Abstract
Heterotrimeric G-proteins relay signals between membrane-bound receptors and downstream effectors. Little is known, however, about the regulation of Gα subunit localization within the natural endogenous environment of a specialized signaling cell. Here we show, using live Drosophila flies, that light causes massive and reversible translocation of the visual Gqα to the cytosol, associated with marked architectural changes in the signaling compartment. Molecular genetic dissection together with detailed kinetic analysis enabled us to characterize the translocation cycle and to unravel how signaling molecules that interact with Gqα affect these processes. Epistatic analysis showed that Gqα is necessary but not sufficient to bring about the morphological changes in the signaling organelle. Furthermore, mutant analysis indicated that Gqβ is essential for targeting of Gqα to the membrane and suggested that Gqβ is also needed for efficient activation of Gqα by rhodopsin. Our results support the ‘two-signal model’ hypothesis for membrane targeting in a living organism and characterize the regulation of both the activity-dependent Gq localization and the cellular architectural changes in Drosophila photoreceptors.
Keywords: G-protein/localization/membrane attachment/rhabdomere/vision
Introduction
In many signaling cascades, heterotrimeric (αβγ) G-proteins function as molecular transducers that relay signals from cell surface receptors to downstream effectors. To ensure specificity, effective concentrations and speed of interactions, the signaling components usually are restricted to the membrane domain. One of the main determinants of this localization is the dynamic lipid modification of G-proteins by S-acylation, commonly referred to as palmitoylation. All α-subunits of heterotrimeric G-proteins (with the exception of transducin) are modified reversibly by palmitoylation on cysteine residues at the N-terminus of the protein (reviewed in Mumby, 1997; Wedegaertner, 1998; Chen and Manning, 2001). This modification has been equated with targeting and anchorage of the G-protein to the plasma membrane, and influences its functional interaction with relevant receptors and effectors (Wedegaertner et al., 1993; Edgerton et al., 1994; Hepler et al., 1996; Wise et al., 1997; Wedegaertner, 1998; Chen and Manning, 2001; Hughes et al., 2001). In particular, the Gq/G11 family is representative of a wider group of heterotrimeric G-proteins that include Gs, G12 and G13, which are modified only by palmitoylation on N-terminal cysteines of the α-subunit (Wedegaertner et al., 1993; Edgerton et al., 1994; Hepler et al., 1996; Wise et al., 1997; Wedegaertner, 1998).
There are, however, conflicting reports about the relationship between the palmitoylation state of a heterotrimeric G-protein and its cellular localization (Resh, 1999; Chen and Manning, 2001). Results range from persistent membrane localization of the α-subunit (Edgerton et al., 1994; Mumby et al., 1994; Huang et al., 1999; Hughes et al., 2001) to activity-dependent translocation from the membrane to the cytosol as a result of depalmitoylation (Milligan and Unson, 1989; Ransnas et al., 1989; Wedegaertner et al., 1993, 1996; Wedegaertner and Bourne, 1994; Terakita et al., 1996; Wise et al., 1997; Narita, 1999). These apparent conflicting findings might be due to the inherent differences among various cell lines and between transfected cell cultures and the original physiological environment of the G-protein (discussed in Jones et al., 1997). Indeed, most previous studies have been carried out using different cell cultures that were transfected with the analyzed proteins. However, in two marine invertebrate visual systems, the phenotype of Gqα translocation has been described previously in vivo (Terakita et al., 1996; Narita et al., 1999). While a great deal was learnt in previous studies, little is known about the mechanism and control of the dynamic localization of G-proteins within their endogenous physiological environment.
The extensively studied Drosophila visual system, combined with the large repertoire of Drosophila visual mutants, offers a unique opportunity to study in vivo signaling by Gq in sensory neurons in general and the effects of other signaling molecules on its translocation cycle in particular. In contrast to cell cultures, this specialized system is comprised of highly polarized and compartmentalized cells that sequester the phototransduction machinery in a specific membrane organelle—the rhabdomere (Minke and Hardie, 2000; Hardie and Raghu, 2001). This signaling organelle is functionally equivalent to the vertebrate rod photoreceptor outer segment, as both are the structural unit responsible for the utmost sensitivity of the photoreceptor cells, capable of detecting single photons. At the biochemical level, however, each system uses a different cascade to translate light into an electrical signal (Hardie and Raghu, 2001).
Drosophila phototransduction is initiated upon activation of rhodopsin by light and proceeds through a photoreceptor-specific Gq protein (DGq), which activates phospholipase C (PLC) (Devary et al., 1987; Scott et al., 1995). In turn, the latter activates downstream effectors that culminate in the opening of the trp and trpl channels, depolarization of the photoreceptor cell and a rise in cellular calcium (Hardie and Raghu, 2001). Drosophila photoreceptors contain extraordinarily high amounts of signaling molecules per cell. For example, each photoreceptor cell contains ∼30 × 106 copies of rhodopsin and ∼3 × 106 copies of DGqα (Hardie and Raghu, 2001). This high copy number, as well as the specificity of the signaling molecules for the photoreceptor cells, enabled us to utilize the live, whole fly in the present studies.
Here we show that in Drosophila photoreceptors, activation of DGq by light causes a massive, but reversible, translocation of the α-subunit to the cytosol. Intriguingly, we also observed activity-dependent architectural changes that are specific to the signaling compartment of the photoreceptor. Epistatic analysis of these light-dependent changes shows that DGqα is necessary but not sufficient to bring about these changes. Our detailed analysis of the translocation and recovery kinetics of DGqα in wild-type flies together with the use of specific visual mutants enabled us to determine how other signaling components influence these processes. Our study provides a functional and morphological analysis of the voyages undertaken by a G-protein in vivo, which may have implications for other sensory transduction systems and for a variety of proteins that undergo reversible anchoring to the cell membrane.
Results
Light-dependent translocation of the DGqα protein
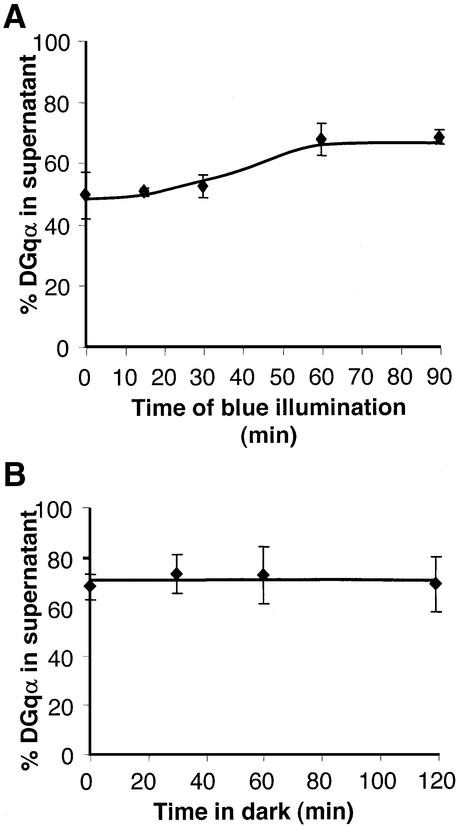
The distribution of DGqα between membrane and cytosol can be determined readily and accurately by separation of the membranes from the cytosol followed by western blot analysis (Figure 1A). We have chosen to employ kinetic analysis of both the translocation of DGqα from the membrane to the cytosol and its recovery back to the membrane in order to characterize fully this cycle in wild-type flies. By comparing the kinetics in wild-type flies and specific visual mutants, we could elucidate the main determinants of this process. The western blot analysis shows that 80% of DGqα is in the membrane fraction of dark-adapted wild-type flies (Figure 1A). Activation by light (see Materials and methods) results in progressive and massive translocation of DGqα from the membrane to the cytosol. Analysis of the translocation process showed apparent first-order reaction kinetics, reaching a plateau after 50 min. At this time point, 50% of the total DGqα has moved to the cytosol, which roughly translates to 1.5 × 106 molecules of DGqα per cell (Hardie and Raghu, 2001). The apparent rate constant of the translocation is 0.055/min (Figure 1A, inset). Our findings of activity-dependent translocation of DGqα are in agreement with previously reported experiments (Wedegaertner et al., 1993; Terakita et al., 1996; Wise et al., 1997; Narita et al., 1999) and differ from the reports of persistent membrane localization of Gqα (Edgerton et al., 1994; Hughes et al., 2001).
Fig. 1. Reversible, light-dependent translocation of DGqα in wild-type flies. (A) Western blot analysis shows the localization of DGqα in the pellet (P) and supernatant (S) fractions as a function of illumination times of dark-adapted flies. Time points on the graph show amounts of DGqα in the supernatant as a percentage of the total DGqα in membrane + supernatant. Inset: linearization on a logarithmic scale indicating that the reaction follows first-order kinetics with an apparent rate constant of 0.055/min. T = percentage of DGqα that has translocated (compared with dark-adapted flies). Tmax = maximal percentage of DGqα that translocated. Data points represent mean values ± SE from eight independent experiments. (B) Time-dependent recovery of DGqα from the cytosol to the membrane. Time points are mean amounts of DGqα in the supernatant (as a percentage of total) ± SE from four independent experiments. Inset: linearization of the recovery gives a rate constant of 0.03/min. T, Tmax = as above.
Any physiologically relevant event such as the activity-dependent translocation of DGqα would be expected to be fully reversible once the activating stimulus is turned off. To examine the recovery process, flies illuminated until maximum translocation had occurred were illuminated briefly with orange light to photoconvert the active meta-rhodopsin to the inactive rhodopsin. Following this treatment, the flies were kept in the dark for various times and assayed for DGqα localization (Figure 1B). The recovery also follows apparent first-order kinetics with a rate constant of 0.03/min (Figure 1B, inset). After 120 min in the dark, we observed a maximal return of DGqα to the membrane, reaching the level of the dark-adapted flies (80% in the membrane fraction and 20% in the cytosol). The recovery is thus slower than the activity-dependent translocation, but is fast enough to account for a readily reversible process.
Past and recent work has shown that in vertebrate photoreceptors, transducin moves along the photoreceptor cell (Brann and Cohen, 1987; Philp et al., 1987; Whelan and McGinnis, 1988; Sokolov et al., 2002), reminiscent of the results shown above. Because of the nature of the experimental procedures used, it was not determined whether translocation from the membrane to the cytosol was involved. The mechanism of translocation of Gqα described in the present study probably differs from that of transducin, as the latter is not palmitoylated and instead is modified by stable myristoylation.
Depalmitoylation of Gq was shown to be the underlying cause of its translocation from membrane to cytosol. A method previously used to show this was incubating membranes or isolated proteins containing acylated cysteines (and in particular palmitoylated Gq) with neutral hydroxylamine, resulting in specific cleavage of the thioester bond and consequently, translocation of the protein to the soluble fraction (Degtyarev et al., 1993; Wedegaertner et al., 1993; Pepperberg et al., 1995; Iiri et al., 1996; Terakita et al., 1996; Jones et al., 1997). We therefore incubated isolated membranes of fly heads with 1 M hydroxylamine in Tris buffer pH 8.0 for 30 min at 30°C. This treatment caused translocation of ∼50% of the DGqα to the soluble fraction, while incubation with the equivalent buffer lacking hydroxylamine did not result in appreciable translocation (data not shown). These results suggest that the in vivo translocation of the DGqα to the cytosol is the result of thioester bond cleavage, as indeed was shown previously for transfected Gq in cell lines.
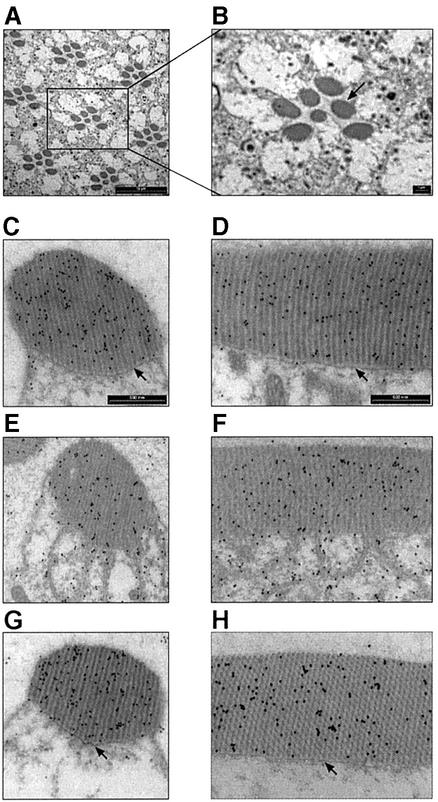
In an attempt to see whether the translocation of DGqα can be observed at the structural level of the cell, we applied transmission electron microscopy (TEM) using immunogold staining of DGqα with specific antibodies (Figure 2). In dark-adapted flies, most of the DGqα was located in the rhabdomere (Figure 2C and D), corroborating the results detailed above. Illumination for 1 h with blue light caused vast amounts of DGqα to translocate to the cytosol (Figure 2E and F). DGqα was also detected far away from the rhabdomere (data not shown), showing that it can diffuse away from its point of origin. Consistent with the results shown in Figure 1B, keeping the flies for 2 h in the dark following illumination restored DGqα’s localization to the rhabdomere, as in the dark-adapted state (Figure 2G and H).
Fig. 2. Light-dependent translocation of DGqα and rhabdomeral architectural changes shown by immunogold EM labeling. (A and B) Low magnification cross-sections of the Drosophila compound eye for orientation. Scale bar = 10 µm in (A) and 1 µm in (B). The seven dark oval structures of each visual unit (ommatidium) are the rhabdomeres, the signaling compartment of the photoreceptor cell, composed of tightly packed microvilli. (C, E and G) Cross-sections of a single rhabdomere. (D, F and H) A longitudinal section along the axis of the rhabdomere. Scale bar = 500 nm in (C–H). DGqα is localized by immunogold labeling, seen as dark dots. (C and D) Dark-adapted flies. DGqα is localized to the rhabdomeres. (E and F) Flies illuminated for 60 min with blue light. DGqα spreads out of the rhabdomere. In addition, remarkable architectural changes are observed in the cell. The boundary between the rhabdomere and the rest of the cell disappears (marked with arrows in C and D). Microvilli tendrils resembling a house painter’s brush are seen intruding into the cytosol. (G and H) Flies that had been illuminated for 60 min and subsequently kept for 2 h in the dark. DGqα localization and the architectural changes are completely reversed. Similar observations were made in six different flies.
Light-dependent architectural changes in the photoreceptor
Intriguingly, we also found that illumination apparently caused disruption of the rhabdomeral boundary (marked with arrows in Figure 2C and D), leading to partial collapse of the rhabdomere into the cytosol, resembling a house painter’s brush. Even though the participants and forces that constrain the rhabdomere are incompletely understood, the submembrane cortical cytoskeleton actin and its associated proteins are major contributors (Chang and Ready, 2000). These striking architectural changes were very prominent after 60 min of illumination (Figure 2E and F) but were already obvious after 15 min of illumination (data not shown). The architectural changes were completely reversible, as the rhabdomere’s structure after 2 h recovery in the dark was indistinguishable from that of the dark-adapted photoreceptors (Figure 2G, H, C and D, respectively).
To see whether these morphological changes indeed correlate with changes in the cytoskeleton, we used fluorescent confocal microscopy of Rh1–GFP–moe flies. In these transgenic flies, a GFP–moesin chimera expressed in the photoreceptors (see Materials and methods) marks the actin cytoskeleton (Edwards et al., 1997; Chang and Ready, 2000). In dark-adapted flies (Figure 3A), the actin signal is localized to the rhabdomere. In contrast, illumination for 60 min (Figure 3B) results in profound changes in the distribution of the cortical actin to the cytosol outside the rhabdomere, in a pattern resembling that seen in Figure 2E.
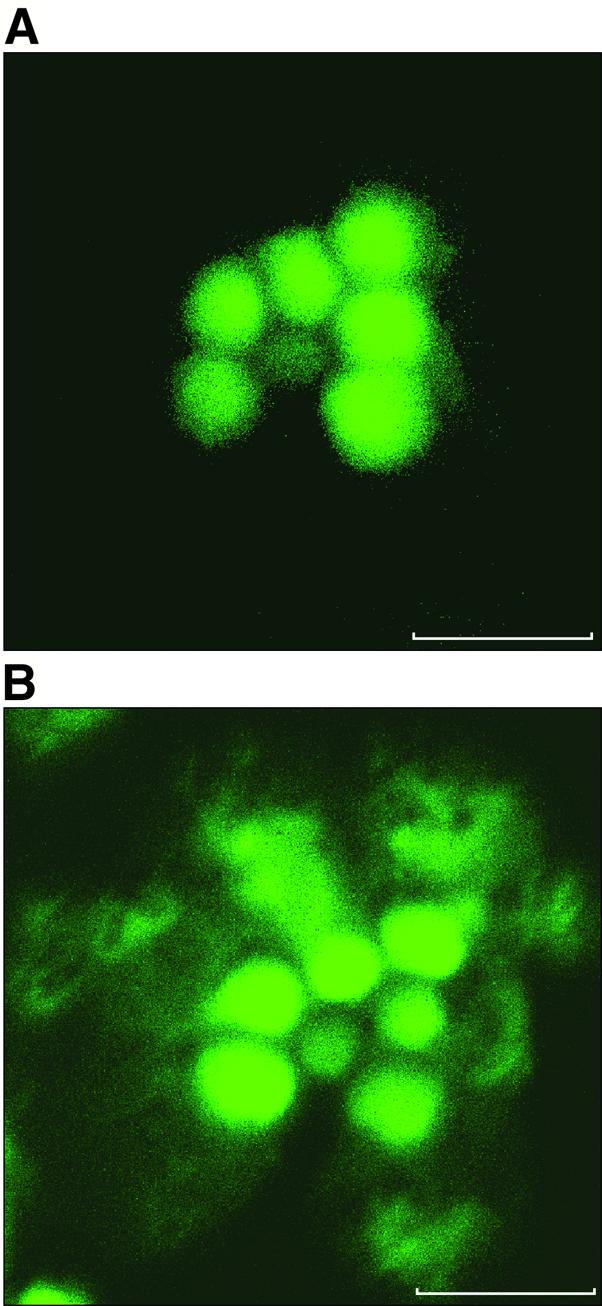
Fig. 3. Light-dependent redistribution of actin in the photoreceptor. Shown are confocal microscopy cross-sections of a single ommatidium in living isolated Drosophila retina. The orientation of the ommatidium is as in Figure 2B. Scale bar = 5 µm. The fluorescence images show GFP–moe marking the actin cytoskeleton (see Materials and methods). (A) Ommatidium of a retina isolated from a dark-adapted fly. (B) Illumination for 60 min results in profound changes in the distribution of the cortical actin to the cytosol outside the rhabdomere, in a pattern resembling that seen in Figure 2E. Similar observations were made in nine different flies.
Epistatic analysis of the architectural changes
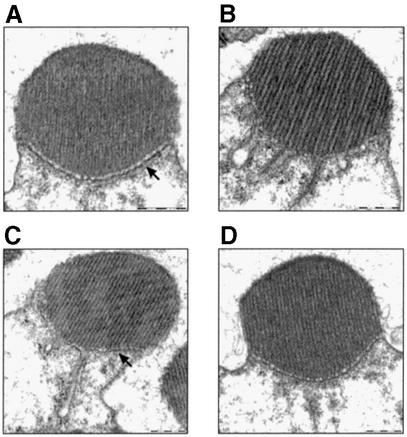
To investigate these architectural changes further, we turned to ultrastructural EM of the photoreceptor cell. As a control, we illuminated wild-type flies with white light to make sure the changes are not an artifact of illumination with blue light, and saw that the same phenomenon is observed with either treatment (Figure 4B). Characterization of the recovery process revealed that 30 min of incubation in the dark (after 60 min of blue illumination) resulted in full recovery in some rhabdomeres (data not shown) and partial recovery in others (Figure 4C). Incubation for 60 min in the dark resulted in full recovery in all the rhabdomeres (Figure 4D), showing that the recovery of both DGqα localization (Figure 1B) and the morphological changes proceed on a similar time scale.
Fig. 4. Ultrastructural analysis of the light-dependent architectural changes. Ultrastructural EM cross-sections of a single rhabdomere from wild-type flies. Scale bars = 500 nm. (A) Dark-adapted. (B) After illumination for 60 min with white light, resulting in the same architectural changes as with blue light (Figure 2) and causing the border of the rhabdomere (marked with an arrow in A) to disappear. (C and D) Flies that had been illuminated for 60 min and subsequently kept for 30 and 60 min, respectively, in the dark. Partial morphological recovery is achieved after 30 min (C) with partial return of the rhabdomere’s border (marked with an arrow). After 60 min (D), full morphological recovery is achieved. Similar observations were made in four different flies.
We next used the powerful genetics of Drosophila for epistatic analysis of the activity-dependent architectural changes. The Gαq1 mutant, a severe hypomorph in DGqα, exhibited a complete block of this phenomenon (Figure 5A and B), showing that DGqα is necessary for these changes to occur. We proceeded with two mutants, null for effectors downstream of DGqα, norpAP24, a PLC-null mutant (Pearn et al., 1996), and trpP343, a null mutant for the eye-specific TRP calcium channel (Scott et al., 1997). Both the norpAP24 mutant (Figure 5C and D) and the trpP343 mutant (Figure 5E and F) blocked the morphological changes, indicating that functional DGqα is not sufficient to activate this transformation. Because both of these mutants inhibit the massive, light-induced rise in intracellular calcium, it is logical that calcium may play a role in eliciting the architectural changes.
Fig. 5. Epistatic analysis of the light-dependent architectural changes. (A–F) Ultrastructural EM cross-sections of a single rhabdomere. (G–H) Immunogold EM labeling of DGqα as in Figure 2. Scale bar = 500 nm in all panels. (A and B) The near absence of DGqα in the Gαq1 mutant abolishes the light-dependent morphological changes, showing that DGqα is necessary for the morphological transformation. However, these changes are also blocked in the norpAP24 mutant (C and D) and in the trpP343 mutant (E and F), showing that DGqα is not sufficient to bring about these changes. As the trpP343 mutant also blocks the translocation of DGqα outside the rhabdomere (G and H), it seems that the morphological changes are required to enable DGqα to migrate outside of the rhabdomere. Similar observations were made in four or five different flies.
Intriguingly, in contrast to wild-type flies, in the trpP343 mutant, the DGqα protein does not spread out of the rhabdomere after illumination (Figure 5G and H). However, in this mutant, DGqα does translocate from the membrane to the soluble fraction, as measured by western blot analysis (data not shown). It therefore appears that the translocation of DGqα as seen in wild-type flies (Figure 2) proceeds in two phases: translocation from the membrane to the rhabdomeric cytosol, followed by migration of DGqα out of the rhabdomere.
Constitutively active rhodopsin results in persistent localization of DGqα to the cytosol
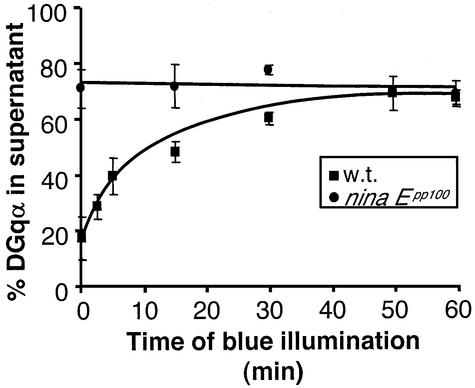
To learn more about the translocation process of DGqα, we used the Drosophila ninaEpp100 mutant in which rho dopsin is constitutively active (T.D.Zars and D.R.Hyde, in preparation). In this mutant, ∼70% of the α-subunit is in the cytosol, with no noticeable difference between dark-adapted and blue-illuminated flies (Figure 6). The localization of DGqα in this mutant does not change and is equal to the maximal translocation achieved in illuminated wild-type flies. Therefore, the in vivo translocation of DGqα is probably caused directly by rhodopsin activation. This is consistent with previous studies of activity-dependent Gq translocation in cell cultures (Wedegaertner et al., 1993; Edgerton et al., 1994; Hepler et al., 1996; Wise et al., 1997; Hughes et al., 2001).
Fig. 6. Constitutively active rhodopsin in the ninaEpp100 mutant is associated with localization of DGqα to the cytosol. In the ninaEpp100 mutant, rhodopsin constitutively activates DGqα. The percentage of DGqα in the cytosol of dark-adapted ninaEpp100 flies (circles) is similar to that obtained after prolonged activation of the wild-type flies (squares). Illumination of the mutant flies does not promote further translocation of DGqα to the cytosol. The graph shows the mean amount of DGqα in the supernatant (as a percentage of total) ± SE from three independent experiments. The graph of wild-type flies is identical to Figure 1A and is shown for comparison.
DGqβ is critical for the translocation cycle of DGqα
The βγ dimer binds the inactive Gα (charged with GDP) and plays an important role in localization and interaction of the α-subunit with upstream and downstream signaling components (Degtyarev et al., 1994; Iiri et al., 1996; Wedegaertner, 1998; Resh, 1999). Previous work employed Gqα mutations to disrupt the interaction between the α-subunit and the βγ complex in cell cultures (Evanko et al., 2000). While these mutant Gqα proteins were localized mainly to the cytosol, this approach could not resolve the question of whether this steady-state localization was due to an increase in the rate of the translocation reaction or an inhibition of the recovery back to the membrane. The Drosophila visual system offers a different approach to study the role of the β-subunit by using the Gβe1 mutant, which expresses small amounts of the eye-specific DGqβ (Dolph et al., 1994).
We found that in the Gβe1 mutant, the kinetics of the light-dependent translocation of DGqα, and in particular the subsequent recovery in the dark, were markedly affected (Figure 7). Dark-adapted Gβe1 flies have only 50% of DGqα in the membrane fraction (Figure 7A), as compared with the 80% found in the membranes of wild-type flies (Figure 1A). Additionally, the mutant exhibits a considerable time lag from the onset of illumination until detectable translocation is observed. In wild-type flies, massive translocation was observed already after 5 min of activation (Figure 1A), while little if any translocation was detected in the Gβe1 mutant even after 15 min of illumination (Figure 7A). These results complement the electrophysiological phenotype observed in the Gβe1 mutant (Dolph et al., 1994). In this work, both the increased latency of the electrophysiological response and the absence of light-dependent GTPγS binding indicated that the β-subunit is essential for the efficient activation of DGqα by rhodopsin. The latter could explain the slow onset of translocation depicted in Figure 7A. In contrast to the effective recovery observed in wild-type flies (Figure 1B), in Gβe1 flies no recovery of DGqα localization back to the membrane was detected, even after 2 h in the dark (Figure 7B). These results show that a functional βγ complex is essential for facilitating the rapid return of DGqα to the membrane following activation. Both the lack of recovery and the decreased presence of DGqα in the membranes of dark-adapted flies indicate a key role of the βγ complex in targeting the α-subunit to the plasma membrane. Therefore, our results suggest that the steady-state cytosolic localization of the mutant Gq observed by Evanko et al. (2000) was most probably due to inhibition of Gqα targeting to the membrane rather than an increase of translocation from the membrane to the cytosol. Our findings lead to the conclusion that in the environment of Drosophila photoreceptors, the βγ complex in fact plays a dual role. It is needed for efficient activation and translocation of DGqα to the cytosol and it is essential for the recovery of the translocated DGqα back to the membrane.
Fig. 7. Severe reduction of Gβ inhibits the translocation of DGqα and abolishes its recovery. (A) The Gβe1 mutant contains low levels of DGqβ. Only 50% of DGqα is localized to the membranes of Gβe1 dark-adapted flies as compared with 80% in wild-type flies (Figure 1). In contrast to the massive initial translocation observed in wild-type flies, in Gβe1 flies there is an obvious lag in the translocation kinetics of DGqα The graph shows the mean amount of DGqα in the supernatant (as a percentage of total) ± SE from four independent experiments. (B) Gβe1 flies were illuminated for 60 min and then incubated in the dark for various times. Even prolonged incubation of Gβe1 flies in the dark (2 h) produced no discernible recovery. The graph shows the mean amount of DGqα in the supernatant (as a percentage of total) ± SE from four independent experiments.
Positively charged patches in the N-terminus of DGqα
The translocation cycle of DGqα can also be influenced by additional factors. We have shown recently that all human Gα proteins that undergo palmitoylation as their only lipid modification (including Gq) contain a novel basic, positively charged motif in their N-termini (Kosloff et al., 2002). This structural motif was suggested to participate together with the βγ complex in the recovery of these Gα subunits back to the membrane. It is therefore notable that of the 34 residues in the N-terminal α-helix of DGqα, 11 are basic, positively-charged residues (Figure 8A). We therefore constructed a homology model of DGqα and mapped the electrostatic potential onto its molecular surface (Figure 8B). This model shows that the α-helical N-terminus of DGqα also contains this basic motif (marked with blue arrows). As shown for human Gα proteins, this positively charged structural motif is opposite to the residues of DGqα that interact with the βγ subunits. Hence, the positive patches are free to interact with the negatively charged inner face of the plasma membrane, regardless of whether DGqα is in the monomeric or trimeric form. As discussed in Kosloff et al. (2002), this basic motif is therefore another signal that may regulate the translocation cycle of DGqα, together with the βγ subunits.
Fig. 8. Positive patches in the N-terminus of DGqα (A) Of the 34 residues in the N-terminus of DGqα, 11 are basic, positively charged residues (marked in blue). (B) A three-dimensional model of DGqα mapping the electrostatic potential onto its Connolly molecular surface. The approximate orientation towards the βγ subunits is based on the crystal structures of the heterotrimer (PDB IDs 1GP2 and 1GOT). Superimposed over the molecular surface of DGqα are the electrostatic equipotential contours at +1 (blue mesh) and –1 kT/e (red mesh). As shown by the equipotential contour meshes, the positive patches extend a positive potential (marked with blue arrows), reaching significantly beyond the molecular surface of the protein.
DGqα translocation is independent of PLC and downstream signaling
In Drosophila photoreceptors, PLC plays a key role in both excitation and termination of the response to light (Cook et al., 2000). DGqα activates the PLC enzyme, while PLC accelerates the hydrolysis of GTP, thereby leading to response termination (Berstein et al., 1992). In the absence of PLC, DGqα remains in the activated (GTP-bound) state much longer than in wild-type flies (Cook et al., 2000).
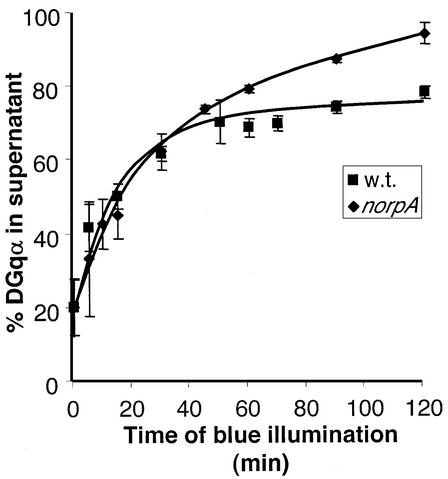
To test potential effects of PLC on the localization of DGqα, we studied the translocation reaction in the norpAP24 mutant. We found that in this mutant, the initial rate of DGqα translocation during the first 30 min is very similar to that of wild-type flies (Figure 9). Therefore, the translocation is independent of signaling downstream of the PLC effector and is triggered only through activation by rhodopsin. A difference between wild-type and norpAP24 flies becomes apparent only after a longer illumination time. While in wild-type the translocation reaches a plateau at a value of ∼70% of DGqα in the cytosol, in the norpAP24 mutant the translocation does not reach a plateau and proceeds almost to completion (>95% of DGqα is localized to the cytosol after 120 min). Kinetic analysis of the recovery process indicates that this difference is due, at least in part, to slower recovery in norpAP24 (data not shown).
Fig. 9. The translocation of DGqα is independent of PLC and downstream signaling. In the absence of PLC (in the norpAP24 mutant), the initial kinetics of DGqα translocation to the cytosol (diamonds) are very similar to those of wild-type flies (squares). Differences emerge only after prolonged illumination, resulting in translocation that proceeds almost to completion. The graph shows the mean amount of DGqα in the supernatant (as a percentage of total) ± SE from five independent experiments. The graph of wild-type flies is identical to Figure 1A and is shown for comparison.
Discussion
In this work, we investigated the dynamic localization of the Gqα subunit in a neuronal, compartmentalized cell. The physiological environment of the Drosophila visual system stands in contrast to the heterologous expression of Gqα in tissue culture cells. These cells have various ratios and compositions of Gα-interacting molecules that affect the translocation process and could account for the conflicting reports in previous studies. On the other hand, the large amount of photoreceptor-specific signaling molecules in Drosophila eyes combined with the availability of visual mutants constitute a unique and powerful experimental system. This system enabled us to characterize the kinetics of the DGqα translocation cycle in its natural setting and thereby to investigate how other signaling molecules interact with DGqα to regulate this process.
We found that in live Drosophila flies, there is a massive light-dependent translocation of DGqα from the membrane to the soluble fraction. This translocation is time dependent and follows reversible first-order reaction kinetics. Further evidence, together with previous studies of Gq localization in transfected cell lines, suggests that this activity-dependent translocation is due to depalmitoylation of DGqα resulting from activation of the G-protein by rhodopsin. Accordingly, in a constitutively active rhodopsin mutant, the major part of DGqα was present in the cytosol regardless of illumination. Thus, it is evident that DGqα translocation is a sensitive indicator of activated rhodopsin, regardless of whether this activation is achieved by light or by mutation. Interestingly, in a PLC-null mutant, the initial rate of DGqα translocation is similar to the rate in wild-type flies. This result indicates that DGqα translocation is due to its interaction with the receptor and is neither dependent on nor influenced by phototransduction steps at the level of PLC or downstream of it. The fact that the extent of DGqα translocation is higher in the norpAP24 mutant, reaching >95%, apparently is the result of slower recovery. Presumably, this is due to the absence of PLC, which normally accelerates GTP hydrolysis. This leaves DGqα active for a longer period in the norpAP24 mutant (Cook et al., 2000). While it is important to characterize the enzymes that underlie the translocation cycle and its regulation, technical difficulties unfortunately have hindered this effort (Wedegaertner, 1998; Resh, 1999; Chen and Manning, 2001). The finding that the regulation of DGqα translocation is confined to the interaction of the G-protein with the receptor should simplify future analysis of this process.
Another insight into the mechanism of the translocation cycle of DGqα was obtained by analysis of the Gβe1 mutant. The importance of the interactions between the α- and β-subunits in membrane localization was noted previously in several studies, but left unresolved which part of the translocation cycle is affected (Evanko et al., 2000). Our results indicate that the major determinant of the persistent cytosolic localization of such mutant α-subunits was probably a severe inhibition of the recovery process. In fact, our kinetic analysis showed that the βγ complex plays a dual role. First, it is required for proper presentation of the G-protein to the receptor in order to achieve efficient activation of the α-subunit. This notion is in line with the lack of light-dependent GTPγS binding shown for the Gβe1 mutant (Dolph et al., 1994). Secondly, we found that the βγ complex is essential for efficient targeting of DGqα to the membrane. The mechanistic explanation for the latter has been described by the ‘two-signal model’ (reviewed in Wedegaertner, 1998; Resh, 1999). This model suggests that more than one membrane attachment signal determines the membrane localization of peripheral membrane proteins such as G-proteins. In the case of α-subunits such as DGqα, the two signals are lipid modification of the α-subunit and its interaction with the βγ complex, which contain lipid modification on the γ-subunit. Our results showed that in the Gβe1 mutant, recovery of the soluble DGqα back to the membrane was not observed, even after 2 h in the dark. Under the same conditions, full recovery was obtained in the wild-type flies. This indeed demonstrates that in vivo the βγ complex is a critical signal for targeting of the α-subunit to the membrane of the signaling compartment. An additional insight gained from our experiments is that dissociation of DGqα from the βγ complex is not sufficient to cause its translocation. As a significant amount of DGqα is membrane bound in the Gβe1 mutant in the dark, it is conceivable that activation by the receptor and exchange of the bound GDP for GTP are also needed to facilitate the translocation reaction. Additionally, the three-dimensional model of DGqα identified a basic, positively charged motif in its N-terminus, a signal that can participate in the targeting of the α-subunit to the plasma membrane and assist in its subsequent palmitoylation (Kosloff et al., 2002). This structural motif can be an additional regulatory signal in the translocation cycle of DGqα, adding another signal to the two-signal model in addition to the βγ complex and the palmitoylation of DGqα.
We observed and characterized light-dependent architectural changes in the Drosophila photoreceptor cell, associated with redistribution of the cortical actin cytoskeleton. We showed that DGqα is necessary, but not sufficient, to bring about the morphological changes, while these changes are needed to allow the spread of DGqα from the cytosol of the rhabdomere to the cell interior. A possible physiological role for these reversible architectural changes is to facilitate efficient recovery of damaged phototransduction components after prolonged illumination, and can enable the relocation of signaling molecules within the compartmentalized photoreceptor cell. The translocation of DGqα, combined with the light-dependent morphological changes we observed, raises new possibilities for cross-talk between the DGq protein and other cellular processes and effectors. Indeed, several such novel effectors have been reported recently (Bence et al., 1997; Carman et al., 1999). Additional work is needed to characterize further this morphological phenomenon and its relevance to additional signaling molecules.
The removal of DGqα from the vicinity of the other phototransduction components might accelerate signal termination in the short term and contribute to long-term adaptation in the longer time frame (Selinger et al., 1993; Wedegaertner and Bourne, 1994). Such a contribution to adaptation was shown recently to be associated with the redistribution of transducin in vertebrate photoreceptors (Sokolov et al., 2002). A similar redistribution was also reported in earlier studies (Brann and Cohen, 1987; Philp et al., 1987; Whelan and McGinnis, 1988). These results suggest that although the two phototransduction cascades differ greatly in their signaling components and mechanisms, the general phenotype of translocation evolved in both visual systems. A recent study (Bahner et al., 2002) reported that light causes translocation of the Drosophila TRPL channels and that this contributes to long-term adaptation. This translocation of TRPL and its recovery back to the rhabdomere follow a similar time course to the translocation and recovery of DGqα shown in the present work. As the amount of DGqα was shown to determine directly the sensitivity of the photoreceptor to light (Scott et al., 1995), it seems that the DGqα translocation we observe is an additional direct contributor to long-term adaptation. It is also possible that the light-induced morphological changes play a part in the previously observed translocation of the TRPL channel, an integral membrane protein, but this problem needs further study.
In conclusion, our in vivo studies suggest a putative model of DGqα translocation and its regulation in Drosophila phototransduction, depicted in Figure 10. This model complements and extends previous studies in cell lines, indicating that the substrate for depalmitoylation and hence translocation to the cytosol is the G-protein α-subunit, after it has been activated by the receptor, loaded with GTP and dissociated from the βγ subunits (Resh, 1999). The present study provides in vivo evidence supporting the ‘two-signal model’ for membrane attachment of G-proteins. As palmitoylation has been shown to modify a plethora of proteins extending beyond G-proteins (Mumby, 1997; Resh, 1999), our results might be applicable to other palmitoylated proteins in vivo.
Fig. 10. Putative model of the light-dependent translocation cycle of DGqα in Drosophila photoreceptors. The numbers represent discreet steps in the translocation cycle of DGqα. (1) Light-activated rhodopsin activates DGq by catalyzing the exchange of GDP for GTP. This causes the GTP-bound α-subunit to dissociate from the βγ subunits. The dissociated βγ subunits are not shown. (2) The activated α-subunit proceeds to activate its effector, PLC. The latter, in turn, accelerates the turn off (GTPase) of the DGqα protein. (3) Alternatively, the activated α-subunit is subject to depalmitoylation and translocates to the cytosol. (4) The inactivated DGqα returns to the membrane in a βγ-dependent process, followed by palmitoylation and attachment to the membrane (according to the ‘two-signal model’).
Materials and methods
Fly stocks
Drosophila melanogaster of the following strains were used: wild-type, Oregon-R w; Gaq1, a severe hypomorph for DGqα (Scott et al., 1995) (from C.S.Zuker); norpAP24, a null mutant for the eye-specific PLCβ (Pearn et al., 1996) (from W.L.Pak); ninaEpp100, a constitutively active dominant rhodopsin mutant (from D.R.Hyde); Gβe1, a severe hypomorph mutant of the eye-specific DGqβ (Dolph et al., 1994) (from C.S.Zuker); and trpP343, a null mutant for the eye-specific TRP calcium channel (Scott et al., 1997) (from W.L.Pak). Rh1–GFP–moe, GFP–moe (a chimeric protein coupling GFP and the actin-binding domain of moesin) (Edwards et al., 1997) was expressed in the rhabdomeres by crossing P[UAS-GFP–moe] transgenic flies (from D.Kiehart and R.Montague) with Rh1–Gal4 flies.
Assay of light-dependent DGqα localization
In Drosophila flies, the photopigment is thermostable and photoreversible. By applying blue light (λ <490 nm), inactive rhodopsin is converted to active meta-rhodopsin (80%) and, by applying orange light (λ >570 nm), rhodopsin is regenerated (100%) (Selinger et al., 1993). In each experiment, flies were dark adapted overnight prior to the experiments. Live flies, in glass vials covered with aluminum foil, were subjected to illumination with activating blue light (18 W white light lamp with a Schott BG 28 wide band filter, 1 mm thick, 12 cm away from the flies) for various times at 22°C. Control experiments using white light showed similar results. Termination was carried out by moving the flies to 4°C in the dark and promptly separating the fly heads. Ten flies were used for each time point. The fly heads were homogenized in 1 ml of hypotonic homogenization buffer (HEPES 20 mM pH 7.6, leupeptin 20 µg/ml, pepstatin A 1 µg/ml, o-phenanthroline 0.35 mg/ml, N-ethylmaleimide 15 mM). Membranes and cytosol were separated by centrifugation (15 800 g for 15 min at 4°C). The pellet was washed, centrifuged again and the supernatants were combined. Ultracentrifugation at 150 000 g for 30 min did not precipitate additional DGqα proteins or change the distribution between the fractions. The proteins were precipitated by 5% trichloroacetic acid (TCA), run on 10% SDS–polyacrylamide gels and subjected to quantification as described below.
In recovery experiments, after illumination of the flies with blue light to achieve maximal translocation of DGqα (60 min), the flies were illuminated for 5 min with orange light (Schott OG 570 edge filter) to inactive the photo-pigment. These flies were then incubated in the dark at 22°C for various times. Subsequent steps were as described above.
Quantification of DGqα
After separation by SDS–PAGE, western blotting was carried out using an anti-DGqα polyclonal antibody raised in rabbit against the C-terminal decapeptide of the protein as described in Palczewski et al. (1993). This antibody was shown to be highly specific and hardly detected any background bands. Quantitation of the enhanced chemiluminescence (ECL) was carried out either using a Fuji LAS-1000 system or by exposure to film, scanning and using the NIH image software (version 1.62). To reduce the variance due to the experimental procedure, the amounts of DGqα in each fraction were calculated as a percentage of the total DGqα in both the pellet and supernatant of each treatment. In the graphs, only the percentage in the supernatant is shown (out of 100%).
Electron microscopy
Flies were dark adapted and illuminated as detailed above. For immunogold EM analysis, the heads were separated, cut longitudinally in two and fixed for 12 h in a solution of 4% paraformaldehyde and 0.05% glutaraldehyde in 0.1 M phosphate buffer pH 7.4. The head sections were washed three times in the same phosphate buffer and dehydrated in ethanol. The half heads were infiltrated by LR white resin and polymerized in gelatin capsules at 50°C for 24 h. Thin sections were cut and placed on nickel grids. The sections were blocked for 5 min with 5% goat serum in 10 mM Tris–HCl buffer pH 8.2 (containing 0.9% NaCl, 0.5% bovine serum albumin, 0.1% Tween-20 and 20 mM NaN3) and incubated for 1 h at room temperature with the same primary antibody used for the western blotting (diluted 1:80). After washing with the Tris–HCl buffer described above, the grids were incubated for 1 h with a goat anti-rabbit secondary antibody conjugated to either 18 or 20 nm gold particles, washed again and stained with saturated aqueous uranyl acetate and lead citrate.
For ultrastructural EM, fixation was done in a solution of 1.5% paraformaldehyde and 2.5% glutaraldehyde in 0.1 M phosphate buffer pH 7.4. The head sections were washed three times in the same phosphate buffer. After fixation, eyes were post-fixed with 1% osmium tetroxide for 4 h, dehydrated in ethanol and propylene oxide, and embedded in epon. Thin sections were cut and stained with saturated aqueous uranyl acetate and lead citrate.
Sections were observed and photographed with a Technai-12 transmission electron microscope (Philips) equipped with a Mega-view II CCD camera.
Fluorescent confocal microscopy
The retina from Rh1–GFP–moe flies was isolated from the cornea and brain and kept in Ringer’s solution as described previously (Peretz et al., 1994). A cross-section of a single ommatidium was visualized using a Fluoview 300 confocal microscope (Olympus). Optical sections were obtained from the upper region of the ommatidia at a depth of 6–10 µm from the tip of the ommatidium. The fluorescence images arise from excitation with blue light of GFP–moe, a marker for the actin cytoskeleton (see above).
Electrostatic surface map of DGqα
A three-dimensional homology model of DGqα and the electrostatic potential distribution around it were produced as described previously (Kosloff et al., 2002). Visualization of the results was done by mapping the electrostatic potential on the Connolly surface of the protein and by adding the electrostatic potential surfaces as equipotential contour meshes using the InsightII package (Accelrys).
Acknowledgments
Acknowledgements
We thank D.Kiehart, R.Montague, C.Zuker and W.Pak for specific Drosophila mutants, M.Bar-Yaakov and D.Shalev for technical assistance, and the late M.Shramm for helpful discussion. This work was supported by grants from the NIH (EY-03529 to Z.S and B.M., R01-EY-12426 to D.R.H.), the ISF (to Z.S. and B.M.), the BSF (to Z.S. and B.M.), The Moscona foundation (to Z.S. and B.M.), the German–Israel Foundation (to B.M.), the Foundation for Fighting Blindness (to D.R.H.) and the Minerva Foundation (to Z.S. and B.M.).
References
- Bahner M., Frechter,S., Da Silva,N., Minke,B., Paulsen,R. and Huber,A. (2002) Light-regulated subcellular translocation of Drosophila TRPL channels induces long-term adaptation and modifies the light-induced current. Neuron, 34, 83–93. [DOI] [PubMed] [Google Scholar]
- Bence K., Ma,W., Kozasa,T. and Huang,X.Y. (1997) Direct stimulation of Bruton’s tyrosine kinase by Gq-protein α-subunit. Nature, 389, 296–299. [DOI] [PubMed] [Google Scholar]
- Berstein G., Blank,J.L., Jhon,D.Y., Exton,J.H., Rhee,S.G. and Ross,E.M. (1992) Phospholipase C-β1 is a GTPase-activating protein for Gq/11, its physiologic regulator. Cell, 70, 411–418. [DOI] [PubMed] [Google Scholar]
- Brann M.R. and Cohen,L.V. (1987) Diurnal expression of transducin mRNA and translocation of transducin in rods of rat retina. Science, 235, 585–587. [DOI] [PubMed] [Google Scholar]
- Carman C.V., Parent,J.L., Day,P.W., Pronin,A.N., Sternweis,P.M., Wedegaertner,P.B., Gilman,A.G., Benovic,J.L. and Kozasa,T. (1999) Selective regulation of Gαq/11 by an RGS domain in the G protein-coupled receptor kinase, GRK2. J. Biol. Chem., 274, 34483–34492. [DOI] [PubMed] [Google Scholar]
- Chang H.Y. and Ready,D.F. (2000) Rescue of photoreceptor degeneration in rhodopsin-null Drosophila mutants by activated Rac1. Science, 290, 1978–1980. [DOI] [PubMed] [Google Scholar]
- Chen C.A. and Manning,D.R. (2001) Regulation of G proteins by covalent modification. Oncogene, 20, 1643–1652. [DOI] [PubMed] [Google Scholar]
- Cook B., Bar-Yaacov,M., Cohen Ben-Ami,H., Goldstein,R.E., Paroush,Z., Selinger,Z. and Minke,B. (2000) Phospholipase C and termination of G-protein-mediated signalling in vivo. Nat. Cell Biol., 2, 296–301. [DOI] [PubMed] [Google Scholar]
- Degtyarev M.Y., Spiegel,A.M. and Jones,T.L. (1993) Increased palmitoylation of the Gs protein α subunit after activation by the β-adrenergic receptor or cholera toxin. J. Biol. Chem., 268, 23769–23772. [PubMed] [Google Scholar]
- Degtyarev M.Y., Spiegel,A.M. and Jones,T.L. (1994) Palmitoylation of a G protein αI subunit requires membrane localization not myristoylation. J. Biol. Chem., 269, 30898–30903. [PubMed] [Google Scholar]
- Devary O., Heichal,O., Blumenfeld,A., Cassel,D., Suss,E., Barash,S., Rubinstein,C.T., Minke,B. and Selinger,Z. (1987) Coupling of photoexcited rhodopsin to inositol phospholipid hydrolysis in fly photoreceptors. Proc. Natl Acad. Sci. USA, 84, 6939–6943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolph P.J., Man-Son-Hing,H., Yarfitz,S., Colley,N.J., Deer,J.R., Spencer,M., Hurley,J.B. and Zuker,C.S. (1994) An eye-specific Gβ subunit essential for termination of the phototransduction cascade. Nature, 370, 59–61. [DOI] [PubMed] [Google Scholar]
- Edgerton M.D., Chabert,C., Chollet,A. and Arkinstall,S. (1994) Palmitoylation but not the extreme amino-terminus of Gqα is required for coupling to the NK2 receptor. FEBS Lett., 354, 195–199. [DOI] [PubMed] [Google Scholar]
- Edwards K.A., Demsky,M., Montague,R.A., Weymouth,N. and Kiehart,D.P. (1997) GFP–moesin illuminates actin cytoskeleton dynamics in living tissue and demonstrates cell shape changes during morphogenesis in Drosophila. Dev. Biol., 191, 103–117. [DOI] [PubMed] [Google Scholar]
- Evanko D.S., Thiyagarajan,M.M. and Wedegaertner,P.B. (2000) Interaction with Gβγ is required for membrane targeting and palmitoylation of Gαs and Gαq. J. Biol. Chem., 275, 1327–1336. [DOI] [PubMed] [Google Scholar]
- Hardie R.C. and Raghu,P. (2001) Visual transduction in Drosophila. Nature, 413, 186–193. [DOI] [PubMed] [Google Scholar]
- Hepler J.R., Biddlecome,G.H., Kleuss,C., Camp,L.A., Hofmann,S.L., Ross,E.M. and Gilman,A.G. (1996) Functional importance of the amino terminus of Gqα. J. Biol. Chem., 271, 496–504. [DOI] [PubMed] [Google Scholar]
- Huang C., Duncan,J.A., Gilman,A.G. and Mumby,S.M. (1999) Persistent membrane association of activated and depalmitoylated G protein α subunits. Proc. Natl Acad. Sci. USA, 96, 412–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes T.E., Zhang,H., Logothetis,D.E. and Berlot,C.H. (2001) Visualization of a functional Gαq–green fluorescent protein fusion in living cells. J. Biol. Chem., 276, 4227–4235. [DOI] [PubMed] [Google Scholar]
- Iiri T., Backlund,P.S.,Jr, Jones,T.L., Wedegaertner,P.B. and Bourne,H.R. (1996) Reciprocal regulation of Gsα by palmitate and the βγ subunit. Proc. Natl Acad. Sci. USA, 93, 14592–14597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones T.L., Degtyarev,M.Y. and Backlund,P.S.,Jr (1997) The stoichiometry of Gαs palmitoylation in its basal and activated states. Biochemistry, 36, 7185–7191. [DOI] [PubMed] [Google Scholar]
- Kosloff M., Elia,N. and Selinger,Z. (2002) Structural homology discloses a bi-functional structural motif at the N-termini of Gα proteins. Biochemistry, 41, 14518–14523. [DOI] [PubMed] [Google Scholar]
- Milligan G. and Unson,C.G. (1989) Persistent activation of the α subunit of Gs promotes its removal from the plasma membrane. Biochem. J., 260, 837–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minke B., and Hardie,R.C. (2000) Genetic dissection of Drosophila phototransduction. In Stavenga,D.G., van der Hope,D.J.N. and Pugh,E. (eds), Molecular Mechanisms in Visual Transduction. Elsevier, North Holland, The Netherlands, pp. 449–525.
- Mumby S.M. (1997) Reversible palmitoylation of signaling proteins. Curr. Opin. Cell Biol., 9, 148–154. [DOI] [PubMed] [Google Scholar]
- Mumby S.M., Kleuss,C. and Gilman,A.G. (1994) Receptor regulation of G-protein palmitoylation. Proc. Natl Acad. Sci. USA, 91, 2800–2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita K., Suzuki,T., Ohtsu,K., Seidou,M., Kito,Y. and Tsukahara,Y. (1999) Structural and functional differences of two forms of GTP-binding protein, Gq, in the cephalopod retina. Comp. Biochem. Physiol. B Biochem. Mol. Biol., 123, 319–327. [DOI] [PubMed] [Google Scholar]
- Palczewski K., Buczylko,J., Lebioda,L., Crabb,J.W. and Polans,A.S. (1993) Identification of the N-terminal region in rhodopsin kinase involved in its interaction with rhodopsin. J. Biol. Chem., 268, 6004–6013. [PubMed] [Google Scholar]
- Pearn M.T., Randall,L.L., Shortridge,R.D., Burg,M.G. and Pak,W.L. (1996) Molecular, biochemical and electrophysiological characterization of Drosophila norpA mutants. J. Biol. Chem., 271, 4937–4945. [DOI] [PubMed] [Google Scholar]
- Pepperberg D.R., Morrison,D.F. and O’Brien,P.J. (1995) Depalmitoylation of rhodopsin with hydroxylamine. Methods Enzymol., 250, 348–361. [DOI] [PubMed] [Google Scholar]
- Peretz A., Suss-Toby,E., Rom-Glas,A., Arnon,A., Payne,R. and Minke,B. (1994) The light response of Drosophila photoreceptors is accompanied by an increase in cellular calcium: effects of specific mutations. Neuron, 12, 1257–1267. [DOI] [PubMed] [Google Scholar]
- Philp N.J., Chang,W. and Long,K. (1987) Light-stimulated protein movement in rod photoreceptor cells of the rat retina. FEBS Lett., 225, 127–132. [DOI] [PubMed] [Google Scholar]
- Ransnas L.A., Svoboda,P., Jasper,J.R. and Insel,P.A. (1989) Stimulation of β-adrenergic receptors of S49 lymphoma cells redistributes the α subunit of the stimulatory G protein between cytosol and membranes. Proc. Natl Acad. Sci. USA, 86, 7900–7903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resh M.D. (1999) Fatty acylation of proteins: new insights into membrane targeting of myristoylated and palmitoylated proteins. Biochim. Biophys. Acta, 1451, 1–16. [DOI] [PubMed] [Google Scholar]
- Scott K., Becker,A., Sun,Y., Hardy,R. and Zuker,C. (1995) Gqα protein function in vivo: genetic dissection of its role in photoreceptor cell physiology. Neuron, 15, 919–927. [DOI] [PubMed] [Google Scholar]
- Scott K., Sun,Y., Beckingham,K. and Zuker,C.S. (1997) Calmodulin regulation of Drosophila light-activated channels and receptor function mediates termination of the light response in vivo. Cell, 91, 375–383. [DOI] [PubMed] [Google Scholar]
- Selinger Z., Doza,Y.N. and Minke,B. (1993) Mechanisms and genetics of photoreceptors desensitization in Drosophila flies. Biochim. Biophys. Acta, 1179, 283–299. [DOI] [PubMed] [Google Scholar]
- Sokolov M., Lyubarsky,A.L., Strissel,K.J., Savchenko,A.B., Govardovskii,V.I., Pugh,E.N. and Arshavsky,V.Y. (2002) Massive light-driven translocation of transducin between the two major compartments of rod cells. A novel mechanism of light adaptation. Neuron, 34, 95–106. [DOI] [PubMed] [Google Scholar]
- Terakita A., Takahama,H., Tamotsu,S., Suzuki,T., Hariyama,T. and Tsukahara,Y. (1996) Light-modulated subcellular localization of the α-subunit of GTP-binding protein Gq in crayfish photoreceptors. Vis. Neurosci., 13, 539–547. [DOI] [PubMed] [Google Scholar]
- Wedegaertner P.B. (1998) Lipid modifications and membrane targeting of Gα. Biol. Signals Recept., 7, 125–135. [DOI] [PubMed] [Google Scholar]
- Wedegaertner P.B. and Bourne,H.R. (1994) Activation and depalmitoylation of Gsα. Cell, 77, 1063–1070. [DOI] [PubMed] [Google Scholar]
- Wedegaertner P.B., Chu,D.H., Wilson,P.T., Levis,M.J. and Bourne,H.R. (1993) Palmitoylation is required for signaling functions and membrane attachment of Gqα and Gsα. J. Biol. Chem., 268, 25001–25008. [PubMed] [Google Scholar]
- Wedegaertner P.B., Bourne,H.R. and von Zastrow,M. (1996) Activation-induced subcellular redistribution of Gsα. Mol. Biol. Cell, 7, 1225–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan J.P. and McGinnis,J.F. (1988) Light-dependent subcellular movement of photoreceptor proteins. J. Neurosci. Res., 20, 263–270. [DOI] [PubMed] [Google Scholar]
- Wise A., Parenti,M. and Milligan,G. (1997) Interaction of the G-protein G11α with receptors and phosphoinositidase C: the contribution of G-protein palmitoylation and membrane association. FEBS Lett., 407, 257–260. [DOI] [PubMed] [Google Scholar]