Abstract
Grancalcin, one of the penta-EF-hand Ca2+ binding proteins, is expressed at high levels in polymorphonuclear granulocytes (neutrophils). EF-hand proteins are implicated in the regulation of diverse processes including cell migration, apoptosis, and mobilization of neutrophil effector functions. To determine the role of grancalcin in vivo, we inactivated the gene encoding grancalcin (Gca) in embryonic stem cells and generated grancalcin-deficient mice. Homozygous Gca mutants appeared healthy and reproduced normally. Leukocyte recruitment into the peritoneal cavity upon induction of inflammation was not significantly affected by the absence of grancalcin. The mutants also resisted systemic fungal infection similarly to wild-type mice, and in vitro killing of Staphylococcus aureus by inflammatory cells was not significantly impaired. While marginally increased survival rates of mutants faced with endotoxic shock may indicate a contribution of grancalcin to immunopathogenesis, it is not essential for vital leukocyte effector functions required to control microbial infections.
Neutrophils (polymorphonuclear granulocytes) are phagocytic leukocytes which are rapidly recruited from the bloodstream to the site of tissue damage or infection and which dominate the early response against invading microbes. Neutrophil activation during phagocytosis of microbes leads to respiratory burst and the formation of reactive oxygen intermediates by the NADPH oxidase of phagocytic cells (16). Concomitantly, degradative enzymes from the neutrophil granules are released into the phagocytic vacuole, where they serve as effectors in the killing and digestion of engulfed microbes (13). The critical role of the NADPH oxidase in the mobilization of microbicidal activity is well documented through genetic diseases in humans (15) and the corresponding mouse models (7, 12). The analysis of mice deficient in neutrophil granule proteases elastase and cathepsin G not only demonstrated the vital role of these proteases in antimicrobial immunity but also revealed their contribution to immunopathogenesis in a model of lipopolysaccharide (LPS)-induced shock (19).
Grancalcin (encoded by Gca) was identified as a cytosolic protein that translocates to the granule membrane upon neutrophil activation (3, 18). It belongs to a group of EF-hand Ca2+ binding proteins including calpain, Alg-2, sorcin, and grancalcin, which have structural rather than functional similarity (10). While calpain has proteolytic activity with effects on platelet function (2) and cell migration through organization of the cytoskeleton (4), Alg-2 has been linked with the induction of apoptosis (20). Little is known about the function of sorcin, which can associate with the cardiac ryanodine receptor and which may modulate Ca2+ channel activity (9, 11).
The primary structure of grancalcin includes an N-terminal domain of approximately 50 amino acids that is acetylated (9), of which 14 amino acids are removed posttranslationally in the mature protein (3). The remaining 170 amino acids constitute five motifs with features of EF hands. Crystallographic studies showed that the protein is predominantly α helical, with eight α helices and two short two-stranded β sheets between the loops of the paired EF hands and a “disordered” N-terminal domain. The fifth EF hand engages in the formation of grancalcin homodimers (8). The binding of Ca2+ induces a conformational change that is thought to cause exposure of hydrophobic amino acids, binding to membrane lipids and release from the grancalcin binding partner l-plastin, which can cross-link F-actin and may modulate cell adhesion (9).
To determine the role of grancalcin in vivo, we generated mice lacking this protein. The phenotypic analysis shows that leukocyte development, migration in response to inflammatory stimuli in vivo, and the ability to control microbial infection are not critically affected by the absence of grancalcin. A marginal effect on leukocyte function may be indicated by partial resistance of the mutants to LPS-induced endotoxic shock.
MATERIALS AND METHODS
Gene targeting and generation of grancalcin-deficient mice.
The mouse grancalcin (Gca) cDNA was isolated from a mouse spleen λ zap cDNA library by using a 692-bp BamHI/EcoRI fragment of the human grancalcin cDNA (3). The mouse grancalcin cDNA sequence was determined and used to design primers for screening a P1 genomic library by PCR (Genome Systems, St. Louis, Mo.). A restriction fragment containing part of the genomic Gca gene was subcloned, and the locations of exons were mapped by PCR and sequence analysis. Exon numbers were assigned on the basis of the human (accession no. NM_012198) and mouse (accession no. NW_000176) genome sequences available at http://www.ncbi.nlm.nih.gov/.
The targeting vector for inactivation of the mouse Gca gene was constructed by insertion of a loxP-flanked neomycin resistance gene into a PstI site in exon 4, disrupting the second EF-hand motif near the N terminus. Skipping this modified exon during the splicing process would lead to a frameshift mutation. A herpes simplex virus thymidine kinase gene was included at the 3′ end to permit selection against random integration of the targeting vector. E14-1 mouse embryonic stem (ES) cells were transfected and selected as described previously (14).
Chimeric mice were generated by microinjection of homologous recombinant E14-1 ES cells into C57BL/6 blastocysts. Chimeras transmitting the mutation were mated with 129Sv mice (129S2) to establish a mutant strain on the 129 background (129S2/P2), which was used for further analysis. The mouse colony was maintained in a nonbarrier facility. For fungal infection experiments animals were transferred to microisolator cages and treated with antibiotics (enrofloxacin; 50 mg/liter; Bayer) in the drinking water to prevent opportunistic bacterial superinfections. Experiments on animals were carried out according to regulations under the Home Office (United Kingdom) Animals (Scientific Procedures) Act 1986.
cDNA synthesis and reverse transcription-PCR (RT-PCR).
cDNA was synthesized by using bone marrow RNA as a template with Superscript reverse transcriptase (Gibco/BRL). Synthesis was primed with both oligo(dT) and a primer specific for the 3′ end of the grancalcin gene coding sequence (GcaRev2 [5′-CACAAAGTGCAGCTGTCCC-3′]) to avoid synthesis through the extensive 1.9-kb 3′ untranslated region of the grancalcin gene and to obtain cDNA containing the grancalcin gene coding region. Grancalcin cDNA was amplified with GCAfor1 (5′-CTGACAGCTACTCCCCTGCC-3′) and GCArev1 (5′-CAGCTCATGATGTTCTACTGTGCC-3′). GAPDH cDNA was amplified with primers Gapd-for1 (5′-ACCACCAACTGCTTAGCCCC-3′) and Gapd-rev1 (5′-ATACTTGGCAGGTTTCTCCAGG-3′).
Induction of sterile peritonitis.
Three percent thioglycolate broth was injected intraperitoneally at 0.7 ml/20 g of body weight. Animals were killed by CO2 asphyxiation, and peritoneal exudate cells were recovered by peritoneal lavage with 8 to 10 ml of phosphate-buffered saline-heparin or Hanks' buffered saline-heparin.
Induction of endotoxic shock.
Escherichia coli serotype 0111:B4 LPS and d-galactosamine (both from Sigma Chemical) were injected intraperitoneally in 200 μl of sterile saline. Mice were monitored for endotoxemia twice daily for up to 4 days.
Western blotting.
Cell lysates were generated and Western blotting was carried out as described previously (16). Grancalcin protein was detected with a rabbit antiserum raised against the synthetic multiple antigenic peptide molecule SYSPADDSMWTYFTAB (AltaBioscience, Birmingham, United Kingdom), derived from the grancalcin coding sequence constituting tentative exon 2 (cDNA nucleotides 145 to 189) upstream of the mutation. The binding of the antiserum was revealed with horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin G (Sigma Chemical), and the blot was developed by enhanced chemiluminescence followed by exposure to chemiluminescence film (Amersham Pharmacia Biotech).
Fluorescence conjugates and flow cytometry.
Fluorochrome-conjugated reagents to detect phosphatidylserine on apoptotic cells (annexin V), Gr-1 (Ly-6G), and CD11b were obtained from PharMingen (San Diego, Calif.), and the antibody against F4/80 was obtained from Serotec Ltd. (Oxford, United Kingdom). Stained cell populations were analyzed with a FACScan flow cytometer (Becton Dickinson, Inc., Mountain View, Calif.). For Ca2+ flux measurements, bone marrow cells were stained with anti-Gr-1-phycoerythrin in Hanks' buffered saline supplemented with 1 mM MgCl2, 1 mM CaCl2, 25 mM HEPES, and 0.5% bovine serum albumin, washed in the same medium, and loaded with Indo-1 (3 μM) in the presence of 4 mM probenecid for 30 min at 37°C. Cells were analyzed on a LSR flow cytometer (Becton Dickinson, Inc.), and changes in the ratio of violet emission/blue emission were recorded over 3 min.
Neutrophil function and microbicidal activity.
Experiments were carried out as described previously (19). Neutrophil activation of the respiratory burst was measured by using dihydrorhodamine as a fluorescent probe. Degranulation and shape change were assessed by flow cytometry by determining changes in side and forward light scatter. Aspergillus fumigatus spores were injected intravenously into mice, which were maintained in microisolator cages and under prophylactic antibiotic treatment to prevent superinfection with opportunistic bacterial pathogens. Fungal load in the kidneys was determined by isolation of both kidneys 6 days after infection. Organs were homogenized in phosphate-buffered saline, and serial dilutions were plated on Luria-Bertani (LB) agar plates containing chloramphenicol. Colonies were enumerated after overnight incubation at 37°C. The microbicidal activity of leukocytes in vitro was assessed by incubating peritoneal exudate cells with opsonized Staphylococcus aureus at a ratio of 10:1. After 10 min extracellular bacteria were lysed by addition of lysostaphin (10 U/ml), and the cells were incubated further to permit the killing of the ingested microbes. Cells were lysed in distilled water, and the number of viable bacteria was determined by colony formation on LB agar plates.
Lactoferrin release was determined by enzyme-linked immunosorbent assay as a measure of neutrophil degranulation. Total bone marrow cells were resuspended in Dulbecco's modified Eagle medium (DMEM)-25 mM HEPES. Cells (107) in a volume of 0.6 ml were briefly centrifuged in 24-well plates to promote adhesion. Cells were then stimulated for 30 min at 37°C, and the supernatant was harvested and centrifuged. Microtiter plates were coated with serial dilutions of the supernatant, and lactoferrin was detected with rabbit anti-human lactoferrin, followed by anti-rabbit immunoglobulin G alkaline phosphatase (Sigma Chemical). Purified human lactoferrin (Sigma Chemical) was used as a standard.
Apoptosis assays.
Bone marrow and peritoneal exudate cells were incubated in DMEM-10% fetal calf serum-20 mM HEPES, pH 7, at 37°C and 5% CO2 and stained with anti-Gr-1 and annexin V. Granulocytes were gated successively via light scatter and Gr-1 fluorescence, and the fraction of annexin V-binding cells was determined.
Nucleotide sequence accession number.
The GenBank accession number of the mouse grancalcin cDNA determined in this study is AF518325.
RESULTS AND DISCUSSION
Generation of grancalcin-deficient mice.
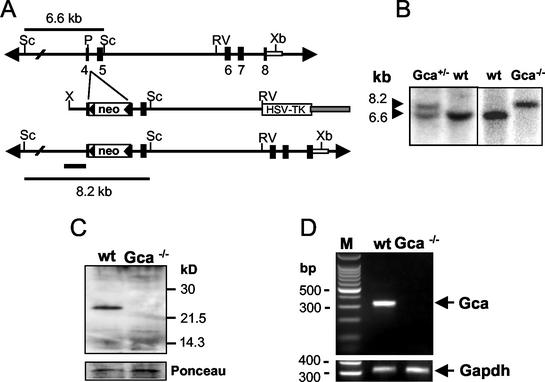
The sequence of the mouse grancalcin cDNA (GenBank accession no. AF518325) revealed an open reading frame encoding 220 amino acids; the open reading frame had 81% identity and 88% similarity to that of the human grancalcin gene (GCA). In line with the reported tissue distribution, the grancalcin protein was readily detectable by Western blotting in bone marrow cells and at lower levels in peritoneal macrophages, while spleen and thymus were negative (data not shown). The strategy for inactivation of the mouse grancalcin gene (Gca) by homologous recombination in mouse ES cells is shown in Fig. 1A. The targeting event mediates insertion of the resistance marker into exon 4 and disruption of the open reading frame at codon 96 and a 1.6-kb shift of the ScaI restriction fragment. The mutation leads to disruption of the second EF hand in the grancalcin protein. The structure of the targeted locus was confirmed by using a number of different restriction enzymes and probes (data not shown). Western blot analysis of bone marrow leukocytes using a polyclonal rabbit antiserum raised against an exon 2-encoded peptide sequence in EF hand 1 confirmed the inactivation of the Gca gene (Fig. 1C). Skipping modified exon 4 during splicing could pose a potential problem. However, splicing exon 3 to exon 5 would cause a frameshift mutation and thus nonfunctional mRNA. Also, such an aberrant protein should be detectable by the antiserum raised against a peptide encoded by exon 2. Neither full-length nor truncated mRNA was detectable by RT-PCR using primers flanking the insertion in exon 4, providing further evidence for functional inactivation of the Gca gene. Mice lacking grancalcin (Gca−/−) were born at a frequency of 21%, slightly below the 25% expected from F1 heterozygous matings (Gca+/+, 58 mice; Gca+/−, 102 mice; Gca−/−, 43 mice). Gca−/− mice reproduced normally and appeared healthy without obvious abnormalities. Spontaneous infections or signs of inflammatory disease were not observed macroscopically or by histopathological examination (data not shown).
FIG. 1.
Generation of grancalcin (Gca)-deficient mice. (A) Targeted inactivation of the grancalcin gene (Gca) in mouse ES cells. Shown is the structure of the Gca gene locus (top), the targeting vector (middle), and the Gca locus after homologous recombination (bottom). Exons 4 to 8 encode EF hands 2 to 5. The insertion of the neo gene into exon 4 disrupts EF hand 2 near its N terminus. Skipping exon 4 during splicing would result in a frameshift mutation. Open boxes, neomycin resistance and herpes simplex virus thymidine kinase (HSV-TK) genes; Sc, ScaI; P, PstI; RV, EcoRV; Xb, XbaI; X, XhoI. (B) Southern blot analysis of ScaI-restricted genomic DNA from the targeted ES cell clone used to generate chimeras (left) and of genomic DNA from a wild-type (wt) and Gca-deficient mouse (right). The probe fragments used are indicated in panel A (bars). (C) Western blot analysis of grancalcin expression in bone marrow of control and mutant mice. Membranes were probed with rabbit anti-mouse grancalcin antiserum raised against a synthetic peptide sequence in the first EF hand. Bottom, Ponceau S-stained membranes to confirm equivalent sample loading for wild-type and mutant mice. (D) RT-PCR of bone marrow RNA to determine levels of mRNA encoding grancalcin (302-bp product [top]) and GAPDH (309-bp product [bottom]) as a control. Grancalcin primers were specific for sequences in exons 2 and 5 flanking the mutated exon. No functional or aberrantly spliced mRNA is detectable in the mutants. Lane M, marker.
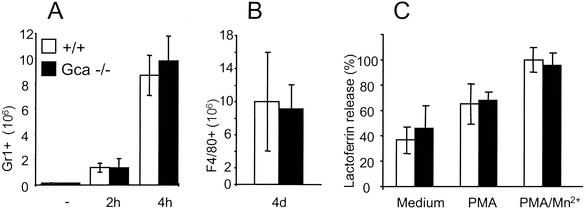
The absence of grancalcin affected neither the generation of mature neutrophils in the bone marrow (data not shown) nor neutrophil or macrophage recruitment in response to the sterile inflammatory stimulus thioglycolate (Fig. 2). Also, neutrophils showed normal granularity as assessed by light side scatter analysis (mean wild-type level, 23.7 ± 0.9; mean Gca−/− level, 24.8 ± 0.8). Stimulation with phorbol-12-myristate-13-acetate of heat-killed S. aureus induced a normal respiratory burst (data not shown) and degranulation similarly in both mutants and controls as indicated by the loss in mean side scatter values for neutrophils (wild-type levels, 17.4 and 17.1; Gca−/− levels, 16.6 and 17.5). Release of the secondary granule component lactoferrin in response to stimulation in mutant mice was similar to that in normal mice (Fig. 2). Also, Ca2+ flux in response to stimulation with fMLP or calcium ionophores such as ionomycin and A-23187 in grancalcin-deficient neutrophils was not affected (data not shown).
FIG. 2.
Leukocyte recruitment and degranulation in grancalcin-deficient mice. (A and B) Neutrophil (A) and macrophage (B) recruitment in sterile peritonitis. PMN granulocytes and macrophages were obtained by peritoneal lavage at the time points indicated after injection of thioglycolate and identified by flow cytometry as Ly-6G- (Gr-1) or F4/80-positive cells, respectively (n = 3). (C) Degranulation of purified bone marrow neutrophils, as indicated by release of the secondary granule component lactoferrin upon a 30-min incubation at 37°C with or without additional stimulation. One hundred percent release corresponds to approximately 300 ng of lactoferrin/ml. PMA, phorbol-12-myristate-13-acetate.
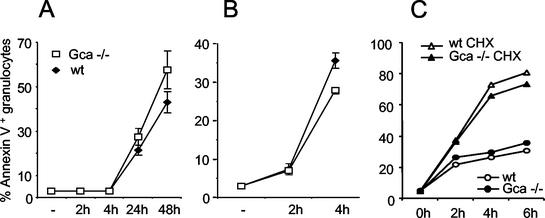
Since Ca2+ binding proteins of the penta-EF-hand family have been implicated in the control of apoptosis (17), we tested whether this process was affected in the mutants. Grancalcin-deficient neutrophils, however, entered apoptosis spontaneously in vitro or upon induction with cycloheximide (Fig. 3). Although minor differences in the frequency of apoptotic neutrophils between mutant and control mice were found, the direction of the effect varied depending on the source of the cells and the nature of the stimulus. No consistent trend toward resistance or facilitated apoptosis in the absence of grancalcin became apparent.
FIG. 3.
Apoptosis of grancalcin-deficient neutrophils. (A and B) Spontaneous (A) and cycloheximide (CHX)-induced (B) apoptosis of bone marrow granulocytes (Gr-1+, annexin V-positive cells). wt, wild type. (C) Spontaneous (circles) or cycloheximide-induced (triangles) apoptosis of inflammatory granulocytes obtained by thioglycolate-induced peritonitis.
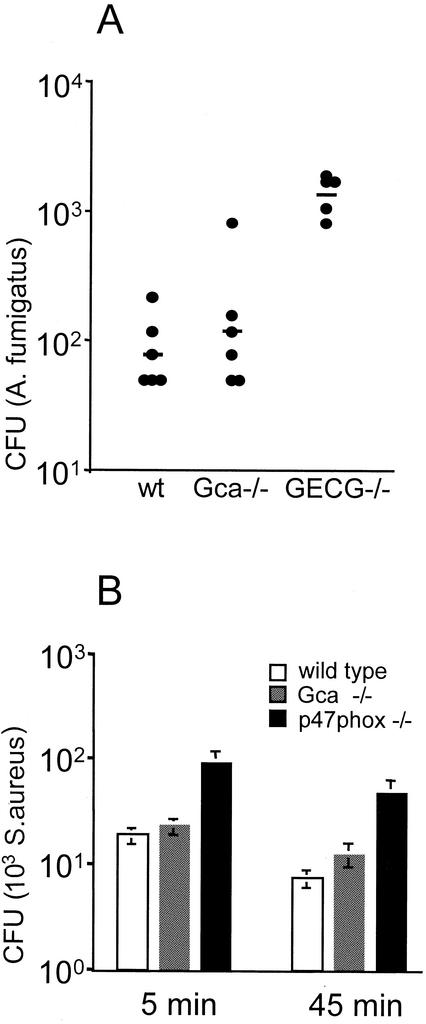
We have shown previously that the absence of neutrophil granule serine proteases leads to profound susceptibility to systemic A. fumigatus infection (19). We used this stringent test for neutrophil function to assess whether the absence of grancalcin affected the ability to control A. fumigatus in vivo. Figure 4 shows the microbial load in the kidneys of wild-type mice and grancalcin-deficient and granule protease (elastase and cathepsin G)-deficient mice. While the protease-deficient mice are impaired in their ability to restrict fungal growth, grancalcin mutants were not significantly affected and the microbial load was similar to that in wild-type mice. Gca mutants also resisted systemic infection with Candida albicans or S. aureus similarly to wild-type mice (data not shown). In line with the normal antimicrobial activity in vivo mutant leukocytes showed efficient killing of S. aureus in vitro (Fig. 4B). While p47phox mutants deficient in the respiratory burst (6) show impaired killing of ingested S. aureus, the bactericidal activity of grancalcin-deficient cells is similar to that of controls. The marginally increased numbers of viable S. aureus cells at the later time point in the mutants could perhaps reflect a role for grancalcin in the mobilization of the killing machinery. This minor difference, however, does not seem to affect host resistance to infection significantly, as indicated by the in vivo infection models described above.
FIG. 4.
Control of A. fumigatus and S. aureus in the absence of grancalcin. (A) Fungal load in kidneys of wild-type (wt) and grancalcin-deficient mice (n = 6) 6 days after intravenous infection with 106 A. fumigatus spores (viability, >95%). Horizontal lines, geometric means. One outlier (60 CFU) was excluded from the granulocyte elastase-cathepsin G double-mutant (GECG−/−) group as this value is not in line with the normal distribution and inconsistent with the homogeneous response normally seen in these animals. (B) Killing of ingested S. aureus 5 or 45 min after lysis of extracellular bacteria by addition of lysostaphin. Cells from p47phox-deficient mice, which are impaired in their microbicidal activity due to a defective respiratory burst, served as a control.
Mice lacking elastase and cathepsin G are relatively resistant to LPS-induced shock (19), indicating the contribution of neutrophils and their products to the progression of this pathogenic response. We chose the low-dose LPS model of endotoxic shock (5) to determine whether grancalcin contributed to this immunopathogenic syndrome. One hundred nanograms of LPS coinjected with the sensitizer d-galactosamine induced lethal shock in 26 of 26 wild-type mice, while approximately 40% of the mutants (10 of 26; P = 0.0002) survived. While the possibility of substrain differences between the control and mutant mouse strains cannot be fully excluded, a role for grancalcin may thus become apparent in resistance to endotoxic shock.
Neutrophil granules contain an array of proteolytic and microbicidal enzymes, which are released into the phagocytic vacuole, in parallel with the mobilization of the respiratory burst. The respiratory burst is necessary, but not sufficient, to generate protective microbicidal activity. The release of the granule contents, including elastase and cathepsin G, is essential (13). The observation that neutrophil activation was associated with translocation of the cytosolic grancalcin to the granule membrane led to the suggestion of a role for this protein in the mobilization of the microbicidal activity of neutrophils (3, 17). While the lack of the respiratory burst (12) or the absence of myeloperoxidase (1) or granule proteases (19) increases susceptibility to experimental microbial infections in mice, the absence of grancalcin does not significantly impair host immunity to microbial pathogens. Thus, microbicidal activity of phagocytes can be mobilized efficiently in the absence of grancalcin, and this protein seems to play a minor role, if any, in this process.
The observed resistance of Gca mutant mice to LPS-induced shock suggests, however, that grancalcin can contribute to the mobilization of the destructive potential of leukocytes under certain conditions. Effects of grancalcin on the release of pathogenic products and/or the modulation of neutrophil activation or apoptosis upon interaction with the endothelium could account for the relative protection of Gca mutants from endotoxic shock. While our study shows that potent protective neutrophil functions can be mobilized in the absence of grancalcin, a subtle role for this protein perhaps in the control of leukocyte activation or protection against specific microbial pathogens cannot be ruled out.
Acknowledgments
We thank Marco Novelli (Department of Histopathology, UCL) for help with histopathological analysis and Gemma Lee for technical assistance.
This work was supported by the Wellcome Trust.
REFERENCES
- 1.Aratani, Y., H. Koyama, S. Nyui, K. Suzuki, F. Kura, and N. Maeda. 1999. Severe impairment in early host defense against Candida albicans in mice deficient in myeloperoxidase. Infect. Immun. 67:1828-1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Azam, M., S. S. Andrabi, K. E. Sahr, L. Kamath, A. Kuliopulos, and A. H. Chishti. 2001. Disruption of the mouse μ-calpain gene reveals an essential role in platelet function. Mol. Cell. Biol. 21:2213-2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boyhan, A., C. M. Casimir, J. K. French, C. G. Teahan, and A. W. Segal. 1992. Molecular cloning and characterization of grancalcin, a novel EF-hand calcium-binding protein abundant in neutrophils and monocytes. J. Biol. Chem. 267:2928-2933. [PubMed] [Google Scholar]
- 4.Dourdin, N., A. K. Bhatt, P. Dutt, P. A. Greer, J. S. Arthur, J. S. Elce, and A. Huttenlocher. 2001. Reduced cell migration and disruption of the actin cytoskeleton in calpain-deficient embryonic fibroblasts. J. Biol. Chem. 276:48382-48388. [DOI] [PubMed] [Google Scholar]
- 5.Galanos, C., M. A. Freudenberg, and W. Reutter. 1979. Galactosamine-induced sensitization to the lethal effects of endotoxin. Proc. Natl. Acad. Sci. USA 76:5939-5943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harbord, M., M. Novelli, B. Canas, D. Power, C. Davis, J. Godovac-Zimmermann, J. Roes, and A. W. Segal. 2002. Ym1 is a neutrophil granule protein that crystallizes in p47phox-deficient mice. J. Biol. Chem. 277:5468-5475. [DOI] [PubMed] [Google Scholar]
- 7.Jackson, S. H., J. I. Gallin, and S. M. Holland. 1995. The p47phox mouse knock-out model of chronic granulomatous disease. J. Exp. Med. 182:751-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jia, J., Q. Han, N. Borregaard, K. Lollike, and M. Cygler. 2000. Crystal structure of human grancalcin, a member of the penta-EF-hand protein family. J. Mol. Biol. 300:1271-1281. [DOI] [PubMed] [Google Scholar]
- 9.Lollike, K., A. H. Johnsen, I. Durussel, N. Borregaard, and J. A. Cox. 2001. Biochemical characterization of the penta-EF-hand protein grancalcin and identification of L-plastin as a binding partner. J. Biol. Chem. 276:17762-17769. [DOI] [PubMed] [Google Scholar]
- 10.Maki, M., S. V. Narayana, and K. Hitomi. 1997. A growing family of the Ca2+-binding proteins with five EF-hand motifs. Biochem. J. 328:718-720. [PMC free article] [PubMed] [Google Scholar]
- 11.Meyers, M. B., V. M. Pickel, S. S. Sheu, V. K. Sharma, K. W. Scotto, and G. I. Fishman. 1995. Association of sorcin with the cardiac ryanodine receptor. J. Biol. Chem. 270:26411-26418. [DOI] [PubMed] [Google Scholar]
- 12.Pollock, J. D., D. A. Williams, M. A. Gifford, L. L. Li, X. Du, J. Fisherman, S. H. Orkin, C. M. Doerschuk, and M. C. Dinauer. 1995. Mouse model of X-linked chronic granulomatous disease, an inherited defect in phagocyte superoxide production. Nat. Genet. 9:202-209. [DOI] [PubMed] [Google Scholar]
- 13.Reeves, E. P., H. Lu, H. L. Jacobs, C. G. Messina, S. Bolsover, G. Gabella, E. O. Potma, A. Warley, J. Roes, and A. W. Segal. 2002. Killing activity of neutrophils is mediated through activation of proteases by K+ flux. Nature 416:291-297. [DOI] [PubMed] [Google Scholar]
- 14.Roes, J., and K. Rajewsky. 1993. Immunoglobulin D (IgD)-deficient mice reveal an auxiliary receptor function for IgD in antigen-mediated recruitment of B cells. J. Exp. Med. 177:45-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roos, D. 1994. The genetic basis of chronic granulomatous disease. Immunol. Rev. 138:121-157. [DOI] [PubMed] [Google Scholar]
- 16.Segal, A. W., and A. Abo. 1993. The biochemical basis of the NADPH oxidase of phagocytes. Trends Biochem. Sci. 18:43-47. [DOI] [PubMed] [Google Scholar]
- 17.Teahan, C., P. Rowe, P. Parker, N. Totty, and A. W. Segal. 1987. The X-linked chronic granulomatous disease gene codes for the beta-chain of cytochrome b-245. Nature 327:720-721. [DOI] [PubMed] [Google Scholar]
- 18.Teahan, C. G., N. F. Totty, and A. W. Segal. 1992. Isolation and characterization of grancalcin, a novel 28-kDa EF-hand calcium-binding protein from human neutrophils. Biochem. J. 286:549-554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tkalcevic, J., M. Novelli, M. Phylactides, J. P. Iredale, A. W. Segal, and J. Roes. 2000. Impaired immunity and enhanced resistance to endotoxin in the absence of neutrophil elastase and cathepsin G. Immunity 12:201-210. [DOI] [PubMed] [Google Scholar]
- 20.Vito, P., E. Lacana, and L. D'Adamio. 1996. Interfering with apoptosis: Ca2+-binding protein ALG-2 and Alzheimer's disease gene ALG-3. Science 271:521-525. [DOI] [PubMed] [Google Scholar]