in which MAPK activation by HRG had been blocked by preincubation with PD98059. We also immunoprecipitated PR from C4HD cells growing in 2.5% ChFCS and used it as a source of PR in the in vitro phosphorylation assay. As shown in Fig. 6, PR obtained from cells not stimulated with MPA or HRG is transcriptionally inactive. We then incubated MAPK, immunoprecipitated from C4HD cells treated as described above, with the transcriptionally inactive PR, and performed an in vitro phosphorylation assay in presence of [γ-32P]ATP. At the end of incubation, proteins were subjected to SDS-PAGE, and the upper part of the gel containing PR was dried and revealed by autoradiography. As shown in Fig. 8A (lane 1) and D, nonactivated MAPK obtained from C4HD cells growing in 2.5% serum did not induce PR phosphorylation. However, MAPKs activated by HRG treatment of C4HD cells were able to induce a strong in vitro phosphorylation of both PRA and PRB isoforms (Fig. 8A, lane 2, and the histogram of panel D). Further evidence of MAPK direct involvement in PR phosphorylation is provided by the finding that when MAPKs were immnunoprecipitated from C4HD cells in which their HRG-induced activation had been abolished by PD98059, no phosphorylation of PR was observed (Fig. 8A, lane 3, and the histogram of panel D). The lower part of the gel in which MAPKs were resolved was transferred onto nitrocellulose and, to control MAPK activity, we performed Western blot with an antisera specific for the dually phosphorylated, active form of this kinase. Figure 8B shows the strong degree of activation of MAPK immunoprecipitated from HRG-treated C4HD cells (lane 2). This filter was stripped and reprobed with an anti-total p42/p44 MAPK antibody to show that an almost equal amount of total MAPK was present in immunoprecipitates incubated with the PR (Fig. 8C). We then performed the same experimental approach in T47D cells. MAPKs activated by HRG treatment were also able to induce in vitro phosphorylation of PRA and PRB isoforms in T47D cells (Fig. 8A, lane 2, and the histogram of panel D). Therefore, our results indicate that both hPR and mouse PR can be phosphorylated in vitro by HRG-activated MAPK. As further confirmation of these findings, we performed another experimental strategy. On one hand, we transiently transfected C4HD cells with an HA-tagged p42 MAPK expression vector, and 48 h after transfection cells were treated with HRG, were left untreated, or were subjected to preincubation with PD98059 before HRG stimulation. p42 MAPK from each cell treatment was then immunoprecipitated with an anti-HA antibody. On the other hand, we transfected C4HD cells with a Flag epitope-tagged PRB expression vector and then immunoprecipitated PRB from C4HD cells growing in 2.5% ChFCS with an anti-Flag antibody. We then performed the in vitro phosphorylation assay described above with the immunoprecipitated p42 MAPK and PRB. As shown in Fig. 9A (lane 2) and D, HRG-activated p42 MAPK induced strong phosphorylation of PRB. The same results were obtained when transfections were performed in T47D cells (Fig. 9A and D).
FIG. 7.
HRG and MPA treatment of T47D cells induced hPR Ser294 phosphorylation. T47D cells were incubated for 10 min with 10 nM MPA or HRG at 20 ng/ml, preincubated for 30 min with 10 μM PD98059 prior to HRG treatment, or were left untreated and growing in serum-free medium. A total of 100 μg of from cell lysates was immunoblotted with an anti-phospho Ser294 hPR antibody (upper panel). The membrane was stripped and hybridized with an anti-hPR antibody (bottom panel).
FIG. 8.
MAPKs induce PR phosphorylation in vitro. (A) C4HD and T47D cells were treated with HRG for 10 min, pretreated with PD98059 for 30 min prior to HRG treatment, or remained untreated. Cells were lysed and MAPKs were immunoprecipitated from 4-mg protein extracts from each cell treatment with an anti-p42/p44 MAPK antibody. On the other hand, transcriptionally inactive PR was immunoprecipitated from 4 mg of protein from cells growing in ChFCS (C4HD) or in serum-free DMEM (T47D) with an anti-PR antibody. The immunoprecipitated PR was then subjected to an in vitro phosphorylation assay with MAPKs immunoprecipitated from cells subjected to each of the treatments described above. Kinase reactions were separated by SDS-PAGE. The upper part of the gels, containing the PR, was dried, and phosphorylation of PR was visualized by autoradiography. A representative experiment of a total of three performed for each cell type is shown. (B) The lower part of the gel was transferred onto nitrocellulose, and an immunoblot was performed with an anti-phospho MAPK antibody. (C) The filter was stripped and hybridized with anti-total p42/p44 MAPK antibody to show that almost equal amounts of total MAPKs were present in immunoprecipitates incubated with the PR. (D) The levels of PR bands phosphorylation (see panel A) were quantitated by densitometry and normalized to the amount of the immunoprecipitated MAPKs (see panel C). In the histogram is shown the HRG-induced increase in PR phosphorylation. The data shown represent the means of three independent experiments ± the SEM. Significance for columns b versus a and columns c versus b, P < 0.001.
FIG. 9.
Transfected p42 MAPK can phosphorylate PR in vitro. (A) C4HD and T47D cells were transiently transfected with an HA-tagged p42 MAPK expression vector and, 48 h later, cells were treated with HRG for 10 min, pretreated with PD98059 for 30 min prior to HRG treatment, or were left untreated. Cells were lysed, and p42 MAPK was immunoprecipitated from 4-mg protein extracts from each cell treatment. As a source of transcriptionally inactive PR, C4HD and T47D cells were transfected with a Flag-tagged hPRB expression vector and, 48 h later, PR was immunoprecipitated from 4 mg of protein from lysates of cells growing in ChFCS (C4HD) or in serum-free DMEM (T47D) by using an anti-Flag antibody. The immunoprecipitated PR was then subjected to an in vitro phosphorylation assay with p42 MAPK immunoprecipitated from cells subjected to each of the treatments described above. Kinase reactions were separated by SDS-PAGE. The upper part of the gels, containing the PR, was dried, and phosphorylation of the PR was visualized by autoradiography. A representative experiment of a total of three performed for each cell type is shown. (B) The lower part of the gel was transferred onto nitrocellulose, and an immunoblot was performed with an anti-phospho MAPK antibody. (C) The filter was stripped and hybridized with an antibody anti-total p42/p44 MAPK. (D) The levels of PRB band phosphorylation (see panel A) were quantitated by densitometry and normalized to the amount of immunoprecipitated MAPKs (see panel C). In the histogram is shown the HRG-induced increase in PRB phosphorylation. The data represent the means of thee independent experiments ± the SEM. Significance for columns b versus a and columns c versus b, P < 0.001.
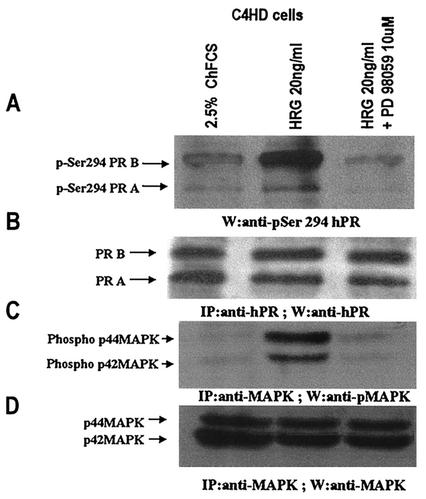
To directly assess whether MAPK could induce phosphorylation of Ser294, we immunoprecipitated MAPK from extracts of C4HD cells treated or untreated with HRG for 10 min and from C4HD cells preincubated with PD98059 before HRG addition. Immunoprecipitated MAPK were then incubated with transcriptionally inactive PR obtained, as described in Fig. 8, from protein extracts of C4HD cells growing in 2.5% ChFCS. An in vitro phosphorylation assay, in the absence of [γ-32P]ATP, in which the reaction contained 100 μM cold ATP instead, was also performed. At the end of incubation, proteins were subjected to SDS-PAGE. Gels were transferred onto nitrocellulose and in the upper part of the gels immunoblots were performed with the anti-phospho Ser294 PR antibody. The lower part of the gel was revealed with an antiserum specific for phosphorylated MAPK. As shown in Fig. 10A (lane 2), MAPK immunoprecipitated from HRG-treated C4HD cells induced phosphorylation of PR Ser294 residue. Neither inactive MAPK, obtained from C4HD cells growing in 2.5% serum, nor MAPK immunoprecipitated from C4HD cells in which their HRG-induced activation had been abolished by PD98059 induced phosphorylation of Ser294 (Fig. 10A, lanes 1 and 3, respectively).
FIG. 10.
MAPKs induce PR Ser294 phosphorylation in vitro. C4HD cells were treated with HRG for 10 min, pretreated with PD98059 for 30 min prior to HRG treatment, or remained untreated. Cells were lysed, and MAPKs were immunoprecipitated from 4-mg protein extracts from each cell treatment by using an anti-p42/p44 MAPK antibody. In contrast, transcriptionally inactive PR was immunoprecipitated from 4 mg of protein from C4HD cells growing in ChFCS by using an anti-PR antibody. The immunoprecipitated PR was then subjected to a cold in vitro phosphorylation assay as described in Materials and Methods. (A) Proteins were separated by electrophoresis, gels were transferred onto nitrocellulose, and in the upper part of the gels immunoblots were performed with anti-phospho Ser294 PR antibody. (B) The filter was stripped and reprobed with anti-PR antibody. (C) The lower part of the gel was revealed with anti-phospho MAPK antibody. (D) The filter was stripped and hybridized with anti-total p42/p44 MAPK antibody.
DISCUSSION
We have found a novel mechanism of PR activation by HRG, a ligand of the type I RTKs. HRG was able to exquisitely regulate biochemical attributes of PR in a way that mimicked PR activation by progestins, cognate PR ligands. Assessment of the molecular mechanisms underlying PR transactivation by HRG highlighted a key role for a functional ErbB-2 and MAPK activation. One of the most salient aspects of the present study lies in the fact that we demonstrated that HRG-activated MAPKs can phosphorylate both hPR and mouse PR in vitro.
HRG induces PR transcriptional activity.
It has long been acknowledged that progestin treatment of PR results in a profound loss of absolute receptor protein mass (14, 49, 57, 70, 71). In the present study, we found that HRG was able to mimic progestin effect on PR protein expression. Thus, HRG downregulated PR protein levels both in mouse mammary C4HD tumor cells and in human breast cancer T47D cells. As reported with progestin treatment of human breast cancer cells (14), HRG induced a similar degree of downregulation of PRA and PRB in the two cell types. Ligand-independent activation of PR by modulators of kinases and phosphatases has been found in the course of studies of molecular mechanisms involved in PR activation. Thus, okadaic acid, an inhibitor of protein phosphateses 1 and 2, and 8-bromo-cyclic AMP (cAMP), an activator of cAMP-dependent protein kinase A, have been shown to potently stimulate chicken PR (cPR)-mediated transcription in the absence of progesterone (19, 77). In accordance with the present findings, cPR abundance was decreased by 8-bromo-cAMP treatment (19). Interestingly, vanadate, an inhibitor of phosphotyrosine phosphatases, induced cPR-mediated transcription in the absence of progestins (77). This finding is in line with those we have shown here in which signals originating in pathways requiring tyrosine phosphorylation, such as RTK activation, are able to induce PR transactivation. hPR appears to be less susceptible than cPR to ligand-independent activation by modulators of protein phosphorylation. Thus, treatment of T47D cells with 8-bromo-cAMP or okadaic acid alone had no measurable effect on PR expression at the protein level or in PR number and did not stimulate hPR transcriptional activity (7). However, both compounds augmented hPR-mediated target gene transcription when added together with progestins (7, 22). Additionally, accumulated evidence has clearly shown convergence between steroid hormones and RTK signaling pathways. The bidirectional nature of this interaction is to be noted, in which steroid hormones activate RTKs or their downstream signaling pathways (4, 5, 9, 39, 45, 58) and, conversely, where RTK ligands are able to modulate steroid receptor transcriptional activity (32, 33, 51, 60, 64). Thus, in accordance with our present findings, another type I RTK ligand, EGF, was found to decrease progestins binding after 2 h of treatment of T47D, MCF-7, and ZR75-1 breast cancer cells (62) and to induce transactivation of cPRA (74). In contrast, IGF-I, a ligand of the type II RTKs, caused PR upregulation in uterine cells and in MCF-7 cells (1, 12).
We found in this work that HRG treatment of C4HD and T47D cells for 2 h induced an upward shift of PRA and PRB isoforms on SDS-gels similar to the one observed with MPA. Progestin-induced changes in the electrophoretic mobility of PR have long been associated with an increase in PR phosphorylation levels (3, 7, 14, 22, 67, 72, 75). Particularly, in hPR multiple serine residues are phosphorylated in a complex and orderly process that takes place in at least three distinct steps: first, basal phosphorylation in the absence of hormone; next, a rapid net increase in phosphorylation that occurs within a few minutes of progestin addition, and (iii) finally, a slower phosphorylation that promotes an upshift of PR on SDS-gels (7, 22, 36, 67, 75). This later stage of PR phosphorylation, associated with the PR upshift, occurs after PR binding to DNA, suggesting that it may represent a phosphorylation step involved in the regulation of transcriptional activity (7, 22, 67). Changes in the electrophoretic mobility of PR induced by transactivators have not been reported. For example, two agents that potentiate the activation of hPR by R5020, such as 8-bromo-cAMP and okadaic acid, did not affect the change in mobility of PR on SDS-gels induced by R5020 (22). Therefore, HRG-induced upshift of PR on SDS-gels was the first of our findings leading us to speculate that phosphorylation could be involved in the mechanism of PR transactivation by HRG.
In most target cells and tissues, in the absence of hormone, PR distributes between the cytoplasm and the nucleus, whereas hormone treatment stimulates the major amount of cellular PR to bind tightly to the nucleus (14, 22, 24, 46). We found that HRG treatment of C4HD and T47D cells resulted in a significant increase in PR nuclear localization. The neurotransmitter dopamine, which induces ligand-independent activation of cPR (56), was also found to induce a shift in receptor localization to the nucleus identical to the one caused by progesterone (56).
It is also well acknowledged that progestins induce PR binding to specific PREs, a prerequisite for PR-induced transcriptional activation. We found that in both cell types, HRG was able to promote PR binding to a PRE, with a band shifting pattern very similar to that seen after MPA treatment. Regarding hPR, Beck et al. (7) reported that the capacity of 8-bromo-cAMP and of okadaic acid to augment progestin-induced transcriptional activity was not the result of these agents enhancing PR-DNA binding activity, since they were not able either to stimulate PR binding to a PRE in the absence of hormone or to enhance R5020-induced PR-DNA binding activity.
Here we found that HRG treatment of C4HD and T47D cells transiently transfected with a PRE2-tk-CAT reporter plasmid promoted strong activation of CAT. HRG-induced PR transactivation is mediated by PR, since the response to HRG is completely abolished by the antiprogestin RU486. In addition, the activation of PR by HRG cannot be due to a hypothetical HRG-induced synthesis of progesterone, since neither C4HD nor T47D cells expressed progesterone. As happened with HRG stimulation of PR binding to a PRE, HRG-induced PR transactivation was abolished by treatment with ASODNs to ErbB-2, by blockage of HRG binding to and activation of ErbB-3, and by preincubation of cells with PD98059, a specific MEK1/MAPK inhibitor. These latter findings demonstrate that HRG-induced transactivation of PR requires both functional ErbB-2 and ErbB-3, as well as MAPK activation. ErbB-2 involvement in HRG activity was an expected result, since we had previously found that blockage of ErbB-2 expression with ASODNs completely abolished the HRG proliferative effect in C4HD cells (4, 5). In addition, several studies have established that ErbB-2 is a critical component in mediating HRG-induced breast cancer cell growth (8, 27, 41). We demonstrated here that both ErbB-3 and ErbB-4 play a role in HRG proliferative effects. However, the functional heterodimer in the molecular mechanism of HRG-induced activation of PR appears to be ErbB-2/ErbB-3, since abolishment of HRG binding to ErbB-4 did not inhibit HRG effects on PR. The fact that MAPKs are involved in the transactivation of PR by HRG adds further evidence to previous findings demonstrating, first, that phosphorylation plays a role in both ligand-dependent and ligand-independent activation of PR and, second, that MAPK could be involved in PR phosphorylation. Multiple lines of evidence suggest that MAPK-induced phosphorylation plays a role in PR function. Thus, Lange et al. (40) demonstrated that progestin-induced PR downregulation depended on MAPK activation. They also found that mutation of Ser294, an MAPK consensus site in hPR, completely prevented ligand-dependent receptor downregulation, suggesting that the requirement for MAPK resulted from a direct effect on PR phosphorylation. We found here that MAPK activation appears not to be an absolute requirement for ligand (i.e., MPA)-induced activation of PR, suggesting that direct phosphorylation of PR plays a different role in the molecular mechanism involved in ligand-dependent and -independent receptor activation. Recently, Shen et al. (64) mimicked the activation of molecules downstream of GF-initiated signaling pathways by overexpressing MAPK kinase kinase 1 (MEKK1). MEKK1 is an activator of MAPKs. Unlike our results showing that HRG, a strong activator of MAPKs in C4HD and T-47D cells, is able to induce PR activation, MEKK1 expression did not result in ligand-independent activation of hPRB but increased progestin-mediated transcription. Still another difference with our findings is that PR protein levels remained unchanged by MEKK1 overexpression. We demonstrated here that HRG induced rapid phosphorylation of the PR Ser294 residue in T47D cells. Blockage of MAPK activity with PD98059 resulted in the abolishment of HRG capacity to phosphorylate Ser294, clearly indicating MAPK involvement in this process. Similarly, Shen et al. (64) reported that MEKK1 overexpression resulted in the phosphorylation of Ser294 in the hPRB; MEK1-MEK2 inhibitors blocked phosphorylation of Ser294 and attenuated PR transcriptional hyperactivity in response to MEKK1 plus R5020. Furthermore, these authors showed that EGF, another well-characterized MAPK activator, induced rapid phosphorylation of Ser294 in hPR. In contrast to our present findings indicating that MPA was in fact able to exert the same rapid induction of Ser294 phosphorylation as HRG, no phosphorylation of hPRB Ser294 was detected by Shen et al. (64) after 10 min of R5020 treatment. Our findings showing that blockage of MAPK activity results in the abolishment of HRG's capacity to phosphorylate the Ser294 residue and that HRG-activated MAPKs are able to phosphorylate Ser294 in vitro suggest that Ser294 plays a role in the mechanism of HRG-induced, ligand-independent activation of PR.
Even though ours is the first report assessing HRG effects on PR activity, Pietras et al. (51) have demonstrated that HRG is able to transactivate ER. In accordance with our present findings, these researchers found that HRG regulates several biochemical properties of ER that are affected in the physiological activation of ER by estrogens. The striking similarity between these results and ours reveals that HRG's regulatory effect on ER activity also holds true for PR. Since the molecular mechanisms of ER activation were not determined by Pietras et al. (51), it remains to be elucidated whether HRG works through common signal transduction pathways to induce ER and PR transactivation. In another study (60), treatment of breast cancer cells with HRG also resulted in a profound decrease of ER protein. However, HRG was found to inhibit ER transcriptional activation (60).
We found here that the progestin antagonist RU486 significantly inhibited HRG-induced proliferation of C4HD and T47D cells. This evidence of a role for PR in mediating the physiologic actions of HRG in breast cancer cells unravels the other scenario of the coupling of GF and steroid receptor signaling pathways, indicating the necessity for steroid receptors in the mitogenic actions of GFs. This kind of cross talk has been demonstrated for EGF and the ER. Thus, EGF has been found to mimic the effect of estrogen in the mouse reproductive tract in terms of increased DNA synthesis, as well as of increased phosphorylation and nuclear retention of the ER (31). These effects were blocked by the ER antagonist ICI 160,384 (32). In addition, ER knockout mice were found to lack an estrogen-like response to EGF (18).
MAPK phosphorylates hPR and mouse PR in vitro.
We have provided here the first demonstration that HRG-activated MAPKs can phosphorylate both hPR and mouse PR in vitro. To our knowledge, there is only one other report demonstrating that Xenopus PR can be phosphorylated in vitro by recombinant active p42 MAPK (2). Therefore, those findings with Xenopus PR, together with our findings with mammalian PR, provide the first experimental evidence to the long-sustained hypothesis that PR is a substrate for active MAPKs in vitro. MAPKs activation by GFs has long been acknowledged. We therefore propose a mechanism in which rapid activation of MAPK by GFs could be involved in phosphorylation of PR in vivo. Further studies are required to elucidate whether this MAPK-induced PR phosphorylation takes place in the nucleus and whether it is required for full transcriptional activity of the receptor. Phosphorylation of another member of the nuclear receptor superfamily, ER, by MAPKs was described long ago by Chambon and coworkers (33). These authors demonstrated that recombinant hER was phosphorylated in vitro by activated MAPK purified from Xenopus oocytes (33). In addition, several serine/threonine kinases have been found to phosphorylate purified cPR or hPR in vitro. Among them, cAMP-dependent protein kinase A phosphorylates cPR in vitro on Ser528 (19). Interestingly, this is not one of the identified basal or hormone-dependent phosphorylation sites in the cPR. Cdk2 phosphorylates hPR in vitro at a total of eight sites, five of which have been confirmed in vivo (Ser162, Ser190, Ser213, Ser400, and Ser676) (36, 76), and casein kinase II phosphorylates hPR in vitro on the authentic Ser81 site (74).
In summary, our findings provide further support to the hypothesis that, in breast cancers expressing steroid hormone receptors but resistant to endocrine therapies, control over tumor growth is assumed by GFs that are able to transactivate steroid receptors. Phosphorylation of steroid receptors by GF-activated MAPK appears to play a key role in the molecular mechanism of steroid hormone receptor transactivation.
Acknowledgments
This work was supported by grants from the National Scientific Council of Argentina (CONICET; PID 4188/96), from the National Agency of Scientific Promotion of Argentina (IDB 802/OC-AR PICT 0503402), from the Centro Argentino Brasilero de Biotecnología, and from The Ramón Carrillo-Orturo Oñativia Fellowship from the Public Health Ministry of Argentina awarded to P. V. Elizalde.
We thank C. Lanari for providing the MPA-induced mammary tumor model.
REFERENCES
- 1.Aronica, S. M., and B. S. Katzenellenbogen. 1991. Progesterone receptor regulation in uterine cells: stimulation by estrogen, cyclic adenosine 3′,5′-monophosphate, and insulin-like growth factor I and suppression by antiestrogens and protein kinase inhibitors. Endocrinology 128:2045-2052. [DOI] [PubMed] [Google Scholar]
- 2.Bagowski, C. P., J. W. Myers, and J. E. Ferrell, Jr. 2001. The classical progesterone receptor associates with p42MAPK and is involved in phosphatidylinositol 3-kinase signaling in Xenopus oocytes. J. Biol. Chem. 276:37708-37714. [DOI] [PubMed] [Google Scholar]
- 3.Bai, W., B. G. Rowan, V. E. Allgood, B. W. O'Malley, and N. L. Weigel. 1997. Differential phosphorylation of chicken progesterone receptor in hormone-dependent and ligand-independent activation. J. Biol. Chem. 272:10457-10463. [DOI] [PubMed] [Google Scholar]
- 4.Balañá, M. E., L. Labriola, M. Salatino, F. Movsichoff, G. Peters, E. H. Charreau, and P. V. Elizalde. 2001. Activation of ErbB-2 via a hierarchical interaction between ErbB-2 and type I insulin-like growth factor receptor in mammary tumor cells. Oncogene 20:34-47. [DOI] [PubMed] [Google Scholar]
- 5.Balañá, M. E., R. Lupu, L. Labriola, E. H. Charreau, and P. V. Elizalde. 1999. Interactions between progestins and heregulin (HRG) signaling pathways: HRG acts as mediator of progestins proliferative effects in mouse mammary adenocarcinomas. Oncogene 18:6370-6379. [DOI] [PubMed] [Google Scholar]
- 6.Baserga, R. 1995. The insulin-like growth factor I receptor: a key to tumor growth. Cancer Res. 55:249-252. [PubMed] [Google Scholar]
- 7.Beck, C. A., N. L. Weigel, and D. P. Edwards. 1992. Effect of hormone and cellular modulators of protein phosphorylation on transcriptional activity, DNA binding, and phosphorylation of human progesterone receptors. Mol. Endocrinol. 6:607-620. [DOI] [PubMed] [Google Scholar]
- 8.Beerli, R. R., D. Graus-Porta, K. Woods-Cook, X. Chen, Y. Yarden, and N. E. Hynes. 1995. Neu differentiation factor activation of ErbB-3 and ErbB-4 is cell specific and displays a differential requirement for ErbB-2. Mol. Cell. Biol. 15:6496-6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boonjarantanakornkit, V., M. Porter Scott, V. Ribon, L. Sherman, S. M. Anderson, J. L. Maller, W. T. Miller, and D. P. Edwards. 2001. Progesterone receptor contains a proline-rich motif that directly interacts with SH3 domains and activates c-SRC family tyrosine kinases. Mol. Cell 8:269-280. [DOI] [PubMed] [Google Scholar]
- 10.Braunsberg, H., N. G. Coldham, R. E. Learke, S. K. Cowan, and W. Wong. 1987. Actions of progestogen on human breast cancer cells: mechanisms of growth stimulation and inhibition. Eur. J. Cancer Clin. Oncol. 23:563-571. [DOI] [PubMed] [Google Scholar]
- 11.Bunone, G., P. A. Briand, R. J. Miksicek, and D. Picard. 1996. Activation of the unliganded estrogen receptor by EGF involves the MAP kinase pathway and direct phosphorylation. EMBO J. 15:2174-2183. [PMC free article] [PubMed] [Google Scholar]
- 12.Cho, H., S. M. Aronica, and B. S. Katzenellenbogen. 1994. Regulation of progesterone receptor gene expression in MCF-7 breast cancer cells: a comparison of the effects of cyclic adenosine 3′,5′-monophosphate, estradiol, insulin growth factor I, and serum factors. Endocrinology 134:658-664. [DOI] [PubMed] [Google Scholar]
- 13.Clarke, C. L., and R. L. Sutherland. 1990. Progestin regulation of cellular proliferation. Endocrinol. Rev. 11:266-302. [DOI] [PubMed] [Google Scholar]
- 14.Clemm, D. L., L. Sherman, V. Boonyaratanakornkit, W. T. Schrader, N. L. Weigel, and D. P. Edwards. 2000. Differential hormone-dependent phosphorylation of progesterone receptor A and B forms revealed by a phosphoserine site-specific monoclonal antibody. Mol. Endocrinol. 14:52-65. [DOI] [PubMed] [Google Scholar]
- 15.Cohen, G. B., R. Ren, and D. Baltimore. 1995. Modular binding domains in signal transduction proteins. Cell 80:237-248. [DOI] [PubMed] [Google Scholar]
- 16.Colomer, R., R. Lupu, S. S. Bacus, and E. P. Gelmann. 1994. ErbB-2 antisense oligonucleotides inhibit the proliferation of breast carcinoma cells with erbB-2 oncogene amplification. Br. J. Cancer 70:819-825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coso, O. A., S. Montaner, C. Fromm, J. C. Lacal, R. Prywes, H. Teramoto, and J. S. Gutkind. 1997. Signaling from G protein-coupled receptors to the c-Jun promoter involves the MEF2 transcription factor. J. Biol. Chem. 272:20691-20697. [DOI] [PubMed] [Google Scholar]
- 18.Curtis, S. W., T. Washburn, C. Sewall, R. Di Augustine, J. Lindzey, J. F. Couse, and K. S. Korach. 1996. Physiological coupling of growth factor and steroid receptor signaling pathways: estrogen receptor knockout mice lack estrogen-like response to epidermal growth factor. Proc. Natl. Acad. Sci. USA 93:12626-12630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Denner, L. A., N. L., Weigel, B. L. Maxwell, W. T. Schrader, and B. W. O'Malley. 1990. Regulation of progesterone receptor-mediated transcription by phosphorylation. Science 250:1740-1743. [DOI] [PubMed] [Google Scholar]
- 20.Denner, L. A., N. L. Weigel, W. T. Schrader, and B. W. O'Malley. 1989. Hormone-dependent regulation of chicken progesterone receptor deoxyribonucleic acid binding and phosphorylation. Endocrinology 125:3051-3058. [DOI] [PubMed] [Google Scholar]
- 21.Dran, G., I. A. Luthy, A. A. Molinolo, F. Montecchia, E. H. Charreau, C. D. Pasqualini, and C. Lanari. 1995. Effect of medroxyprogesterone acetate (MPA) and serum factors on cell proliferation in primary cultures of an MPA-induced mammary adenocarcinoma. Breast Cancer Res. Treatment 35:173-186. [DOI] [PubMed] [Google Scholar]
- 22.Edwards, D. P., N. L. Weigel, S. K. Nordeen, and C. A. Beck. 1993. Modulators of cellular protein phosphorylation alter the trans-activation function of human progesterone receptor and the biological activity of progesterone antagonists. Breast Cancer Res. Treatment 27:41-56. [DOI] [PubMed] [Google Scholar]
- 23.Egan, S. E., and R. A. Weinberg. 1993. The pathway to signal achievement. Nature 365:781-783. [DOI] [PubMed] [Google Scholar]
- 24.El-Ashry, D., S. A. Oñate, S. K. Nordeen, and D. P. Edwards. 1989. Human progesterone receptor complexed with the antagonist RU486 binds to hormone response elements in a structurally altered form. Mol. Endocrinol. 3:1545-1558. [DOI] [PubMed] [Google Scholar]
- 25.Fiddes, R. J., P. W. Janes, S. P. Silversten, R. L. Sutherland, E. A. Musgrove, and R. J. Daly. 1998. Inhibition of the MAP kinase cascade blocks heregulin-induced cell cycle progression in T-47D human breast cancer cells. Oncogene 16:2803-2813. [DOI] [PubMed] [Google Scholar]
- 26.Gass, E. K., S. A. Leonhardt, S. K. Nordeen, and D. P. Edwards. 1998. The antagonists RU486 and ZK98299 stimulate progesterone receptor binding to deoxyribonucleic acid in vitro and in vivo but have distinct effects on receptor conformation. Endocrinology 139:1905-1919. [DOI] [PubMed] [Google Scholar]
- 27.Graus-Porta, D., R. R. Beerli, and N. E. Hynes. 1995. Single-chain antibody-mediated intracellular retention of ErbB-2 impairs Neu differentiation factor and epidermal growth factor signaling. Mol. Cell. Biol. 15:1182-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Graus-Porta, D., R. R. Beerli, J. M. Daly, and N. E. Hynes. 1997. ErbB-2, the preferred heterodimerization partner of all ErbB receptors, is a mediator of lateral signaling. EMBO J. 16:1647-1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Groshong, S. D., G. L. Owen, B. Grimison, I. E. Schauer, M. C. Todd, T. A. Langan, R. A. Sclafani, C. A. Lange, and K. B. Horwitz. 1997. Biphasic regulation of breast cancer cell growth by progesterone: role of the cyclin-dependent kinase inhibitor, p21 and p27 (kip1). Mol. Endocrinol. 11:1593-1607. [DOI] [PubMed] [Google Scholar]
- 30.Horwitz, K. B., and P. S. Alexander. 1983. In situ photolinked nuclear progesterone receptors of human breast cancer cells: subunit molecular weights after transformation and translocation. Endocrinology 113:2195-2201. [DOI] [PubMed] [Google Scholar]
- 31.Ignar-Trowbridge, D. M., C. T. Teng, K. A. Ross, M. G. Parker, K. S. Korach, and J. A. McLachlan. 1993. Peptide growth factors elicit estrogen-dependent transcriptional activation of an estrogen-responsive element. Mol. Endocrinol. 7:992-998. [DOI] [PubMed] [Google Scholar]
- 32.Ignar-Trowbridge, D. M., K. G. Nelson, M. C. Bidwell, S. W. Curtis, T. F. Washburn, J. A. McLachlan, and K. S. Korach. 1992. Coupling of dual signaling pathways: epidermal growth factor action involves the estrogen receptor. Proc. Natl. Acad. Sci. USA 89:4658-4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kato, S., H. Endoh, Y. Masuhiro, T. Kitamoto, S. Uchiyama, H. Sasaki, S. Masushige, Y. Gotoh, E. Nishida, H. Kawashima, D. Metzger, and P. Chambon. 1995. Activation of the estrogen receptor through phosphorylation by mitogen-activated protein kinase. Science 270:1491-1494. [DOI] [PubMed] [Google Scholar]
- 34.Kavanaugh, W. M., and L. T. Williams. 1994. An alternative to SH2 domains for binding tyrosine phosphorylated proteins. Science 266:1862-1865. [DOI] [PubMed] [Google Scholar]
- 35.Kiss, R., R. J. Paridaens, J. C. Heuson, and A. J. Danguy. 1986. Effect of progesterone on cell proliferation on the MXT mouse hormone-sensitive mammary neoplasm. J. Natl. Cancer Inst. 77:173-178. [PubMed] [Google Scholar]
- 36.Knotts, T. A., R. O. Orkiszewski, R. G. Cook, D. P. Edwards, and N. L. Weigel. 2001. Identification of a phosphorylation site in the hinge region of the human progesterone receptor and additional amino-terminal phosphorylation sites. J. Biol. Chem. 276:8475-8483. [DOI] [PubMed] [Google Scholar]
- 37.Kraus, M. H., W. Issing, T. Miki, N. C. Popescu, and S. A. Aaronson. 1989. Isolation and characterization of ERBB3, a third member of the ERBB/epidermal growth factor receptor family: evidence for overexpression in a subset of human mammary tumors. Proc. Natl. Acad. Sci. USA 86:9193-9197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lanari, C., E. Kordon, A. A. Molinolo, C. D. Pasqualini, and E. H. Charreau. 1989. Mammary adenocarcinomas induced by medroxyprogesterone acetate: hormone dependence and EGF receptors of BALB/c in vivo sublines. Int. J. Cancer 43:845-850. [DOI] [PubMed] [Google Scholar]
- 39.Lange, C. A., J. K. Richer, T. Shen, and K. B. Horwitz. 1998. Convergence of progesterone and epidermal growth factor signaling in breast cancer. J. Biol. Chem. 273:31308-31316. [DOI] [PubMed] [Google Scholar]
- 40.Lange, C. A., T. Shen, and K. B. Horwitz. 2000. Phosphorylation of human progesterone receptors at serine-294 by mitogen-activated protein kinase signals their degradation by the 26S proteosome. Proc. Natl. Acad. Sci. USA 97:1032-1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lewis, G. D., J. A. Lofgren, A. E. McMurtrey, A. Nuijens, B. M. Fendly, K. D. Bauer, and M. X. Sliwkowski. 1996. Growth regulation of human breast and ovarian tumor cells by heregulin: evidences for the requirement of ErbB-2 as a critical component in mediating heregulin responsivness. Cancer Res. 56:1457-1465. [PubMed] [Google Scholar]
- 42.Lowenstein, E. J., R. J. Daly, A. G. Batzer, W. Li, B. Margolis, R. Lammers, A. Ullrich, E. Y. Skolnik, D. Bar-Sagi, and J. Schlessinger. 1992. The SH2 and SH3 domain-containing protein GrbB-2 links receptor tyrosine kinases to ras signaling. Cell 70:431-442. [DOI] [PubMed] [Google Scholar]
- 43.Manni, A., B. Badger, C. Wright, J. R. Ahmed, and L. M. Demers. 1987. Effects of progestins on growth of experimental breast cancer in culture: interaction with estradiol and prolactin and involvement of the polyamine pathway. Cancer Res. 47:3066-3071. [PubMed] [Google Scholar]
- 44.Marte, B. M., D. Graus-Porta, M. Jescke, D. Favor, N. E. Hynes, and D. Taverna. 1995. NDF/heregulin activates MAP kinase and p70/p85 S6 kinase during proliferation or differentiation of mammary epithelial cells. Oncogene 10:167-175. [PubMed] [Google Scholar]
- 45.Migliaccio, A., D. Piccolo, G. Castoria, M. Di Domenico, A. Bilancio, M. Lombardi, W. Gong, M. Beato, and F. Auricchio. 1998. Activation of the Src/p21 ras/Erk pathway by progesterone receptor via cross-talk with estrogen receptor. EMBO J. 17:2008-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mockus, M. B., and K. B. Horwitz. 1983. Progesterone receptors in human breast cancer: stoichiometric translocation and nuclear receptor processing. J. Biol. Chem. 258:4778-4783. [PubMed] [Google Scholar]
- 47.Molinolo, A. A., C. Lanari, E. H. Charreau, N. Sanjuan, and C. D. Pasqualini. 1987. Mouse mammary tumors induced by medroxiprogesterone acetate: immunochemistry and hormonal receptors. J. Natl. Cancer. Inst. 79:1341-1350. [PubMed] [Google Scholar]
- 48.Montecchia, M. F., C. Lamb, A. A. Molinolo, I. A. Luthy, P. Pazos, E. H. Charreau, S. Vanzulli, and C. Lanari. 1999. Progesterone receptor involvement in independent tumor growth in MPA-induced murine mammary adenocarcinomas. J. Steroid Biochem. Mol. Biol. 68:11-21. [DOI] [PubMed] [Google Scholar]
- 49.Mullik, A., and B. S. Katzenellenbogen. 1986. Progesterone receptor synthesis and degradation in MCF-7 human breast cancer cells as studied by dense amino acid incorporation. J. Biol. Chem. 261:13236-13243. [PubMed] [Google Scholar]
- 50.Pelicci, G., L. Lanfrancone, F. Grignani, J. McGlade, F. Cavallo, G. Forni, I. Nicoletti, F. Grignani, T. Pawson, and P. G. Pelicci. 1992. A novel transforming protein (SHC) and SH2 domain is implicated in mitogenic signal transduction. Cell 70:93-104. [DOI] [PubMed] [Google Scholar]
- 51.Pietras, R. J., J. Arboleda, D. M. Reese, N. Wongvipat, M. D. Pegram, L. Ramos, C. M. Groman, M. G. Parker, M. X. Slikowski, and D. J. Slamon. 1995. Her-2 tyrosine kinase pathway targets estrogen receptor and promotes hormone-independent growth in human breast cancer cells. Oncogene 10:2435-2446. [PubMed] [Google Scholar]
- 52.Pinkas-Kramaski, R., I. Alroy, and Y. Yarden. 1997. Human epidermal growth factor receptor cDNA sequence and aberrant expression of the amplified gene in A431 epidermoid carcinoma cells. J. Mamm. Gland Biol. Neoplasia 2:97-107. [Google Scholar]
- 53.Plowman, G. D., G. S. Whitney, M. G. Neubauer, J. M. Green, V. L. McDonald, G. J. Todaro, and M. Shoyab. 1990. Molecular cloning and expression of an additional epidermal growth factor receptor-related gene. Proc. Natl. Acad. Sci. USA 87:4905-4909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Plowman, G. D., J. M. Culouscou, G. S. Whitney, J. M. Green, G. W. Carlton, L. Foy, M. G. Neubauer, and M. Shoyab. 1993. Ligand-specific activation of HER4/p180erbB-4, a fourth member of the epidermal growth factor receptor family. Proc. Natl. Acad. Sci. USA 90:1746-1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Poletti, A., and N. L. Weigel. 1993. Identification of a hormone-dependent phosphorylation site adjacent to the DNA-binding domain of the chicken progesterone receptor. Mol. Endocrinol. 7:241-246. [DOI] [PubMed] [Google Scholar]
- 56.Power, R. F., S. K. Mani, J. Codina, O. M. Conneely, and B. W. O'Malley. 1991. Dopaminergic and ligand-independent activation of steroid hormone receptors. Science 254:1636-1939. [DOI] [PubMed] [Google Scholar]
- 57.Read, L. D., C. E. Snider, J. S. Miller, G. L. Green, and B. S. Katzenellenbogen. 1988. Ligand-modulated region of progesterone receptor messenger ribonucleic acid and protein in human breast cancer cell lines. Mol. Endocrinol. 2:263-271. [DOI] [PubMed] [Google Scholar]
- 58.Richer, J. K., C. A. Lange, N. G. Manning, G. Owen, R. Powell, and K. B. Horwitz. 1998. Convergence of progesterone with growth factor and cytokine signaling in breast cancer. J. Biol. Chem. 273:31317-31326. [DOI] [PubMed] [Google Scholar]
- 59.Riese, D. J., II, and D. F. Stern. 1998. Specificity within the EGF family/ErbB receptor family signaling network. Bioessays 20:41-48. [DOI] [PubMed] [Google Scholar]
- 60.Saceda, M., T. Grunt, R. Colomer, M. Lippman, R. Lupu, and M. Martin. 1996. Regulation of estrogen receptor concentration and activity by an erbB/HER ligand in breast carcinoma cell lines. Endocrinology 137:4322-4330. [DOI] [PubMed] [Google Scholar]
- 61.Sartorius, C. A., M. Y. Melville, A. R. Hovland, L. Tung, G. S. Takimoto, and K. B. Horwitz. 1994. A third transactivation function (AF3) of human progesterone receptors locate in the unique N-terminal segment of the B-isoform. Mol. Endocrinol. 8:1347-1360. [DOI] [PubMed] [Google Scholar]
- 62.Sarup, J. C., K. V. S. Rao, and C. F. Fox. 1988. Decreased progesterone binding and attenuated progesterone action in cultured human breast carcinoma cells treated with epidermal growth factor. Cancer Res. 48:50711-55078. [PubMed] [Google Scholar]
- 63.Sepp-Lorenzino, L., I. Eberhard, Z. Ma, C. Cho, H. Serve, F. Liu, N. Rosen, and R. Lupu. 1996. Signal transduction pathways induced by heregulin in MDA-MB-453 breast cancer cells. Oncogene 12:1679-1687. [PubMed] [Google Scholar]
- 64.Shen, T., K. B. Horwitz, and C. A. Lange. 2001. Transcriptional hyperactivity of human progesterone receptors is coupled to their ligand-dependent downregulation by mitogen-activated protein kinase-dependent phosphorylation of serine 294. Mol. Cell. Biol. 21:6122-6131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shyamala, G., W. Schneider, and W. Schott. 1990. Developmental regulation of murine mammary progesterone receptor gene expression. Endocrinology 126:2882-2889. [DOI] [PubMed] [Google Scholar]
- 66.Takimoto, G. S., A. R. Hovland, D. M. Tasset, M. Y. Melville, L. Tung, and K. B. Horwitz. 1996. Role of phosphorylation in DNA binding and transcriptional functions of human progesterone receptors. J. Biol. Chem. 271:13308-13316. [DOI] [PubMed] [Google Scholar]
- 67.Takimoto, G. S., D. M. Tasset, A. C. Eppert, and K. B. Horwitz. 1992. Hormone-induced progesterone receptor phosphorylation consists of sequential DNA-independent and DNA-dependent stages: analysis with zinc finger mutants and the progesterone antagonist ZK98299. Proc. Natl. Acad. Sci. USA 89:3050-3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tzahar, E., H. Waterman, X. Chen, G. Levkowitz, D. Karunagaran, S. Lavi, B. J. Ratzkin, and Y. Yarden. 1996. A hierarchical network of interreceptor interactions determines signal transduction by Neu differentiation factor/neuregulin and epidermal growth factor. Mol. Cell. Biol. 16:5276-5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ullrich, A., L. Coussens, J. S. Hayflick, T. J. Dull, A. Gray, A. W. Tam, J. Lee, Y. Yarden, T. A. Liebermann, J. Schlessinger, J. Downward, E. L. V. Mayes, N. Whittle, M. D. Waterfield, and P. H. Seeburh. 1984. Human epidermal growth factor receptor cDNA sequence and aberrant expression of the amplified gene in A431 epidermoid carcinoma cells. Nature 309:418-425. [DOI] [PubMed] [Google Scholar]
- 70.Wei, L. L., N. L. Krett, M. D. Francis, D. F. Gordon, W. M. Wood, B. W. O'Malley, and K. B. Horwitz. 1988. Multiple human progesterone receptor messenger ribonucleic acids and their autoregulation by progestin agonist and antagonists in breast cancer cells. Mol. Endocrinol. 2:62-72. [DOI] [PubMed] [Google Scholar]
- 71.Wei, L. L., P. L. Sheridan, N. L. Krett, M. D. Francis, D. O. Toft, D. P. Edwards, and K. B. Horwitz. 1987. Immunologic analysis of human breast cancer progesterone receptors: structure, phosphorylation, and processing. Biochemistry 26:6262-6272. [DOI] [PubMed] [Google Scholar]
- 72.Weigel, N. L. 1996. Steroid hormone receptors and their regulation by phosphorylation. Biochem. J. 319:657-667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yamamoto, T., S. Ikawa, and T. Akiyama. 1986. An erbB-related gene, c-erbB-2, encodes a possible receptor protein similar to the epidermoid growth factor receptor. Nature 319:230-234. [DOI] [PubMed] [Google Scholar]
- 74.Zhang, Y., C. A. Beck, A. Poletti, D. P. Edwards, and N. L. Weigel. 1994. Identification of phosphorylation sites unique to the B form of human progesterone receptor. J. Biol. Chem. 269:31034-31040. [PubMed] [Google Scholar]
- 75.Zhang, Y., C. A. Beck, A. Poletti, D. P. Edwards, and N. L. Weigel. 1995. Identification of a group of Ser-Pro motif hormone-inducible phosphorylation sites in the human progesterone receptor. Mol. Endocrinol. 9:1029-1040. [DOI] [PubMed] [Google Scholar]
- 76.Zhang, Y., C. A. Beck, A. Poletti, J. P. Clement IV, P. Prendergast, T. Yip, T. W. Hutchens, D. P. Edwards, and N. L. Weigel. 1997. Phosphorylation of human progesterone receptor by cyclin-dependent kinase 2 on three sites that are authentic basal phosphorylation sites in vivo. Mol. Endocrinol. 11:823-832. [DOI] [PubMed] [Google Scholar]
- 77.Zhang, Y., W. Bai, V. E. Allgood, and N. L. Weigel. 1994. Multiple signaling pathways activate the chicken progesterone receptor. Mol. Endocrinol. 8:577-584. [DOI] [PubMed] [Google Scholar]