Abstract
In recent years, a role for AMPA receptors as modulators of presynaptic functions has emerged. We have investigated the presence of AMPA receptor subunits and the possible dynamic control of their surface exposure at the presynaptic membrane. We demonstrate that the AMPA receptor subunits GluR1 and GluR2 are expressed and organized in functional receptors in axonal growth cones of hippocampal neurons. AMPA receptors are actively internalized upon activation and recruited to the surface upon depolarization. Pretreatment of cultures with botulinum toxin E or tetanus toxin prevents the receptor insertion into the plasma membrane, whereas treatment with α-latrotoxin enhances the surface exposure of GluR2, both in growth cones of cultured neurons and in brain synaptosomes. Purification of small synaptic vesicles through controlled-pore glass chromatography, revealed that both GluR2 and GluR1, but not the GluR2 interacting protein GRIP, copurify with synaptic vesicles. These data indicate that, at steady state, a major pool of AMPA receptor subunits reside in synaptic vesicle membranes and can be recruited to the presynaptic membrane as functional receptors in response to depolarization.
Keywords: AMPA/GluR1/GluR2/growth cone/synaptic vesicles
Introduction
Glutamate is the major excitatory neurotransmitter in the brain. At mature glutamatergic synapses, the response to glutamate is transduced by two different ion-permeable receptors, which are clustered in the portion of the postsynaptic membrane facing the presynaptic active zone: the N-methyl-d-aspartate (NMDA) receptors and the AMPA receptors. The NDMA receptors are largely inactive at resting membrane potential and require membrane depolarization to become activated, and the AMPA receptors open upon glutamate binding and mediate most of the depolarizing effect of this neurotransmitter. Thus AMPA receptors, by directly controlling the majority of excitatory synaptic transmission, play a major role in the storage of information in brain (Wisden and Seeburg, 1993; Hollmann and Heinemann, 1994; Nakanishi et al., 1998; Scannevin and Huganir, 2000; Malinow and Malenka, 2002).
Recently, evidence has been accumulating indicating that, in addition to their well-established postsynaptic action, AMPA receptors also mediate presynaptic effects (Nicoll et al., 2000). Presynaptic AMPA receptors have been shown to modulate synaptic transmission, by depressing the release of inhibitory transmitters in the adult cerebellum (Satake et al., 2000). Furthermore, glutamate, by acting on AMPA/kainate receptors during synaptogenesis, regulates the actin-based motility of axonal filopodia implicated in synapse formation (Chang and De Camilli, 2001). Finally, lack of activation of glutamate receptors during synaptogenesis prevents the functional maturation of the presynaptic machinery (Bacci et al., 2001). As a result, a novel role for AMPA receptors as modulators of the presynaptic structure and function at both the developing and the mature synapse is emerging.
One of the interesting features of postsynaptic AMPA receptors is their regulated dynamic recycling between the plasma membrane and intracellular pools (Carroll et al., 2001; Sheng and Lee, 2001; Malinow and Malenka, 2002). As the number of surface-exposed postsynaptic AMPA receptors regulates the efficiency of synaptic transmission, this recycling is thought to play a major role in finely tuning synaptic strength during development and synaptic plasticity phenomena. In particular, receptor internalization, occurring in response to various extracellular stimuli, plays a potential role in the rapid attenuation of neurotransmission during long-term depression, whereas receptor insertion into the plasmalemma would represent one mechanism by which synaptic strength is increased during long-term potentiation (Carroll et al., 2001; Sheng and Lee, 2001; Malinow and Malenka, 2002).
The molecular processes involved in AMPA receptor internalization have been clearly identified (Carroll et al., 2001). Receptor internalization occurs via clathrin-mediated endocytosis (Carroll et al., 1999; Man et al., 2000; Wang and Linden, 2000), driven by activation of either protein kinase C, calcineurin or protein phosphatase 1, depending on the cell type (reviewed in Carroll et al., 2001). Once internalized, the receptors are either transported for degradation or stabilized in intracellular vesicles through binding to AMPA receptor interacting proteins (Dong et al., 1999; Carroll et al., 2001). The phosphorylation state of AMPA receptor subunits appears to strictly regulate this process (Chung et al., 2000; Malinow and Malenka, 2002). On the other hand, the mechanisms responsible for the delivery of AMPA receptors to the synaptic membrane are much less clear. This process is thought to be mediated by exocytosis of receptor-containing vesicles, regulated by neuronal activity (Lledo et al., 1998; Maletic-Savatic et al., 1998; Broutman and Baudry, 2001; Liao et al., 2001; Passafaro et al., 2001; Shi et al., 2001).
In this study, we have investigated the traffic of presynaptic AMPA receptors, taking advantage of the extensive characterization of the vesicular trafficking in the nerve terminal (Greengard et al., 1993; Jahn and Sudhof, 1999; Cremona and De Camilli, 1997; Lin and Scheller, 2000). We demonstrate that, at steady state, a major pool of GluR2 and GluR1 subunits is associated with synaptic vesicle membranes and that AMPA receptors are recruited to the presynaptic membrane in response to depolarization, through clostridial toxin- sensitive exocytosis.
Results
Expression of AMPA receptor subunits in axonal growth cones of cultured hippocampal neurons
A panel of antibodies directed against either the C- or the N-terminus of various AMPA receptor subunits (GluR1, GluR2/3, GluR2 N-ter) was used to investigate the expression of these proteins in primary cultures of hippocampal neurons. When tested on homogenates of adult rat brain as well as on cell extracts of mature hippocampal cultures, all the antibodies recognized a single band with the expected molecular mass (see Supplementary data, available at The EMBO Journal Online). Immunofluorescence of mature neuronal cultures with the anti-GluR1 and -GluR2/3 antibodies resulted in a typical staining of the somatodendritic compartment and of dendritic spines (Wyszynski et al., 2002). Application of antibodies directed against the N-terminal domain of GluR2 to living neurons, followed by immunofluorescence for the bound antibodies, specifically stained neuronal cell bodies and dendrites, consistent with the in vivo exposure of this AMPA receptor subunit on the somatodendritic plasma membrane (not shown). Antibodies directed against the AMPA receptor subunit GluR4 produced a faint staining in cell extracts of mature hippocampal cultures by western blot analysis and did not produce any detectable signal by immunofluorescence.
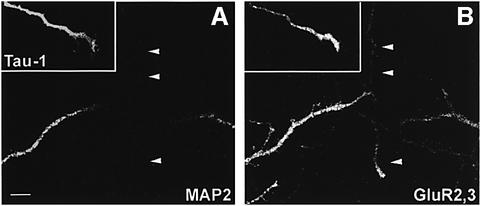
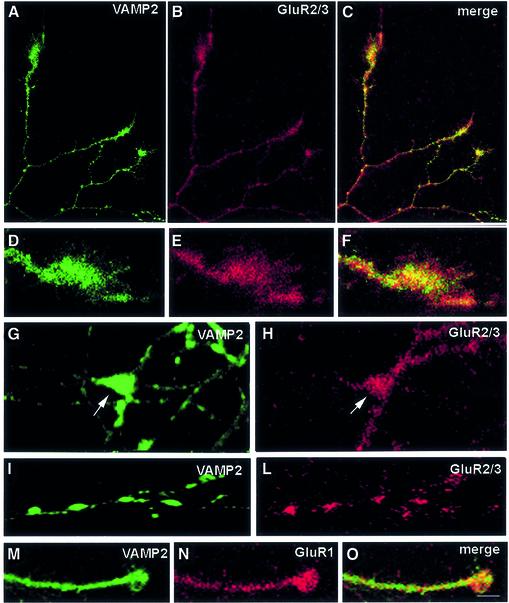
Once the specificity of the antibodies had been assessed, we used them to investigate the possible presence of AMPA receptor subunits in the axonal compartment of cultured hippocampal neurons. Five to seven days in vitro (DIV) old cultures were double stained with the antibodies to AMPA receptor subunits GluR2/3 and with antibodies directed against the synaptic vesicle protein synaptobrevin/VAMP2. GluR2/3 antibodies labeled with variable intensity, axonal growth cones (Figure 1), which could be identified by the lack of staining with antibodies against the somatodendritic marker MAP2 (Figure 1A) or by immunoreactivity for the axonal protein tau-1 (insert). Axonal growth cones were also characterized by the presence of small synaptic vesicle clusters (Figure 2A and D), as previously described (Mundigl et al., 1993). The percentage of GluR2/3-positive growth cones was 77 ± 14%. GluR2/3 immunoreactivity was still present in the growth cone after it has contacted a target dendrite (Figure 2G and H, arrow). GluR2/3 immunoreactivity was also detectable in packages of synaptic vesicles which travel along the axon (Figure 2I and L; Kraszewski et al., 1995; Ahmari et al., 2000). A percentage of axonal growth cones (42 ± 7.3%) was also found to be positive upon staining with antibodies to GluR1 (Figure 2M–O) (see also Craig et al., 1993).
Fig. 1. Immunocytochemical localization of GluR2/3 in axonal growth cones of cultured hippocampal neurons. Seven day old neurons double labeled for GluR2/3 (B) and for the somatodendritic marker MAP2 (A). GluR2/3 immunoreactivity is detectable in a MAP2-negative axonal process (arrowheads). Inserts show a GluR2/3-positive process immunoreactive for the axonal protein tau-1. Scale bar: 12 µm.
Fig. 2. Immunocytochemical localization of GluR2/3 and GluR1 in axonal compartments of hippocampal neurons. (A–F) Seven day old neurons double labeled for GluR2/3 (B and E) and for the synaptic vesicle protein synaptobrevin/VAMP2 (A and D). GluR2/3 immunoreactivity is detectable along the axon and in the growth cone, identified by the presence of synaptic vesicle clusters. (G–L) Ten day old neurons double labeled for GluR2/3 (H and L) and for synaptobrevin/VAMP2 (G and I). GluR2/3 is still detectable in the axonal growth cone (G and H, arrow) contacting the postsynaptic dendrite. Note the presence of GluR2/3 immunostaining in the dendritic shaft of the postsynaptic neuron. GluR2/3 immunoreactivity is also detectable in corresponding packages of synaptic vesicles which travel along the axon (I and L). (M–O) Five day old neurons double labeled for GluR1 (N) and for synaptobrevin/VAMP2 (M). Scale bars: A–C, 15 µm; D–F, 6 µm; G–L, 1.8 µm; M–O, 6.6 µm.
Functional activation of AMPA receptors in axonal growth cones
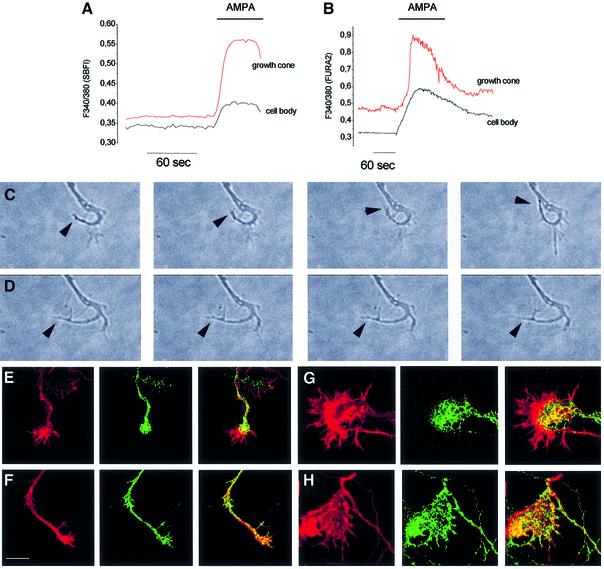
To investigate whether the presence of AMPA receptor subunits in the growth cone is accompanied by the expression of functional AMPA receptors on the plasma membrane, 5–7 DIV old neurons were loaded with the sodium-sensitive dye SBFI and stimulated with 100 µM AMPA, applied in the presence of 50 µM cyclothiazide. Exogenous application of the agonist resulted in a [Na+]i transient both in the somatodendritic region and in the axonal growth cone (Figure 3A). The identification of the growth cone was carried out by morphological criteria and by retrospective immunocytochemical labeling with antibodies aginst tau-1 or MAP2 at the end of the recording session (see Figure 1). The growth cone response to AMPA was specifically blocked by the AMPA receptor antagonist CNQX (data not shown). A CNQX-sensitive response to AMPA was also recorded in growth cones of neurons loaded with the calcium-sensitive dye FURA-2 (Figure 3B), consistent with the activation of voltage-gated calcium channels upon AMPA receptor activation, as previously described (Fischer et al., 2000). The percentage of axonal growth cones responding to AMPA was found to be 75 ± 4.2%. These data indicate that functional glutamate receptors are present in the membrane of axonal growth cones.
Fig. 3. Functional AMPA receptors in axonal growth cones of hippocampal neurons. Temporal analysis of [Na+]i (A) or [Ca2+]i (B) changes (measured as variations in the 340/380 fluorescence ratio) in single neurons loaded with either SBFI (A) or Fura-2 (B), following stimulation with 100 µM AMPA in the presence of 50 µM cyclothiazide. Recordings are carried out from the cell body and from the axonal growth cone, as indicated. (C and D) Time-lapse recordings of the peripheral portion of the axon, carried out under control conditions (C) or after the application of 100 µM AMPA (D). Note that application of AMPA almost completely inhibits the growth cone motility. Arrowheads point to the leading edge of the growth cone. Both in panels C and D, the timing between consecutive images is 4 s (images 1–3) and 40 s (images 3 and 4). (E–H) Immunocytochemical staining of an axonal growth cone (E and F) and of giant growth cone (G and H) stained for synaptobrevin/VAMP2 (green) and for F-actin (red, TRITC–phalloidin). (E and G) Control conditions; (F and H) neurons treated with 100 µM AMPA for 5 min. Note that the vesicle-rich core of the growth cones becomes disorganized upon AMPA treatment. Scale bars: C and D, 6 µm; E–H, 9 µm.
It has been recently described that glutamate receptor activation regulates the actin-based motility of both dendritic spines and axonal filopodia (Fischer et al., 2000; Chang and De Camilli, 2001). To test whether the activation of AMPA receptors affects the motility of the axonal growth cone, time-lapse recordings of the peripheral portion of the axon were carried out under control conditions or after the application of 100 µM AMPA. The series of images shown in Figure 3C confirms previous studies indicating that axonal growth cones are highly motile structures, endowed with filopodia which repeatedly extend and retract (Zheng et al., 1996). Application of AMPA almost completely inhibited motility (Figure 3D), as previously reported in growth cones of mouse spinal cord neurons (Owen and Bird, 1997), dendritic growth cones (Mattson et al., 1988) and filopodia of hippocampal neurons (Chang and De Camilli, 2001). Removal of AMPA reversed the inhibition, arguing against a non-specific effect due to neuronal damage (data not shown).
Application of AMPA to cultured neurons also affected the distribution of synaptic vesicles within the growth cone. Whereas in control cultures synaptic vesicles, labeled by antibodies against synaptobrevin/VAMP2, were most frequently retained in the core of the axonal growth cone (Figure 3E, 72% of growth cones; Mundigl et al., 1993, 1998), a vesicle relocation up to the tips of growth cone filopodia could be detected upon AMPA application (Figure 3F, 81% of growth cones). The AMPA-induced relocation of synaptic vesicles was even more clearly detectable in the giant growth cones that are occasionally found in hippocampal cultures. The large flat geometry of these growth cones makes them especially suitable for high-resolution light microscopy (Mundigl et al., 1993, 1998). Figure 3H shows the disorganization of the organelle-rich core of the growth cone consequent to AMPA stimulation (compare with control, Figure 3G). Synaptic vesicle markers become detectable at the periphery of the growth cone, coinciding with patches of filamentous actin (Figure 3H). Quantification of the growth cone area immunoreactive for synaptobrevin/VAMP2, normalized to the total growth cone area, revealed a two-fold increase (197 ± 15%) in AMPA-treated cultures compared with controls (P < 0.01).
Regulated recruitment of AMPA receptors to the plasma membrane of growth cones
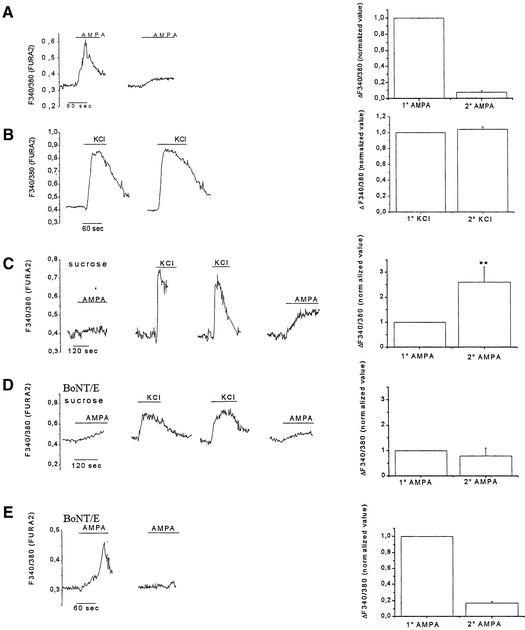
In neurons loaded with either FURA-2 (Figure 4A) or SBFI (not shown), a second stimulation with 100 µM AMPA, carried out in the presence of cyclothiazide, 10–15 min after the first AMPA application, resulted in a significantly smaller growth cone response (see right panel for quantitation). On the other hand, equivalent [Ca2+]i responses were recorded from neurons subjected to two subsequent challenges with 50 mM KCl (Figure 4B and relative quantitation). These results indicate that a specific reduction of functional AMPA receptors on the growth cone surface occurs in response to receptor activation, suggesting that the exposure of AMPA receptors on the axonal plasma membrane may be dynamically regulated.
Fig. 4. Regulated delivery of functional AMPA receptors into the axonal plasma membrane. (A) Subsequent stimulations with 100 µM AMPA in the presence of 50 µM cyclothiazide lead to a reduced [Ca2+]i responsivity of the growth cone in Fura-2-loaded cultures. (B) Equivalent [Ca2+]i responses in a growth cone repetitively stimulated with 50 mM KCl. (C) Two subsequent stimulations with 50 mM KCl, carried out in 320 mM sucrose, lead to an enhancement of the growth cone response to 100 µM AMPA. (D) No increase in the growth cone response to AMPA takes place, upon repetitive KCl stimulation in high sucrose, in cultures intoxicated with 80 nM BoNTE. (E) Subsequent stimulations with 100 µM AMPA lead to a reduced growth cone responsivity in BoNTE-intoxicated cultures.
We then tested the possibility that AMPA receptors could be recruited to the axonal growth cone plasma membrane upon stimulation, as previously indicated for postsynaptic AMPA receptors (Shi et al., 1999; Liao et al., 2001). To this aim, FURA-2-loaded neurons were repetitively stimulated with 50 mM KCl in the presence of high concentrations of extracellular sucrose, a condition known to inhibit clathrin-mediated endocytosis (Daukas and Zigmond, 1985; Heuser and Anderson, 1989). As shown in Figure 4C, a significantly larger response to AMPA was detected in the growth cone after KCl stimulation. To investigate whether the enhancement of the response to AMPA could be produced by insertion of new receptors into the plasma membrane from an intracellular vesicular pool, we performed the same experiment after impairing the fusion of intracellular vesicles with the plasma membrane using clostridial toxins. Figure 4D shows that treatment with 80 nM botulinum toxin E (BoNTE), which proteolytically cleaves the t-SNARE SNAP25, completely prevented the enhancement of AMPA response consequent to depolarization. The same treatment did not impair receptor internalization (Figure 4E), thus arguing against a generalized neurotoxic effect of BoNTE (see right panels for quantitation). Similar results were obtained after intoxicating mature neurons with tetanus toxin (TeNT), which proteolytically cleaves the v-SNARE synaptobrevin/VAMP2. The extent of the second response to AMPA, normalized to the first one, was: 2.19 ± 0.62, n = 7, P < 0.05, controls; 0.86 ± 0.27, n = 5, P > 0.1, BoNTE-treated; 1.06 ± 0.7, n = 5, P > 0.1, TeNT-treated. These data point to a regulated insertion of AMPA receptors into the axonal plasma membrane.
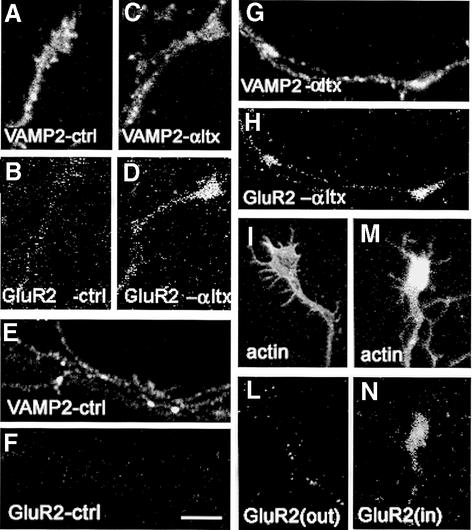
The regulated, vesicular recruitment of AMPA receptors to the plasma membrane of growth cones was further supported by immunofluorescence experiments carried out in the presence of α-latrotoxin, a potent stimulator of synaptic vesicle exocytosis (Ceccarelli et al., 1988; Schiavo et al., 2000; Sudhof, 2001). When applied to cultured hippocampal neurons in the absence of extracellular calcium, α-latrotoxin not only induces synaptic vesicle exocytosis, but also prevents synaptic vesicle endocytosis (Pennuto et al., 2002). To determine whether the α-latrotoxin-induced exocytosis of synaptic vesicles was paralleled by an increase in the number of AMPA receptor subunits recruited to the surface of growth cones, antibodies directed against the extracellular portion of the GluR2 subunits were added to living neurons, either under control conditions or after stimulation with the toxin. Neurons were then fixed and double stained for the synaptic vesicle protein synaptobrevin/VAMP2 (Figure 5). Under control conditions, virtually no staining for the extracellular portion of GluR2 could be detected (Figure 5B), possibly due to the low amount of receptor subunits present on the cell surface at steady state. Upon α-latrotoxin treatment, a specific staining of growth cones could be detected (Figure 5D), indicating that the massive fusion of synaptic vesicles was accompanied by the insertion of AMPA receptor subunits into the plasma membrane. A significant staining for the extracellular portion of GluR2 subunits was also detected, upon α-latrotoxin treatment, corresponding to synaptic vesicle packages traveling along the axon (Figure 5H). To detect the possible internalization of GluR subunits, cultures were incubated with antibodies directed against the extracellular portion of the GluR2 during stimulation with α-latrotoxin in the presence of extracellular calcium, a condition which enhances synaptic vesicle fusion and membrane retrieval (Ceccarelli et al., 1988). After removal of surface-bound antibodies by acid-strip protocol (Figure 5L; Passafaro et al., 2001), antibodies to GluR2 could be detected upon fixation and permeabilization of the neurons (Figure 5N), further confirming that GluR subunits can be internalized from the growth cone surface upon membrane recycling. A surface GluR2 staining was also detectable in cultures stimulated by repetitive application of 50 mM KCl in the presence of high concentrations of extracellular sucrose (data not shown), i.e. the same experimental treatment which enhances AMPA responsivity. These data further support the existence of a vesicle-mediated recruitment of receptor subunits to the growth cone plasma membrane and open up the possibility that synaptic vesicles may represent the organelles mediating this delivery.
Fig. 5. Exposure of GluR2 on the growth cone surface upon neuronal stimulation. (A and B) Five day old control neurons were incubated at 4°C with antibodies against the extracellular portion of GluR2 (B), fixed and double stained for synaptobrevin/VAMP2 (A). Virtually no surface GluR2 staining is detectable on the growth cone. (C and D) Culture incubated at 4°C with antibodies against the extracellular portion of GluR2 (D) upon stimulation with 0.1 nM α-latrotoxin (αltx), applied in the absence of extracellular calcium, fixed and double stained for synaptobrevin/VAMP2 (C). A surface GluR2 staining is detectable. (E–H) Ten day old neurons were incubated at 4°C with antibodies against the extracellular portion of GluR2 (F and H) and double labeled for synaptobrevin/VAMP2 (E and G) either in the presence (G and H) or in the absence (E and F) of α-latrotoxin. Note the selective surface staining for GluR2, specifically upon α-latrotoxin treatment. (I and L) Acid-strip protocol applied to cultures treated as in panel D, allows the removal of membrane-bound antibodies (L). (M and N) Cultures stimulated with αltx in the presence of extracellular calcium, subjected to acid-strip protocol, permeabilized and stained for internalized antibodies (N). Note the labeling of growth cones, revealing internalization of surface GluR2 subunits. I and M: double staining of growth cones with FITC–phalloidin. Scale bars: A–D, 7.1 µm; E–H, 4.2 µm; I–N, 7.1 µm.
Regulated recruitment of AMPA receptors to synaptosomal plasma membrane
To determine whether stimuli which promote the insertion of AMPA receptor subunits into the surface of axonal growth cones are also efficient in enhancing the delivery of receptor subunits to the plasma membrane of mature synapses, synaptosomes purified from adult rat forebrain were stimulated with 0.1 nM α-latrotoxin for 5, 10 or 15 min in the absence of extracellular calcium. A biotinylation labeling of cell surface proteins was then carried out, and biotinylated proteins were separated from non-biotinylated, intracellular proteins by using avidin-coated beads. Western blots prepared from biotinylated and non-biotinylated protein fractions were then probed with antibodies against GluR2/3, the NMDA receptor subunit NR1 or the integral synaptic vesicle protein synaptophysin. As a control, immunolabeling was performed with antibodies to the intracellular protein hsp60 or to TI-VAMP, a marker for vesicles involved in neurite outgrowth (Martinez-Arca et al., 2001). The latter bands, which provide an index of synaptosome permeabilization, are present exclusively in the non-biotinylated fraction, thus excluding synaptosome damage and biotinylation of intracellular proteins as a consequence of the experimental treatments. α-latrotoxin treatment produced an increase in biotinylated (cell surface) synaptophysin, as previously described (Torri Tarelli et al., 1990), but not in biotinylated TI-VAMP (Figure 6). The lack of TI-VAMP biotinylation is not attributable to the short intravesicular/extracellular tail of the protein, as synaptobrevin/VAMP2, which has an intravesicular/extracellular tail which is shorter than TI-VAMP, is effectively biotinylated (not shown), thus supporting a specific effect of α-latrotoxin on synaptic vesicle exocytosis. An increase in cell surface GluR2/3, similar or even higher compared with synaptophysin, could be detected after α-latrotoxin treatment, in the absence of any significant increase in biotinylated NR1 (Figure 6). These results indicate that a vesicular delivery of GluR2/3, but not of NR1, to the plasma membrane is facilitated by stimuli which are known to promote synaptic vesicle exocytosis.
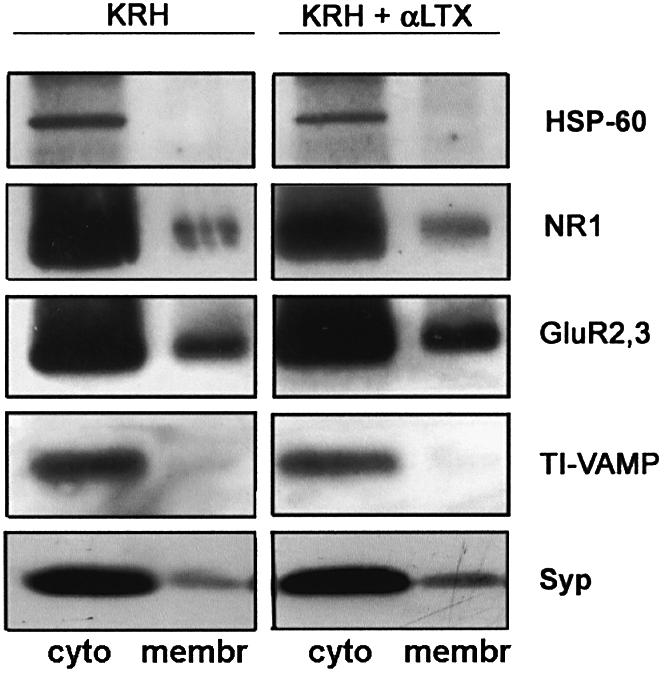
Fig. 6. Surface exposure of GluR2 in synaptosome stimulated with α-latrotoxin. Biotinylation labeling of cell surface proteins in control or α-latrotoxin-stimulated synaptosomes, followed by separation of biotinylated (membr) from non-biotinylated (cyto) proteins by avidin-coated beads and analysis by western blotting. α-latrotoxin treatment produces an increase in biotinylated (membrane) synaptophysin and GluR2/3. No increase in surface TI-VAMP or in surface NR1 is detectable, thus indicating that a vesicular delivery of GluR2/3, but not of NR1, to the plasma membrane is induced by α-latrotoxin stimulation. No biotinylation of hsp60 occurs, thus excluding synaptosomal damage.
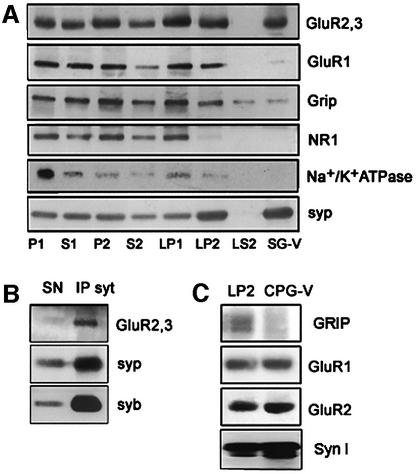
The increase in surface exposure of GluR2/3 subunits could in principle be produced by dendritic vesicular organelles fusing with the postsynaptic membrane. As resealed, metabolically active postsynaptic compartments are also present in the synaptosomal fraction, we carried out a subcellular fractionation of rat forebrain homogenates by differential centrifugation, up to the isolation of a highly purified synaptic vesicle fraction (Huttner et al., 1983). The AMPA receptor subunits GluR2/3 and GluR1, the GluR2/3 binding partner GRIP (GluR2 interacting protein), the NMDA receptor subunit NR1, the plasma membrane marker Na+/K+ ATPase and the synaptic vesicle protein synaptophysin were all clearly detectable in the crude synaptosomal fraction (P2) (Figure 7A). However, only GluR2/3 and, to a lesser extent, GluR1 subunits were found to be present, together with synaptophysin, in a synaptic vesicle fraction purified by continuous sucrose density gradient (Figure 7A). As a further step of purification, an immunoisolation of synaptic vesicles was carried out starting from the latter fraction, using magnetic beads coated with antibodies directed against the synaptic vesicle protein synaptotagmin. Synaptophysin, synaptobrevin/VAMP2 and the AMPA receptor subunits GluR2/3 (Figure 7B) and GluR1 (not shown) co-enriched in the immunoisolated fraction. Finally, the immunolabeling of a highly purified synaptic vesicle fraction prepared via permeation chromatography on controlled-pore glass (CPG-V), revealed that GluR2/3 (not shown) and, more specifically, GluR2 (Figure 7C), copurify with the synaptic vesicle-specific protein synapsin I, indicating that, at steady state, a major pool of GluR2/3 subunits resides in synaptic vesicle membranes. GluR1, but not GRIP, was also detectable in the same fraction (Figure 7C). Moreover, synaptic vesicles (or synaptic vesicle precursors) immunoisolated from a growth cone particle-enriched fraction prepared from embryonic tissue (Lohse et al., 1996) contain GluR2 (see Supplementary data), thus indicating that similar mechanisms are responsible for AMPA receptor subunit delivery to both the growth cone and the mature presynaptic membranes.
Fig. 7. Distribution of AMPA receptor subunits in subcellular fractions from synaptosomes. (A) The AMPA receptor subunits GluR2/3 and GluR1, the GluR2/3 binding partner GRIP, the NMDA receptor subunit NR1, the plasma membrane marker Na+/K+ ATPase and the synaptic vesicle protein synaptophysin are all clearly detectable in the crude synaptosome fraction (P2). The crude synaptic vesicle fraction (LP2) obtained upon hypo-osmotic lysis of synaptosomes and differential centrifugation, contains GluR2/3, synaptophysin, GluR1 and GRIP. GluR2/3 and, at a much lesser extent, GluR1 and GRIP, are present, together with synaptophysin, in a synaptic vesicle fraction purified by continuous sucrose density gradient (SG-V). (B) Western blot analysis of a vesicular fraction immunoisolated from the SG-V fraction, using magnetic beads coated with antibodies directed against synaptotagmin. Note that synaptophysin (syp), synaptobrevin/VAMP2 (syb) and GluR2/3 are co-enriched in the immunoisolated fraction. (C) Western blot analysis of a highly purified synaptic vesicle fraction prepared via CPG-V reveals the presence of GluR2 and GluR1, together with synapsin I (syn I), in synaptic vesicles. Note the lack of GRIP immunoreactivity in the purified synaptic vesicles.
Discussion
AMPA receptors are hetero-oligomeric complexes formed by different combinations of the four subunits GluR1– GluR4 (Wisden and Seeburg, 1993; Hollmann and Heinemann, 1994). Whereas GluR4 is mainly expressed during development, the other subunits associate in the adult nervous system to form two distinct populations of receptors, GluR1/GluR2 and GluR2/GluR3, which are clustered on the postsynaptic plasma membrane facing the active zone (Wenthold et al., 1996). As the amount of postsynaptic AMPA receptors available for binding to presynaptically released glutamate contributes to the tuning of synaptic strength and plays a major role in synaptic plasticity phenomena, the regulation of the traffic of glutamate receptor subunits to and from the plasma membrane has been widely investigated. The currently accepted model predicts a subunit-specific regulation of AMPA receptor delivery to the postsynaptic membrane, with receptors cycling between intracellular pools and the membrane surface, either constitutively or during plasticity phenomena (Passafaro et al., 2001; Shi et al., 2001). According to this model, the existence of a tubulovesicular membrane compartment, which may serve as a reservoir for the dendritic recycling of AMPA receptors, has been recently identified in the postsynaptic compartment (Lee et al., 2001).
Recent results, indicating a function of AMPA receptors in the regulation of the motility of presynaptic filopodia during synaptogenesis (Chang and De Camilli, 2001) and in the modulation of neurotransmitter release in the mature brain (Satake et al., 2000), prompted us to investigate the possible presence of AMPA receptor subunits in the axonal compartment of cultured hippocampal neurons. We have demonstrated that axonal growth cones of developing neurons contain AMPA receptor subunits. These data com plement recent findings showing that GRIP and liprin-α, a major binding partner of GRIP, are present in the axon and colocalize in axonal growth cones (Wyszynski et al., 2002). AMPA receptor subunits are assembled in functional receptors, as the axonal growth cones respond to the application of the agonist with the activation of Na+ and Ca2+ fluxes. As GluR2 subunits confer their associated ion channels with Ca2+-impermeability, this leaves voltage-activated calcium channels as the most likely source of glutamate receptor-mediated calcium influx, as previously described (Verderio et al., 1994; Fischer et al., 2000). The functional consequences of AMPA receptor activation in the distal axonal arbor of developing neurons are both an inhibition of the growth cone motility and a regional redistribution of synaptic vesicles within the growth cone.
Similarly to their postsynaptic counterparts, which are inserted into synaptic membranes from an intracellular pool, through a Brefeldin A- (Broutman and Baudry, 2001) and tetanus toxin- (Maletic-Savatic et al., 1998; Lu et al., 2001) sensitive pathway, AMPA receptors on the axonal plasma membrane appear to be dynamically regulated. The rapid depolarization-induced redistribution of functional AMPA receptors in the growth cone surface, and its impairment by clostridial toxins, point to the involvement of a SNARE-dependent, vesicular mechanism in receptor delivery to the axonal plasma membrane. Although the ionic changes produced by AMPA stimulation do not allow the identification of the specific receptor subunits recruited to the plasma membrane and accounting for the functional response, we detected an increase in cell surface GluR2 induced by stimulation. This indicates that at least GluR2 may be involved in mediating the enhancement of the functional response to AMPA. Other AMPA receptor subunits, like GluR1, may undergo a similar, regulated recruitment. The latter possibility appears very likely, based on our demonstration of a presynaptic subcellular localization of GluR2 and GluR1.
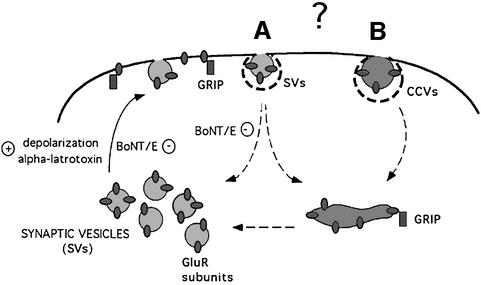
Once established that AMPA receptors are not stable on the axonal membrane, we aimed at identifying the intracellular organelles potentially involved in receptor trafficking in and out of the plasma membrane, taking advantage of the extensive knowledge available on vesicular trafficking in presynaptic terminals (Greengard et al., 1993; Jahn and Sudhof, 1999; Cremona and De Camilli, 1997; Lin and Scheller, 2000). We show that, in presynaptic terminals, a major pool of GluR2 subunits reside, at steady state, in highly purified small synaptic vesicles isolated from synaptosomes by a procedure which involves permeation chromatography on controlled-pore glass as a final purification step (Huttner et al., 1983). The fact that GluR1 is also detectable in highly purified synaptic vesicles, opens the possibility that the various AMPA receptor subunits may undergo a similar regulated recruitment. On the other hand, the lack of immunoreactivity for the GluR2/3 interacting protein GRIP on purified synaptic vesicles is consistent with the possibility that either GRIP plays a role in stabilizing the receptor in the plasma membrane or GRIP interacts with receptors contained in intracellular vesicular pools distinct from synaptic vesicles (Carroll et al., 2001; Malinow and Malenka, 2002) (Figure 8).
Fig. 8. Cartoon illustrating possible trafficking pathways of AMPA receptor subunits in presynaptic compartments. AMPA receptor subunits are present in ‘bona fide’ synaptic vesicles and can be recruited on the surface by depolarization or stimulation with α-latrotoxin. Botulinum toxin E (BoNT/E) impairs the insertion of receptors into the plasma membrane. Internalization of receptors from the cell surface may occur via clathrin-dependent pathways involving either synaptic vesicles (A) or different populations of clathrin coated vesicles (CCVs) (B). The possible recycling of AMPA receptor subunits via tubulo-vesicular endosomes (Lee et al., 2001) is illustrated. The scheme also depicts the alternative localizations of GRIP, either on the plasma membrane, for the surface stabilization of AMPA receptors, or on intracellular vesicular pools distinct from synaptic vesicles, for regulating receptor availability to the plasma membrane.
Consistent with a localization on synaptic vesicles, AMPA receptor subunits are recruited to both the synaptosomal and the growth cone plasma membrane by stimulation with α-latrotoxin, an agent which promotes the exocytosis of synaptic vesicles from nerve terminals (Ceccarelli et al., 1988; Schiavo et al., 2000; Sudhof, 2001). We cannot, however, at present, exclude the possibility that the AMPA receptor subunit-containing organelles represent a different population of vesicles, with size equivalent to synaptic vesicles, endowed with synaptobrevin/VAMP2 and synaptotagmin on their membranes and sensitive to α-latrotoxin. Interestingly, however, we have detected the presence of GluR2/3 on vesicles immunoisolated from LS1 fractions using antibodies against vesicular neurotransmitter transporters (U.Schenk and M.Matteoli, unpublished observations), further supporting the possibility that the AMPA receptor subunit-containing organelles are in fact bona fide synaptic vesicles.
What would be the significance of a recruitment of AMPA receptors to the presynaptic membrane of mature synapses, via a synaptic vesicle-mediated process? One could speculate that recirculating AMPA receptor subunits in nerve terminals via synaptic vesicles might be a very efficient way to capitalize on a highly regulated vesicular pathway already existing in the neuron. Synaptic vesicles, which continuously fuse with the plasma membrane as a consequence of spontaneous neuronal activity, may represent the organelles which are best suited to continuously and constitutively insert AMPA receptors into the membrane. On the other hand, the evoked synaptic vesicle exocytosis may provide the pathway responsible for the activity-dependent presynaptic delivery of AMPA receptor subunits to the surface.
Materials and methods
Hippocampal cell cultures
Primary neuronal cultures were prepared from the hippocampi of 18-day-old fetal rats as described by Bartlett and Banker (1984). The dissociated cells were plated onto glass coverslips coated with poly-l-lysine at densities ranging from 250 000 to 400 000 cells/cm2. After a few h, the coverslips were transferred to dishes containing a monolayer of cortical glial cells (Bartlett and Banker, 1984). The cells were maintained in MEM (Gibco) without sera, supplemented with N2 (Gibco), 2 mM glutamine and 1 mg/ml bovine serum albumin (neuronal medium).
Immunocytochemistry
The cultures were fixed and stained as previously described (Verderio et al., 1994). The images were acquired using a Bio-Rad MRC-1024 confocal microscope equipped with LaserSharp 3.2 software. For staining on living neurons, cultures were incubated with antibodies to the GluR2-Nter (1–5 µg/ml), on ice for 30 min. For α-latrotoxin stimulation, cultures were incubated in the simultaneous presence of 0.1 nM toxin and GluR2-Nter antibody for 15 min in Krebs–Ringer solution buffered with HEPES (KRH) (150 mM NaCl, 5 mM KCl, 1.2 mM MgSO4, 2 mM CaCl2, 10 mM glucose, 10 mM HEPES/NaOH pH 7.4) at 37°C or for 30 min in KRH without calcium at 37°C. When necessary, acid-strip protocol was carried out as previously described (Passafaro et al., 2001), in order to remove surface-bound antibodies. After a few washes in KRH, cultures were fixed and stained with secondary antibodies. Actin was visualized by TRITC- or FITC-conjugated phalloidin. Quantitations were performed using NIH Image software.
Calcium and sodium imaging
Cultured cells were loaded for 60–90 min at 37°C with 5 µM FURA-2 pentacetoxy-methylester or 20–40 µM SBFI pentacetoxy-methylester in KRH, washed in the same solution, and transferred to the recording chamber of an inverted microscope (Axiovert 100; Zeiss, Oberkochen, Germany) equipped with a calcium imaging unit. For the assays, a modified CAM-230 dual wavelength microfluorometer (Jasco, Tokyo, Japan) was used as a light source. The experiments were performed using an Axon Imaging Workbench 2.2 equipped with a PCO SuperVGA SensiCam (Axon Instruments, Foster City, CA). The ratio values in discrete areas of interest were calculated from sequences of images in order to obtain temporal analyses. The images were acquired at 1–3 340/380 ratios/s. The experiments were performed in a static bath at 28–30°C. The increases in calcium were quantified by measuring the peak and/or area of the response. For intoxication experiments, neurons were pretreated with 80 nM BoNTE for 2 h, as previously described (Verderio et al., 1999).
Biochemical techniques
Biotinylation experiments. Synaptosomes were prepared as described in Huttner et al. (1983). Samples of 200 µg were pelleted and resuspended in KRH, KRH-Ca2+ or KRH-Ca2+ with 0.1 nM α-latrotoxin and left at room temperature for 5, 10 or 15 min. To stop the experimental treatments the samples were diluted eight times in KRH or KRH-Ca2+and put on ice. For biotinylation, samples were incubated at 4°C with 1 mg/ml biotin-EZ link (Pierce) for 20 min. To remove and inactivate biotin residues, synaptosomes were centrifuged and washed in phosphate-buffered saline containing 50 mM glycine. Proteins were extracted in 140 mM KCl, 20 mM HEPES–KOH pH 7.4, 2 mM EDTA, 1% (v/v) Triton X-100 for 1 h. Triton insoluble material was removed by centrifugation at 700 g for 3 min. The isolation of biotinylated proteins was performed with Streptavidin–Sepharose (Pierce) at 4°C overnight. Biotinylated proteins were subsequently analyzed by SDS electrophoresis and western blotting.
Synaptosome fractionation and synaptic vesicle purification. The purification of synaptic vesicles from rat forebrain was carried out as described (Huttner et al., 1983). Briefly, 15 rat brains were homogenized in a glass-teflon homogenizer in ice-cold buffered sucrose (320 mM sucrose/5 mM HEPES–NaOH buffer pH 7.4). The homogenate was centrifuged at 800 g for 10 min, the pellet (P1) was discarded and the supernatant (S1) was collected and centrifuged for 15 min at 9200 g. The supernatant (S2) was removed and the pellet was washed, resuspended in buffered sucrose and recentrifuged for 15 min at 10 200 g. The pellet (P2′) was resuspended in buffered sucrose and lysed by hypo-osmotic shock as described (Huttner et al., 1983). The lysate was then centrifuged at 25 000 g for 20 min and the supernatant (LS1) was collected. LS1 was centrifuged at 165 000 g for 2 h and the pellet (LP2) was resuspended in 40 mM sucrose and layered on top of a linear sucrose gradient (50–800 mM sucrose). Sucrose gradient centrifugation was performed for 5 h at 65 000 g. The material banding at 200–400 mM sucrose was collected (SG-V) and loaded onto a CPG chromatography column (180 × 2 cm; GLY03000B beads, Electro-Nucleonics Inc., Fiarfield, NJ) equilibrated in ‘glycine buffer’. Highly purified synaptic vesicles (CPG-V) eluted as a major peak following a flowthrough peak constituted of small synaptic membranes (Huttner et al., 1983). Immunoisolation of synaptic vesicles from the SG-V fraction was carried out with Dynabeads M-450 rat anti-mouse IgG1 (Dynal, Oslo, Norway) according to the manufacturer’s instructions.
Materials
Monoclonal antibodies against synaptobrevin/VAMP2, synaptophysin and synaptotagmin were from Synaptic System (Goettingen, Germany); antibodies to Na+/K+ ATPase were kindly provided by Dr M.Caplan (Yale University, CT). Antibodies against TI-VAMP were a kind gift of Dr Thierry Galli (INSERM, Paris). Polyclonal antibodies against GluR1 and GluR2/3 were purchased from Chemicon (Temecula, CA); monoclonal antibodies to GluR2-Nter were from PharMingen (PharMingen Europe) or Chemicon (Temecula, CA), whereas monoclonal antibodies to NR1 were from PharMingen (PharMingen Europe). Antibodies against GRIP were kindly provided by Dr Morgan Sheng (MIT, MA) and antibodies to MAP2 were a kind gift of Dr A.Matus (Basel, Switzerland). Monoclonal antibodies against tau-1 were from Boehringer Mannheim (Germany). The secondary Abs conjugated to FITC, Texas Red or peroxidase were obtained from Jackson Immunoresearch Laboratories (West Grove, PA). α-latrotoxin was kindly provided by Dr Flavia Valtorta (DIBIT, Milano) and AMPA was purchased from Tocris Neuramin (Bristol, UK).
Supplementary data
Supplementary data are available at The EMBO Journal Online.
Acknowledgments
Acknowledgements
We wish to thank Drs M.Passafaro (CNR, Milano) and M.Francolini (University of Milano) for discussion. We also thank Drs T.Galli (INSERM, Paris), M.Caplan (Yale University), M.Sheng (MIT, Boston) and A.Matus (Basel, Switzerland) for the generous gift of antibodies. This work has been supported by EC (QLGR3-CT-2000-01343, Coordinator E.Scarfone) and by HFSPO (RGY0027/2001) to M.M., by Telethon Italia (grant no. 1042 to M.M. and 1131 to F.B.) and by MURST-PRIN 2001 to F.B. and M.M.
References
- Ahmari S.E., Buchanan,J. and Smith,S.J. (2000) Assembly of presynaptic active zones from cytoplasmic transport packets. Nat. Neurosci., 3, 445–451. [DOI] [PubMed] [Google Scholar]
- Bacci A., Coco,S., Pravettoni,E., Schenk,U., Armano,S., Frassoni,C., Verderio,C., De Camilli,P. and Matteoli,M. (2001) Chronic blockade of glutamate receptors enhances presynaptic release and downregulates the interaction between synaptophysin–synaptobrevin- vesicle-associated membrane protein 2. J. Neurosci., 21, 6588–6596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett W.P. and Banker,G.A. (1984) An electron microscopic study of the development of axons and dendrites by hippocampal neurons in culture. I. Cells which develop without intercellular contacts. J. Neurosci., 4, 1944–1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broutman G. and Baudry,M. (2001) Involvement of the secretory pathway for AMPA receptors in NMDA-induced potentiation in hippocampus. J. Neurosci., 21, 27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll R.C., Beattie,E.C., Xia,H., Luscher,C., Altschuler,Y., Nicoll,R.A., Malenka,R.C. and von Zastrow,M. (1999) Dynamin-dependent endocytosis of ionotropic glutamate receptors. Proc. Natl Acad. Sci. USA, 96, 112–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll R.C., Beattie,E.C., von Zastrow,M. and Malenka,R.C. (2001) Role of AMPA receptor endocytosis in synaptic plasticity. Nat. Rev. Neurosci., 2, 315–324. [DOI] [PubMed] [Google Scholar]
- Ceccarelli B., Hurlbut,W.P. and Iezzi,N. (1988) Effect of α-latrotoxin on the frog neuromuscular junction at low temperature. J. Physiol., 402, 195–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S. and De Camilli,P. (2001) Glutamate regulates actin-based motility in axonal filopodia. Nat. Neurosci., 4, 787–793. [DOI] [PubMed] [Google Scholar]
- Chung H.J., Scannevin,U.H., Zhang,X. and Huganir,R.L. (2000) Phosphorylation of the AMPA receptor subunit GluR2 differentially regulates its interaction with PDZ domain-containing proteins. J. Neurosci., 20, 7258–7267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig A.M., Blackstone,C.D., Huganir,R.L. and Banker,G. (1993) The distribution of glutamate receptors in cultured rat hippocampal neurons: postsynaptic clustering of AMPA-selective subunits. Neuron, 10, 1055–1068. [DOI] [PubMed] [Google Scholar]
- Cremona O. and De Camilli,P. (1997) Synaptic vesicle endocytosis. Curr. Opin. Neurobiol., 7, 323–330. [DOI] [PubMed] [Google Scholar]
- Daukas G. and Zigmond,S.H. (1985) Inhibition of receptor-mediated but not fluid-phase endocytosis in polymorphonuclear leukocytes. J. Cell Biol., 101, 1673–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H., Zhang,P., Song,I., Petralia,R.S., Liao,D. and Huganir,R.L. (1999) Characterization of the glutamate receptor-interacting proteins GRIP1 and GRIP2. J. Neurosci., 19, 6930–6941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer M., Kaech,S., Wagner,U., Brinkhaus,H. and Matus,A. (2000) Glutamate receptors regulate actin-based plasticity in dendritic spines. Nat. Neurosci., 3, 887–894. [DOI] [PubMed] [Google Scholar]
- Greengard P., Valtorta,F., Czernik,A.J. and Benfenati,F. (1993) Synaptic vesicle phosphoproteins and regulation of synaptic function. Science, 259, 780–785. [DOI] [PubMed] [Google Scholar]
- Heuser J.E. and Anderson,R.G. (1989) Hypertonic media inhibit receptor-mediated endocytosis by blocking clathrin-coated pit formation. J. Cell Biol., 108, 389–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollmann M. and Heinemann,S. (1994) Cloned glutamate receptors. Annu. Rev. Neurosci., 17, 31–108. [DOI] [PubMed] [Google Scholar]
- Huttner W.B., Schiebler,W., Greengard,P. and De Camilli,P. (1983) Synapsin I (protein I), a nerve terminal-specific phosphoprotein. III. Its association with synaptic vesicles studied in a highly purified synaptic vesicle preparation. J. Cell Biol., 96, 1374–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn R. and Sudhof,T.C. (1999) Membrane fusion and exocytosis. Annu. Rev. Biochem., 68, 863–911. [DOI] [PubMed] [Google Scholar]
- Kraszewski K., Mundigl,O., Daniell,L., Verderio,C., Matteoli,M. and De Camilli,P. (1995) Synaptic vesicle dynamics in living cultured hippocampal neurons visualized with CY3-conjugated antibodies directed against the lumenal domain of synaptotagmin. J. Neurosci., 15, 4328–4342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.H., Valtschanoff,J.G., Kharazia,V.N., Weinberg,R. and Dheng,M. (2001) Biochemical and morphological characterization of an intracellular membrane compartment containing AMPA receptors. Neuropharmacology, 41, 680–692. [DOI] [PubMed] [Google Scholar]
- Liao D., Scannevin,R.H. and Huganir,R. (2001) Activation of silent synapses by rapid activity-dependent synaptic recruitment of AMPA receptors. J. Neurosci., 21, 6008–6017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin R.C. and Scheller,R.H. (2000) Mechanisms of synaptic vesicle exocytosis. Annu. Rev. Cell. Dev. Biol., 16, 19–49. [DOI] [PubMed] [Google Scholar]
- Lledo P.M., Zhang,X., Sudhof,T.C., Malenka,R.C. and Nicoll,R.A. (1998) Postsynaptic membrane fusion and long-term potentiation. Science, 279, 399–403. [DOI] [PubMed] [Google Scholar]
- Lohse K., Helmke,S.M., Wood,M.R., Quiroga,S., de la Houssaye,B.A., Miller,V.E., Negre-Aminou,P. and Pfenninger,K.H. (1996) Axonal origin and purity of growth cones isolated from fetal rat brain. Brain Res. Dev. Brain Res., 96, 83–96. [DOI] [PubMed] [Google Scholar]
- Lu W., Man,H., Ju,W., Trimble,W.S., MacDonald,J.F. and Wang,Y.T. (2001) Activation of synaptic NMDA receptors induces membrane insertion of new AMPA receptors and LTP in cultured hippocampal neurons. Neuron, 29, 243–254. [DOI] [PubMed] [Google Scholar]
- Maletic-Savatic M., Koothan,T. and Malinow,R. (1998) Calcium-evoked dendritic exocytosis in cultured hippocampal neurons. Part II: mediation by calcium/calmodulin-dependent protein kinase II. J. Neurosci., 18, 6814–6821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinow R. and Malenka,R.C. (2002) AMPA receptor trafficking and synaptic plasticity. Annu. Rev. Neurosci., 25, 103–126. [DOI] [PubMed] [Google Scholar]
- Man H.Y., Lin,J.W., Ju,W.H., Ahmadian,G., Liu,L., Becker,L.E., Sheng,M. and Wang,Y.T. (2000) Regulation of AMPA receptor-mediated synaptic transmission by clathrin-dependent receptor internalization. Neuron, 25, 649–662. [DOI] [PubMed] [Google Scholar]
- Martinez-Arca S. et al. (2001) A common exocytotic mechanism mediates axonal and dendritic outgrowth. J. Neurosci., 21, 3830–3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson M.P., Dou,P. and Kater,S.B. (1988) Outgrowth-regulating action of glutamate in isolated hippocampal pyramidal neurons. J. Neurosci., 8, 2087–2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundigl O., Matteoli,M., Daniell,L., Thomas-Reetz,A., Metcalf,A., Jahn,R. and De Camilli,P. (1993) Synaptic vesicle proteins and early endosomes in cultured hippocampal neurons: differential effects of Brefeldin A in axon and dendrites, J. Cell Biol., 122, 1207–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundigl O., Ochoa,G.C., David,C., Slepnev,V.I., Kabanov,A. and De Camilli,P. (1998) Amphiphysin I antisense oligonucleotides inhibit neurite outgrowth in cultured hippocampal neurons. J. Neurosci., 18, 93–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi S., Nakajima,Y., Masu,M., Ueda,Y., Nakahara,K., Watanabe,D., Yamaguchi,S., Kawabata,S. and Okada,M. (1998) Glutamate receptors: brain function and signal transduction. Brain Res. Rev., 26, 230–235. [DOI] [PubMed] [Google Scholar]
- Nicoll R.A., Frerking,M. and Schmitz,D. (2000) AMPA receptors jump the synaptic cleft. Nat. Neurosci., 3, 527–529. [DOI] [PubMed] [Google Scholar]
- Owen A.D. and Bird,M.M. (1997) Role of glutamate in the regulation of the outgrowth and motility of neurites from mouse spinal cord neurons in culture. J. Anat., 191, 103–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passafaro M., Piech,V. and Sheng,M. (2001) Subunit-specific temporal and spatial patterns of AMPA receptor exocytosis in hippocampal neurons. Nat. Neurosci., 4, 917–926. [DOI] [PubMed] [Google Scholar]
- Pennuto M., Dunlap,D., Contestabile,A., Benfenati,F. and Valtorta,F. (2002) FRET detection of synaptophysin I and VAMP2 interactions during exocytosis from single live synapses. Mol. Biol. Cell, 8, 2706–2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satake S., Saitow,F., Yamada,J. and Konishi,S. (2000) Synaptic activation of AMPA receptors inhibits GABA release from cerebellar interneurons. Nat. Neurosci., 3, 551–558. [DOI] [PubMed] [Google Scholar]
- Scannevin R.H. and Huganir,R.L. (2000) Postsynaptic organization and regulation of excitatory synapses. Nat. Rev. Neurosci., 1, 133–141. [DOI] [PubMed] [Google Scholar]
- Schiavo G., Matteoli,M. and Montecucco,C. (2000) Neurotoxins affecting neuroexocytosis. Physiol. Rev., 80, 717–766. [DOI] [PubMed] [Google Scholar]
- Sheng M. and Lee,S.H. (2001) AMPA receptor trafficking and the control of synaptic transmission. Cell, 107, 825–828. [DOI] [PubMed] [Google Scholar]
- Shi S.H., Hayashi,Y., Petralia,R.S., Zaman,S.H., Wenthold,R.J., Svobodok,K. and Malinow,R. (1999) Rapid spine delivery and redistribution of AMPA receptors after NMDA receptor activation. Science, 284, 1811–1816. [DOI] [PubMed] [Google Scholar]
- Shi S.H., Hayashi,Y., Esteban,J.A. and Malinow,R. (2001) Subunit-specific rules governing AMPA receptor trafficking to synapses in hippocampal pyramidal neurons. Cell, 105, 331–343. [DOI] [PubMed] [Google Scholar]
- Sudhof T.C. (2001) α-latrotoxin and its receptors: neurexins and CIRL/latrophilins. Annu. Rev. Neurosci., 24, 933–962. [DOI] [PubMed] [Google Scholar]
- Torri-Tarelli F., Villa,A., Valtorta,F., De Camilli,P., Greengard,P. and Ceccarelli,B. (1990) Redistribution of synaptophysin and synapsin I during α-latrotoxin-induced release of neurotransmitter at the neuromuscular junction. J. Cell Biol., 110, 449–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.T. and Linden,D.J. (2000) Expression of cerebellar long-term depression requires postsynaptic clathrin-mediated endocytosis. Neuron, 25, 635–647. [DOI] [PubMed] [Google Scholar]
- Wenthold R.J., Petralia,R.S., Blahos,J.,II and Niedzielski,A.S. (1996) Evidence for multiple AMPA receptor complexes in hippocampal CA1/CA2 neurons. J. Neurosci., 16, 1982–1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisden W. and Seeburg,P.H. (1993) Mammalian ionotropic glutamate receptors. Curr. Opin. Neurobiol., 3, 291–298. [DOI] [PubMed] [Google Scholar]
- Wyszynski M., Kim,E., Dunah,A.W., Passafaro,M., Valtschanoff,J.G., Serra-Pages,C., Streuli,M., Weinberg,R.J. and Sheng,M. (2002) Interaction between GRIP and liprin-alpha/SYD2 is required for AMPA receptor targeting. Neuron, 34, 39–52. [DOI] [PubMed] [Google Scholar]
- Verderio C., Coco,S., Fumagalli,G. and Matteoli,M. (1994) Spatial changes in calcium signaling during the establishment of neuronal polarity and synaptogenesis. J. Cell Biol., 126, 1527–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verderio C., Coco,S., Bacci,A., Rossetto,O., De Camilli,P., Montecucco,C. and Matteoli,M. (1999) Tetanus toxin blocks the exocytosis of synaptic vesicles clustered at synapse but not of synaptic vesicles in isolated axons. J. Neurosci., 19, 6723–6732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J.Q., Poo,M.M. and Connor,J.A. (1996) Calcium and chemotropic turning of nerve growth cones. Perspect. Dev. Neurobiol., 4, 205–213. [PubMed] [Google Scholar]