Abstract
In Leishmania tarentolae, all mitochondrial tRNAs are encoded in the nuclear genome and imported from the cytosol. It is known that tRNAGlu(UUC) and tRNAGln(UUG) are localized in both cytosol and mitochondria. We investigated structural differences between affinity-isolated cytosolic (cy) and mitochondrial (mt) tRNAs for glutamate and glutamine by mass spectrometry. A unique modification difference in both tRNAs was identified at the anticodon wobble position: cy tRNAs have 5-methoxycarbonylmethyl-2- thiouridine (mcm5s2U), whereas mt tRNAs have 5- methoxycarbonylmethyl-2′-O-methyluridine (mcm5Um). In addition, a trace portion (4%) of cy tRNAs was found to have 5-methoxycarbonylmethyluridine (mcm5U) at its wobble position, which could represent a common modification intermediate for both modified uridines in cy and mt tRNAs. We also isolated a trace amount of mitochondria-specific tRNALys(UUU) from the cytosol and found mcm5U at its wobble position, while its mitochondrial counterpart has mcm5Um. Mt tRNALys and in vitro transcribed tRNAGlu were imported much more efficiently into isolated mitochondria than the native cy tRNAGlu in an in vitro importation experiment, indicating that cytosol-specific 2-thiolation could play an inhibitory role in tRNA import into mitochondria.
Keywords: Leishmania tarentolae/mitochondria/post-transcriptional modification/tRNA/wobble position
Introduction
The importation of nuclear-encoded tRNA into mitochondria occurs in a variety of organisms, including protozoa (Simpson et al., 1989; Hancock and Hajduk, 1990; Schneider and Marechal-Drouard, 2000), yeast (Tarassov and Martin, 1996) and plants (Dietrich et al., 1996). In the case of mammalian mitochondria, a set of tRNA genes sufficient to decipher the mitochondrial genetic code is encoded in mitochondrial (mt) DNA, which it had been thought would preclude the importation of nuclear-encoded tRNA—although cytosolic (cy) 5S rRNA (Yoshinari et al., 1994; Magalhaes et al., 1998), the RNA component of RNase MRP, which is involved in mt RNA processing (Li et al., 1994), and perhaps the RNA component of RNase P (Puranam and Attardi, 2001), are known to be imported into mammalian mitochondria. Recently, however, the first known instance of tRNA import into mammalian mitochondria was reported in marsupials (Dorner et al., 2001); the imported tRNALys is encoded in the nuclear genome, while the mt tRNALys gene seems to represent a pseudogene in the mt DNA. It has also been found that yeast tRNALys can be imported in vitro into human mitochondria in the presence of a yeast protein factor (Kolesnikova et al., 2000). The foregoing evidence suggests that tRNA importation into mitochondria can be a common process in a wide range of organisms, including mammals.
In the kinetoplastid protists Leishmania tarentolae and Trypanosoma brucei, no tRNA genes are encoded in the mt DNA, and a complete set of mt tRNAs encoded in the nuclear genome is imported from the cytosol (Simpson et al., 1989; Kapushoc et al., 2000; Rubio et al., 2000; Tan et al., 2002). The importation of tRNAs into mitochondria of Leishmania and Trypanosoma has been investigated both in vivo and in vitro, but the mechanism is not yet well understood. While it appears to involve protein receptors in the membrane and to require ATP hydrolysis (Lima and Simpson, 1996; Mahapatra and Adhya, 1996; Adhya et al., 1997; Rubio et al., 2000; Entelis et al., 2001), there are several differences from the well-studied protein import pathway. In some cases, RNA import determinants apparently include the tertiary structure as well as the size of the RNA molecule (Rubio et al., 2000). The substrate for importation has been proposed to be a 5′-extended (Hancock et al., 1992) or dicistronic precursor (LeBlanc et al., 1999); however, a recent study showed that five specific mt tRNAs in L.tarentolae are 5′- and 3′-end processed prior to exiting the nucleus, indicating that the importation substrate is the end-processed tRNA (Kapushoc et al., 2000).
Leishmania tarentolae tRNAs can be classed into three major groups based on their subcellular localization: group I tRNAs that reside mainly in the cytosol; group II tRNAs that reside mainly in mitochondria; and group III tRNAs that are shared between both cytosol and mitochondria. Preliminary characterization results indicate that there are ∼20 tRNAs of group I, nine of group II and 24 of group III (Kapushoc et al., 2000, 2002). It should be noted, however, that the above classification is based on the relative intensities of specific tRNA spots visualized in northern analysis of equal amounts of cy and mt tRNA; it does not take into account the relative amounts of total RNA in each compartment. Variations between the extent of the mitochondrial or cytosolic localization of tRNAs within each class were also observed (Kapushoc et al., 2002). Although the specificity of the import in vitro of several tRNAs into isolated mitochondria has been shown to resemble the specificity observed in vivo, the mechanism(s) determining the observed distinctive subcellular distribution patterns of different tRNAs largely remains to be elucidated.
As previously proposed by Rusconi and Cech (1996), in species such as trypanosomatid protists, in which all the tRNAs required for mitochondrial translation are imported, the subcellular localization of tRNAs is likely to involve a negative determinant mechanism. In this model, a signal(s) embedded in the tRNA structure and sequence would inhibit import into mitochondria. This model can be contrasted to a positive determinant mechanism where some import signal(s) or positive determinant directs import. According to the negative-determinant mechanism, it can be speculated that cy tRNA-specific modification of group III tRNA would function as a negative determinant for the importation.
It is unclear whether group III tRNAs are distributed passively between mitochondria and cytosol in a concentration-dependent manner or in a more active mode involving some protein factors. Hypothesizing that structural differences induced by specific post-transcriptional modifications of tRNA may be involved, we have analyzed nucleotide modifications in purified cy and mt tRNAs that read purine-ending codons with adenosine at the second position, NAR (R; purine) codons; tRNAGln for CAR, tRNAGlu for GAR and tRNALys for AAR. In general, these tRNAs have the same modified uridine at the wobble position (Sprinzl et al., 1998). In L.tarentolae, tRNAs for glutamate (UUC) and glutamine (UUG) are classified in group III tRNAs which are shared between the cytosol and mitochondria, whereas tRNALys(UUU) is classified in group II which are more mitochondria specific. We purified and analyzed these three tRNAs from both cytosol and mitochondria to investigate the relationship between modification difference and subcellular localization. Based on our findings, we propose that one means by which subcellular tRNA distribution is regulated is a negative regulation of tRNA importation into the mitochondrion by cytosol specific post-transcriptional modification.
Results
Purification of cytosolic and mitochondrial tRNAs from L.tarentolae
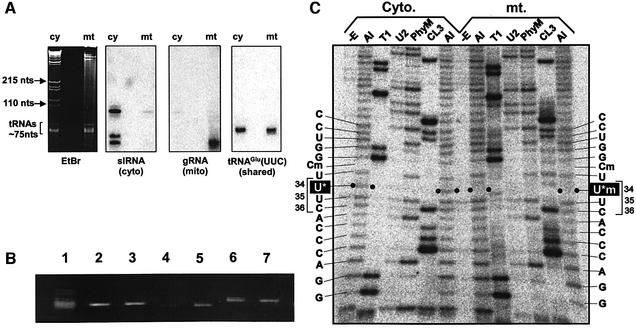
Crude tRNA fractions isolated from the cytosolic and mitochondrial cell compartments were subjected to northern hybridization using DNA probes for the cytosolic spliced leader RNA (slRNA) and a mitochondrial guide RNA (Grps-12 gRNA) to verify the quality of each tRNA fraction. The results indicated that cross-contamination was <3% in each fraction (Figure 1A). It has been shown that tRNAGlu(UUC) and tRNAGln(UUG) are localized in both cytosol and mitochondria, and were classified as group III tRNAs according to Kapushoc et al. (2002). Northern analysis showed tRNAGlu to be shared approximately equally between mitochondria and cytosol when equal amounts of RNA were loaded (Figure 1A), verifying its classification as a group III tRNA.
Fig. 1. Fractionation and isolation of cy and mt tRNAs. (A) Northern analysis of L.tarentolae RNA. Cy and mt RNAs (as indicated) were separated in denaturing 8% polyacrylamide gels. The ethidium bromide-stained gel is shown on the left, with the sizes and positions of several marker RNAs indicated. The results of northern hybridizations with oligonucleotide probes specific for several RNAs are shown: slRNA, the spliced leader RNA; gRNA, the RPS12 block I guide RNA; tRNAGlu(UUC), the glutamic tRNA analyzed in this study. (B) Gel-purified cy and mt tRNAs: cy total tRNA, cy tRNAGlu, mt tRNAGlu, cy tRNAGln, mt tRNAGln, cy tRNALys and mt tRNALys in lanes 1–7, respectively. (C) RNA sequencing of purified cy and mt tRNAsGlu by the method of Donis-Keller (1980) for comparison of the cy (left) and mt (right) tRNA sequences. Each 3′-labeled tRNA was partially digested with an RNase: RNase T1 for G; RNase U2 for A; RNase PhyM for A/U; and RNase CL3 for C. The digests were electrophoresed with an undigested control (–E) and alkaline-treated ladder (Al). Filled circles indicate a band corresponding to position 34 on the alkaline ladder of cy tRNAGlu (U*). No band appears at the same position on the alkaline ladder of mt tRNAGlu, indicating the presence of a 2′-O-methyluridine derivative (U*m).
As described in Materials and methods, specific mt tRNAs were isolated from total mt tRNA, and specific cytosolic cy tRNAs were isolated from total cy tRNA. To isolate group III tRNAs from cytosol and mitochondria by a solid phase DNA probe method, several DNA probes for isolating tRNAGlu and tRNAGln were designed according to the reported DNA sequences (Kapushoc et al., 2002) and screened on the basis of hybridization efficiency by dot hybridization (Kumazawa et al., 1992; data not shown). We also designed a DNA probe for tRNALys as a representative mitochondria-specific group II tRNA. As shown in Figure 1B, three tRNA species were isolated successfully from both cy and mt tRNA fractions using the ‘chaplet’ column solid-phase probing method (see Materials and methods). From the total RNA, we purified 2.28 A260unit (91.2 µg) of cy tRNAGlu, 0.079 A260unit (3.2 µg) of mt tRNAGlu, 1.14 A260unit (45.6 µg) of cy tRNAGln, 0.026 A260unit (1.0 µg) of mt tRNAGln, 0.059 A260unit (2.4 µg) of cy tRNALys and 0.081 A260unit (3.2 µg) of mt tRNALys, respectively. Although tRNALys is classified in group II, it was shown that ∼4% of tRNALys is distributed in the cytosol according to northern analysis (Kapushoc et al., 2002).
Difference in anticodon wobble base modification revealed by RNA sequencing
3′-[α-32P]pCp-labeled purified tRNAs were analyzed by the enzymatic digestion method of Donis-Keller (1980) (Figure 1C). The sequencing confirmed that the isolated cy and mt tRNAs were indeed tRNAGlu(UUC), tRNAGln(UUG) and tRNALys(UUU), respectively (data not shown). As shown in Figure 1C, the ladder patterns for both the cy and mt tRNAGlu were identical, with the exception of a single prominent difference: the mitochondrial anticodon wobble base (position 34) is modified differently from its cytosolic counterpart. This is evident from the fact that while an alkaline-hydrolyzed band occurs at this position in the cy tRNAGlu(UUC) sequence, no such band appears in the mitochondrial counterpart (indicated by filled circles in Figure 1C), suggesting that 2′ OH in the ribose portion of the mitochondrial anticodon wobble nucleotide is methylated (Uchida and Egami, 1971). Likewise, missing bands at position 32 indicate the presence of 2′-O-methylcytidine (Cm) in both tRNAs, because this position is C in the tRNAGlu DNA sequence. The same ladder pattern was observed in both mt tRNAGln and mt tRNALys (data not shown), indicating that mt tRNAs from these three tRNA species have in common 2′-O-methylated modified uridine at the wobble position, while each of the cy tRNAs has a non-2′-O-methylated modified uridine at the same position.
Chemical structures of anticodon wobble nucleosides of the cytosolic and mitochondrial tRNAs as determined by mass spectrometric analysis
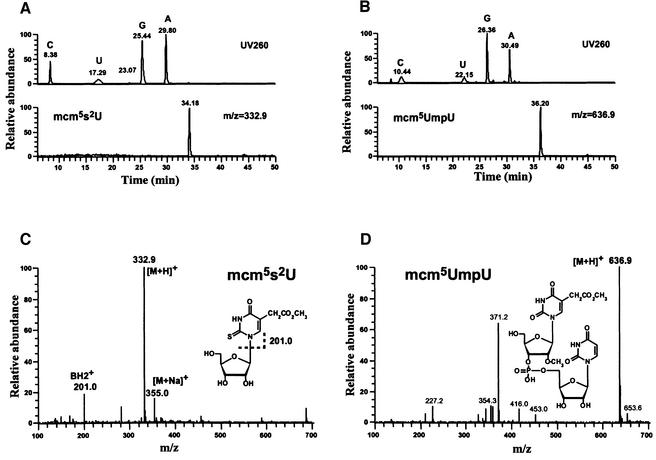
Nucleoside analysis by liquid chromatography/mass spectrometry (LC/MS) was employed to determine the chemical structures of the anticodon wobble bases in cy and mt tRNAs (Figure 2). In this analysis, we looked for modified uridines likely to be found at the wobble position of the tRNAs on the basis of information available in the literature (Pomerantz and McCloskey, 1990; Motorin and Grosjean, 1998) and the RNA Modification Database (http://medlib.med.utah.edu/RNAmods/). It was found that cy tRNAGlu and cy tRNAGln commonly possess the modified base mcm5s2U (5-methoxycarbonylmethyl-2-thiouridine) (Baczynskyj et al., 1968). The retention time (RT) of the identified mcm5s2U corresponded well to the value reported in the literature (Pomerantz and McCloskey, 1990) (Figure 2A). In the mass spectrum (Figure 2C), both the proton adduct [M + H]+ and sodium adduct [M + Na]+ of mcm5s2U were identified. In addition, the base fragment ion [BH2]+ (m/z 201), which is known to be generated by spontaneous dissociation, was observed. Analysis of purified mt tRNAGlu and mt tRNAGln, on the other hand, revealed the presence of mcm5Um (5-methoxycarbonylmethyl-2′-O-methyluridine) (Diamond et al., 1993) in the form of a dimer with the second base of the anticodon (mcm5UmpU) (Figure 2B and D). The methyl modification at 2′ OH in the ribose of the wobble uridine led to incomplete digestion by P1 nuclease. This result is consistent with the direct enzymatic sequencing (Figure 1C). When the nucleotide component of group II tRNALys was analyzed, its wobble nucleoside was shown to have mcm5U (5-methoxycarbonylmethyluridine) (Tumaitis and Lane, 1970) instead of mcm5s2U, which was found in two group III cy tRNAs. Mt tRNALys was shown to possess mcm5Um, the same wobble modification as the two group III mt tRNAs. To verify the unique presence of mcm5s2U and mcm5Um in cy and mt tRNAs for glutamate and glutamine, we searched for mcm5s2U in the mitochondrial fraction and mcm5UmpU in the cytosolic fraction; the result was negative (data not shown), and thus mcm5s2U and mcm5Um are cytosol-specific and mitochondria-specific base modifications, respectively, in tRNAGlu and tRNAGln in L.tarentolae. Our analysis also revealed several other commonly modified nucleosides in both forms of tRNAs: dihydro uridine (D), pseudouridine (Ψ), 2′-O-methylcytidine (Cm) and 5-methylcytidine (m5C) (data not shown). Cm was detected mainly in a dimer form (CmpU), similar to the case of mcm5UmpU (data not shown).
Fig. 2. LC/MS nucleoside analysis of tRNAGlu. (A and B) Chromatograms for cy tRNAGlu and mt tRNAGlu, respectively. Top: UV chromatograms for nucleosides. Bottom: mass chromatograms for modified uridines with mass filters at m/z 332.9 and 636.9 to detect, respectively, mcm5s2U in cy tRNAGlu and a dimer form of mcm5Um with the adjacent uridine (mcm5UmpU) in mt tRNAGlu. (C and D) Mass spectra for (C) mcm5s2U in cy tRNAGlu and for (D) mcm5UmpU in mt tRNAGlu. The chemical structure of each nucleoside is shown within the spectrum.
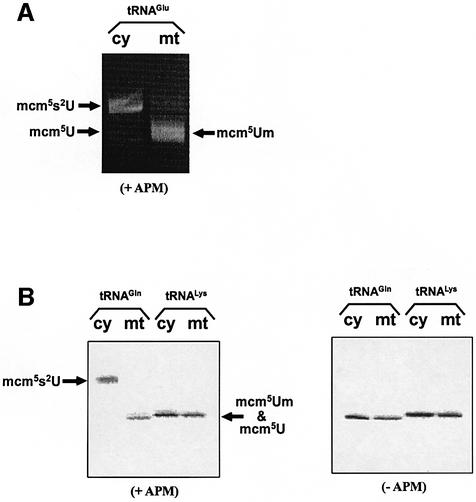
To confirm the presence of the 2-thiolated nucleoside mcm5s2U in cy tRNAGlu and cy tRNAGln and its absence in mt tRNAGlu, mt tRNAGln, mt tRNALys and cy tRNALys, the purified tRNAs were electrophoresed in an acrylamide gel containing [(N-acryloylamino)phenyl] mercuric chloride (APM). It has been reported that the mercuric compound in the gel interacts specifically with the tRNA containing the thiocarbonyl group (Igloi, 1988), thereby retarding tRNA migration. As shown in Figure 3A, cy tRNAGlu clearly exhibited retardation due to the presence of the thiolated nucleoside mcm5s2U, while no retardation was observed in the case of the mitochondrial counterpart in the APM gel. In addition, the same retardation was observed in cy tRNAGln, while no retardation was found in mt tRNAGln and mt tRNALys or cy tRNALys (Figure 3B). It was thus confirmed that the presence of the thiolated nucleoside is specific to group III cy tRNAGlu and cy tRNAGln.
Fig. 3. APM gel electrophoresis of cy and mt tRNAs visualized by ethidium bromide staining. (A) Cy and mt tRNAsGlu on an APM gel. (B) Cy and mt tRNAsGln and cy and mt tRNALys on a polyacrylamide gel with (left) or without (right) APM. 5′-32P-labeled tRNAs separated on the gels were visualized by the imaging analyzer (Fuji photosystem). Only cy tRNAGlu and cy tRNAGln showed apparent retardation on the APM gel.
RNA fragment analysis by mass spectrometry
Additional confirmation of the anticodon wobble nucleotides of cy and mt tRNAs was provided by LC/MS fragment analysis of RNase T1-digested tRNAs (see Supplementary data, available at The EMBO Journal Online). Many RNA fragments derived from the digestion of purified cy and mt tRNAsGlu by RNase T1 were detected as negative ions (Supplementary figure 1). Most of the expected fragments, including some modifications (D, Ψ, m5C and Cm), were observed with both tRNAs. The detected fragments are listed in Table I. In each case, the anticodon-containing 11mer fragments, the longest RNA fragment in the tRNAs (Table I), should contain the distinct modified nucleosides observed in the nucleoside analysis. The largest RNA fragments were found as multiply charged ions with 2–5 negative charges at RT 26.04 and 26.15 for cy and mt tRNAGlu, respectively (Supplementary figure 1). The observed masses of the anticodon-containing fragments were determined from the multiply charged ions to be 3568.7 ± 1.4 Da for cy tRNAGlu and 3566.1 ± 1.2 Da for mt tRNAGlu, respectively (Table I; Supplementary table 1). The difference of two units in the masses corresponds well to the expected mass difference between the modified nucleotides mcm5s2U (332.3 Da) and mcm5Um (330.3 Da). Taking these findings together with the Donis–Keller sequencing and nucleoside analysis results, the RNA sequences of the anticodon-containing fragments were determined to be CmU(mcm5s2U)UCACCCAGp for cy tRNAGlu and CmU(mcm5Um)UCACCCAGp for mt tRNAGlu.
Table I. RNase T1-digested fragments of cy and mt tRNAGlu discerned in this study with calculated and observed masses.
| Fragment no.a | Sequenceb | Position | Calculated mass(average) | Observed massc |
|
|---|---|---|---|---|---|
| Cy | Mt | ||||
| 1 | AGp | 44–45; 71–72 | 692.4 | 692.3 | 692.2 |
| 2(3′ term) | CCA | 73–75 | 877.6 | 877.2 | 877.2 |
| 3 | AUGp | 5–7 | 998.6 | 998.3 | 998.2 |
| 4 | UAGp | 8–10 | 998.6 | 998.3 | 998.2 |
| 5d | U(Ψ)CGp | 53–56 | 1280.7 | 1280.2 | 1280.1 |
| 6 | DDAGp | 19–22 | 1308.8 | 1308.2 | 1308.1 |
| 7(5′ term) | pUCCGp | 1–4 | 1359.7 | 1359.3 | 1359.1 |
| 8 | AUm5Cm5CGp | 46–50 | 1637.0 | 1637.8 | 1637.2 |
| 9 | UAUCGp | 65–69 | 1610.0 | 1610.8 | 1610.2 |
| 10 | AUUCCCGp | 57–63 | 2220.3 | 2221.0 | 2220.0 |
| 11 | UAUAACGp | 11–17 | 2268.4 | 2268.6 | 2267.8 |
| 12 | AACACCUGp | 23–30 | 2572.6 | 2572.6 | 2572.2 |
| 13 | CmU(mcm5s2U)UCACCCAGp | 32–42 | 3568.2 | 3568.7 | nd |
| 14 | CmU(mcm5U)UCACCCAGp | 32–42 | 3552.2 | 3552.3 | nd |
| 15 | CmU(mcm5Um)UCACCCAGp | 32–42 | 3566.2 | nd | 3566.1 |
aFragment numbers correspond to the numbers in Supplementary figure 1 (upper chromatograms). Numbers 2 and 7 are the 3′- and 5′-terminal fragments.
bAbbreviations for modified nucleosides: D, dihydrouridine; Ψ, pseudouridine; m5C, 5-methylcytidine; Cm, 2′-O-methylcytidine; mcm5U, 5-methoxycarbonylmethyluridine; mcm5s2U, 5-methoxycarbonylmethyl-2-thiouridine; and mcm5Um, 5-methoxycarbonylmethyl-2′-O-methyluridine.
cnd, not detected.
dPseudouridine was predicted from enzymatic RNA sequencing analysis (data not shown).
In the case of tRNAGln and tRNALys, the anticodon-containing fragments were also determined as described in Supplementary table 1. No modification difference other than that of the anticodon wobble position was found for group III tRNAGlu and tRNAGln (Figure 4; Table I). However, in the case of group II tRNALys, another modification difference was seen; 2′-O-methylated cytidine at position 32 was identified as a mitochondrial-specific modification in addition to the wobble modification difference (Figure 4; Supplementary table 1).
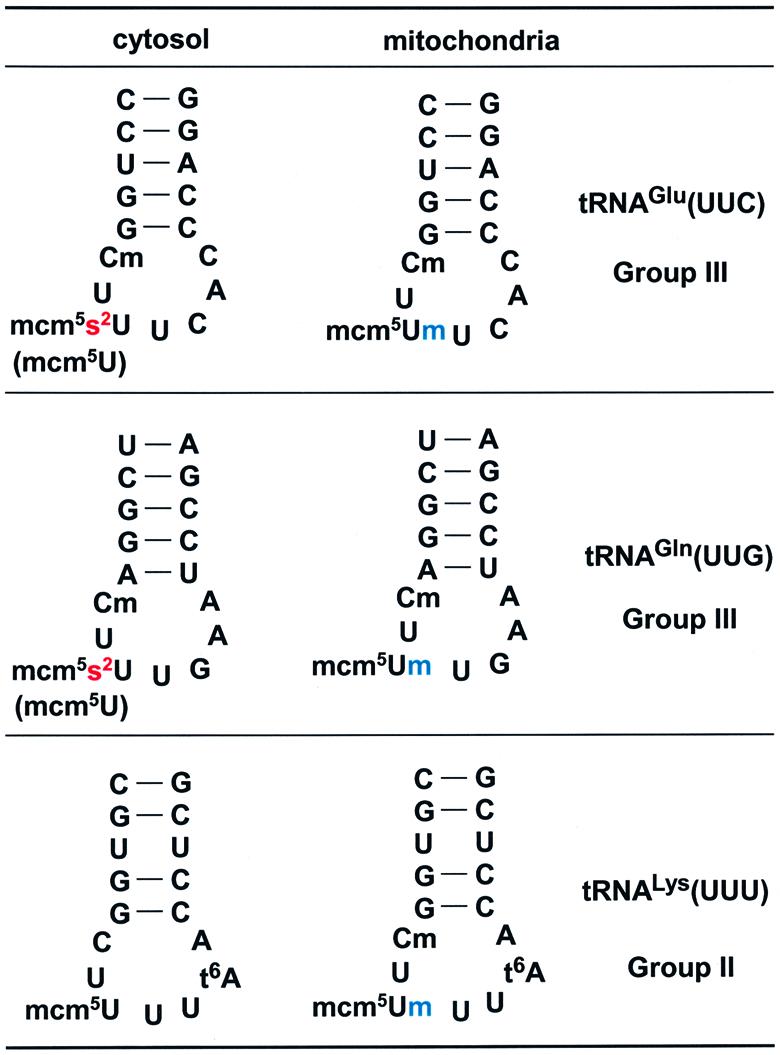
Fig. 4. Comparison of RNA sequences in the anticodon arms of cy and mt tRNAs. The anticodon wobble modifications are mcm5s2U for both cy tRNAGlu and cy tRNAGln, while they are mcm5Um for the mitochondrial counterparts. In addition, a trace amount of tRNAs with mcm5U modification is found in both cy tRNAGlu and cy tRNAGln. For tRNALys, the anticodon wobble modifications are mcm5U and mcm5Um for cy and mt tRNAs, respectively. In tRNALys, Cm modification at position 32 was found in mt tRNA but not in the cytosolic counterpart.
Presence of a modification intermediate in cytosolic tRNAs for glutamate and glutamine
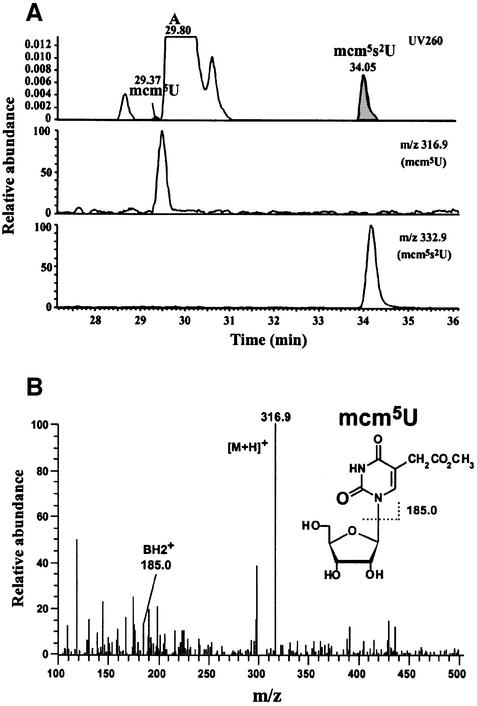
Another notable finding was that a trace amount of a modified base, mcm5U, was detected specifically in cy tRNAGlu at RT 29.37 (Figure 5A) with ions of the proton adduct (m/z 316.9) and base fragment (m/z 185) (Figure 5B). mcm5U has also been found to be the wobble modification of cy tRNALys of group II (see Supplementary table 1). Since we consider that mcm5U is a modification intermediate for mcm5s2U in cy tRNAsGlu, side chain modification at position 5 in the uracil base would precede the thiolation at position 2. This premise is substantiated by the fact that s2U was not detected in the nucleoside analysis of cy tRNAsGlu, unless specific oxidization of s2U to be converted to U has occurred. The amount of mcm5U was estimated to be 4.3% of the total cy tRNAGlu by quantifying the UV peak areas for both mcm5U and mcm5s2U (Figure 5A), since both modified uridines should have similar coefficients of molecular absorption. To confirm that mcm5U actually exists at the wobble position of cy tRNAsGlu, we searched for the anticodon-containing fragment with mcm5U digested by RNase T1 within the spectra obtained in the LC/MS analysis of cy tRNAGlu. The mass spectrum for the anticodon-containing fragment of cy tRNAGlu was deconvoluted to detect CmU(mcm5U)UCACCCAGp (3552 Da) along with CmU(mcm5s2U)UCACCCAGp (3568 Da) (Table I; Supplementary figure 1 and table 1). In addition, we also found a trace amount of mcm5U from cy tRNAGln(UUG) by nucleoside analysis (data not shown), suggesting that mcm5U is a common modification intermediate of cy tRNAGlu and tRNAGln.
Fig. 5. Detection of mcm5U in cy tRNAGlu as the modification intermediary for both mcm5s2U and mcm5Um. (A) LC/MS nucleoside analysis chromatograms for cy tRNAGlu: Top: UV trace at 260 nm in the region of 27–36 min. Middle and bottom: mass chromatograms for m/z 316.9 and 332.9 to detect, respectively, mcm5U (RT 29.37) and mcm5s2U (RT 34.05). The longer retention time of each nucleotide in the mass chromatogram compared with that in the UV trace is due to a time lag between the UV detector and mass spectrometer in the LC/MS system. (B) Mass spectrum for mcm5U at RT 29.37 with the chemical structure of mcm5U. The proton adduct and base fragment are detected.
It can be noted that mcm5U can also serve as a modification intermediate of mcm5Um in mt tRNAGlu, tRNAGln and tRNALys. Cy tRNAs with mcm5U modification potentially are direct precursors of mt tRNAs prior to the importation.
In vitro importation activity of purified cytosolic tRNAGlu
Given the absence of tRNAGlu with 2-thiolated modified uridine at the wobble position in mitochondria (Figure 3), we speculate that only cy tRNAGlu with the mcm5U modification is imported into mitochondria, while the mature cytosolic form with mcm5s2U is not imported. This suggests that 2-thiolation at the wobble position is a negative determinant against tRNA import into mitochondria. This speculation is also supported by the fact that the cytosolic form of mitochondria-specific tRNALys has mcm5U as the wobble modification, which is regarded as the counterpart of the modification intermediate for tRNAGlu and tRNAGln. We investigated this by comparing the in vitro importation activity of the mature form of cy tRNAGlu with mitochondria-specific tRNALys as well as in vitro transcribed tRNAGlu without any post-transcriptional modification.
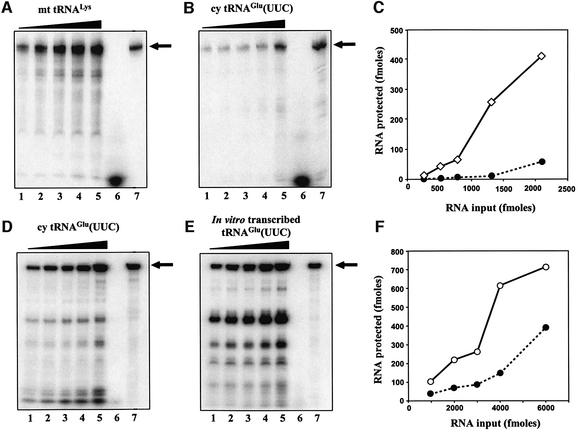
First, we compared the importation efficiency of native cy tRNAGlu and mt tRNALys to see the effect of 2-thiolation at the wobble position. Both of the purified tRNAs were 3′ end-labeled with [5′-32P]pCp, and the efficiency of their in vitro importation into isolated mitochondria was determined by a nuclease protection assay (Rubio et al., 2000). As shown in Figure 6A–C, mt tRNALys exhibited import kinetics comparable with those of the mt tRNAIle studied previously (Rubio et al., 2000), while cy tRNAGlu was imported into mitochondria much less efficiently than mt tRNALys, demonstrating that cy tRNAGlu with 2-thiolation is not an appropriate substrate for the tRNA import machinery. Then, to estimate the effect of post-transcriptional modification including 2-thiolation on tRNA import, the importation efficiency of native cy tRNAGlu was compared with that of the T7 transcript. The import of the T7-transcribed tRNA, which saturated ∼12% of the input level (Figure 6D–F), showed efficient kinetics. In contrast, the native cy tRNAGlu was imported at a significantly lower level, suggesting that the post-transcriptional modifications in cy tRNAGlu have an inhibitory effect on importation.
Fig. 6. (A and B) In vitro importation assays of affinity-purified mt tRNALys and cy tRNAGlu. Both pCp-labeled tRNAs were gel purified and resuspended at the same concentration and specific activity. Lanes 1–5: increasing concentrations (263, 526, 789, 1315 and 2105 fmol) of tRNA incubated with mitochondria and digested with MNase. Lane 6: 1000 fmol of tRNA digested with MNase. Lane 7: 10 fmol of the input RNA (IN). (D and E) In vitro importation assays of cy tRNAGlu and its transcript. Lanes 1–5: increasing concentrations (1000, 2000, 3000, 4000 and 6000 fmol) of RNA incubated with mitochondria and digested with MNase. Lane 6: 1000 fmol of tRNA digested with MNase. Lane 7: 19 fmol of the input RNA (IN). The RNAs were resolved by electrophoresis on 7 M urea/10% acrylamide gels for (A) and (B), or 7 M urea/8% acrylamide gels for (D) and (E). The migration of the full-length RNAs is indicated by an arrow. (C and F) The results showing nuclease protection of the pCp-labeled tRNAs incubated with isolated L.tarentolae mitochondria. Full-length RNAs protected from nuclease digestion were quantitated for native mt tRNALys (diamonds), native cy tRNAGlu (filled circles) and T7-transcribed tRNAGlu (open circles).
Discussion
In our study of group III tRNAGlu(UUC) and tRNAGln(UUG), and a group II tRNALys(UUU) in L.tarentolae, a correlation between the subcellular localization and the compartment-specific post-transcriptional modifications of tRNA was discovered. For the tRNAGlu and tRNAGln isolated from cytosol, the anticodon wobble bases were modified to mcm5s2U, and for the counterparts recovered from mitochondria, the anticodon wobble bases were modified to mcm5Um. In terms of decoding activity, it is known that both the 2-thio group and the 2′-O-methyl group of uridine derivatives stabilize the C3′-endo form of the anticodon wobble position in the recognition of cognate codons (Watanabe et al., 1979; Yokoyama et al., 1985), and confer ribosome-binding activity (Ashraf et al., 1999; Yasukawa et al., 2001; Suzuki et al., 2002). Thus, it is possible that tRNAGlu and tRNAGln alternatively utilize these two different wobble modifications for their compartmentalization in the cell. In addition, it is also advantageous that both modifications are generated from the same intermediate mcm5U.
Apart from a functional contribution in translation, the modification differences may function in tRNA sorting between the cytosol and the mitochondrion. There is precedent for RNA modifications affecting subcellular localization. The subcellular localization of eukaryotic mRNA and UsnRNA (uridine-rich small nuclear RNA) has been investigated extensively (Mattaj and Englmeier, 1998). After transcription in the nucleus, mRNA and UsnRNA post-transcriptionally undergo monomethylguanosine (MMG) cap formation at the 5′ end. The MMG cap functions as a nuclear export signal to the cytosol where mRNA is translated on the ribosome. In the case of UsnRNA, the MMG cap is hypermodified to a trimethylguanosine (TMG) cap in the cytosol, which results in import into the nucleus. Thus, the shuttle motion of the UsnRNA between nucleus and cytosol is governed by the post-transcriptional methylation of the cap structure. Furthermore, it has been reported that post-transcriptional modification of tRNA enhances its nuclear export activity (Kutay et al., 1998). These facts suggest that post-transcriptional modification plays a fundamental role in the subcellular targeting/localization of RNA.
It is difficult to assess, however, if the compartment-specific post-transcriptional modification is the cause or the result of localization. Mitochondria-specific modification of nuclear-encoded tRNAs has been reported in several organisms. In the bean, Phaseolus vulgaris, 2′-O-methyl modification of G at position 18 (Gm) of tRNALeu(NAG) and (NAA) was found to be specific to mt tRNA (Marechal-Drouard et al., 1988, 1990). In T.brucei, the mt tRNALys, tRNALeu and tRNATyr were shown by nuclease sensitivity analysis to possess a modified cytidine (possibly Cm) at position 32, while the cytosolic counterparts lacked such modifications (Schneider et al., 1994). However, unspliced mutant tRNATyr without methylation at position 32 was imported efficiently in mitochondria (Schneider et al., 1994), suggesting that Cm modification as well as intron removal is not necessary for mt tRNA importation in this species.
In our investigation of the L.tarentolae tRNAs, although three mt tRNAs commonly possess 2′-O-methylation at the anticodon wobble position (Figure 4), it is difficult to imagine that the mitochondrial specific 2′-O-methylation serves as a positive determinant for tRNA importation, considering that the transcribed tRNA without modification was shown to be imported efficiently into mitochondria using an in vitro importation assay (Figure 6D–F), in addition to the case of Trypanosoma tRNA discussed above (Schneider et al., 1994). Since no cy tRNA with 2′-O-methylated modified uridine was observed, 2′-O-methylation of mt tRNAs is likely to be a post-import process.
Thus the negative-determinant hypothesis (Rusconi and Cech, 1996) provides the most plausible explanation for the results of our study, linking post-transcriptional modification and mitochondrial import of tRNAs. The cytosol-specific tRNA modification, 2-thiolation at the wobble position, would serve as an inhibitory signal for the tRNA importation. Indeed, we showed that cy tRNALys, a representative of group II tRNAs, has non-thiolated modified uridine (mcm5U), which is consistent with the observation that only 4% of cellular tRNALys is localized in cytosol (Kapushoc et al., 2002). In addition, group III cy tRNAs contained a trace amount (4.3%) of mcm5U as the common modification intermediate for both cy and mt tRNAs. Cy tRNAs with mcm5U can be regarded as the tRNA import substrates. If the mature form of cy tRNAs with mcm5s2U was imported into mitochondrion, an enzyme that efficiently oxidizes the 2-thiolated uridine of the imported cy tRNAs must exist; however, this is unlikely since neither was mt tRNA having a 2-thiolated uridine detected in our analysis nor has such an enzymatic activity yet been reported.
Another instance of mitochondria-specific wobble modification in L.tarentolae was reported in another group III tRNATrp (Alfonzo et al., 1999; Crain et al., 2002). The wobble base C34 in cytosolic tRNATrp is edited to U34 in mitochondria following the importation. Further modification of the U34 is thought to enable decoding of the mitochondria-specific tryptophan codons (UGG and UGA) (Alfonzo et al., 1999).
As discussed above, a modification difference at the wobble position of tRNAs is correlated with the subcellular localization of tRNAs. The regulatory mechanism for controlling the subcellular distribution of group III tRNAs, however, most probably varies among the regulated tRNA species. Thus, investigations of modification differences between cy and mt group III tRNAs, such as Val(CAC), Leu(CAG), Arg(ACG), Asp(GTC), His(GTG) and Tyr(GTA), would be necessary to clarify the whole picture of the tRNA sorting mechanism in L.tarentolae.
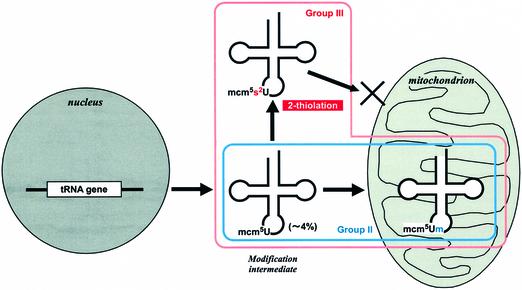
We propose a model in Figure 7 for the subcellular distribution of L.tarentolae tRNAs with NAG codons associated with wobble modifications. The precursor tRNAs are transcribed from nuclear genes and exported into the cytosol with the mcm5U modification at the wobble position. In the case of group III tRNAGlu and tRNAGln, cytosolic tRNA is matured by 2-thiolation of the modification intermediate and localized in the cytosol. A portion of the modification intermediate is imported into the mitochondrion where 2′-O-methylation of mcm5U occurs. We also propose that the thiolation is a negative determinant for tRNA importation, assuming the existence of some protein factors that inhibit tRNA import by recognizing 2-thiolated uridine. For group II tRNALys, since the modification intermediate, cy tRNALys with mcm5U, is not 2-thiolated in the cytosol, the transcribed tRNA enters into the mitochondrion where the wobble base is 2′-O-methylated.
Fig. 7. Schematic depiction of the proposed subcellular distribution mechanism for tRNAs that read NAG codons in L.tarentolae. Precursor tRNAs are transcribed from genes in the nucleus and are exported into the cytosol with the mcm5U modification at the wobble position. Cytosolic tRNA is matured by 2-thiolation of the modification intermediate and localized in the cytosol. A portion of the modification intermediate is imported into the mitochondrion, where 2′-O-methylation of mcm5U follows.
The in vitro importation experiments provide evidence for a possible involvement of post-transcriptional modification in the mature cy tRNAGlu in inhibition of import. Although the 2-thio group of mcm5s2U is considered to be one of the negative determinant candidates, our investigation does not provide sufficient data to show that it is solely responsible for the inhibition of import. Moreover, the in vitro system may not reflect satisfactorily the in vivo situation, as there may be protein factor(s) in vivo that interacts specifically with the cy tRNAGlu and cy tRNAGln by recognizing the 2-thiocarbonyl group, thus inhibiting the import of these species. Further work is required to investigate the specific role of the cytosolic 2-thiolation of tRNAs.
Materials and methods
Leishmania tarentolae culture, fractionation and RNA isolation
Leishmania tarentolae cells were grown at 27°C in BHI medium (Difco) supplemented with 10 µg/ml hemin (Calbiochem). Mitochondria were prepared by hypotonic cell lysis followed by purification from 20–35% Renografin (Bracco) gradients (Braly et al., 1974). Cytosolic fractions were obtained from cell lysates that were cleared by two successive centrifugations at 15 000 g for 15 min each (Kapushoc et al., 2000). RNA was isolated from each fraction using the guanidinium thiocyanate/phenol/chloroform method (Chomczynski and Sacchi, 1987).
Northern analysis
A 2 µg aliquot of each RNA was separated on an 8% polyacrylamide gel containing 7 M urea. Gels were stained with ethidium bromide for visualization prior to transferring the RNAs to Zeta-Probe membranes (Bio-Rad). Various RNAs were detected by hybridization to oligonucleotides that were 5′ end-labeled with [γ-32P]ATP. The following oligonucleotides were used: S-3315 (5′-GTTCCGGAAGTTTCGCAT AC-3′) to detect the spliced leader RNA; S-3316 (5′-GTCTTCCTC TGAATGCGTAAGCG-3′) to detect the RPS12-I guide RNA; and S-3226 (5′-CTCCGATACCGGGAATCCAAC-3′) to detect cy and mt tRNAGlu. Filters were exposed to a phosphor imaging screen for visualization with a Storm PhosphorImager, and analyzed with ImageQuant software (Amersham Biosciences).
Purification of individual cy and mt tRNAs
Cy and mt tRNA fractions were enriched by anion-exchange column chromatography using DEAE–Sepharose Fast Flow (Amersham Biosciences; 1 × 45 cm) with a linear gradient of NaCl and MgCl2; 500 ml of elution buffer A (20 mM Tris–HCl pH 7.5, 200 mM NaCl and 8 mM MgCl2) and elution buffer B (20 mM Tris–HCl pH 7.5, 450 mM NaCl and 16 mM MgCl2) with gravitational flow. The tRNA fractions were combined, precipitated with ethanol and dissolved in a binding buffer (1.2 M NaCl, 30 mM HEPES–KOH pH 7.5 and 15 mM EDTA). From 3420 A260unit (136.8 mg) of cy and 172 A260unit (6.88 mg) of mt total RNA fractions, 308 A260unit (12.3 mg) of cy and 15.0 A260unit (0.60 mg) of mt tRNA fractions were obtained, respectively. Individual tRNAGlu, tRNAGln and tRNALys were isolated by an improved solid-phase DNA probe method (which we have named ‘chaplet’ column chromatography). 3′-biotinylated DNA probes for purification of individual cy and mt tRNAs used in this study are as follows: tRNAGlu, 5′-TGGGTGA AAGCCAGGTGTTCTAACCGTTAT-3′; tRNALys, 5′-CGCACTCCGT GGGGCTCGAACCCACGTCCA-3′; and tRNAGln, 5′-GGATTCAAA GTCCGAAGTGATAACCACTAC-3′. Each DNA probe (∼80 µg) was immobilized on 200 µl of avidin–Sepharose (50% slurry, Amersham-Pharmacia) and packed in a small ‘chaplet’ column. Three columns were connected in tandem. The tRNA fraction dissolved in the binding buffer from cytosol or mitochondria was circulated through the chaplet column by a peristaltic pump at a temperature of 65°C to entrap each tRNA. After washing out non-specifically bound tRNAs with a wash buffer (0.6 M NaCl, 15 mM HEPES–KOH pH 7.5 and 7.5 mM EDTA), each tRNA was eluted from the column with a low-salt buffer (20 mM NaCl, 0.5 mM HEPES–KOH pH 7.5 and 0.25 mM EDTA) at 65°C. The final yields of tRNAs are shown in Results. A detailed description of the method will be reported elsewhere.
Mass spectrometry for analysis of post-transcriptional modification
An LCQ ion-trap (IT) mass spectrometer (ThermoFinnigan) equipped with an electrospray ionization (ESI) source and a MAGIC 2002 liquid chromatography system (Michrom BioResources) was used to analyze nucleosides and RNase T1-digested RNA. Purified tRNAs (0.01–0.05 A260unit/0.05–2.5 µg in each case) were digested into nucleosides at 37°C for 1 h in 10 µl of a reaction mixture containing 20 mM HEPES–KOH pH 8.0, 10 µg/ml nuclease P1 and 9 U/ml bacterial alkaline phosphatase. The hydrolysates were analyzed by LC/MS as follows. An ODS reversed-phase column with a 3 × 10 mm pre-column cartridge (Inertsil ODS-3, 2.1 × 250 mm; GL Sciences) was connected online to the electrospray interface. The conditions for the chromatography were determined as described previously by Pomerantz and McCloskey (1990) with slight modification. The solvent system consisted of 5 mM NH4OAc pH 5.3 in 0.5% acetonitrile (solvent A) and 60% acetonitrile (solvent B), and the column was developed at a flow rate of 150 µl/min by a multistep linear gradient: 1–25% B in 0–25 min, 25–66% B in 25–37 min and 66–99% B in 37–45 min. The chromatographic effluent was conducted directly into the ESI ion source without splitting. Positive ions were scanned over an m/z range from 103 to 750 throughout the separation under the following conditions: flow rate of sheath gas, 100 arb; capillary temperature, 250°C; spray voltage, 4.25 kV.
APM gel electrophoresis
The presence of the 2-thio derivative in cy tRNAGlu was verified by the retardation of electrophoresis in acrylamide in the presence of the phenyl-mercuric compound developed by Igloi (1988). Purified mt tRNAs were analyzed using a 10% acrylamide gel containing 7 M urea and 0.05 mg/ml APM, which was kindly provided by Mr Naoki Shigi and Yukinori Yamamoto (University of Tokyo).
RNA sequencing
Purified tRNAGlu was sequenced by Donis–Keller’s enzymatic digestion method (Donis-Keller, 1980). The 3′ end of the tRNA was labeled with [5′-α-32P]pCp (75 MBq/A; Amersham Biosciences) and T4 RNA ligase (Toyobo, Osaka). Partial enzymatic digestion was carried out for the following base-specific RNases: T1 (Amersham Biosciences); U2 (Seikagaku Kogyo, Tokyo); PhyM (Amersham Biosciences); and CL3 (Roche Molecular Biochemicals). The digested fragments were electrophoresed separately in lanes of 12 or 15% acrylamide gel with the undigested control and alkaline-hydrolyzed ladder.
In vitro transcription
T7 in vitro run-off transcription reactions were carried out using a linearized plasmid as a template and T7 RNA polymerase, NTPs and the appropriate buffer (Milligan et al., 1987; Cunningham and Ofengand, 1990).
Assay of in vitro importation of RNA into isolated mitochondria
Isolated or in vitro transcribed tRNAs were 3′ end-labeled by ligation of [5′-32P]pCp (NEN) using T4 RNA ligase in the buffer provided (Invitrogen). All tRNAs were gel purified by electrophoresis through a denaturing 7 M urea/10% acrylamide gel. The RNAs were eluted in 200–400 µl of 300 mM sodium acetate and 0.1 mM EDTA at room temperature overnight. The purified RNAs were precipitated with 3 vols of ethanol at –20°C, followed by centrifugation at 12 000 g for 30 min. The pellets were suspended at the same concentration and specific activity in either TE (10 mM Tris–HCl pH 8.0 and 1 mM EDTA pH 8.0) or water. The concentration of RNA in the solution was measured with a GeneQuant spectrophotometer (Amersham Biosciences). In vitro RNA importation assays were performed in a 10 µl reaction volume containing radioactive 3′ end-labeled RNAs, 1 mg of mitochondria (∼40 µg of protein), 0.5 M sucrose, 20 mM Tris–HCl pH 8.0, 1 mM ATP, 2 mM DTT, 10 mM MgCl2, 0.63 mM creatine phosphate and 22.5 mg/ml creatine phosphokinase (ATP regeneration system). After incubation at 27°C for 5 min, 100 U of micrococcal nuclease (MNase) (Roche) and 5 mM CaCl2 were added to digest the RNAs that were not imported into the mitochondria. MNase was then inhibited by the addition of 10 mM EGTA pH 8. To isolate protected RNAs, the mitochondrial pellets were washed with 0.5 M sucrose/20 mM Tris–HCl pH 8.0, suspended in 90 µl of 10 mM Tris–HCl pH 8.0, 1 mM EDTA and 0.1% SDS, and extracted with 100 µl of water-saturated phenol pH 4.5. RNAs were precipitated with 2.5 vols of ethanol and 300 mM sodium acetate pH 5.2. The radioactively labeled RNAs were separated by electrophoresis through a 7 M urea/8% (or 10%) acrylamide gel. After electrophoresis, the gels were dried onto Whatman 3MM chromatography paper and exposed to a phosphor screen (Amersham Biosciences). The nuclease-protected radioactively labeled RNAs resolved in the denaturing polyacrylamide gels were visualized using the Storm Gel and Blot Imaging System (Amersham Biosciences) and quantitated with ImageQuant analysis software (Amersham Biosciences).
Supplementary data
Supplementary data are available at The EMBO Journal Online.
Acknowledgments
Acknowledgements
This work was supported by the Human Frontier Science Program (grant RG0349).
References
- Adhya S., Ghosh,T., Das,A., Bera,S.K. and Mahapatra,S. (1997) Role of an RNA-binding protein in import of tRNA into Leishmania mitochondria. J. Biol. Chem., 272, 21396–21402. [DOI] [PubMed] [Google Scholar]
- Alfonzo J.D., Blanc,V., Estevez,A.M., Rubio,M.A.T. and Simpson,L. (1999) C to U editing of the anticodon of imported mitochondrial tRNA(Trp) allows decoding of the UGA stop codon in Leishmania tarentolae. EMBO J., 18, 7056–7062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashraf S.S., Sochacka,E., Cain,R., Guenther,R., Malkiewicz,A. and Agris,P.F. (1999) Single atom modification (O→S) of tRNA confers ribosome binding. RNA, 5, 188–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baczynskyj L., Biemann,K. and Hall,R.H. (1968) Sulfur-containing nucleoside from yeast transfer ribonucleic acid: 2-thio-5(or 6)-uridine acetic acid methyl ester. Science, 159, 1481–1483. [DOI] [PubMed] [Google Scholar]
- Braly P., Simpson,L. and Kretzer,F. (1974) Isolation of kinetoplast–mitochondrial complexes from Leishmania tarentolae. J. Protozool., 21, 782–790. [DOI] [PubMed] [Google Scholar]
- Chomczynski P. and Sacchi,N. (1987) Single-step method of RNA isolation by acid guanidinium thiocyanate–phenol–chloroform extraction. Anal. Biochem., 162, 156–159. [DOI] [PubMed] [Google Scholar]
- Crain P.F., Alfonzo,J.D., Rozenski,J., Kapushoc,S.T., McCloskey,J.A. and Simpson,L. (2002) Modification of the universally unmodified uridine-33 in a mitochondria-imported edited tRNA and the role of the anticodon arm structure on editing efficiency. RNA, 8, 752–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham P.R. and Ofengand,J. (1990) Use of inorganic pyrophosphatase to improve the yield of in vitro transcription reactions catalyzed by T7 RNA polymerase. Biotechniques, 9, 713–714. [PubMed] [Google Scholar]
- Diamond A.M. et al. (1993) Dietary selenium affects methylation of the wobble nucleoside in the anticodon of selenocysteine tRNA([Ser]Sec). J. Biol. Chem., 268, 14215–14223. [PubMed] [Google Scholar]
- Dietrich A., Small,I., Cosset,A., Weil,J.H. and Marechal-Drouard,L. (1996) Editing and import: strategies for providing plant mitochondria with a complete set of functional transfer RNAs. Biochimie, 78, 518–529. [DOI] [PubMed] [Google Scholar]
- Donis-Keller H. (1980) Phy M: an RNase activity specific for U and A residues useful in RNA sequence analysis. Nucleic Acids Res., 8, 3133–3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorner M., Altmann,M., Paabo,S. and Morl,M. (2001) Evidence for import of a lysyl-tRNA into marsupial mitochondria. Mol. Biol. Cell, 12, 2688–2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entelis N.S., Kolesnikova,O.A., Dogan,S., Martin,R.P. and Tarassov,I.A. (2001) 5S rRNA and tRNA import into human mitochondria: comparison of in vitro requirements. J. Biol. Chem., 276, 45642–45653. [DOI] [PubMed] [Google Scholar]
- Hancock K. and Hajduk,S.L. (1990) The mitochondrial tRNAs of Trypanosoma brucei are nuclear encoded. J. Biol. Chem., 265, 19208–19215. [PubMed] [Google Scholar]
- Hancock K., LeBlanc,A.J., Donze,D. and Hajduk,S.L. (1992) Identification of nuclear encoded precursor tRNAs within the mitochondrion of Trypanosoma brucei. J. Biol. Chem., 267, 23963–23971. [PubMed] [Google Scholar]
- Igloi G.L. (1988) Interaction of tRNAs and of phosphorothioate-substituted nucleic acids with an organomercurial. Probing the chemical environment of thiolated residues by affinity electrophoresis. Biochemistry, 27, 3842–3849. [DOI] [PubMed] [Google Scholar]
- Kapushoc S.T., Alfonzo,J.D., Rubio,M.A. and Simpson,L. (2000) End processing precedes mitochondrial importation and editing of tRNAs in Leishmania tarentolae. J. Biol. Chem., 275, 37907–37914. [DOI] [PubMed] [Google Scholar]
- Kapushoc S.T., Alfonzo,J.D. and Simpson,L. (2002) Differential localization of nuclear-encoded tRNAs between the cytosol and mitochondrion in Leishmania tarentolae. RNA, 8, 57–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolesnikova O.A., Entelis,N.S., Mireau,H., Fox,T.D., Martin,R.P. and Tarassov,I.A. (2000) Suppression of mutations in mitochondrial DNA by tRNAs imported from the cytoplasm. Science, 289, 1931–1933. [DOI] [PubMed] [Google Scholar]
- Kumazawa Y., Yokogawa,T., Tsurui,H., Miura,K.-i. and Watanabe,K. (1992) Effect of the higher-order structure of tRNAs on the stability of hybrids with oligodeoxyribonucleotides: separation of tRNA by an efficient solution hybridization. Nucleic Acids Res., 20, 2223–2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutay U., Lipowsky,G., Izaurralde,E., Bischoff,F.R., Schwarzmaier,P., Hartmann,E. and Gorlich,D. (1998) Identification of a tRNA-specific nuclear export receptor. Mol. Cell, 1, 359–369. [DOI] [PubMed] [Google Scholar]
- LeBlanc A.J., Yermovsky-Kammerer,A.E. and Hajduk,S.L. (1999) A nuclear encoded and mitochondrial imported dicistronic tRNA precursor in Trypanosoma brucei. J. Biol. Chem., 274, 21071–21077. [DOI] [PubMed] [Google Scholar]
- Li K., Smagula,C.S., Parsons,W.J., Richardson,J.A., Gonzalez,M., Hagler,H.K. and Williams,R.S. (1994) Subcellular partitioning of MRP RNA assessed by ultrastructural and biochemical analysis. J. Cell Biol., 124, 871–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima B.D. and Simpson,L. (1996) Sequence-dependent in vivo importation of tRNAs into the mitochondrion of Leishmania tarentolae. RNA, 2, 429–440. [PMC free article] [PubMed] [Google Scholar]
- Magalhaes P.J., Andreu,A.L. and Schon,E.A. (1998) Evidence for the presence of 5S rRNA in mammalian mitochondria. Mol. Biol. Cell, 9, 2375–2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahapatra S. and Adhya,S. (1996) Import of RNA into Leishmania mitochondria occurs through direct interaction with membrane-bound receptors. J. Biol. Chem., 271, 20432–20437. [DOI] [PubMed] [Google Scholar]
- Marechal-Drouard L., Weil,J.H. and Guillemaut,P. (1988) Import of several tRNAs from the cytoplasm into the mitochondria in bean Phaseolus vulgaris. Nucleic Acids Res., 16, 4777–4788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marechal-Drouard L., Neuburger,M., Guillemaut,P., Douce,R., Weil,J.H. and Dietrich,A. (1990) A nuclear-encoded potato (Solanum tuberosum) mitochondrial tRNA(Leu) and its cytosolic counterpart have identical nucleotide sequences. FEBS Lett., 262, 170–172. [DOI] [PubMed] [Google Scholar]
- Mattaj I.W. and Englmeier,L. (1998) Nucleocytoplasmic transport: the soluble phase. Annu. Rev. Biochem., 67, 265–306. [DOI] [PubMed] [Google Scholar]
- Milligan J.F., Groebe,D.R., Witherell,G.W. and Uhlenbeck,O.C. (1987) Oligoribonucleotide synthesis using T7 RNA polymerase and synthetic DNA templates. Nucleic Acids Res., 15, 8783–8798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motorin Y. and Grosjean,H. (1998) Chemical structures and classification of posttranscriptionally modified nucleosides in RNA. In Grosjean,H. and Benne,R. (eds), Modification and Editing of RNA. ASM Press, Washington, DC, pp. 543–549.
- Pomerantz S.C. and McCloskey,J.A. (1990) Analysis of hydrolyzates by LC/MS. Methods Enzymol., 193, 796–824. [DOI] [PubMed] [Google Scholar]
- Puranam R.S. and Attardi,G. (2001) The RNase P associated with HeLa cell mitochondria contains an essential RNA component identical in sequence to that of the nuclear RNase P. Mol. Cell. Biol., 21, 548–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio M.A., Liu,X., Yuzawa,H., Alfonzo,J.D. and Simpson,L. (2000) Selective importation of RNA into isolated mitochondria from Leishmania tarentolae. RNA, 6, 988–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusconi C.P. and Cech,T.R. (1996) The anticodon is the signal sequence for mitochondrial import of glutamine tRNA in Tetrahymena. Genes Dev., 10, 2870–2880. [DOI] [PubMed] [Google Scholar]
- Schneider A. and Marechal-Drouard,L. (2000) Mitochondrial tRNA import: are there distinct mechanisms? Trends Cell Biol., 10, 509–513. [DOI] [PubMed] [Google Scholar]
- Schneider A., McNally,K.P. and Agabian,N. (1994) Nuclear-encoded mitochondrial tRNAs of Trypanosoma brucei have a modified cytidine in the anticodon loop. Nucleic Acids Res., 22, 3699–3705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson A.M., Suyama,Y., Dewes,H., Campbell,D.A. and Simpson,L. (1989) Kinetoplastid mitochondria contain functional tRNAs which are encoded in nuclear DNA and also contain small minicircle and maxicircle transcripts of unknown function. Nucleic Acids Res., 17, 5427–5445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprinzl M., Horn,C., Brown,M., Ioudovitch,A. and Steinberg,S. (1998) Compilation of tRNA sequences and sequences of tRNA genes. Nucleic Acids Res., 26, 148–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T., Suzuki,T., Wada,T., Saigo,K. and Watanabe,K. (2002) Taurine as a constituent of mitochondrial tRNAs: new insights into the functions of taurine and human mitochondrial diseases. EMBO J., 21, 6581–6589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan T.H., Pach,R., Crausaz,A., Ivens,A. and Schneider,A. (2002) tRNAs in Trypanosoma brucei: genomic organization, expression and mitochondrial import. Mol. Cell. Biol., 22, 3707–3716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarassov I.A. and Martin,R.P. (1996) Mechanisms of tRNA import into yeast mitochondria: an overview. Biochimie, 78, 502–510. [DOI] [PubMed] [Google Scholar]
- Tumaitis T.D. and Lane,B.G. (1970) Differential labelling of the carboxymethyl and methyl substituents of 5-carboxymethyluridine methyl ester, a trace nucleoside constituent of yeast transfer RNA. Biochim. Biophys. Acta, 224, 391–403. [DOI] [PubMed] [Google Scholar]
- Uchida T. and Egami,F. (1971) In Boyer,P.D. and Krebs,E.G. (eds), The Enzymes., Vol. 4. Academic Press, New York, NY, p. 205.
- Watanabe K., Yokoyama,S., Hansske,F., Kasai,H. and Miyazawa,T. (1979) CD and NMR studies on the conformational thermostability of 2-thioribothymidine found in the T ΨC loop of thermophile tRNA. Biochem. Biophys. Res. Commun., 91, 671–677. [DOI] [PubMed] [Google Scholar]
- Yasukawa T., Suzuki,T., Ishii,N., Ohta,S. and Watanabe,K. (2001) Wobble modification defect in tRNA disturbs codon–anticodon interaction in a mitochondrial disease. EMBO J., 20, 4794–4802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama S., Watanabe,T., Murao,K., Ishikura,H., Yamaizumi,Z., Nishimura,S. and Miyazawa,T. (1985) Molecular mechanism of codon recognition by tRNA species with modified uridine in the first position of the anticodon. Proc. Natl Acad. Sci. USA, 82, 4905–4909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshinari S., Koike,T., Yokogawa,T., Nishikawa,K., Ueda,T., Miura,K. and Watanabe,K. (1994) Existence of nuclear-encoded 5S-rRNA in bovine mitochondria. FEBS Lett., 338, 137–142. [DOI] [PubMed] [Google Scholar]