Abstract
Targeted disruption of the retinoblastoma gene in mice leads to embryonic lethality in midgestation accompanied by defective erythropoiesis. Rb−/− embryos also exhibit inappropriate cell cycle activity and apoptosis in the central nervous system (CNS), peripheral nervous system (PNS), and ocular lens. Loss of p53 can prevent the apoptosis in the CNS and lens; however, the specific signals leading to p53 activation have not been determined. Here we test the hypothesis that hypoxia caused by defective erythropoiesis in Rb-null embryos contributes to p53-dependent apoptosis. We show evidence of hypoxia in CNS tissue from Rb−/− embryos. The Cre-loxP system was then used to generate embryos in which Rb was deleted in the CNS, PNS and lens, in the presence of normal erythropoiesis. In contrast to the massive CNS apoptosis in Rb-null embryos at embryonic day 13.5 (E13.5), conditional mutants did not have elevated apoptosis in this tissue. There was still significant apoptosis in the PNS and lens, however. Rb−/− cells in the CNS, PNS, and lens underwent inappropriate S-phase entry in the conditional mutants at E13.5. By E18.5, conditional mutants had increased brain size and weight as well as defects in skeletal muscle development. These data support a model in which hypoxia is a necessary cofactor in the death of CNS neurons in the developing Rb mutant embryo.
The retinoblastoma tumor suppressor is an important regulator of the cell cycle, differentiation, and apoptotic death (reviewed in references 13, 29, and 46). Germ line mutations in the RB gene predispose individuals to bilateral retinoblastoma as well as osteosarcoma (reviewed in reference 13). Somatic inactivation of RB contributes to the development of these tumor types as well as prostate, breast, lung, and bladder cancer. Disruption of the retinoblastoma pathway function through direct RB mutation or mutation of upstream regulators of pRB, such as CDK4 or p16INK4a, is thought to occur in the vast majority of human cancers (46).
Studies involving the targeted disruption of Rb in mice have provided significant insight into the function of pRB in normal development and tumor suppression. Mice heterozygous for an Rb mutation develop pituitary and thyroid tumors, which exhibit loss of the remaining wild-type Rb allele (21, 47). Homozygosity for an Rb mutation causes embryonic lethality near embryonic day 14.5 (E14.5) (9, 22, 26). Rb-null embryos are pale and exhibit defects in fetal liver erythropoiesis. Absence of pRB function also causes dramatic defects in the lens, central nervous system (CNS), and peripheral nervous systems (PNS). In these tissues, both inappropriate S-phase entry and high levels of apoptosis are evident (9, 22, 26, 27, 34).
Extensive analyses of the molecular pathways contributing to these phenotypes have been carried out using compound mutant analysis. pRB binds to members of the E2F family of transcription factors to regulate G1-to-S-phase progression (reviewed in reference 10). Compound mutant analysis involving deletion of both Rb and either E2f1 or E2f3 supports a critical role of these transcription factors in Rb function (44, 54). Mutation of both Rb and E2f1 or E2f3 led to reduced levels of the inappropriate S-phase entry and apoptosis in the CNS and lens. The erythropoietic defect was also partially rescued in these compound mutants, extending embryo survival until nearly E17.5. Compound mutant analysis has also implicated the p53 tumor suppressor in apoptosis in the Rb-deficient CNS and lens but not PNS (33, 34). Loss of p53 specifically inhibited apoptosis in the setting of Rb deficiency; inappropriate S-phase and embryonic lethality were not affected. The specific signals that mediate p53 activation in the Rb−/− embryos have not been determined, although it is known that deregulated E2f activity can cause p53 activation in other systems. In cell culture, E2f1 overexpression leads to proliferation and apoptosis that is partially p53 dependent (24, 40, 49). One possible mediator of p53 activation downstream of E2f members is the E2f target ARF, which regulates p53 by inhibiting MDM2 (39) and is induced in mouse embryonic fibroblasts upon E2f1 overexpression (5). However, loss of Arf does not significantly inhibit the CNS or lens apoptosis in Rb mutant embryos (45).
The analysis of chimeric animals composed of both wild-type and Rb-null cells has demonstrated that both the development of erythroid cells and the death of Rb−/− neurons can be rescued (30, 32, 48). With respect to inhibition of apoptosis in the CNS, it is possible that neighboring cells provide survival signals in the form of a secreted factor or perhaps involving cell-cell contacts that allow nearby Rb-deficient cells to survive (30). An alternative possibility is that the survival of CNS neurons is due to the absence of a proapoptotic factor normally present in germ line Rb−/− embryos. Specifically, the normal development of the hematopoietic system in these chimeras may relieve hypoxic stress on the embryo, thus eliminating a critical signal for apoptosis.
The cause of the defect in erythropoiesis in Rb−/− embryos is not known, although it has been proposed that the Rb-deficient fetal liver may not provide an environment supportive of erythrocyte development. Rb mutant fetal livers are hypocellular, have high levels of apoptosis, and produce a deficit of mature, enucleated erythrocytes. However, in Rb+/+:Rb−/− chimeras mature enucleated red blood cells derived from Rb-deficient cells are present (32, 48). Also, fetal livers from Rb-null embryos can reconstitute a lethally irradiated host (20), again suggesting that the environment in which the erythrocytes develop is a critical factor. Given that Rb−/− neurons in chimeras are not prone to apoptosis, we hypothesized that hypoxia downstream of defective erythropoiesis may contribute to p53-dependent apoptosis in the Rb-null CNS.
Upregulation of p53 protein in neurons has been demonstrated upon ischemic injury in vivo (28, 50) and upon hypoxia treatment of neurons cultured in vitro (3, 53). Also, neurons cultured from p53-deficient animals showed resistance to hypoxia-mediated cell death (18), supporting the idea that hypoxia can lead to p53-dependent apoptosis in neurons. It is also possible that hypoxia could contribute to p53-dependent apoptosis in the Rb−/− lens or p53-independent apoptosis in the Rb−/− PNS. In the Rb−/− PNS, the mechanism of apoptosis appears mechanistically distinct from that in the CNS. Apoptosis in this tissue is not affected on a p53-null background (33), and loss of E2f1 or E2f3 only partially reduces the PNS apoptosis in the absence of Rb function (44, 54).
To determine if hypoxia due to the erythropoietic defect was required for apoptosis in the Rb-null embryos, we used a conditional gene-targeting approach to remove Rb from the CNS, PNS, and lens while maintaining normal erythropoiesis. This was carried out using the Cre-loxP system in which Cre expression was driven by regulatory elements from the rat nestin gene (4, 11, 43). In this system, Cre is expressed efficiently in nervous system progenitor cells as well as in other tissues, leading to deletion of Rb in the CNS, PNS, and lens. As such, we were able to examine the fate of Rb−/− cells in these tissues in the presence of an intact hematopoietic system.
MATERIALS AND METHODS
Mice and generation of embryos.
Male Nes-Cre1 mice were initially crossed to Rb2lox/2lox mice. Male offspring expressing Cre were crossed to Rb2lox/2lox females. Details on PCR genotyping reactions are available upon request. The morning of plug detection was taken as E0.5, and embryos were collected throughout development. Embryos were dissected from the mother, the yolk sac was collected for genotyping, and embryos were fixed overnight in 3.7% formaldehyde in PBS. Embryos were processed and embedded in paraffin, and 4-μm-thick sections were cut.
Northern blotting.
Whole-brain tissue was quickly dissected from E13.5 embryos and frozen on dry ice. Tissue was homogenized in Trizol reagent (Invitrogen), and total RNA was isolated following the manufacturer's instructions. Northern blotting with 10 μg of total RNA was performed using standard methods. Vascular endothelial growth factor (VEGF) and lactate dehydrogenase A (LDH-A) cDNAs were used as probes. VEGF cDNA was a gift of Volker Haase and LDH-A cDNA was obtained through reverse transcription-PCR. Probe labeling was performed using the Prime-It II random primer labeling kit (Stratagene) and hybridization was performed using ExpressHyb solution (Clontech).
Southern blotting.
Genomic DNA was isolated from E13.5 whole brains following digestion of tissue with proteinase K and extraction with phenol-chloroform. Genomic DNA was digested with PstI and Acc65I, run on a 0.8% agarose gel, transferred to a nylon membrane (Hybond N+; Amersham), and hybridized with a 32P-labeled internal probe as described previously (22).
Western blotting.
Whole embryo brain, dorsal root ganglia (DRG), and ocular lens were dissected from E13.5 embryos, and tissue was frozen on dry ice. For DRG microdissection, the E13.5 spinal cord was separated out, and ganglia subsequently were removed from the spinal cord and pooled. Skeletal muscle was dissected from E18.5 embryos. Tissue was lysed in a solution containing 100 mM NaCl, 100 mM Tris (pH 8), 1% NP-40, and Complete protease inhibitor cocktail (Roche). Protein was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to a polyvinylidene difluoride membrane (Immobilon). Blots were first probed with an antibody to pRB (1/1,000; Pharmingen). Blots were then stripped and reprobed with an antibody to actin (1/1,000; Santa Cruz). Horseradish peroxidase-conjugated secondary antibodies (Jackson Immunochemicals) were used at a 1/5,000 dilution. Enhanced chemiluminescence (ECL+; Amersham) was used for signal detection before exposing blots to film.
Apoptosis and BrdU staining in embryos.
For bromodeoxyuridine (BrdU) analysis, pregnant females received intraperitoneal injections of BrdU (Sigma) at 30 μg/g of body weight 1 h before animal sacrifice and embryo dissection. Staining for BrdU was performed as described previously (45). For analysis of apoptosis, terminal deoxynucleotidyltransferase-mediated dUTP-end labeling (TUNEL) was used (14). Paraffin sections were rehydrated, treated with proteinase K, and incubated in TUNEL mixture including biotin-labeled dUTP (Roche) and recombinant terminal deoxynucleotidyltransferase (Invitrogen). Detection of incorporation of biotin-labeled dUTP was done using the ABC kit (Vector Labs) and detection with DAB (Vector Labs).
RESULTS
Expression of hypoxia-inducible genes in Rb−/− CNS.
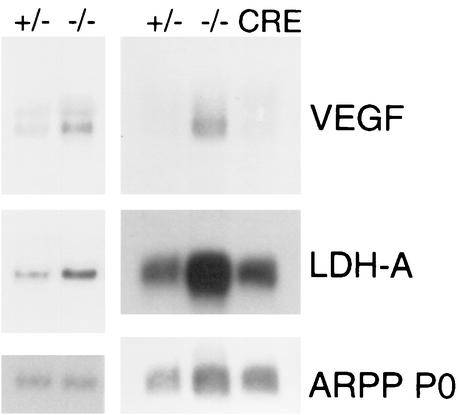
In order to determine whether the CNS in Rb−/− embryos may be under hypoxic stress that could contribute to p53-dependent apoptosis, we examined the expression of the hypoxia-inducible genes coding for VEGF and LDH-A in microdissected brain tissue using Northern blotting. As shown in Fig. 1, increased expression of VEGF and LDH-A was observed in CNS tissue of E13.5 Rb−/− embryos compared to controls. Our observation of upregulation of these genes provides indirect evidence that these animals may be under hypoxic stress. Given that hypoxia would be expected to be secondary to the defective erythropoiesis in the Rb−/− embryo, we sought to conditionally eliminate Rb in the nervous system and determine the function of Rb mutant cells.
FIG. 1.
Expression of hypoxia-inducible genes in Rb−/− CNS. Northern blotting showing expression levels of the hypoxia-inducible genes VEGF and LDH-A in Rb+/−, Rb−/−, and conditional Rb mutant (CRE) E13.5 whole-brain tissue. ARPP P0 was used as a loading control.
Generation of conditional Rb mutants.
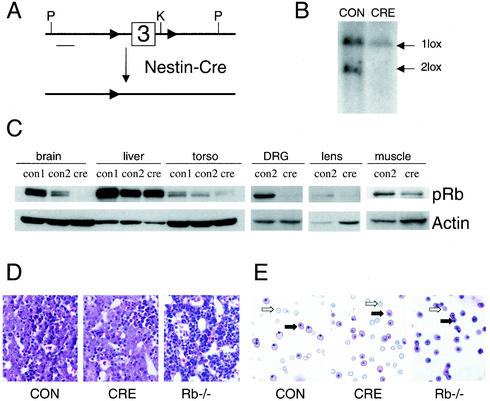
To remove Rb conditionally, the endogenous Rb locus was targeted to introduce loxP sites surrounding exon 3. Generation of mice with both alleles containing loxP sites flanking Rb exon 3 (termed Rb2lox/2lox) will be described elsewhere (J. Sage and T. Jacks, unpublished data). We bred Rb2lox/2lox mice to Nes-Cre1 transgenic mice in which Cre expression is driven by regulatory elements of the rat nestin gene (4, 11, 43) (Fig. 2A). The expression of Cre in this strain has been shown to begin prior to E9, resulting in almost complete Cre-mediated recombination in the midgestation brain as well as in the germ line (4, 11). Cre-mediated recombination has been observed at lower efficiency in other tissues, including skeletal muscle (11). All crosses were performed with the male bearing the Cre transgene due to previously reported imprinting effects with the Nes-Cre1 transgene (4, 43). Male Rb2lox/+ mice carrying the Cre transgene were crossed to Rb2lox/2lox females. Due to Cre expression in the germ line, conditional Rb mutants arising from this cross carried the Nes-Cre1 transgene and had the genotype Rb1lox/2lox at the Rb locus. Littermates with genotype Rb1lox/2lox that lacked the Cre transgene served as controls. Southern blot analysis demonstrated recombination in the E13.5 brain of conditional mutants bearing the Nes-Cre1 transgene (Fig. 2B). We confirmed by Western blot that pRB was indeed absent from whole-embryo brain at E13.5 in conditional mutants with the Cre transgene, but levels of pRB in the liver and torso of Cre-expressing animals were similar to those observed in controls (Fig. 2C). We also documented removal of pRB in the PNS, by performing Western blots on microdissected DRG from E13.5 embryos. Nestin is normally expressed in the developing mouse lens (51), and microdissection of E13.5 lenses from conditional mutants and subsequent Western blot analysis showed decreased pRB levels in conditional mutants expressing the Nes-Cre1 transgene (Fig. 2C). Thus, this strategy allowed us to delete Rb from several tissues in which apoptosis and inappropriate S-phase occurs in germ line Rb−/− embryos.
FIG. 2.
Conditional removal of Rb in developing nervous system and lens with rescue of erythropoietic defect. (A) Schematic representation of Rb conditional knockout allele. Cre-mediated recombination removes exon 3 of Rb in tissues expressing Cre from regulatory elements of the rat nestin gene. Abbreviations: P, PstI site, K, KpnI site. (B) Southern blot of genomic DNA from E13.5 whole brain with the genotype Rb1lox/2lox lacking the cre transgene (CON) or bearing the Nes-Cre1 transgene (CRE). Genomic DNA was digested with PstI and the KpnI isoschizomer Acc65I. The bottom 6.5-kb band is the Rb2lox unrecombined allele, while the larger 9-kb band is the recombined Rb1lox allele. (C) Western blot showing tissue-specific loss of Rb conditional mutants carrying the Nes-Cre1 transgene. Tissue is from E13.5 embryos except for skeletal muscle, which was from E18.5 embryos. CON1 refers to control embryos with two copies of the Rb gene product (genotype Rb2lox/+), while CON2 refers to embryos with one copy of the Rb gene product (genotype Rb1lox/2lox). CRE refers to conditional mutants (genotype Rb1lox/2lox) carrying the Nes-Cre1 transgene. Torso is the remainder of embryo lacking the head, liver, and heart. (D) Hematoxylin-eosin-stained sagittal section show normal liver hematopoiesis in conditional-mutant animals, while Rb−/− livers show decreased cellularity and pycnotic nuclei. (E) Peripheral blood smear from E13.5 control, conditional-mutant, or Rb-null embryos. Note the normal ratio of enucleated definitive erythrocytes (open arrows) to nucleated erythrocytes (solid arrows) in the conditional-mutant smear, while Rb −/− smears have very few enucleated erythrocytes. See text for quantitation.
Normal erythropoietic development in conditional-mutant animals.
As shown in Fig. 2D, conditional Rb mutants had normal liver cellularity and levels of apoptosis compared to controls. They were also normally superficially vascularized, in contrast to the pale appearance of Rb−/− embryos (not shown). As demonstrated previously, peripheral blood smears from Rb−/− embryos have predominantly nucleated erythrocytes (93.6% ± 3.1% nucleated; n = 4) (unless otherwise noted, results are means ± standard deviations). Rb conditional mutants, however, exhibit normal production of enucleated erythrocytes (Fig. 2E). 57.4% ± 6.1% of erythrocytes from conditional mutants were nucleated (n = 4), which is similar to the frequency seen in controls (55.1% ± 4.5% nucleated; n = 3). Importantly, while overexpression of VEGF and LDH-A was observed in germ line Rb−/− CNS tissue, levels of these hypoxia-inducible genes were normal in conditional-mutant tissue, supporting the conclusion that the conditional-mutant embryos were not under hypoxic stress (Fig. 1). These data indicated that erythropoiesis was normal in conditional mutants and allowed us to focus on the phenotypic consequences of loss of Rb function in normoxic CNS, PNS and lens.
Rescue of midgestation apoptosis but not S-phase entry in CNS.
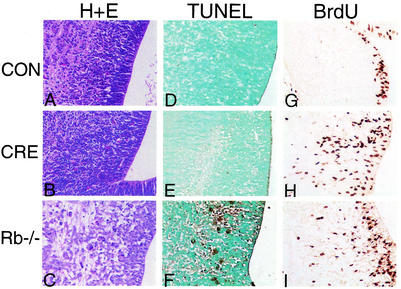
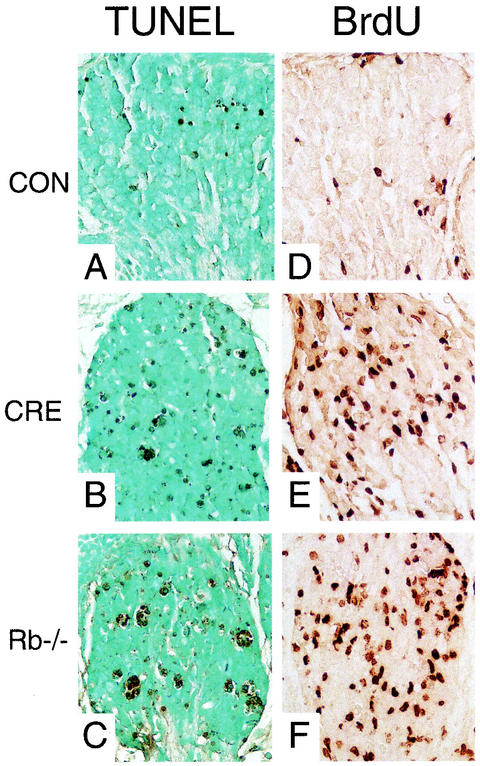
We first determined the phenotype of conditional-mutant animals at E13.5, which is the time point that Rb-null embryos normally exhibit high levels of CNS apoptosis. Strikingly, levels of apoptosis in the conditional-mutant embryos were similar to those in controls and dramatically lower than in the Rb−/− CNS (Fig. 3 and 4). The data shown are for the hindbrain, where apoptosis in Rb-null embryos is particularly high; however, equivalent suppression of apoptosis was observed throughout the conditional-mutant brain (data not shown). These data suggest that the death of Rb−/− neurons may be dependent on a hypoxic state induced by defective erythropoiesis. In contrast to the dramatic suppression of CNS apoptosis, the levels of ectopic S-phase determined by BrdU incorporation away from the ventricular zone were similar in conditional mutants compared to Rb-null embryos (Fig. 3 and 4).
FIG. 3.
Apoptosis and S-phase entry in midsagittal sections of hindbrain and fourth ventricle from control, conditional Rb mutant, and Rb-null E13.5 embryos. Hematoxylin and eosin stain (H+E) (A to C) and TUNEL staining (D to F) show apoptotic cells at a magnification of ×40. Rb-null (C and F) hindbrain has numerous darkly staining apoptotic bodies, while apoptosis in conditional-mutant (B and E) hindbrain is similar to that observed in controls (A and D). (G to I) BrdU analysis of S-phase entry at a magnification of ×40. In controls (G), BrdU-positive cells are restricted to the ventricular zone, while in conditional-mutant (H) or Rb-null (I) sections, extensive ectopic BrdU-positive cells are seen in the intermediate zone.
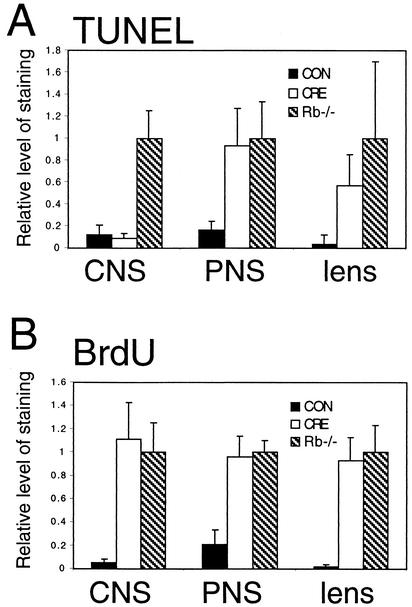
FIG. 4.
Quantitation of apoptosis and S-phase entry in Rb mutant and conditional-mutant embryos. (A) Conditional mutation of Rb leads to apoptosis in the PNS and lens but not in the CNS. Apoptotic cells were quantified as the number of TUNEL-positive nuclei per area of tissue measured in the hindbrain adjacent to the fourth ventricle, DRG, and ocular lens. Apoptosis is expressed relative to the amount seen in Rb-null embryos, which was set to 1.0. Standard deviation is indicated by error bars. (B) Extent of inappropriate S-phase entry is similar between Rb-null embryos and conditional mutants in the lens, CNS, and PNS. For the CNS, ectopic S-phase was quantified as BrdU-positive cells outside of the ventricular zone and quantified per area of tissue measured. For the PNS, overall S-phase in the DRG was quantified per area of tissue measured. For the lens, BrdU-positive cells in the lens fiber compartment were quantified per area of tissue measured. S-phase entry is expressed relative to the amount seen in Rb-null embryos, which was set to 1.0. Standard deviation is indicated by error bars. All data are from 5 to 10 groups of embryos of a given genotype.
Apoptosis in conditional-mutant PNS and lens.
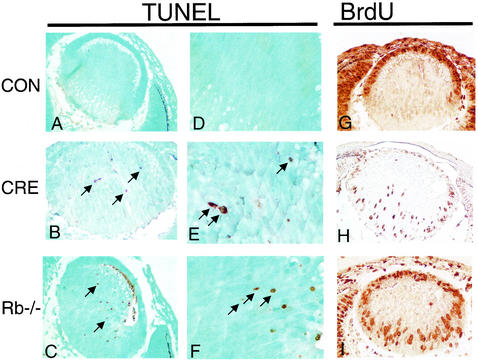
We next determined the phenotype of the conditional Rb mutant PNS and lens. In contrast to the CNS, levels of apoptosis in the conditional-mutant PNS were similar to those seen in Rb−/− embryos (Fig. 4 and 5). The data shown are for the DRG, but similar results were obtained from the trigeminal ganglia (data not shown). The DRG neurons of conditional mutants underwent inappropriate S-phase entry comparable to Rb−/− embryos (Fig. 4 and 5). In the normal lens, epithelial cells proliferate at the outer edge of the tissue and migrate posteriorly before exiting the cell cycle. Following cell cycle exit, they migrate into the interior of the lens and differentiate into lens fiber cells. BrdU-positive cells are not normally present in the lens fiber cell compartment (Fig. 6). However, in both Rb conditional-mutant and Rb-null lenses, ectopic BrdU-positive cells were readily apparent (Fig. 6). Apoptosis in the lens fiber cell compartment in Rb-null E13.5 lenses has been shown to be p53 dependent (34). Interestingly, in contrast to control lens sections, conditional-mutant lenses had significant levels of apoptosis. Apoptosis in the conditional-mutant lens was comparable, though quantitatively lower than apoptosis observed in the Rb-null embryo (Fig. 4 and 6). Thus, in the lens and PNS, loss of Rb appears to be sufficient to cause apoptosis even in the presence of a normal hematopoietic system. These data underscore the mechanistic differences in the apoptotic programs in the Rb-deficient CNS, PNS, and lens.
FIG. 5.
Apoptosis and S-phase entry in sections of DRG from control, conditional Rb mutant, and Rb-null E13.5 embryos. (A to C) TUNEL staining of E13.5 DRG at a magnification of 40×. Note the darkly staining apoptotic cells seen at increased levels in the conditional-mutant (B) and Rb-null (C) sections. (D to F) BrdU staining of E13.5 DRG at a magnification of 40× shows extensive S-phase activity in both conditional-mutant (E) and Rb-null (F) ganglia.
FIG. 6.
Apoptosis and S-phase entry in transverse sections of ocular lens from control, conditional Rb mutant, and Rb-null E13.5 embryos. TUNEL staining for apoptosis at a magnification of ×40 (A to C) or ×100 (D to F) demonstrates numerous darkly stained apoptotic nuclei (arrows) in both conditional-mutant (B and E), and Rb-null lens (C and F) sections, but not in controls (A). BrdU staining at a magnification of ×40 (G to I) demonstrates ectopic S-phase entry in the lens fiber cell compartment in lenses from both conditional mutants (H) and Rb-null (I) embryos, whereas BrdU-positive cells are restricted from this compartment in control sections (G).
Rescue of development with skeletal muscle defects in Rb conditional mutants.
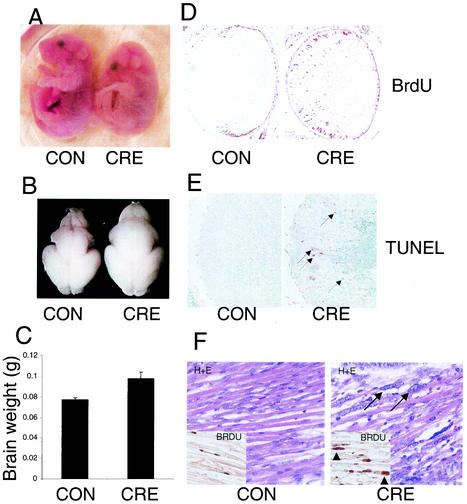
To determine if the normal erythroid development would affect the survival of Rb conditional mutants, we collected embryos throughout gestation and determined the frequency of different genotypes recovered. Conditional Rb mutant embryos were found in Mendelian ratios throughout embryonic development, including those collected at E18.5 (data not shown). At E18.5, conditional-mutant embryos were not visibly pale but could be clearly distinguished from their littermates by their hunched appearance (Fig. 7A). Conditional-mutant embryos were observed to be born alive but died within 30 min following birth. We were interested in the consequence of aberrant S-phase activity in the E18.5 brain, due to the observed increased S-phase entry without apoptosis in earlier developmental stages. At E18.5, brains from conditional Rb mutant animals were visibly larger than littermate controls (Fig. 7B). Recently a telencephalon-specific Rb knockout was generated, which had shown cell survival in the telencephalon and increased cortical size (12). In the whole-brain Rb conditional mutants we have described, effects of Rb loss on brain size were not restricted to the telencephalon, as most of the brain appeared enlarged (Fig. 7B and data not shown). Measurements of brain weights (Fig. 7C) indicated that conditional-mutant brains weighed 27% more than controls (P = 0.001). There was no evidence of hydrocephalus in these mutant brains (not shown). We are currently investigating the effects of Rb absence on regional brain development and cortical lamination in these conditional-mutant brains.
FIG. 7.
Phenotype of conditional-mutant embryos at E18.5. (A) Picture of E18.5 control (CON) and conditional-mutant (CRE) animals. Note the hunched appearance of the conditional-mutant embryo. (B) Conditional-mutant brains are visibly larger than those of littermate controls. (C) Measurement of brain weights indicates that conditional-mutant brains weigh 27% more than littermate controls (t test; P = 0.001). (D) BrdU analysis of ocular lens demonstrates continued ectopic S-phase entry in the conditional-mutant (CRE). (E) TUNEL analysis of lens shows high levels of apoptosis (arrows) in conditional-mutant lens (CRE). (F) Hematoxylin-eosin (H+E)-stained sections of axial skeletal muscle from E18.5 control (CON) and conditional-mutant (CRE) animals demonstrating defective muscle differentiation in conditional mutants. Note the enlarged atypical nuclei (arrows) and abnormal rows of adjacent nuclei apparent in the conditional-mutant sections. The inset shows BrdU analysis demonstrating active DNA-synthesis in abnormally large nuclei in conditional mutant (arrowheads).
At E18.5, phenotypes were also observed in the ocular lens and skeletal muscle. In the lens, BrdU analysis indicated that cells continued to enter S-phase ectopically (Fig. 7D). TUNEL analysis indicated that conditional mutants continued to show high levels of apoptosis (Fig. 7E). Importantly, partial recombination in skeletal muscle has been reported with the Nes-Cre1 transgene used here (11), and we also observed decreased pRB levels in conditional-mutant skeletal muscle compared to controls (Fig. 2C). In late stages of development in conditional embryos, impairment of skeletal muscle differentiation was evident (Fig. 7F). Areas of conditional Rb mutant skeletal muscle at E18.5 had strikingly abnormal large nuclei, and the arrangement of myotubes appeared diffuse (Fig. 7f). BrdU analysis demonstrated that some of the abnormally large nuclei apparent in these mutants exhibited inappropriate S-phase activity. (Fig. 7f). We also observed abnormal rows of adjacent nuclei in conditional-mutant myotubes that were not apparent in controls. We have not determined the cause of lethality of the conditional-mutant animals. Perinatal lethality of Rb mutants with development rescued through expression of low levels of Rb with an Rb transgene (52) or with Id2 deficiency (25) had been ascribed to muscle defects affecting respiration. However, given the pertubation in brain development observed in the conditional mutants here, it is also possible that deletion of Rb in the brain may impair nervous system control of respiration or other vital nervous system functions.
DISCUSSION
In this study we have demonstrated that the CNS tissue of Rb−/− embryos shows increased expression of the hypoxia-inducible genes LDH and VEGF, and using a conditional-deletion strategy, we have shown that Rb−/− CNS neurons are spared from apoptosis in an embryo whose erythroid development is normal. Thus, we conclude that hypoxia secondary to the erythroid defect is a likely cofactor in the death of these Rb−/− cells. Importantly, the cell cycle defects associated with Rb mutation in the CNS were still evident in the conditional mutants, supporting the contention that these are cell autonomous. While this work was in preparation, another group reported that conditional mutation of Rb in the telencephalon region of the brain led to inappropriate S-phase entry of Rb-mutant cells without high levels of apoptosis (12). The reason for the strikingly different phenotypes observed in the telencephalon of germ line Rb mutants and telencephalon-specific conditional Rb mutants was not determined. Our study extends the phenotype reported in the conditional-mutant telencephalon and demonstrates cell survival throughout the E13.5 brain, with increased brain size observed at E18.5. In addition, we provide evidence that the germ line mutant Rb brain is under hypoxic stress that is relieved with conditional mutation of Rb. Our findings in the CNS and the results of the telencephalon-specific Rb deletion (12) raised the possibility that apoptosis in other tissues in germ line Rb mutant embryos may also be dependent on signals such as hypoxia carried by the blood. The tissue pattern of Rb deletion using the Nes-Cre1 transgene allowed us to determine if deletion of Rb in the PNS and lens would lead to cell death in the presence of normal hematopoiesis. Consistent with previous work that has uncovered differences in the apoptotic program among different cell types in the Rb−/− embryo, we found significant cell death in the PNS and lens of conditional Rb mutants.
We have previously reported that in E13.5 chimeric embryos composed of both wild-type and Rb-null cells, there was suppression of CNS apoptosis (30) without rescue of inappropriate S-phase entry. There were a number of possible explanations for these findings. For example, in such chimeras, absence of Rb in the cells of the developing CNS might directly trigger the apoptotic program (perhaps as a consequence of cell cycle dysfunction), but this program may be repressed by survival signals stemming from wild-type cells. These survival signals could have originated from cells in the CNS itself in the form of secreted or cell-surface factors. Alternatively, such survival signals could derive from wild-type cells outside of the CNS and be transmitted to the CNS from a distance via the blood. The data from chimeras could also be explained by the existence of proapoptotic factors produced by Rb−/− cells. In this scenario, apoptosis in Rb−/− CNS neurons would be explained by a high-level of such a factor, which could have been produced locally or at a distance. In Rb+/+:Rb−/− chimeras, however, this factor might be diluted below a critical threshold needed for induction of apoptosis.
By using the Nes-Cre1 transgene, we were able to remove Rb throughout the E13.5 brain (Fig. 2c) and observed absence of increased apoptosis in this tissue. The widespread deletion of Rb in the CNS allows us to exclude the possibility that survival signals were sent from wild-type cells in the brain. Also, we can now conclude that CNS apoptosis in Rb−/− embryos is due to the production of a pro-apoptotic signal, and we propose that this signal is hypoxia. Hypoxia has been demonstrated to be an inducer of p53 in neurons and other cell types (3, 16, 28, 50), and there is clear severe anemia and defective development of erythrocytes in Rb−/− embryos. Indeed, the anemia is thought to be responsible for the death of midgestation Rb−/− embryos, and we have demonstrated that conditional Rb mutants with normal erythropoiesis develop until birth. There is also indirect evidence of hypoxia in Rb−/− embryos, with the overexpression of VEGF and LDH-A observed only in germ line Rb mutants but not in the conditional-mutant CNS. We support the hypothesis that the defects in erythropoiesis cause hypoxia, leading to upregulation of VEGF and LDH-A as well as apoptosis. However, it is possible that other unknown cell-extrinsic signals could cause induction of VEGF and LDH-A, and it is still unresolved whether the presence or absence of other signals present in developing Rb−/− blood system might contribute to the death of CNS neurons.
Another unresolved issue is whether Rb-deficient neurons may be sensitized to hypoxia-mediated apoptosis. Rb−/− cells in the CNS show inappropriate S-phase entry that conceivably could make these cells sensitive to hypoxia-induced apoptosis. In cell culture, hypoxia treatment was demonstrated to lead to p53 induction specifically in an S-phase-enriched cell population (19). The mechanism for p53 activation in S-phase was proposed to be via a hypoxia-induced replication arrest and subsequent activation of the ATR kinase upstream of p53 (19). While primary cells in culture have been shown to be resistant to hypoxia-mediated apoptosis, oncogenic transformation of such cells, involving disruption of pRB family function through E1A expression, can confer strong sensitivity to hypoxia-mediated, p53-dependent apoptosis (15). It is possible that loss of cell cycle control in Rb mutants may sensitize these cells to hypoxia-mediated apoptosis. Emerging evidence also suggests that in some systems pRB plays an antiapoptotic role that may be broader than effects of Rb on the cell cycle. For example, exposure of cultured postmitotic neurons to DNA-damaging agents led to rapid phosphorylation of pRB by cyclin-dependent kinases prior to apoptosis (37, 38). Importantly, introduction of a phosphorylation-resistant Rb mutant conferred protection against DNA-damage-induced apoptosis, suggesting that phosphorylation of pRB was important for the progression of apoptosis (31, 37). It has also been proposed that caspase cleavage and subsequent degradation of pRB early in apoptosis is an important event in the execution of apoptosis (8, 23, 42). In cultured neurons and MEFs, introduction of a cleavage-resistant pRB mutant also conferred strong protection against apoptosis (6, 42). pRB-mediated suppression of apoptotic factors may be lost with pRB cleavage or when Rb is constitutively absent through germ line mutation. The proapoptotic signals repressed by pRB are not known; however, in theory such signals could be enhanced in Rb−/− embryos and sensitize neurons to apoptosis. Similarly, Rb−/− MEFs exhibit sensitization to apoptosis induced by DNA damage or growth factor withdrawal (1).
The phenotype of Rb mutant embryos has been used in extensive genetic analyses that have helped define the genetic pathways converging on apoptosis upon Rb loss. In the CNS and lens, E2f1, E2f3, p53, and Apaf1 have all been shown to be required for apoptosis (17, 33, 34, 44, 54). The data presented here may necessitate reinterpretation of these results. For example, given the rescue of both the inappropriate S-phase and apoptosis in the CNS when both E2f1 and Rb were mutated together, it was concluded that inappropriate S-phase through deregulation of E2f activity led to p53 activation (44). Links between deregulated E2F and p53-dependent apoptosis have been found in both cell culture systems and transgenic animals. For example, choroid plexus expression of a fragment of simian virus 40 large T that targets Rb family members but not p53 leads to proliferation and p53-dependent apoptosis, which were both suppressed on an E2f1-deficient background (36). In cell culture, overexpression of E2f1 leads to upregulation of Arf- and p53-dependent apoptosis (5, 24, 40, 49). We subsequently searched for E2F targets that may directly mediate p53 activation in Rb-null embryos and found that the E2F target ARF was not required for p53-dependent apoptosis in the CNS (45). In the CNS, it now appears that the rescue of apoptosis observed with Rb/E2f1 compound mutation may be explained by indirect effects on erythropoiesis. Compound mutation of Rb and E2f1 led to a partial rescue of erythropoiesis at E13.5 (44). However, in contrast to the conditional mutants described here, by E17.0 compound Rb/E2f1 animals appeared anemic and died, indicating that rescue of erythropoiesis was incomplete. We propose that the partial rescue of erythropoiesis in Rb/E2f1 mutants may have been sufficient to reduce the hypoxic stress needed for apoptosis in Rb−/− neurons.
Interestingly, the complete rescue of CNS apoptosis by Rb/E2f1 compound mutation was accompanied by only partial rescue of apoptosis in the PNS (44). Similarly, it was striking that even though apoptosis in the CNS occurred at normal levels in Rb conditional mutants, there was a clear increase in apoptosis in the conditional-mutant PNS. The pathway leading to apoptosis in the Rb−/− PNS is largely undefined. It is known that levels of apoptosis are roughly correlated with levels of inappropriate S-phase entry, that p53 is dispensable for this death, and that caspase3 is required in this system (33, 41). Our present study further confirms that the pathways to apoptosis in the Rb−/− CNS and PNS are functionally distinct. Levels of apoptosis were similar to those in germ line Rb mutants, suggesting that in contrast to CNS neurons, conditional Rb-deficient PNS neurons were not sensitized to apoptosis. The finding that neurons in the PNS were not sensitized to apoptosis induced by the cell-extrinsic signals in germ line Rb mutants indicates that that the apoptotic machinery may be profoundly different in the developing Rb−/− CNS versus PNS. Interestingly, mutation of caspase 3 did not inhibit the CNS apoptosis in germ line Rb−/− embryos but completely inhibited the PNS apoptosis (41). Differences in the importance and amounts of various apoptotic factors such as caspase 3 across different cell types could very well control the life or death of the cell in response to hypoxia or other apoptotic stresses. Therefore, future work elucidating the apoptotic programs activated in response to Rb loss in the different cell types could help us define the basis for these cell type differences. Note that because the Nes-Cre1 transgenic mouse is not specific to the nervous system, we cannot rule out the possibility that deletion of Rb in an unknown tissue compartment could contribute to PNS apoptosis. It will be important to determine the genetics of this p53-independent apoptosis, which appears to be cell autonomous. Because tumors that have mutated p53 are often resistant to chemotherapy, elucidating mechanisms to induce apoptosis in proliferating cells independent of p53 function may be relevant to chemotherapeutic development.
Our findings in the lens further illustrate that the pathway leading to apoptosis upon Rb loss differs in different cell types. p53-dependent apoptosis in Rb conditional-mutant lens still occurred with rescue of the erythropoietic defect, indicating that the pathway upstream of p53 is different in the Rb-null lens versus CNS. In the Rb-deficient lens, previous findings that E2f1 or E2f3 loss led to a rescue of apoptosis point to a role for E2f1 and E2f3 in p53 activation that is more direct than the pathway leading to p53 in the CNS. The findings here related to the Rb-deficient lens agree with our previous observations that adult chimeras composed of Rb−/− and wild-type cells had high levels of apoptosis in the lenses (48), and we also observed high levels of apoptosis in late-gestation E18.5 conditional-mutant lenses (Fig. 7e). Disruption of Rb family function in the lens by expression of the HPV E7 oncoprotein has also been shown to cause lens cell proliferation and apoptosis (35). Apoptosis in this setting was also suppressed by removal of p53 function through E6 expression.
Hypoxia in tumors that have mutated the Rb pathway may indeed be a source of selective pressure for loss of p53 as a means to evade apoptosis. Importantly, hypoxia treatment of oncogenically transformed MEFs in vitro led to selection for cells with mutated p53 (15). In tumors, apoptosis was seen in regions of hypoxia, while p53-null tumors were resistant, suggesting that tumors select for loss of p53 to become resistant to hypoxia-induced apoptosis (15). It may be interesting to search for genetic links between hypoxia and p53-dependent apoptosis in embryos lacking Rb. For example, HIF1α has been implicated in p53 signaling downstream of hypoxia (2, 7), and it would be interesting to know if the pathway to apoptosis in the Rb-deficient CNS is HIF1α dependent. It will also be important to determine if kinases upstream of p53 activation are involved in signaling to p53 in this system. Dissecting the pathways leading to CNS apoptosis in Rb−/− mouse embryos may help to elucidate the pathways that connect hypoxia to p53 activation and selection for p53 mutation in human tumors.
Acknowledgments
A.T. is supported by the Swiss National Science Foundation and Swiss Cancer League. T.J. is an investigator of the Howard Hughes Medical Institute.
REFERENCES
- 1.Almasan, A., Y. Yin, R. E. Kelly, E. Y. Lee, A. Bradley, W. Li, J. R. Bertino, and G. M. Wahl. 1995. Deficiency of retinoblastoma protein leads to inappropriate S-phase entry, activation of E2F-responsive genes, and apoptosis. Proc. Natl. Acad. Sci. USA 92:5436-5440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.An, W. G., M. Kanekal, M. C. Simon, E. Maltepe, M. V. Blagosklonny, and L. M. Neckers. 1998. Stabilization of wild-type p53 by hypoxia-inducible factor 1α. Nature 392:405-408. [DOI] [PubMed] [Google Scholar]
- 3.Banasiak, K. J., and G. G. Haddad. 1998. Hypoxia-induced apoptosis: effect of hypoxic severity and role of p53 in neuronal cell death. Brain Res. 797:295-304. [DOI] [PubMed] [Google Scholar]
- 4.Bates, B., M. Rios, A. Trumpp, C. Chen, G. Fan, J. M. Bishop, and R. Jaenisch. 1999. Neurotrophin-3 is required for proper cerebellar development. Nat. Neurosci. 2:115-117. [DOI] [PubMed] [Google Scholar]
- 5.Bates, S., A. C. Phillips, P. A. Clark, F. Stott, G. Peters, R. L. Ludwig, and K. H. Vousden. 1998. p14ARF links the tumour suppressors RB and p53. Nature 395:124-125. [DOI] [PubMed] [Google Scholar]
- 6.Boutillier, A. L., E. Trinh, and J. P. Loeffler. 2000. Caspase-dependent cleavage of the retinoblastoma protein is an early step in neuronal apoptosis. Oncogene 19:2171-2178. [DOI] [PubMed] [Google Scholar]
- 7.Carmeliet, P., Y. Dor, J. M. Herbert, D. Fukumura, K. Brusselmans, M. Dewerchin, M. Neeman, F. Bono, R. Abramovitch, P. Maxwell, C. J. Koch, P. Ratcliffe, L. Moons, R. K. Jain, D. Collen, E. Keshert, and E. Keshet. 1998. Role of HIF-1alpha in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature 394:485-490. [DOI] [PubMed] [Google Scholar]
- 8.Chen, W. D., G. A. Otterson, S. Lipkowitz, S. N. Khleif, A. B. Coxon, and F. J. Kaye. 1997. Apoptosis is associated with cleavage of a 5 kDa fragment from RB which mimics dephosphorylation and modulates E2F binding. Oncogene 14:1243-1248. [DOI] [PubMed] [Google Scholar]
- 9.Clarke, A. R., E. R. Maandag, M. van Roon, N. M. van der Lugt, M. van der Valk, M. L. Hooper, A. Berns, and H. te Riele. 1992. Requirement for a functional Rb-1 gene in murine development. Nature 359:328-330. [DOI] [PubMed] [Google Scholar]
- 10.Dyson, N. 1998. The regulation of E2F by pRB-family proteins. Genes Dev. 12:2245-2262. [DOI] [PubMed] [Google Scholar]
- 11.Fan, G., C. Beard, R. Z. Chen, G. Csankovszki, Y. Sun, M. Siniaia, D. Biniszkiewicz, B. Bates, P. P. Lee, R. Kuhn, A. Trumpp, C. Poon, C. B. Wilson, and R. Jaenisch. 2001. DNA hypomethylation perturbs the function and survival of CNS neurons in postnatal animals. J. Neurosci. 21:788-797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferguson, K. L., J. L. Vanderluit, J. M. Hebert, W. C. McIntosh, E. Tibbo, J. G. MacLaurin, D. S. Park, V. A. Wallace, M. Vooijs, S. K. McConnell, and R. S. Slack. 2002. Telencephalon-specific Rb knockouts reveal enhanced neurogenesis, survival and abnormal cortical development. EMBO J. 21:3337-3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gallie, B. L., C. Campbell, H. Devlin, A. Duckett, and J. A. Squire. 1999. Developmental basis of retinal-specific induction of cancer by RB mutation. Cancer Res. 59:1731s-1735s. [PubMed] [Google Scholar]
- 14.Gavrieli, Y., Y. Sherman, and S. A. Ben-Sasson. 1992. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J. Cell Biol. 119:493-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Graeber, T. G., C. Osmanian, T. Jacks, D. E. Housman, C. J. Koch, S. W. Lowe, and A. J. Giaccia. 1996. Hypoxia-mediated selection of cells with diminished apoptotic potential in solid tumours. Nature 379:88-91. [DOI] [PubMed] [Google Scholar]
- 16.Graeber, T. G., J. F. Peterson, M. Tsai, K. Monica, A. J. Fornace, Jr., and A. J. Giaccia. 1994. Hypoxia induces accumulation of p53 protein, but activation of a G1-phase checkpoint by low-oxygen conditions is independent of p53 status. Mol. Cell. Biol. 14:6264-6277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo, Z., S. Yikang, H. Yoshida, T. W. Mak, and E. Zacksenhaus. 2001. Inactivation of the retinoblastoma tumor suppressor induces apoptosis protease-activating factor-1 dependent and independent apoptotic pathways during embryogenesis. Cancer Res. 61:8395-8400. [PubMed] [Google Scholar]
- 18.Halterman, M. W., C. C. Miller, and H. J. Federoff. 1999. Hypoxia-inducible factor-1alpha mediates hypoxia-induced delayed neuronal death that involves p53. J. Neurosci. 19:6818-6824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hammond, E. M., N. C. Denko, M. J. Dorie, R. T. Abraham, and A. J. Giaccia. 2002. Hypoxia links ATR and p53 through replication arrest. Mol. Cell. Biol. 22:1834-1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu, N., M. L. Gulley, J. T. Kung, and E. Y. Lee. 1997. Retinoblastoma gene deficiency has mitogenic but not tumorigenic effects on erythropoiesis. Cancer Res. 57:4123-4129. [PubMed] [Google Scholar]
- 21.Hu, N., A. Gutsmann, D. C. Herbert, A. Bradley, W. H. Lee, and E. Y. Lee. 1994. Heterozygous Rb-1 delta 20/+ mice are predisposed to tumors of the pituitary gland with a nearly complete penetrance. Oncogene 9:1021-1027. [PubMed] [Google Scholar]
- 22.Jacks, T., A. Fazeli, E. M. Schmitt, R. T. Bronson, M. A. Goodell, and R. A. Weinberg. 1992. Effects of an Rb mutation in the mouse. Nature 359:295-300. [DOI] [PubMed] [Google Scholar]
- 23.Janicke, R. U., P. A. Walker, X. Y. Lin, and A. G. Porter. 1996. Specific cleavage of the retinoblastoma protein by an ICE-like protease in apoptosis. EMBO J. 15:6969-6978. [PMC free article] [PubMed] [Google Scholar]
- 24.Kowalik, T. F., J. DeGregori, G. Leone, L. Jakoi, and J. R. Nevins. 1998. E2F1-specific induction of apoptosis and p53 accumulation, which is blocked by Mdm2. Cell Growth Differ. 9:113-118. [PubMed] [Google Scholar]
- 25.Lasorella, A., M. Noseda, M. Beyna, Y. Yokota, and A. Iavarone. 2000. Id2 is a retinoblastoma protein target and mediates signalling by Myc oncoproteins. Nature 407:592-598. [DOI] [PubMed] [Google Scholar]
- 26.Lee, E. Y., C. Y. Chang, N. Hu, Y. C. Wang, C. C. Lai, K. Herrup, W. H. Lee, and A. Bradley. 1992. Mice deficient for Rb are nonviable and show defects in neurogenesis and haematopoiesis. Nature 359:288-294. [DOI] [PubMed] [Google Scholar]
- 27.Lee, E. Y., N. Hu, S. S. Yuan, L. A. Cox, A. Bradley, W. H. Lee, and K. Herrup. 1994. Dual roles of the retinoblastoma protein in cell cycle regulation and neuron differentiation. Genes Dev. 8:2008-2021. [DOI] [PubMed] [Google Scholar]
- 28.Li, Y., M. Chopp, Z. G. Zhang, C. Zaloga, L. Niewenhuis, and S. Gautam. 1994. p53-immunoreactive protein and p53 mRNA expression after transient middle cerebral artery occlusion in rats. Stroke 25:849-856. [DOI] [PubMed] [Google Scholar]
- 29.Lipinski, M. M., and T. Jacks. 1999. The retinoblastoma gene family in differentiation and development. Oncogene 18:7873-7882. [DOI] [PubMed] [Google Scholar]
- 30.Lipinski, M. M., K. F. Macleod, B. O. Williams, T. L. Mullaney, D. Crowley, and T. Jacks. 2001. Cell-autonomous and non-cell-autonomous functions of the Rb tumor suppressor in developing central nervous system. EMBO J. 20:3402-3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu, D. X., and L. A. Greene. 2001. Regulation of neuronal survival and death by E2F-dependent gene repression and derepression. Neuron 32:425-438. [DOI] [PubMed] [Google Scholar]
- 32.Maandag, E. C., M. van der Valk, M. Vlaar, C. Feltkamp, J. O'Brien, M. van Roon, N. van der Lugt, A. Berns, and H. te Riele. 1994. Developmental rescue of an embryonic-lethal mutation in the retinoblastoma gene in chimeric mice. EMBO J. 13:4260-4268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Macleod, K. F., Y. Hu, and T. Jacks. 1996. Loss of Rb activates both p53-dependent and independent cell death pathways in the developing mouse nervous system. EMBO J. 15:6178-6188. [PMC free article] [PubMed] [Google Scholar]
- 34.Morgenbesser, S. D., B. O. Williams, T. Jacks, and R. A. DePinho. 1994. p53-dependent apoptosis produced by Rb-deficiency in the developing mouse lens. Nature 371:72-74. [DOI] [PubMed] [Google Scholar]
- 35.Pan, H., and A. E. Griep. 1994. Altered cell cycle regulation in the lens of HPV-16 E6 or E7 transgenic mice: implications for tumor suppressor gene function in development. Genes Dev. 8:1285-1299. [DOI] [PubMed] [Google Scholar]
- 36.Pan, H., C. Yin, N. J. Dyson, E. Harlow, L. Yamasaki, and T. Van Dyke. 1998. Key roles for E2F1 in signaling p53-dependent apoptosis and in cell division within developing tumors. Mol. Cell 2:283-292. [DOI] [PubMed] [Google Scholar]
- 37.Park, D. S., E. J. Morris, R. Bremner, E. Keramaris, J. Padmanabhan, M. Rosenbaum, M. L. Shelanski, H. M. Geller, and L. A. Greene. 2000. Involvement of retinoblastoma family members and E2F/DP complexes in the death of neurons evoked by DNA damage. J. Neurosci. 20:3104-3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park, D. S., E. J. Morris, J. Padmanabhan, M. L. Shelanski, H. M. Geller, and L. A. Greene. 1998. Cyclin-dependent kinases participate in death of neurons evoked by DNA-damaging agents. J. Cell Biol. 143:457-467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pomerantz, J., N. Schreiber-Agus, N. J. Liegeois, A. Silverman, L. Alland, L. Chin, J. Potes, K. Chen, I. Orlow, H. W. Lee, C. Cordon-Cardo, and R. A. DePinho. 1998. The Ink4a tumor suppressor gene product, p19Arf, interacts with MDM2 and neutralizes MDM2's inhibition of p53. Cell 92:713-723. [DOI] [PubMed] [Google Scholar]
- 40.Qin, X. Q., D. M. Livingston, W. G. Kaelin, Jr., and P. D. Adams. 1994. Deregulated transcription factor E2F-1 expression leads to S-phase entry and p53-mediated apoptosis. Proc. Natl. Acad. Sci. USA 91:10918-10922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Simpson, M. T., J. G. MacLaurin, D. Xu, K. L. Ferguson, J. L. Vanderluit, M. A. Davoli, S. Roy, D. W. Nicholson, G. S. Robertson, D. S. Park, and R. S. Slack. 2001. Caspase 3 deficiency rescues peripheral nervous system defect in retinoblastoma nullizygous mice. J. Neurosci. 21:7089-7098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tan, X., S. J. Martin, D. R. Green, and J. Y. Wang. 1997. Degradation of retinoblastoma protein in tumor necrosis factor- and CD95-induced cell death. J. Biol. Chem. 272:9613-9616. [DOI] [PubMed] [Google Scholar]
- 43.Trumpp, A., M. J. Depew, J. L. Rubenstein, J. M. Bishop, and G. R. Martin. 1999. Cre-mediated gene inactivation demonstrates that FGF8 is required for cell survival and patterning of the first branchial arch. Genes Dev. 13:3136-3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tsai, K. Y., Y. Hu, K. F. Macleod, D. Crowley, L. Yamasaki, and T. Jacks. 1998. Mutation of E2f-1 suppresses apoptosis and inappropriate S phase entry and extends survival of Rb-deficient mouse embryos. Mol. Cell 2:293-304. [DOI] [PubMed] [Google Scholar]
- 45.Tsai, K. Y., D. MacPherson, D. A. Rubinson, D. Crowley, and T. Jacks. 2002. ARF is not required for apoptosis in Rb mutant mouse embryos. Curr. Biol. 12:159-163. [DOI] [PubMed] [Google Scholar]
- 46.Weinberg, R. A. 1995. The retinoblastoma protein and cell cycle control. Cell 81:323-330. [DOI] [PubMed] [Google Scholar]
- 47.Williams, B. O., L. Remington, D. M. Albert, S. Mukai, R. T. Bronson, and T. Jacks. 1994. Cooperative tumorigenic effects of germline mutations in Rb and p53. Nat. Genet. 7:480-484. [DOI] [PubMed] [Google Scholar]
- 48.Williams, B. O., E. M. Schmitt, L. Remington, R. T. Bronson, D. M. Albert, R. A. Weinberg, and T. Jacks. 1994. Extensive contribution of Rb-deficient cells to adult chimeric mice with limited histopathological consequences. EMBO J. 13:4251-4259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu, X., and A. J. Levine. 1994. p53 and E2F-1 cooperate to mediate apoptosis. Proc. Natl. Acad. Sci. USA 91:3602-3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yamaguchi, A., M. Taniguchi, O. Hori, S. Ogawa, N. Tojo, N. Matsuoka, S. Miyake, K. Kasai, H. Sugimoto, M. Tamatani, T. Yamashita, and M. Tohyama. 2002. Peg3/Pw1 is involved in p53-mediated cell death pathway in brain ischemia/hypoxia. J. Biol. Chem. 277:623-629. [DOI] [PubMed] [Google Scholar]
- 51.Yang, J., W. Bian, X. Gao, L. Chen, and N. Jing. 2000. Nestin expression during mouse eye and lens development. Mech. Dev. 94:287-291. [DOI] [PubMed] [Google Scholar]
- 52.Zacksenhaus, E., Z. Jiang, D. Chung, J. D. Marth, R. A. Phillips, and B. L. Gallie. 1996. pRb controls proliferation, differentiation, and death of skeletal muscle cells and other lineages during embryogenesis. Genes Dev. 10:3051-3064. [DOI] [PubMed] [Google Scholar]
- 53.Zhu, Y., X. O. Mao, Y. Sun, Z. Xia, and D. A. Greenberg. 2002. p38 mitogen-activated protein kinase mediates hypoxic regulation of Mdm2 and p53 in neurons. J. Biol. Chem. 277:22909-22914. [DOI] [PubMed] [Google Scholar]
- 54.Ziebold, U., T. Reza, A. Caron, and J. A. Lees. 2001. E2F3 contributes both to the inappropriate proliferation and to the apoptosis arising in Rb mutant embryos. Genes Dev. 15:386-391. [DOI] [PMC free article] [PubMed] [Google Scholar]