Abstract
Minichromosomes assembled on the mouse mammary tumor virus (MMTV) promoter in vitro exhibit positioned nucleosomes, one of which covers the binding sites for progesterone receptor (PR) and nuclear factor 1 (NF1). Incorporation of histone H1 into MMTV minichromosomes improves the stability of this nucleosome and decreases basal transcription from the MMTV promoter, as well as its response to either PR or NF1. However, histone H1-containing minichromosomes display better PR binding and support a more efficient synergism between PR and NF1, leading to enhanced transcription initiation. A mutant MMTV promoter lacking positioned nucleosomes does not display enhanced transcriptional synergism in the presence of H1. Binding of PR leads to phosphorylation of H1, which leaves the promoter upon transcription initiation. Thus, H1 assumes a complex and dynamic role in the regulation of the MMTV promoter.
Keywords: H1 phosphorylation/linker histone/nucleosome structure/progesterone receptor/transcriptional synergism
Introduction
The fundamental unit of eukaryotic chromatin is the nucleosome, which consists of a core histone octamer with 147 bp of superhelical DNA wrapped around in 1.65 left-handed turns (Richmond et al., 1984; Luger et al., 1997). A chain of nucleosomes connected by linker DNA builds the 10 nm nucleosomal fiber, which is compacted further in interphase nuclei to form a more irregular 30 nm fiber, stabilized by interactions between nucleosomes and by the so-called linker histones. Linker histones exist in a variety of subtypes (e.g. H1, H1o, H5, etc.; reviewed in Khochbin, 2001; Parseghian and Hamkalo, 2001). Binding of histone H1 stabilizes the nucleosome and protects an additional 20–30 bp of linker DNA against micrococcal nuclease (MNase) digestion to form a structure called the chromatosome (Simpson, 1978). Interactions among linker histones further stabilize and compact the higher order structure of chromatin (Ramakrishnan, 1997; Widom, 1998). As transcriptional activation requires decondensation of chromatin structures (Gregory and Hörz, 1998), histone H1 has long been regarded as a general repressor of transcription (Weintraub, 1985). However, there are indications of a more differential role of H1 in transcriptional regulation (Zlatanova, 1990).
Several years ago, it was shown that H1 regulates individual genes in two different systems: Xenopus laevis and Tetrahymena thermophyla. Disruption of the H1 gene in macronuclei of Tetrahymena displays a specific rather than a global effect on gene expression (Shen and Gorovsky, 1996). In Xenopus, the oocyte and somatic type 5S rRNA genes are transcribed differentially during early development. Repression of oocyte 5S RNA transcription coincides with the appearance of histone H1A during the early gastrula stage (Kandolf, 1994). The increase in histone H1 content specifically restricts TFIIIA-activated transcription of oocyte 5S rRNA but does not significantly influence somatic 5S rRNA gene transcription. Thus, the regulated expression of histone H1 during Xenopus development has a specific and dominant role in mediating the differential expression of the oocyte and somatic 5S rRNA genes (Bouvet et al., 1994). One model proposes that H1 relocates the multiple octamer positions over the oocyte 5S RNA gene, some of which allow TFIIIA binding, to a unique translational position incompatible with TFIIIA binding (Sera and Wolffe, 1998). An effect of linker histone on the distribution of nucleosome positions has also been shown by others (Meersseman et al., 1991). A second model proposes differential nucleosome positioning on somatic and oocyte 5S rRNA genes per se, which favor either H1 or TFIIIA binding (Panetta et al., 1998; Crane-Robinson, 1999). Both models underline the importance of nucleosome positioning for gene regulation.
Although chromatin generally is viewed as an obstacle for transcription that has to be remodeled or modified in the process of transcriptional activation, we have shown previously that a well-organized chromatin template is a necessary prerequisite for the proper activation of the mouse mammary tumor virus (MMTV) promoter (Chávez and Beato, 1997). The MMTV promoter is organized into positioned nucleosomes in vivo (Richard-Foy and Hager, 1987) and in vitro (Perlmann and Wrange, 1988), with a nucleosome covering the five hormone-responsive elements (HREs) and the binding site for nuclear factor 1 (NF1) (Piña et al., 1990; Truss et al., 1995). The binding site for NF1 is not accessible on this positioned nucleosome, and only two of the five HREs can be bound by hormone receptors (Piña et al., 1990; Eisfeld et al., 1997). The MMTV promoter assembled in minichromosomes also exhibits positioned nucleosomes (Venditti et al., 1998) and is activated in a process involving a two-step synergism. First, the hormone receptor binds to the exposed HREs and triggers a chromatin-remodeling event that facilitates access of NF1. Bound NF1 in turn stabilizes an open nucleosomal conformation required for efficient binding of further receptor molecules to the hidden HREs and full transactivation (Di Croce et al., 1999).
A few years ago, it was shown that H1 overexpression in 3T3 cells led to an increase in transcriptional activity of integrated, but not transient transfected MMTV promoters (Gunjan and Brown, 1999). These results suggested that histone H1 played a positive role in MMTV induction depending on its chromatin organization, but did not prove that the effect was a direct consequence of H1 incorporation into the MMTV promoter chromatin. Here, we show that a similar H1-dependent increase in transcriptional activation takes place on MMTV minichromosomes assembled in vitro and that this increase is a direct effect of H1 binding to the MMTV promoter. Addition of H1 to an in vitro chromatin reconstitution system based on extracts from pre-blastodermic Drosophila embryos, which do not contain H1, leads to tightening and stabilization of the nucleosome covering the regulatory elements of the MMTV promoter, which reduces accessibility for restriction enzymes and NF1. However, incorporation of histone H1 in minichromosomes leads to a better binding of PR and to improved synergism between PR and NF1, resulting in enhanced transcription initiation. On a truncated MMTV promoter, which displays no proper nucleosome positioning (Prado et al., 2002), H1 has no positive effect on the synergism between PR and NF1, but rather leads to a general reduction in transcriptional activation. Our results suggest that the positive effect of H1 on transcriptional activation of the MMTV promoter is at least partly due to a more homogeneous nucleosomal organization, which favors PR binding to a larger proportion of MMTV promoters and enhances the functional synergism between PR and NF1. Following PR binding, histone H1 is phosphorylated and is displaced from MMTV promoter chromatin upon activation of transcription initiation.
Results
Incorporation of histone H1 reduces basal transcription and the activation by either PR or NF1
The extract of pre-blastodermic Drosophila embryos (DREX) used for assembly of minichromosomes is known to lack histone H1, but contains HMG-D, a Drosophila member of the HMG 1 protein family. It is only after the mid-blastula transition that HMG-D is replaced progressively by histone H1 (Ner and Travers, 1994). We have confirmed the lack of histone H1 in our extract by western blotting using post-blastoderm extract as a positive control (data not shown). When histone H1 was added at the onset of a chromatin reconstitution on the plasmid pMMTVCAT B-B, an increase of the nucleosome repeat length from 185 bp (without H1) to 210 bp was observed at a calculated ratio of one molecule of H1 per nucleosome (Figure 1). In a chromatin immunoprecipitation (ChIP) experiment with an H1-specific antibody, we confirmed that H1 was incorporated in the reconstituted chromatin over the promoter region (data not shown).
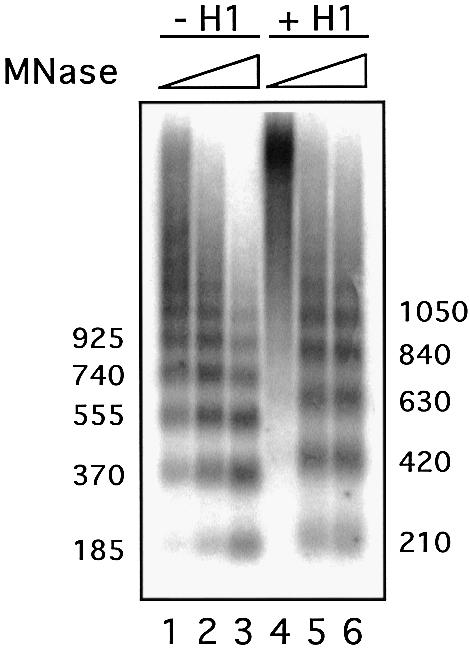
Fig. 1. Incorporation of histone H1 in reconstituted minichromosomes. Effect of H1 on nucleosome spacing. Chromatin assembled with or without histone H1 on pMMTVCAT B-B DNA was digested with micrococcal nuclease (MNase) for increasing times. The resulting DNA fragments were separated by electrophoresis on a 1.3% agarose gel in 1× TBE buffer and stained with ethidium bromide. The numbers on each site indicate the approximate size of the fragments in base pairs as determined by comparison with appropriate size markers.
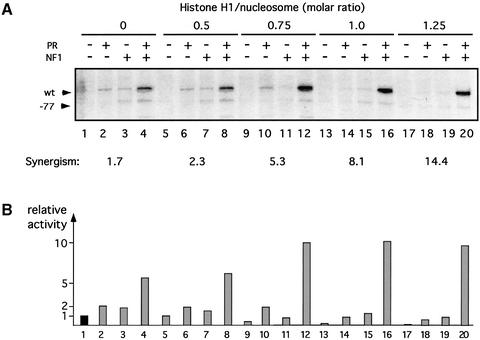
To study the effect of incorporation of histone H1 on the activity of the MMTV promoter chromatin, we used a HeLa nuclear extract for in vitro transcription (Dignam et al., 1983). Increasing concentrations of histone H1 in the minichromosome assembly reactions consistently reduced the basal transcriptional activity of the promoter (Figure 2, lanes 1, 5, 9, 13 and 17) and decreased its response to the addition of either purified PR (Figure 2, lanes 2, 6, 10, 14 and 18) or purified NF1 (Figure 2, lanes 3, 7, 11, 15 and 19). Maximal inhibition reached 60% in the case of PR (compare lanes 2 and 18, and Figure 2B) and 50% in the case of NF1 (compare lanes 3 and 19, and Figure 2B). These findings are in agreement with the postulated repressive effect of linker histones on chromatin transcription and could reflect a more compact or less dynamic structure of the minichromosomes containing H1.
Fig. 2. Influence of histone H1 on transcription from the MMTV promoter. (A) In vitro transcription analysis of MMTV minichromosomes with increasing amounts of histone H1. Chromatin reconstituted with an increasing molar ratio of histone H1 was incubated with purified recombinant PR or/and NF1 as indicated and transcribed in vitro with HeLa nuclear extract. For each template, 25 ng of DNA were used in each reaction. Products were visualized by primer extension analysis and sequencing gel electrophoresis. The positions of the products from the wild-type MMTV promoter (wt) and from a control MMTV promoter lacking the HREs (–77) are indexed on the left. The synergism of PR and NF1 was calculated as: (activity PR + NF1)/(activity PR) + (activity NF1), and is indicated at the bottom. (B) Quantification of the wild-type MMTV transcripts. Transcription signals were quantified in a phosphoimager (Fuji) and are shown in relative units, with the transcription in the absence of H1 and activators (lane 1) set to 1. The numbers on the x-axis refer to the lanes in (A).
Histone H1 incorporation reduces the accessibility of restriction sites in the MMTV promoter
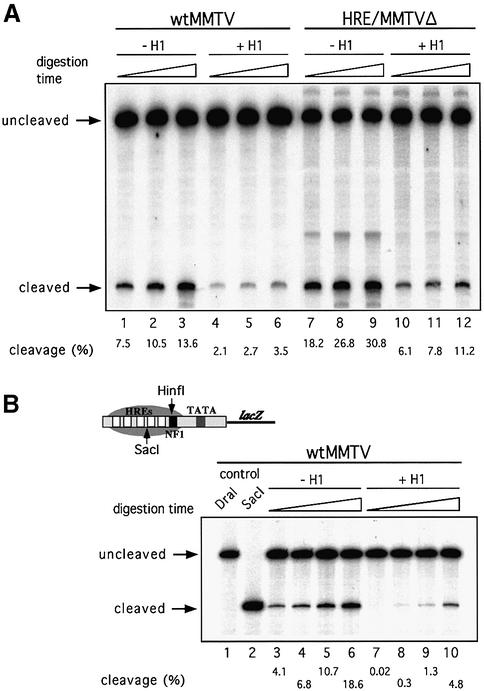
The efficiency of cleavage with restriction enzymes is a measurement of the accessibility of DNA sequences in a chromatin context. In the past, we have used the restriction enzyme HinfI for this kind of analysis, since its recognition sequence overlaps with the NF1-binding site. We have shown that this site is relatively inaccessible in MMTV minichromosomes assembled in DREX compared with naked DNA (Venditti et al., 1998). The incorporation of histone H1 into wild-type MMTV minichromosomes further reduced the accessibility of the HinfI restriction site by a factor of 4 (Figure 3A, compare lanes 1–3 with lanes 4–6). A similar but even more pronounced inhibition of cleavage by histone H1 incorporation was observed with the restriction enzyme SacI, which cleaves near the dyad axis of the promoter nucleosome between HRE2 and HRE3 (Figure 3B). Therefore, the compaction of nucleosome structure by histone H1 affects the complete nucleosomal DNA and is not restricted to a particular region of the DNA superhelix. This increased compaction offers a plausible explanation for the inhibitory effect of histone H1 on basal transcription and on the activation by the individual transcription factors PR or NF1.
Fig. 3. Effect of histone H1 on accessibility for restriction enzymes of promoter DNA sequences in chromatin. (A) Cleavage by HinfI of wild-type MMTV (wtMMTV) and mutant MMTV (HRE/MMTVΔ) promoters in minichromosomes assembled in the presence or absence of histone H1. MMTV minichromosomes (200 ng of DNA) were assembled as described in Materials and methods. After assembly, the samples were digested at 26°C with 50 U of HinfI for 2, 4 or 8 min, and the DNA was purified and restricted with DraI (wtMMTV) or PvuII (HRE/MMTVΔ). The digestion products were analyzed by linear PCR. The positions of the HinfI uncleaved and cleaved fragments are indicated. The amount of cleavage as a percentage of total radioactivity is indicated below the lanes. (B) Cleavage by SacI of the wild-type MMTV promoter in minichromosomes assembled in the presence or absence of histone H1. MMTV minichromosomes (200 ng of DNA) were assembled in DREX, digested at 26°C with 50 U of SacI for 1, 2, 4 or 8 min, and the DNA was purified and restricted with DraI. The digestion products were analyzed by linear PCR. The positions of the SacI uncleaved and cleaved fragments are indicated. The amount of cleavage as a percentage of total radioactivity is indicated below the lanes.
The synergism between PR and NF1 as well as the actual transcription from the activated MMTV promoter are enhanced by histone H1
In contrast to the inhibitory effect of histone H1 on transcriptional activation by isolated PR or NF1, adding both factors together yielded an unexpected result: H1 enhanced the synergism between PR and NF1 as well as the absolute levels of transcription (Figure 2A, lanes 4, 8, 12, 16 and 20). The synergism increased concomitantly with the amount of H1 up to a calculated molar ratio of 1.25 molecules of H1 per nucleosome. Higher molar ratios resulted in aggregation of the templates and a complete loss of transcriptional activity (data not shown). We also observed an increase in overall transcription (compare lane 4 with 8, 12, 16 and 20), though less pronounced than the augmentation of synergism between PR and NF1. This results from the above-mentioned inhibitory effect of H1 on the levels of transcription induced by the single activators, PR and NF1. In 12 independent experiments, the inhibitory effect of H1 on basal transcription was 28% (±16), while the inhibition in the presence of PR alone was 43% (±5) and with NF1 alone 52% (±6). The synergism between PR and NF1 was 3.5-fold (±1.6) in the absence of histone H1 and increased to 8.2-fold (±2.4) in the presence of H1. Most of our studies were performed with commercial histone H1 preparations. However, experiments with recombinant histone H1o and H1.2 isoforms expressed in Escherichia coli (Doenecke et al., 1997) yielded virtually indistinguishable results.
In contrast to the results obtained with minichromosomes, transcription activation on ‘naked’ MMTV DNA was not significantly influenced by the addition of H1 with any combination of activators (data not shown). We conclude that, at the concentrations used in these experiments, H1 has no direct, inhibiting or enhancing effect on the transcriptional machinery as such. This excludes a possible interaction between H1 and PR as a mechanism underlying the positive effects observed with minichromosomes.
Histone H1 increases the proportion of activated MMTV chromatin templates
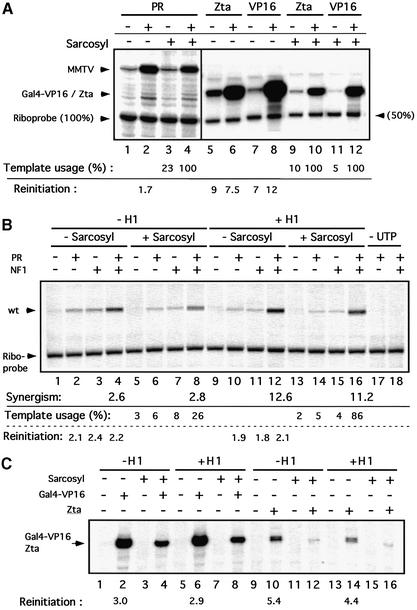
The enhanced transcription from MMTV minichromosome templates containing histone H1 could result from the utilization of a larger proportion of the available promoters for transcription or, alternatively, from a higher efficiency of reinitiation on the same number of promoters. To distinguish between these two possibilities, we studied the effect of sarcosyl, which prevents transcription reinitiation but does not preclude elongation by already engaged RNA polymerase II complexes (Hawley and Roeder, 1985, 1987). The complete transcription reactions lacking UTP were incubated for 30 min before the addition of sarcosyl (final concentration 0.07%), where indicated, and 5 min later the missing UTP was supplemented. Incubation was continued for 30 min before addition of a defined amount of riboprobe as internal standard, followed by quantitation of transcripts by primer extension (Di Croce et al., 1999). With naked DNA templates, 100% of the MMTV promoters were activated by PR, but the efficiency of reinitiation was low. Each template was used only 1.7-fold, whereas two control promoters showed a much higher reinitiation. A Zta-dependent promoter was reinitiated 7.5-fold in the presence of the Zta transactivator (Lieberman and Berk, 1991), and a Gal4 reporter showed a 12-fold reinitiation in the presence of the Gal4-VP16 transactivator (Croston et al., 1991) (Figure 4A).
Fig. 4. Influence of histone H1 on the recruitment of promoters to the active state and on the efficiency of reinitiation. (A) In vitro transcription analysis of free DNA in the presence or absence of sarcosyl. Free reporter DNA (50 ng of template/reaction) for PR (pMMTVCAT B-B), Zta (pZ7E4T CAT) or Gal4-VP16 (pG5E4T CAT) was incubated in the absence of UTP for 30 min with HeLa nuclear extract and purified recombinant PR, Zta or Gal4-Vp16 as indicated. Sarcosyl was added to a final concentration of 0.07% and, after 5 min, transcription was started by addition of UTP. After 30 min, transcription was stopped and a riboprobe was added corresponding to 50 or 100% of the template concentration as indicated. Products were visualized by primer extension analysis and sequencing gel electrophoresis. The positions of the products from the different promoters and the riboprobe are indexed on the left. The template usage was calculated by comparing the signal intensities of the riboprobe with those of the transcription reactions in the presence of 0.07% sarcosyl (single round transcriptions). Comparing the single round transcriptions with the corresponding signals in the absence of sarcosyl (multiple rounds of transcription) yielded the values for the reinitiation rates. (B) In vitro transcription analysis of MMTV minichromosomes in the presence or absence of H1 and sarcosyl. Chromatin reconstituted in the presence or absence of histone H1 (50 ng of template/reaction) was purified via Sephadex G-50 spin columns to remove NTPs. This chromatin showed no transcription signal without addition of UTP (lanes 17 and 18), whereas transcription on non-purified minichromosomes proceeded with internal UTP (data not shown). Purified minichromosomes were incubated in the absence of UTP for 30 min with purified recombinant PR and NF1 as indicated. HeLa nuclear extract was then added for an additional 30 min. Reactions were then processed further with or without sarcosyl addition as in (A). (C) In vitro transcription analysis of pZ7E4T CAT or pG5E4T CAT minichromosomes in the presence or absence of H1 and sarcosyl. Except for the utilization of Gal4-VP16 and Zta instead of PR and NF1, reactions were carried out as in (B).
On minichromosomes assembled in the absence of histone H1, the proportion of MMTV promoters activated in the absence of added factors was 3% in this particular experiment. In the presence of either PR alone or NF1 alone, the proportion was 6 and 8%, respectively, in this particular experiment, and this value increased to 26% in the presence of both factors (Figure 4B, lanes 1–8). The efficiency of reinitiation was low and comparable with that on free DNA templates. On minichromosome templates containing histone H1, the proportion of templates activated by PR alone or NF1 alone was slightly lower, 5 and 4%, respectively, but in the presence of both factors 86% of the templates were activated (Figure 4B, lanes 9–16). The absolute figures varied in three independent experiments, but in all cases H1 inhibited template usage in the presence of either PR or NF1 alone while it increased template usage in the presence of both factors together. Addition of H1 had little effect on the efficiency of reinitiation. Thus, the positive effect of histone H1 on activated transcription from MMTV chromatin templates is due mainly to a better synergism between PR and NF1 in terms of promoter recruitment to the active state.
The complex effect of histone H1 was selective for the MMTV promoter, since the Gal4 reporter promoter assembled in minichromosomes was insensitive to the addition of H1, and the Zta reporter promoter responded to the addition of H1 with a reduction in transcription (Figure 4C). The efficiency of reinitiation on control promoters in chromatin was reduced compared with free DNA (Figure 4, compare C with A) but, as in the case of MMTV promoters, was not signficantly affected by histone H1 (Figure 4C).
The population of nucleosomes on the MMTV promoter is more homogeneous in minichromosomes containing histone H1
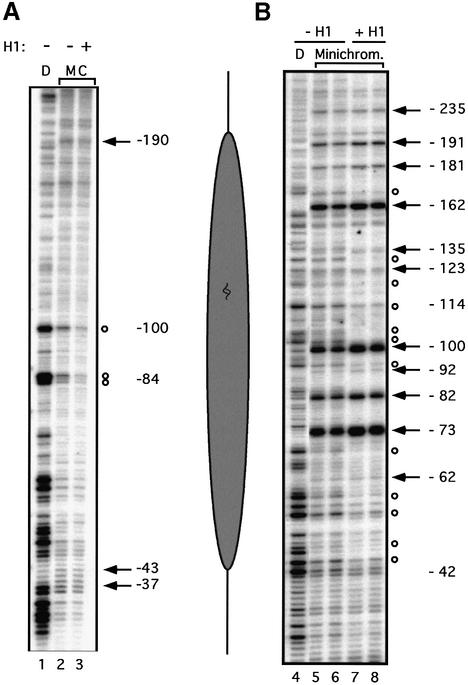
The positioning of a nucleosome along the DNA is defined by the rotational and translational phasing. The rotational phasing refers to the relationship between the nucleosome and the helical periodicity of the DNA, whereas the translational phasing describes the position of the nucleosome relative to a given point along the DNA molecule. We set out to investigate if the incorporation of H1 had any effect on the positioning of the MMTV promoter nucleosome. This nucleosome covers the HREs and has been shown to exhibit preferential translational and rotational phasing in different systems (Richard-Foy and Hager, 1987; Perlmann and Wrange, 1988; Piña et al., 1990; Chávez et al., 1995; Truss et al., 1995; Flaus and Richmond, 1998), including reconstitution with a Drosophila embryo extract (Venditti et al., 1998). There fore, we performed a high resolution analysis of nucleosome structure using MNase footprinting. Confirming previous results (Chávez et al., 1995; Venditti et al., 1998), a protected region flanked by hypersensitive sites at –40 and –190 was detected. Within this region, the protection was less clear over the proximal and distal parts of the footprint and more evident in the central part (Figure 5A), as had been observed with other positioned nucleosomes (Tanaka et al., 1996). In the presence of histone H1, the limits of the protection, as determined by the flanking hypersensitive sites, were the same, but the degree of protection was strikingly more pronounced (compare lanes 2 and 3, see especially the protected bands around –84 and –100, indicated by circles). Thus, we conclude that the MMTV promoter nucleosome is stabilized by the incorporation of histone H1.
Fig. 5. Structural analysis of the promoter nucleosome in the presence or absence of histone H1. (A) High resolution mapping of the translational positioning. Reconstituted minichromosomes were mildly digested with MNase (20 s at 26°C) and the resulting fragments were amplified by linear PCR. Lane D: MNase digestion pattern on naked DNA. Lanes M and C: minichromosomes reconstituted in the presence or absence of H1 (as indicated above) treated with MNase. Cleavage sites protected in chromatin are indicated by open circles; hypersensitive sites are marked by black arrows. The numbers refer to the distance from the start of transcription. The diagram on the right shows the approximate position of the promoter nucleosome. (B) Rotational phasing. The rotational setting of the promoter nucleosome was determined by DNase I digestion of the minichromosomes followed by linear PCR amplification. Lane D: DNase I digestion pattern on naked DNA. Lanes Minichrom.: minichromosomes (with or without H1 as indicated above) digested for 2 min (lanes 5 and 7) and 4 min (lanes 6 and 8) with DNase I. The alternate enhancements (arrows) and protections (circles) show a periodicity of ∼10 bp. The numbers refer to the distance from the transcription start.
To analyze the effect of H1 on the rotational orientation of the double helix on the nucleosome surface, we used DNase I digestion (Figure 5B). In both the absence and presence of histone H1, we observed a pattern of preferential cleavage sites (marked by arrows) spaced by ∼10 bp alternating with protection of cleavage sites (marked by circles), indicative of a preferential rotational phasing of the DNA double helix, as reported previously (Piña et al., 1990; Truss et al., 1995; Venditti et al., 1998). The strong hypersensitive sites at –162, –100, –82 and –73 indicate deformations of the double helix in these regions, with widening of the minor groove particularly around the nucleosome axis. The pattern was similar in the presence of histone H1, but the protections and enhancements were more prominent (compare lanes 5 and 6 with lanes 7 and 8). At the hypersensitive positions, –162, –100 and –82, there is a clear increase in the intensity of the main band accompanied by a decrease in the intensity of the flanking bands. We conclude that the dominant rotational setting on the promoter nucleosome is preserved and stabilized in the presence of H1. Taken together, the structural data suggest a more homogeneous population of nucleosomes in the presence of H1, with a higher percentage of molecules adopting a dominant position.
Histone H1 does not enhance the synergism between PR and NF1 on a truncated MMTV promoter, which does not position nucleosomes
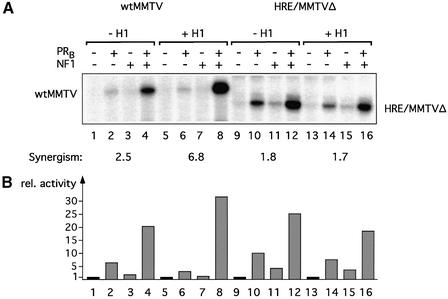
To test the relationship between nucleosome positioning and H1 effects on transcription, we utilized a mutant promoter, HRE/MMTVΔ, in which the region containing the five natural HREs of the MMTV promoter has been replaced by a single canonical HRE. This truncated MMTV promoter does not position nucleosomes (Prado et al., 2002). We compared the accessibility of the NF1-binding site for cleavage by HinfI in minichromosomes containing the HRE/MMTVΔ promoter and on wild-type MMTV minichromosomes (Figure 3A). In the absence of histone H1, the accessibility of this site in the HRE/MMTVΔ promoter chromatin was ∼3-fold higher than in the wild-type chromatin, probably due to the lack of nucleosome positioning (compare lanes 1–3 with lanes 7–9). However, as on the wild-type MMTV promoter, H1 incorporation led to a 3-fold reduction in restriction enzyme cleavage on HRE/MMTVΔ minichromosomes (compare lanes 7–9 with lanes 10–12). Therefore, the difference in accessibility between wild-type MMTV and HRE/MMTVΔ minichromosomes was maintained in the presence of histone H1. In both cases, restriction enzyme access was reliant on ATP-dependent chromatin-remodeling complexes, since reactions performed in the presence of apyrase, which degrades any ATP in the extract (Tsukiyama et al., 1994), almost completely eliminated HinfI cleavage (Venditti et al., 1998; data not shown). Thus, histone H1 has a similar compacting effect on wild-type and HRE/MMTVΔ promoter chromatin.
However, in contrast to the situation with wild-type MMTV, where histone H1 had an enhancing effect on the synergism between PR and NF1 (see also Figure 2A), the synergism between the two factors was not affected by H1 in the HRE/MMTVΔ promoter (Figure 6). Moreover, a clear reduction in overall activation of the HRE/MMTVΔ promoter could be observed in the presence of histone H1, also in contrast to the effect of H1 on overall transcription activation of wild-type MMTV minichromosomes (Figure 6). Thus, the same two factors, PR and NF1, acting on the same core promoter respond to incorporation of H1 into minichromosomes in an opposite way. Most probably, the opposed behavior of the two chromatin templates depends on differences in the nucleosomal organization of their respective promoter sequences.
Fig. 6. Promoter dependency of the H1 effect on transcription. (A) In vitro transcription analysis of wild-type MMTV and HRE/MMTVΔ minichromosomes reconstituted in the presence or absence of histone H1. Chromatin reconstituted with or without histone H1 was incubated with purified recombinant PR and NF1 as indicated and transcribed in vitro with HeLa nuclear extract (50 ng of DNA template/reaction). Products were visualized by primer extension analysis and sequencing gel electrophoresis. The positions of the products for the wild-type MMTV promoter (wtMMTV) and HRE/MMTVΔ are indicated (note that the HRE/MMTVΔ product is 20 bp shorter). (B) Quantification of the transcription signals. Signals were quantified in a phosphoimager (Fuji) and are shown in relative units with wtMMTV control, no H1 and no activator, set to 1. The synergism of PR and NF1 was calculated as: (activity PR + NF1)/(activity PR) + (activity NF1), and is indicated on the top.
Chromatin containing histone H1 binds PR more efficiently
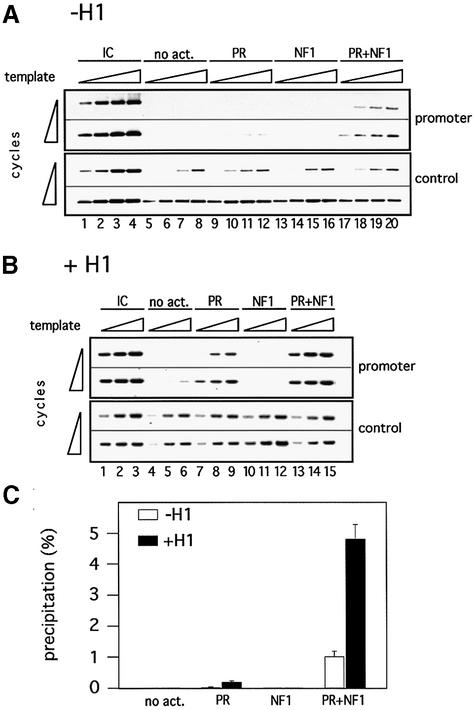
We have shown previously that, at limiting concentrations of PR, NF1 facilitates binding of PR to MMTV minichromosomes lacking histone H1 (Di Croce et al., 1999). We now tested whether we can reproduce these findings by ChIP assays with an antibody against PR and whether the same behavior is observed in the presence of histone H1. In addition to a set of primers amplifying the promoter region, we also used another set of primers corresponding to a distant region of the MMTV minichromosome as a control. Both the promoter and the control set of primers yielded very low background signal, but we increased the number of cycles with the control set of primers in order to generate a visible signal (Figure 7, compare upper and lower panels). Upon incubation of minichromosomes lacking histone H1 with PR, the promoter region yielded a weak signal visible only at high template concentrations and with a large number of cycles, whereas there was no change in the control region (Figure 7A, lanes 9–12). In the presence of NF1, neither the control region nor the promoter region showed significant changes (Figure 7, lanes 13–16). In the presence of both PR and NF1, the control region was unaffected but the promoter region was enriched in the immunoprecipitated material to a higher extent than in the presence of PR alone (Figure 7A, lanes 17–20). Thus the ChIP assay confirms our previous report that NF1 helps PR binding to MMTV chromatin at low concentrations of receptor (Di Croce et al., 1999).
Fig. 7. Incorporation of histone H1 improves PR binding to minichromosomes. (A) ChIP of MMTV minichromosomes with α-PR antibody. Minichromosomes without histone H1 were purified by Sephadex G-50 chromatography and incubated with different activators (as indicated on the top) for 30 min at 30°C. ChIPs were performed in the presence of HeLa nuclear extract (120 µl), but without UTP, under conditions of transcription initiation. The upper panel shows the PCR products obtained for the specific region corresponding to the promoter nucleosome (promoter), where different amounts of precipitated material (0.5, 1, 2 and 4 µl) were PCR amplified for 23 and 26 cycles and compared against 10% of the input material (IC). The lower panel displays the results of PCRs run for 32 and 35 cycles for a control distant region of the plasmid (here the precipitates were compared against 0.1% of the input material). (B) ChIP of histone H1-containing MMTV minichromosomes with α-PR antibody. ChIPs were performed as described in (A), except that the minichromosomes were reconstituted with H1, and only 1, 2 and 4 µl of the precipitates were PCR amplified. (C) Quantification of PR binding in ChIP experiments. PR binding is displayed as a percentage of precipitated template with regard to the input material. Two independent experiments with six data points each were evaluated by densitometric measurements of ethidium bromide-stained gels.
In the presence of histone H1, the ChIP experiments with an antibody against PR detected a stronger binding of PR alone (Figure 7B, lanes 7–9) and an even stronger binding in the presence of PR and NF1 compared with the minichromosomes lacking H1 (Figure 7B, lanes 13–15). Quantitation of the ChIP experiments showed that in both cases the increase in PR binding was 4- to 5-fold in H1-containing minichromosomes (Figure 7C). This effect was selective for the promoter region (Figure 7B, compare upper and lower panels), and offers a basis for the enhanced recruitment of MMTV promoters to the active state and for the functional synergism between these factors in the transcription assay.
Histone H1 is displaced from the promoter upon activation of transcription
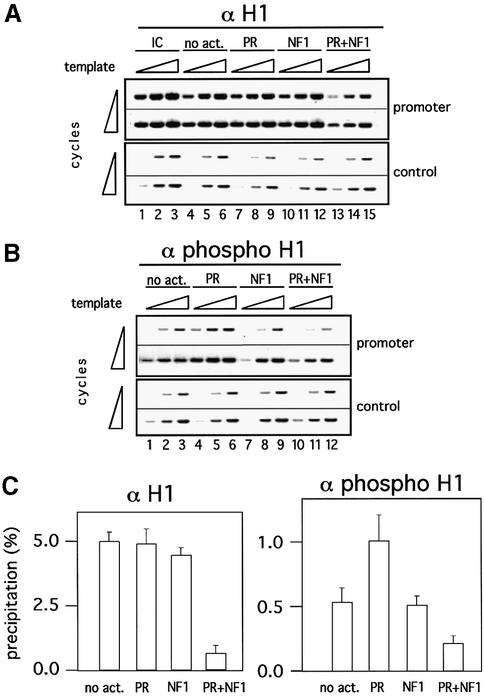
Our finding that incorporation of histone H1 favors activated transcription of the MMTV promoter in chromatin seems in apparent contradiction to the previously reported observations that actively transcribed genes are depleted of histone H1 (Zlatanova et al., 2000). In particular, over the MMTV promoter, a decrease in H1 content has been reported following glucocorticoid induction in cultured cells (Bresnick et al., 1992). To address this issue, we analyzed by ChIP whether the histone H1 content was altered following incubation with PR, NF1 or both. For these experiments, we used an antibody that recognizes histone H1 independently of its state of phosphorylation. We performed these experiments in the presence of HeLa cell extract, but in the absence of UTP to permit transcription initiation while preventing elongation. Under these conditions, a clear and selective H1 depletion over the MMTV promoter was observed upon activation in the presence of PR and NF1 (Figure 8A, upper panel, lanes 13–15). Quantitation of the signals indicates that ∼80% of the histone H1 is lost from the MMTV promoter chromatin after activation of transcription initiation (Figure 8C, left panel). In the presence of PR alone or NF1 alone, no significant reduction in histone H1 content is visible over the promoter region (Figure 8A, upper panel, lanes 7–13, see also left panel in C). No changes in histone H1 content were detected over the control region in the presence of any of the transcription factors (Figure 8A, lower panel). Therefore, we conclude that activation of transcription initiation leads to a selective displacement of histone H1 from the MMTV promoter chromatin.
Fig. 8. Displacement and phosphorylation of histone H1 in the course of transcriptional initiation of the MMTV promoter chromatin. (A) ChIP with α-H1 antibody. ChIPs were performed as described in Figure 7, except that an antibody against histone H1 was used for precipitation. Aliquots of 1, 2 and 4 µl of the precipitates were PCR amplified for 22 and 25 cycles with specific (promoter) and unspecific (control) primers. IC: 10% of input material. (B) ChIP with α-phosphoH1 antibody. ChIPs were performed as described in (A), except that an antibody against phosphorylated histone H1 was used for precipitation, and PCR results for 25 and 28 cycles are shown. (C) Quantification of H1 and phosphorylated H1 binding in ChIP experiments. Factor binding is displayed as a percentage of precipitated template with regard to the input material. The above experiment with six data points for each precipitate was evaluated by densitometric measurement of an ethidium bromide-stained gel.
Binding of PR enhances phosphorylation of histone H1 over the MMTV promoter
Phosphorylation of histone H1 appears to play an important role in glucocorticoid induction of the MMTV promoter (Lee and Archer, 1998; Bhattacharjee et al., 2001). We therefore tested whether there were changes in histone H1 phosphorylation in the course of activation of MMTV minichromosomes in vitro. When an antibody selective for phosphorylated histone H1 is used for ChIP, a 2-fold increased signal over the promoter region was found after incubation with PR (Figure 8B, upper panel, compare lanes 4–6 with lanes 1–3, see also C, right panel). Addition of NF1 alone had no significant effect (Figure 8B, upper panel, lanes 7–9), whereas, in the presence of both PR and NF1, there is a 70% decrease in the proportion of promoters containing phosphorylated histone H1 (Figure 8B, upper panel, lanes 10–12, and C, right panel). The reduction is selective for the MMTV promoter and is not observed over the control region (Figure 8B, lower panel). A separate experiment yielded similar relative values for bound phosphorylated H1 (data not shown). If the signal for phosphorylated H1 is corrected for the 80% displacement of total histone H1 from the promoter observed under these conditions (Figure 8A), there appears to be no significant change in the proportion of phosphorylated histone H1 in the presence of PR and NF1 as compared with PR alone. A possible explanation could be that upon full activation of transcription initiation in the presence of both PR and NF1, histone H1 is displaced from the promoter independently of its phosphorylation state. Alternatively, phosphorylation of H1 could be enhanced further in the presence of PR and NF1, but the proportion of phosphorylated H1, which remains at the promoter nucleosome does not change because phosphorylated H1 is displaced preferentially.
Discussion
Our findings that histone H1 enhances transcriptional activation of the MMTV promoter in minichromosomes assembled in vitro add new insight to the mechanism by which higher order chromatin structure participates in gene regulation by steroid hormones. The role of linker histones in the regulation of individual genes and the underlying molecular mechanisms are largely unknown. There are several studies on genes transcribed by RNA polymerase III, such as the 5S rRNA genes, but virtually no genes transcribed by RNA polymerase II have been studied in detail.
Effect of histone H1 on nucleosome positioning and stability
Using high resolution nuclease mapping, we also found two dominant translational settings with the same rotational orientation on mononucleosomes reconstituted with recombinant histones on MMTV promoter DNA (Spangenberg et al., 1998). Our present studies with MMTV minichromosomes suggest that binding of histone H1 generates a more homogeneous population of nucleosome positions over the promoter. Several criteria support this conclusion. Following H1 binding to MMTV minichromosomes, we observed: (i) reduced access of restriction enzymes to DNA sequences at the edge and at the center of the nucleosome; (ii) more pronounced and better defined nucleosomal cleavage patterns with MNase and DNase I; and (iii) reduced access of NF1 in the transcription assay. These findings suggest that incorporation of histone H1 into chromatin tightens the nucleosome structure by reducing the fluctuation between slightly different translational positions of the histone octamer, leading to a more restricted unique population with a better defined translational setting.
Effect of histone H1 on the synergism between PR and NF1
The incorporation of histone H1 into MMTV minichromosomes compacts the chromatin structure and has the expected negative effect on basal transcription in the absence of sequence-specific factors, as well as on transcription in the presence of either PR or NF1. Unexpectedly, however, H1 has a positive effect on transcription in the presence of both PR and NF1 by enhancing the synergism between these two factors. The enhanced transcriptional synergism depends on the precise nucleosomal organization of the wild-type promoter, as a truncated MMTV promoter that displays no positioned nucleosomes (Prado et al., 2002) does not exhibit an enhanced response to PR and NF1 in the presence of histone H1. It is noteworthy that synergistic activation of this truncated promoter requires the activation domain of NF1 (Prado et al., 2002), which is dispensable for synergism between PR and NF1 on wild-type MMTV chromatin (Di Croce et al., 1999). Therefore, the mechanism of transactivation differs between these two versions of the MMTV promoter: only the promoter which is well organized in chromatin uses NF1 as an architectural factor and responds with enhanced transcription activation to the addition of histone H1. This underlines the significance of the nucleotide sequence of the wild-type promoter for proper chromatin organization, and thus for a physiological response to hormone induction.
In the wild-type promoter chromatin, the central HREs are not accessible for receptor binding unless NF1 binds to its promoter site and maintains an ‘open’ nucleosomal configuration. It is this structural synergism that is enhanced by histone H1 binding. In contrast, the truncated MMTV promoter exhibits a more ‘open’ chromatin structure that already makes the HRE more accessible for receptor binding. This eliminates the contribution of NF1 to receptor binding and thereby the structural synergism between the two factors. Addition of H1 to this promoter only stabilizes the nucleosomes and restricts nucleosome mobility (Pennings et al., 1994; Hill and Imbalzano, 2000), leading to the expected impairment of transcriptional activity.
Recently, experiments similar to those reported here have been performed with minichromosomes assembled on a synthetic promoter made of two estrogen-responsive elements upstream of an adenovirus E4 TATA box (Cheung et al., 2002). In this system, addition of histone H1 leads to a dramatic decrease in the activation of transcription caused by added estrogen receptor α (ERα). On this artificial promoter, histone H1 acts selectively to reduce the overall level of productive transcription initiation by restricting promoter accessibility and preventing the ERα-dependent formation of a stable transcription pre-initiation complex. It is possible that the opposite effect of histone H1 on transcription activation by ERα on the synthetic promoter and by PR and NF1 on the MMTV promoter reflects differential effects of H1 on the binding sites of various transcription factors (Juan et al., 1994). Even so, our results underscore the importance of studying the function of transcription factors or chromatin components in the context of the natural promoter sequences, as the sequence obviously encompasses information for the structure of chromatin and, in particular, for the positioning of nucleosomes and linker histones.
More binding of PR and better synergism in the presence of H1
The results of the ChIP experiments with anti-PR antibodies show that PR binding to the MMTV promoter nucleosome is enhanced in minichromosomes containing histone H1. This effect seems to be in contrast to the reduced transcription observed with H1-containing minichromosomes in response to PR alone. In MMTV mononucleosomes assembled with recombinant histones, we also observed increased binding of PR to the HRE1 upon incorporation of histone H1 (Vicent et al., 2002). Here we see strong binding of PR to the H1-containing MMTV minichromosomes, but no activation of transcription. Thus, recruitment of PR alone is not sufficient to enhance transcription initiation. It seems likely that the tightening and stabilization of the wild-type MMTV promoter nucleosome caused by incorporation of H1 not only enhances binding of PR to the exposed distal HRE1 but also reduces the accessibility of the three ‘inner’ HREs, which are not available for PR binding in a positioned nucleosome (Piña et al., 1990; Eisfeld, 1997). Under these conditions, access of PR to these masked HREs depends completely on the binding of NF1 to the promoter, which stabilizes the ‘open’ nucleosome conformation (Di Croce et al., 1999). This explains why in our ChIP experiments, binding of PR is dramatically enhanced by NF1 in the presence of histone H1.
Histone phosphorylation and displacement
In view of the reported effects of histone H1 phosphorylation and dephosphorylation on the hormonal induction of the MMTV promoter (Lee and Archer, 1998), we analyzed the state of phosphorylation of H1 during the activation process. After incubation with PR, a significant proportion of histone H1 is phosphorylated specifically over the promoter region and not over the control region of the minichromosomes. We cannot determine the proportion of phosphorylated H1 because of the different affinities of the antibodies used for ChIP. However, it seems that PR recruits or activates a kinase, which locally phosphorylates histone H1. Since in the presence of PR alone we do not see transcriptional activation of MMTV chromatin containing histone H1, we must assume that phosphorylation of histone H1 is not sufficient to promote transactivation of the promoter. Moreover, under these conditions, the total amount of H1 on the promoter chromatin does not change. Upon full activation of transcription initiation in the presence of PR and NF1, the majority of histone H1 leaves the promoter.
Our results showing that different isoforms of histone H1 behave similarly in the transcription assay are in agreement with previous reports from gene transfection studies (Gunjan and Brown, 1999), but in apparent contrast to published results suggesting selective effects of hormonal stimulation on the phosphorylation of different histone H1 variants (Banks et al., 2001). However, these latter studies focus on the global dephosphorylation of H1 observed after prolonged exposure (24 h) of cultured cells to dexamethasone (Lee and Archer, 1998), and therefore could be indirect. They do not contradict the rapid effects of progesterone on histone H1 phosphorylation reported here. Indeed, the real question is why prolonged exposure to glucocorticoids precludes the phosphorylation of histone H1 required for hormonal induction (Bhattacharjee et al., 2001).
Our previous in vitro transcription studies indicate that PR recruits the nucleosome remodeling factor (NURF) (Di Croce et al., 1999), and now we show that it may also recruit a linker histone kinase to MMTV promoter chromatin; one wonders about the relationship between these two remodeling steps. It has been reported recently that histone H1 binding blocks chromatin remodeling by ATP-dependent complexes such as SWI/SNF and ACF, and that this inhibition can be overcome by phosphorylation of the linker histones (Horn et al., 2002). Therefore, it seems reasonable to assume that recruitment or activation of the H1 kinase is a prerequisite for the subsequent remodeling of the MMTV chromatin that facilitates access of NF1 and PR to the masked target sites (Di Croce et al., 1999). On the other hand, it is also known that phosphorylation of H1 decreases its affinity for nucleosomes (Lever et al., 2000) and mimics H1 removal (Dou et al., 1999). Therefore, it could facilitate the displacement of H1 from the promoter that accompanies transcription initiation (Zlatanova et al., 2000). Whether later steps in the promoter activation process require additional ATP-dependent remodeling or phosphorylation events remains to be established by ChIP experiments with chromosomal integrated single copies of the MMTV promoter.
Materials and methods
Recombinant proteins and plasmids
Recombinant histidine-tagged human PRB complexed with the agonistic ligand R5020 and pig NF1-C2 were purified as described previously (Di Croce et al., 1999). The Epstein–Barr virus transactivator Zta was expressed and purified as described previously (Lieberman and Berk, 1991). The Gal4-VP16 transactivator was prepared according to Carey et al. (1990a). The plasmid pMMTVCAT B-B contains the MMTV promoter region from –640 to +126 and was used as wild-type MMTV promoter template (Venditti et al., 1998). The plasmid –77 CAT was used as control plasmid, as it contains the MMTV promoter truncated upstream of the NF1 site and therefore lacks the HREs (Chávez and Beato, 1997). The plasmid pHRE/MMTVΔ CAT contains a consensus HRE upstream of the NF1 site of the –77 CAT construct (Prado et al., 2002). The plasmids G5E4T CAT and Z7E4T CAT were described previously (Carey et al., 1990b; Chi et al., 1995).
Chromatin assembly and transcription
Pre-blastodermic Drosophila extracts were prepared as described previously (Bonte and Becker, 1999) and chromatin assembly was performed according to Venditti et al. (1998), except for the addition of calf thymus histone H1 (Sigma) or recombinant, bacterially expressed H1o, H1.2 (gift of D.Doenecke, Göttingen) at a ratio of one molecule per nucleosome, unless otherwise indicated, at the beginning of the assembly reaction. In vitro transcription reactions were performed as described previously (Di Croce et al., 1999).
Restriction enzyme accessibility assay
HinfI and SacI digestions were performed at 26°C using 200 ng of chromatin DNA and 50 U of enzyme in a 50 µl assembly reaction. Reactions were stopped with 20 mM EDTA and 0.5% sarcosyl, and the samples were treated with RNase A and proteinase K. After ethanol precipitation, a second restriction enzyme cleavage was performed using DraI for pMMTVCAT B-B or PvuII for pHRE/MMTVΔ CAT. A 20 ng aliquot of DNA was used as template for linear PCR with oligonucleotide A25 as a primer (Venditti et al., 1998), and the resulting products were analyzed on a 6% sequencing gel.
Chromatin structure analysis and immunoprecipitation
DNase I and MNase digestions were performed according to Venditti et al. (1998), as were the linear PCR amplifications and gel analysis. For the analysis of in vitro reconstituted minichromosomes, a modified version of a protocol for the study of histone acetylation (Chen et al., 1999) is employed. A 120 µl aliquot of a chromatin reconstitution containing pre-blastoderm Drosophila extract and 500 ng of DNA was diluted in transcription reaction buffer to 420 µl. The minichromosomes were then digested for 1 min by addition of 10 µl of 100 mM CaCl2 and 5 µl of 0.2 U/µl MNase (Sigma) and immediately cross-linked with 18 µl of 37% formaldehyde. After another minute, 200 µl of MNase stop solution (50 mM EDTA, 1% sarcosyl) was added, followed by 95 µl of 1 M glycine pH 7.5. After 10 min incubation at room temperature, 750 µl of ChIP dilution buffer (1% Triton X-1000, 2 mM EDTA, 150 mM NaCl, 20 mM Tris pH 8.1) was added and, after removal of an aliquot for input control, pre-cleared with 40 µl of salmon testis DNA–protein A–agarose slurry (Upstate Biotechnology) for 30 min at room temperature. After centrifugation, the supernatant was divided into several (usually four) aliquots and subjected to immunoprecipitation as described previously (Chen et al., 1999). The following antibodies were used: for PR, a mixture of three different antibodies from Santa Cruz Biotechnology (B-30, C-19 and C-20); for H1, clone AE-4 from Upstate Biotechnology; and for phosphorylated H1, polyclonal anti-phospho-histone H1 from Upstate Biotechnology. The PCRs were carried out with Taq DNA polymerase (Stoffel fragment) in the presence of 5 mM MgCl2 under standard conditions (Perkin Elmer). The specific primers generate a 140 bp fragment of the nucleosome B of the MMTV promoter, whereas the unspecific primers give rise to a 140 bp fragment from the opposite side of the pMMTVCAT B-B plasmid within the β-lactamase gene region. PCR products were resolved on 2% agarose gels and stained with ethidium bromide.
Acknowledgments
Acknowledgements
We thank Detlef Doenecke, Göttingen, for the purified H1 variant proteins. The experimental work was supported by a grant from the Deutsche Forschungsgemeinschaft (SFB 397). R.K. was the recipient of a fellowship from the Centre de Regulació Genómica.
References
- Banks G.C., Deterding,L.J., Tomer,K.B. and Archer,T.K. (2001) Hormone-mediated dephosphorylation of specific histone H1 isoforms. J. Biol. Chem., 276, 36467–36473. [DOI] [PubMed] [Google Scholar]
- Bhattacharjee R.N., Banks,G.C., Trotter,K.W., Lee,H.L. and Archer,T.K. (2001) Histone H1 phosphorylation by Cdk2 selectively modulates mouse mammary tumor virus transcription through chromatin remodeling. Mol. Cell. Biol., 21, 5417–5425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonte E. and Becker,P.B. (1999) Preparation of chromatin assembly extracts from preblastoderm Drosophila embryos. Methods Mol. Biol., 119, 187–194. [DOI] [PubMed] [Google Scholar]
- Bouvet P., Dimitrov,S. and Wolffe,A.P. (1994) Specific regulation of Xenopus chromosomal 5S rRNA gene transcription in vivo by histone H1. Genes Dev., 8, 1147–1159. [DOI] [PubMed] [Google Scholar]
- Bresnick E.H., Bustin,M., Marsaud,V., Richard-Foy,H. and Hager,G.L. (1992) The transcriptionally-active MMTV promoter is depeleted of histone H1. Nucleic Acids Res., 20, 273–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey M., Leatherwood,J. and Ptashne,M. (1990a) A potent GAL4 derivative activates transcription at a distance in vitro. Science, 247, 710–712. [DOI] [PubMed] [Google Scholar]
- Carey M., Lin,Y.S., Green,M.R. and Ptashne,M. (1990b) A mechanism for synergistic activation of a mammalian gene by GAL4 derivatives. Nature, 345, 361–364. [DOI] [PubMed] [Google Scholar]
- Chávez S. and Beato,M. (1997) Nucleosome-mediated synergism between transcription factors on the mouse mammary tumor virus promoter. Proc. Natl Acad. Sci. USA, 94, 2885–2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chávez S., Candau,R., Truss,M. and Beato,M. (1995) Constitutive repression and nuclear factor I-dependent hormone activation of the mouse mammary tumor virus promoter in yeast. Mol. Cell. Biol., 15, 6987–6998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Lin,R.J., Xie,W., Wilpitz,D. and Evans,R.M. (1999) Regulation of hormone-induced histone hyperacetylation and gene activation via acetylation of an acetylase. Cell, 98, 675–686. [DOI] [PubMed] [Google Scholar]
- Cheung E., Zarifyan,A.S. and Kraus,W.L. (2002) Histone H1 represses estrogen receptor α transcriptional activity by selectively inhibiting receptor-mediated transcription initiation. Mol. Cell. Biol., 22, 2463–2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi T., Lieberman,P., Ellwood,K. and Carey,M. (1995) A general mechanism for transcriptional synergy by eukaryotic activators. Nature, 377, 254–257. [DOI] [PubMed] [Google Scholar]
- Crane-Robinson C. (1999) How do linker histones mediate differential gene expression? BioEssays, 21, 367–371. [DOI] [PubMed] [Google Scholar]
- Croston G.E., Kerrigan,L.A., Lira,L.M., Marshak,D.R. and Kadonaga,J.T. (1991) Sequence-specific antirepression of histone H1-mediated inhibition of basal RNA polymerase II transcription. Science, 251, 643–649. [DOI] [PubMed] [Google Scholar]
- Di Croce L., Koop,R., Venditti,P., Westphal,H.M., Nightingale,K., Becker,P. and Beato,M. (1999) Two-step synergism between progesterone receptor and the DNA binding domain of NF1 on MMTV minichromosomes. Mol. Cell, 4, 45–54. [DOI] [PubMed] [Google Scholar]
- Dignam J.D., Lebovitz,R.M. and Roeder,R.G. (1983) Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res., 11, 1475–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doenecke D., Albig,W., Bode,C., Drabent,B., Franke,K., Gavenis,K. and Witt,O. (1997) Histones: genetic diversity and tissue-specific gene expression. Histochem. Cell Biol., 107, 1–10. [DOI] [PubMed] [Google Scholar]
- Dou Y., Mizzen,C.A., Abrams,M., Allis,C.D. and Gorovsky,M.A. (1999) Phosphorylation of linker histone H1 regulates gene expression in vivo by mimicking H1 removal. Mol. Cell, 4, 641–647. [DOI] [PubMed] [Google Scholar]
- Eisfeld K., Candau,R., Truss,M. and Beato,M. (1997) Binding of NF1 to the MMTV promoter in nucleosomes: influence of rotational phasing, translational positioning and histone H1. Nucleic Acids Res., 25, 3733–3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaus A. and Richmond,T.J. (1998) Positioning and stability of nucleosomes on MMTV 3′LTR sequences. J. Mol. Biol., 275, 427–441. [DOI] [PubMed] [Google Scholar]
- Gregory P.D. and Hörz,W. (1998) Chromatin and transcription—how transcription factors battle with a repressive chromatin environment. Eur. J. Biochem., 251, 9–18. [DOI] [PubMed] [Google Scholar]
- Gunjan A. and Brown,D.T. (1999) Overproduction of histone H1 variants in vivo increases basal and induced activity of the mouse mammary tumor virus promoter. Nucleic Acids Res., 27, 3355–3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley D.K. and Roeder,R.G. (1985) Separation and partial characterization of three functional steps in transcription initiation by human RNA polymerase II. J. Biol. Chem., 260, 8163–8172. [PubMed] [Google Scholar]
- Hawley D.K. and Roeder,R.G. (1987) Functional steps in transcription initiation and reinitiation from the major late promoter in a HeLa nuclear extract. J. Biol. Chem., 262, 3452–3461. [PubMed] [Google Scholar]
- Hill D.A. and Imbalzano,A.N. (2000) Human SWI/SNF nucleosome remodeling activity is partially inhibited by linker histone H1. Biochemistry, 39, 11649–11656. [DOI] [PubMed] [Google Scholar]
- Horn P.J., Carruthers,L.M., Logie,C., Hill,D.A., Solomon,M.J., Wade,P.A., Imbalzano,A.N., Hansen,J.C. and Peterson,C.L. (2002) Phosphorylation of linker histones regulates ATP-dependent chromatin remodeling enzymes. Nat. Struct. Biol., 9, 263–267. [DOI] [PubMed] [Google Scholar]
- Juan L.-J., Utley,R.T., Adams,C.C., Vettese-Dadey,M. and Workman,J.L. (1994) Differential repression of transcription factor binding by histone H1 is regulated by the core histone amino termini. EMBO J., 13, 6031–6040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandolf H. (1994) The H1A histone variant is an in vivo repressor of oocyte-type 5S gene transcription in Xenopus laevis embryos. Proc. Natl Acad. Sci. USA, 91, 7257–7261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khochbin S. (2001) Histone H1 diversity: bridging regulatory signals to linker histone function. Gene, 271, 1–12. [DOI] [PubMed] [Google Scholar]
- Lee H.L. and Archer,T.K. (1998) Prolonged glucocorticoid exposure dephosphorylates histone H1 and inactivates the MMTV promoter. EMBO J., 17, 1454–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lever M.A., Th’ng,J.P., Sun,X. and Hendzel,M.J. (2000) Rapid exchange of histone H1.1 on chromatin in living human cells. Nature, 408, 873–876. [DOI] [PubMed] [Google Scholar]
- Lieberman P.M. and Berk,A.J. (1991) The Zta trans-activator protein stabilizes TFIID association with promoter DNA by direct protein–protein interaction. Genes Dev., 5, 2441–2454. [DOI] [PubMed] [Google Scholar]
- Luger K., Mäder,A.W., Richmond,R.K., Sargent,D.F. and Richmond,T.J. (1997) Crystal structure of the nucleosome core particle at 2.8 Å resolution. Nature, 389, 251–260. [DOI] [PubMed] [Google Scholar]
- Meersseman G., Pennings,S. and Bradbury,M. (1991) Chromatosome positioning on assembled long chromatin. Linker histones affect nucleosome placement on 5S rDNA. J. Mol. Biol., 220, 89–100. [DOI] [PubMed] [Google Scholar]
- Ner S.S. and Travers,A.A. (1994) HMG-D, the Drosophila melanogaster homologue of HMG 1 protein, is associated with early embryonic chromatin in the absence of histone H1. EMBO J., 13, 1817–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panetta G., Buttinelli,M., Flaus,A., Richmond,T.J. and Rhodes,D. (1998) Differential nucleosome positioning on Xenopus oocyte and somatic 5S RNA genes determines both TFIIIA and H1 binding: a mechanism for selective H1 repression. J. Mol. Biol., 282, 683–697. [DOI] [PubMed] [Google Scholar]
- Parseghian M.H. and Hamkalo,B.A. (2001) A compendium of the histone H1 family of somatic subtypes: an elusive cast of characters and their characteristics. Biochem. Cell Biol., 79, 289–304. [PubMed] [Google Scholar]
- Pennings S., Meersseman,G. and Bradbury,E.M. (1994) Linker histones H1 and H5 prevent the mobility of positioned nucleosomes. Proc. Natl Acad. Sci. USA, 91, 10275–10279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlmann T. and Wrange,Ö. (1988) Specific glucocorticoid receptor binding to DNA reconstituted in a nucleosome. EMBO J., 7, 3073–3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piña B., Brüggemeier,U. and Beato,M. (1990) Nucleosome positioning modulates accessibility of regulatory proteins to the mouse mammary tumor virus promoter. Cell, 60, 719–731. [DOI] [PubMed] [Google Scholar]
- Prado F., Koop,R. and Beato,M. (2002) Accurate chromatin organization of the MMTV promoter determines the nature of the synergism between transcription factors. J. Biol. Chem., 277, 4911–4917. [DOI] [PubMed] [Google Scholar]
- Ramakrishnan V. (1997) Histone H1 and chromatin higher-order structure. Crit. Rev. Eukaryot. Gene Expr., 7, 215–230. [DOI] [PubMed] [Google Scholar]
- Richard-Foy H. and Hager,G.L. (1987) Sequence-specific positioning of nucleosomes over the steroid-inducible MMTV promoter. EMBO J., 6, 2321–2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond T.J., Finch,J.T., Rushton,B., Rhodes,D. and Klug,A. (1984) Structure of the nucleosome core particle at 7 Å resolution. Nature, 311, 532–537. [DOI] [PubMed] [Google Scholar]
- Sera T. and Wolffe,A.P. (1998) Role of histone H1 as an architectural determinant of chromatin structure and as a specific repressor of transcription on Xenopus oocyte 5S rRNA genes. Mol. Cell. Biol., 18, 3668–3680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen X.T. and Gorovsky,M.A. (1996) Linker histone H1 regulates specific gene expression but not global transcription in vivo. Cell, 86, 475–483. [DOI] [PubMed] [Google Scholar]
- Simpson R.T. (1978) Structure of the chromatosome, a chromatin core particle containing 160 base pairs of DNA and all the histones. Biochemistry, 17, 5524–5531. [DOI] [PubMed] [Google Scholar]
- Spangenberg C., Eisfeld,K., Stünkel,W., Luger,K., Flaus,A., Richmonds, T.J., Truss,M. and Beato,M. (1998) The MMTV promoter positioned on a tetramer of histones H3 and H4 binds nuclear factor 1 and OTF1. J. Mol. Biol., 278, 725–739. [DOI] [PubMed] [Google Scholar]
- Tanaka S., Livingstone-Zatchej,M. and Thoma,F. (1996) Chromatin structure of the yeast URA3 gene at high resolution provides insight into structure and positioning of nucleosomes in the chromosomal context. J. Mol. Biol., 257, 919–934. [DOI] [PubMed] [Google Scholar]
- Truss M., Bartsch,J., Schelbert,A., Haché,R.J.G. and Beato,M. (1995) Hormone induces binding of receptors and transcription factors to a rearranged nucleosome on the MMTV promoter in vivo. EMBO J., 14, 1737–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukiyama T., Becker,P.B. and Wu,C. (1994) ATP-dependent nucleosome disruption at a heat-shock promoter mediated by binding of GAGA transcription factor. Nature, 367, 525–532. [DOI] [PubMed] [Google Scholar]
- Venditti P., Di Croce,L., Kauer,M., Blank,T., Becker,P.B. and Beato,M. (1998) Assembly of the MMTV promoter in minichromosomes with positioned nucleosomes precludes NF1 binding but not restriction enzyme cleavage. Nucleic Acids Res., 26, 3657–3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicent G., Meliá,M.J. and Beato,M. (2002) Asymmetric binding of histone H1 stabilizes MMTV nucleosomes and the interaction of progesterone receptor with the exposed HRE. J. Mol. Biol., 324, 501–517. [DOI] [PubMed] [Google Scholar]
- Weintraub H. (1985) Assembly and propagation of repressed and derepressed chromosomal states. Cell, 42, 705–711. [DOI] [PubMed] [Google Scholar]
- Widom J. (1998) Structure, dynamics and function of chromatin in vitro. Annu. Rev. Biophys Biomol. Struct., 27, 285–327. [DOI] [PubMed] [Google Scholar]
- Zlatanova J. (1990) Histone H1 and the regulation of transcription of eukaryotic genes. Trends Biochem. Sci., 15, 273–276. [DOI] [PubMed] [Google Scholar]
- Zlatanova J., Caiafa,P. and Van Holde,K. (2000) Linker histone binding and displacement: versatile mechanism for transcriptional regulation. FASEB J., 14, 1697–1704. [DOI] [PubMed] [Google Scholar]