Abstract
Oxygen plays a key role in energy metabolism. However, there are organisms that survive severe shortfalls in oxygen. Drosophila embryos rapidly arrest development upon severe hypoxia and recover upon restoration of oxygen, even days later. Stabilization of the normally unstable engrailed RNA and protein preserved the localized striped pattern of this embryonic patterning gene during 3 days in hypoxia. Severe hypoxia blocked expression of a heat-shock-inducible lacZ transgene. Cyanide, a metabolic poison, did not immediately block gene expression or turnover, arguing against a passive response to energy limitation. In contrast, nitric oxide, a putative hypoxia signal, induced a reversible arrest of development, gene expression and turnover. Reciprocally, a nitric oxide scavenger allowed continued gene expression and turnover during hypoxia, but it reduced hypoxia tolerance. We suggest that hypoxia-induced stasis preserves the status quo of embryonic processes and promotes survival. Our data implicate nitric oxide as a mediator of this response and provide a system in which to investigate its action.
Keywords: embryonic diapause/hypoxia/nitric oxide/transcription/translation
Introduction
Oxygen plays a central role in metabolism and animal physiology. Oxygen limitation (hypoxia), even if temporary, can result in irreversible cellular damage. Local hypoxia, as occurs in stroke and cardiac infarction, can be deleterious within minutes, and is one of the major causes of morbidity and mortality in humans. Many organisms, however, tolerate extended periods of hypoxia without the devastating damage that occurs in some mammalian tissues (Hochachka, 1986; Donohoe and Boutilier, 1998).
Adaptations that reduce the use of oxygen and improve oxygen delivery to tissues can often accommodate mild hypoxia. Even in severe hypoxia, glycolysis can provide energy in the absence of oxygen; however, metabolic constraints usually limit sustained glycolysis. Conse quently, reducing energy consumption is the most effective strategy in adapting to hypoxia, and hypoxia-tolerant organisms often enter a hypoxia-induced stasis. Some hypoxia-tolerant cells or tissues experience a ≥90% decline in protein synthesis and a decline in membrane permeability, often the major energy-consuming processes in cells. This greatly reduces ATP consumption, which is a crucial step for long-term hypoxia survival (Hochachka et al., 1996; Hochachka and Lutz, 2001).
The discovery of hypoxia-inducible factor 1, or HIF-1, was a stepping stone to the elucidation of a pathway promoting transcriptional responses to hypoxia. HIF-1 is an α/β heterodimeric transcription factor. Under normoxic conditions, HIF-1α undergoes oxygen-dependent prolyl hydroxylation, rendering it a substrate for the VHL-elongin BC ubiquitin ligase, which targets it for degradation (Bruick and McKnight, 2001; Ivan et al., 2001). Hypoxia slows hydroxylation, reducing VHL interaction, and allows accumulation of the now stabilized HIF-1. HIF-1-induced transcription contributes to responses that allow cells to accommodate to at least modest reductions in oxygen (reviewed in Hochachka and Lutz, 2001; Semenza, 2001a,b). Hypoxia-induced transcription is observed in Drosophila at 5% oxygen (Guillemin and Krasnow, 1997; Lavista-Llanos et al., 2002), a condition that allows continued growth and development. On the other hand, severe hypoxia did not induce an HIF-dependent response (Lavista-Llanos et al., 2002), and rapidly arrested growth and development. For example, embryonic cell cycles arrest within 2 min of the imposition of severe hypoxia (Foe and Alberts, 1985; DiGregorio et al., 2001). Consequently, it seems likely that other faster mechanisms might supplement the transcriptional response to hypoxia by triggering a rapid switch to energy conservation upon abrupt imposition of severe hypoxia.
Little is known about the mechanisms eliciting the rapid metabolic downregulation upon sudden reduction of oxygen. While the downregulation could be a passive outcome of the metabolic limitation suffered during hypoxia, a number of observations suggest that an active signaling process contributes (see Discussion). Nitric oxide (NO) was first implicated as a biological signaling molecule because it triggers rapid vasodilation in response to hypoxia in mammals (Ignarro et al., 1987; Palmer et al., 1987). In Drosophila, NO has been reported to mediate aspects of the response to hypoxia, such as hypoxia-induced arrest of S-phase in embryos, and larval behavioral changes (Wingrove and O’Farrell, 1999; DiGregorio et al., 2001).
Drosophila provides a powerful model system to explore the different components of the response to hypoxia. Embryos rapidly arrest development upon removal of oxygen, and resume when oxygen is restored, even if it is days later (Foe and Alberts, 1985; Wingrove and O’Farrell, 1999). Survival implies that the myriad of complex and dynamic events of development arrest and restart in concert. The well-defined and dynamic cascade of expression of the patterning genes encodes spatial and temporal information that directs Drosophila embryogenesis. For example, engrailed (en) is expressed in a striped pattern and it specifies posterior segmental fates. The segmental stripes of en expression are set up early in development by a cascade of transiently expressed pattern regulators (DiNardo et al., 1985). If expression of en is lost, this cascade of interactions cannot be recapitulated in an older embryo. Accordingly, for successful development, the pattern of localized gene expression must either survive hypoxia or be recreated anew by a back-up program of pattern specification.
We examined the responses of Drosophila embryos to severe hypoxia. Our analysis of en RNA and protein distributions, as well as expression of a heat-shock lacZ (hs-lacZ) transgene, showed that severe hypoxia blocks gene expression and turnover of both RNA and protein. Consequently, the usually dynamic expression program of patterning genes was stalled, and the spatial distributions of gene products preserved during the suspension of animation induced by hypoxia. The interruptions in gene expression and product turnover appear to be active responses, as they were not induced by a respiratory poison. We also found that addition of NO phenocopies hypoxia, blocking gene expression and turnover of gene products. Addition of a pharmacological NO scavenger abolished the stabilization of gene products observed in hypoxia and interfered with the downregulation of transcription. Our findings lead us to suggest that severe hypoxia induces an adaptive downregulation of central biological processes and that NO is a candidate mediator of these responses.
Results
Arrest of the patterning cascade in hypoxia
Normally, Drosophila embryos take ∼24 h to hatch into larvae. In severe hypoxia, embryos immediately arrest development as if ‘frozen’ in place, resuming development when oxygen is restored (Foe and Alberts, 1985). To better understand this developmental arrest, we examined the influence of hypoxia on the developmentally dynamic expression of an essential patterning gene, en.
After different periods of hypoxia, embryos (early germ band extended stage, 180 min old) retained the morphology they had upon initial imposition of hypoxia, as has been reported by Foe and Alberts (1985). Development was reactivated and viable flies were produced after the restoration of oxygen (Foe and Alberts, 1985; Wingrove and O’Farrell, 1999; data not shown). In situ hybridization to detect en transcripts (Figure 1A–D) and antibody staining for en protein (En) (Figure 1E–H) showed persistent staining with only slight diminution in intensity after 3 days of hypoxia. The stereotypical refinements of the En expression pattern, like the addition of a fifteenth stripe (DiNardo et al., 1985; Vincent and O’Farrell, 1992), did not occur during hypoxia.
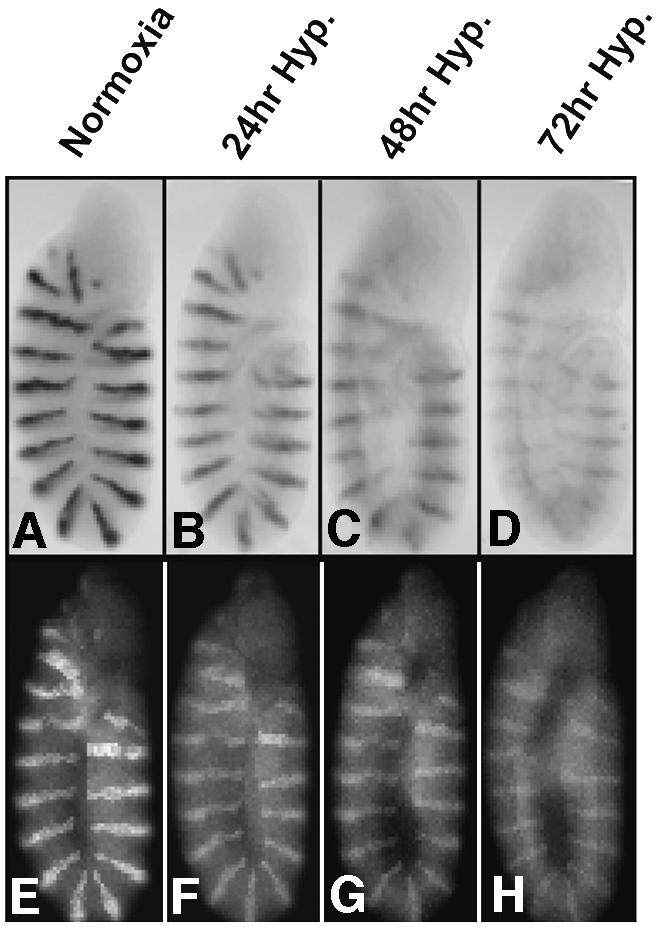
Fig. 1. en RNA and protein persist during prolonged hypoxia. Wild-type 5-h-old embryos were hybridized with an en probe to visualize RNA patterns (A–D), or stained with En antibody to reveal protein distribution (E–H) after exposure to different oxygen environments: normoxia (control) (A, E); 24 h hypoxia (B, F); 48 h hypoxia (C, G); 72 h hypoxia (D, H).
RNA and protein are stabilized during hypoxia
The RNA and protein products of en are normally unstable, a feature that is presumably important for the refinements in the pattern of expression that occur during early embryogenesis. The persistence of the en pattern in hypoxia might be due to stabilization of the gene products or to an unchanging steady state of continued protein synthesis and degradation. Either of these mechanisms could sustain patterning information during periods of hypoxia so that development could be reinitiated upon restoration of oxygen.
We tested the influence of hypoxia on the decay of en RNA and protein following inhibition of transcription or translation, respectively. If active transcription were required to sustain the stripes of en RNA, we would expect them to decay following inhibition of transcription with actinomycin D. After a 4 h treatment with actinomycin D in normoxia, in situ hybridization detected only a faint remnant of an en-striped pattern (Figure 2A). In contrast, when hypoxic embryos were treated for 4 h with actinomycin D, we readily detected the en RNA striped pattern (Figure 2B). Thus, continued transcription is required to sustain the en RNA pattern in normoxia but not in hypoxia. Analysis of another patterning gene, Ultrabithorax (Ubx), showed a similar stabilization during hypoxia (data not shown). We conclude that hypoxia significantly stabilizes en and Ubx transcripts.
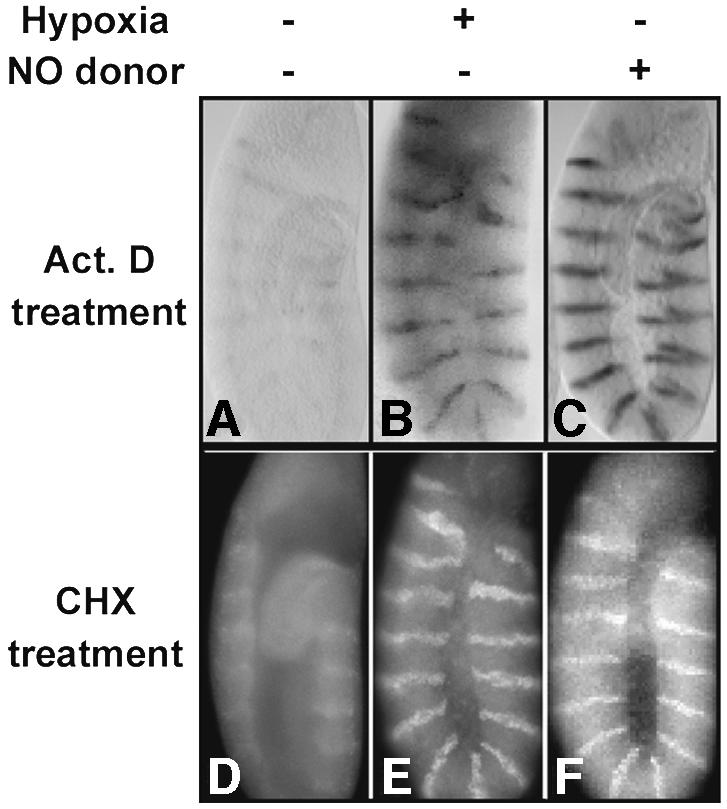
Fig. 2. en RNA and protein are stabilized by hypoxia and by addition of exogenous NO. Wild-type embryos were incubated for 4 h in the presence of actinomycin D (Act. D) (A–C) or cycloheximide (CHX) (D–F) in normoxia (A, D), hypoxia (B, E) or the presence of the NO donor SNAP (C, F). Following incubation, embryos were fixed and en RNA visualized by in situ hybridization (top panels) or En protein detected by antibody staining with anti-En antibody (bottom panels).
To test the requirement for protein synthesis in hypoxia, we inhibited translation with cycloheximide and examined En by antibody staining. In a normoxic environment, En staining was undetectable after 4 h of inhibition of translation with cycloheximide, suggesting that continued translation was required to replace degraded protein (Figure 2D). In contrast, in hypoxia, the En stripes persisted after cycloheximide treatment (Figure 2E). We conclude that En protein, like the RNA, is stabilized during hypoxia.
Abrupt hypoxia blocks de novo gene expression
Given that we did not observe accumulation of en RNA or protein during hypoxia (Figure 1) despite their stabilization (Figure 2), we postulated that RNA and protein synthesis were reduced or blocked by hypoxia. We tested the effect of hypoxia on the ability to accumulate new RNA or protein using a transgenic line carrying a heat-shock-inducible lacZ transgene (hs-lacZ). Northern analysis of embryos heat shocked (45 min at 37°C) in normoxia or hypoxia revealed a strong lacZ RNA signal in the normoxic sample (Figure 3A, lane 1), but no detectable signal in the hypoxic treated embryos (Figure 3A, lane 2). Similarly, an analysis of lacZ RNA expression in individual embryos by in situ hybridization showed a global block to RNA accumulation by hypoxia (data not shown). We conclude that hypoxia blocks the induced accumulation of lacZ transcript.
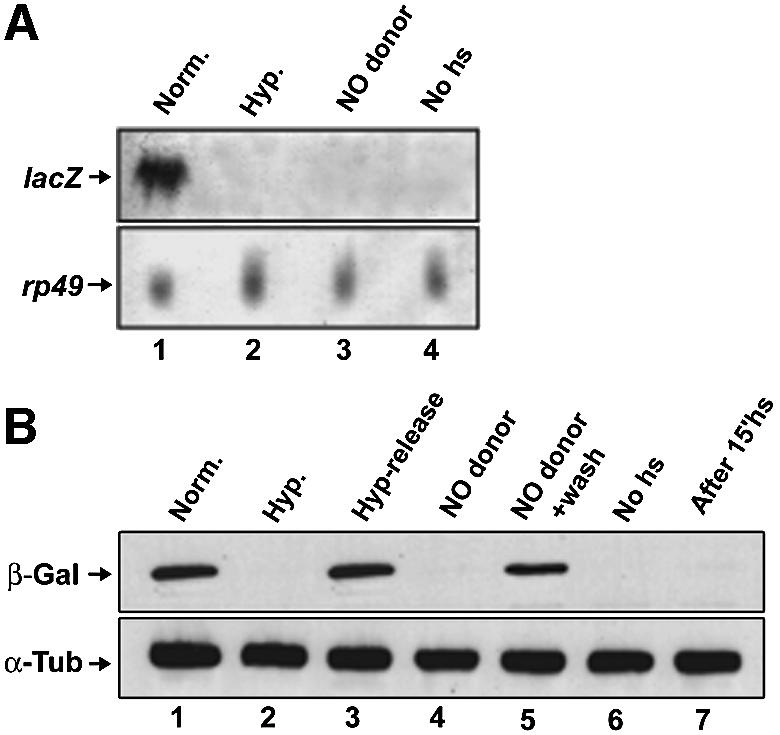
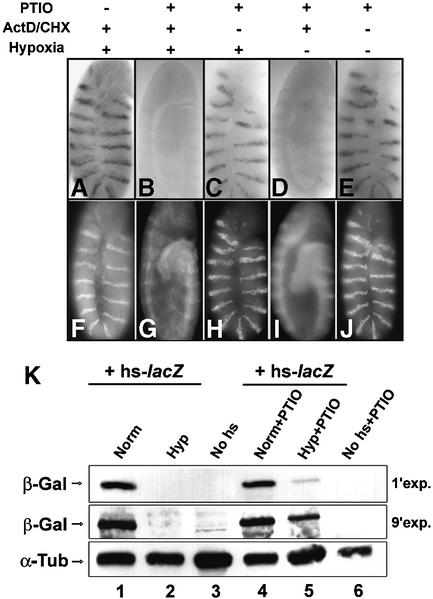
Fig. 3. Severe hypoxia blocks lacZ gene expression. Northern blots (A) and western blots (B) were used to examine the induction of lacZ RNA and β-galactosidase following heat-shock induction of embryos carrying an hsp70-lacZ transgene. (A) Embryos aged to 180 min were heat shocked for 45 min, at 37°C, in normoxia (lane 1), hypoxia (lane 2) or the presence of the NO donor SNAP (lane 3). Lane 4 shows a no-heat-shock control. The lower panel shows the signal from a probe for rp49, which encodes a ribosomal protein, as a loading control. (B) Embryos aged to 180 min were heat shocked for 15 min, at 37°C, in normoxia, then split into thirds, incubated for 75 min in normoxia (lane 1), hypoxia (lane 2) or the presence of the NO donor SNAP (lane 4), and then lysed and blotted for β-galactosidase (β-Gal). To show the reversibility of the block to β-galactosidase accumulation, some embryos of the hypoxic sample were released to normoxia for another 75 min and blotted (lane 3), and some embryos of the SNAP-treated sample were washed and blotted (lane 5). Lane 6 shows embryos without heat shock, and lane 7 shows that there is no detectable β-galactosidase after the initial 15 min heat shock. Samples were immunoblotted for α-tubulin (α-Tub) as a loading control.
To test the influence of hypoxia on translation, we exposed 180-min-old embryos to a short (15 min) heat shock at 37°C in normoxia, at which point no β-galactosidase was detected (Figure 3B, lane 7). Heat-shocked embryos were split into normoxic and hypoxic samples, incubated for 75 min at room temperature in either normoxia or hypoxia, and embryonic extracts analyzed by western blotting (Figure 3B). We detected a strong β-galactosidase signal in normoxic, but not hypoxic, samples (Figure 3B, lane 1 versus 2). To ensure that hypoxic embryos were not dead or otherwise incapacitated, we restored oxygen to some hypoxic embryos, which then accumulated high levels of β-galactosidase in a subsequent 75 min incubation (Figure 3B, lane 3).
The analyses of lacZ show that hypoxia blocks new RNA and protein accumulation. Hypoxia did not destabilize lacZ RNA or β-galactosidase (data not shown), arguing that the effects on RNA and protein accumulation are due to inhibition of synthesis.
NO mimics hypoxia
Several lines of evidence have implicated NO as a mediator of responses to hypoxia in Drosophila (Wingrove and O’Farrell, 1999; DiGregorio et al., 2001). NO is, therefore, a candidate for a mediator of events associated with the hypoxia-induced arrest of development, such as the block to gene expression. If so, we expect that the ectopic addition of NO in normoxia ought to induce responses similar to those normally seen in hypoxia.
To test the ability of NO to induce embryonic stasis, we incubated 180 min embryos (Figure 4A) with or without the NO donor SNAP (S-nitroacetylpenicillamine) and examined them 4 h later. SNAP treatment blocked morphological progression (compare Figure 4B and C). To test reversibility, we removed the SNAP solution after 4 h, washed the embryos and deposited them in standard fly food bottles. Control and hypoxic (4 h exposure) embryos were processed in parallel. After a 4 h arrest by SNAP treatment, 89 ± 0.5% of the embryos survived to eclose as normal fertile adult flies, compared with 85 ± 1% and 90 ± 3% eclosion of hypoxic and control embryos, respectively. Thus, SNAP induces a reversible developmental arrest that resembles the arrest induced by hypoxia.
Fig. 4. An NO donor induces a reversible arrest of development resembling that induced by abrupt and severe hypoxia. A collection of wild-type embryos aged to 180 min was stained with an antibody against α-tubulin. Embryos that were at the germ band extended stage (A) were incubated for 4 h with or without 10 mM SNAP (B and C). Embryos incubated with SNAP were arrested at the precise developmental stage when SNAP was first added (B). In contrast, embryos incubated without SNAP underwent normal development and were 4 h ahead relative to SNAP-treated embryos (C).
As we showed for hypoxia, treatment with SNAP also prevented decay of en RNA and protein after treatment with actinomycin D and cycloheximide, respectively (Figure 2C and F). As an internal control for toxicity, we washed out the SNAP from an aliquot of the treated embryos, and followed DNA synthesis by incorporation of the nucleotide analog BrdU. In situ detection showed incorporation in a pattern typical for S-phase cells in a germ band extended embryo (data not shown).
As shown for hypoxia, treatment with SNAP also blocked induction of lacZ RNA and protein from an hs-lacZ transgene (Figure 3A, lane 1 versus 3, and B, lane 1 versus 4). Embryos accumulated β-galactosidase following removal of SNAP (Figure 3B, lane 5), indicating that the block to β-galactosidase synthesis was reversible. We conclude that SNAP treatment reversibly inhibits lacZ gene expression.
These studies show that an NO donor reversibly arrests development, stabilizes en RNA and protein, and reversibly inhibits expression of an inducible transgene. The ability of the NO donor SNAP to block development was negated by co-incubation with the NO quenching reagent PTIO 2-phenyl-4,4,5,5,-tetramethylinidazoline-1-oxyl-3-oxide. In addition, a different NO donor, NOC-12, also induced an arrest of development (data not shown). Hence, the activity of SNAP appears to be due to its known ability to release NO. From these results we conclude that NO administration in normoxic conditions induces responses similar to those induced by hypoxia.
Inhibition of the electron transport chain with CN does not mimic hypoxia
The simplest model to explain the response to severe hypoxia is that a collapse of cellular energy reserves leads to a passive shutdown of cellular metabolism. Since NO can inhibit the respiratory chain at the cytochrome c oxidase subunit (reviewed in Cooper, 2002), it might similarly induce a collapse of energy reserves and a passive response. If this were the case, an inhibitor of cytochrome c oxidase such as cyanide (CN), which lowers ATP levels even more effectively than hypoxia (DiGregorio et al., 2001), might also mimic hypoxia.
En levels declined rapidly (within 4 h) in embryos treated with CN (data not shown). The pattern of en RNA and protein stripes disappeared rapidly when CN-treated embryos were subjected to a 4 h treatment with actinomycin D or cycloheximide, respectively (Figure 5A and D). Thus, CN treatment, although it arrests embryos, does not stabilize en gene products. To test whether the stasis induced by hypoxia or SNAP can protect embryos from the effect of CN, we subjected embryos to joint treatment. Unlike control embryos, embryos arrested by hypoxia or SNAP treatment preserve en RNA and protein as stable gene products when treated with CN.
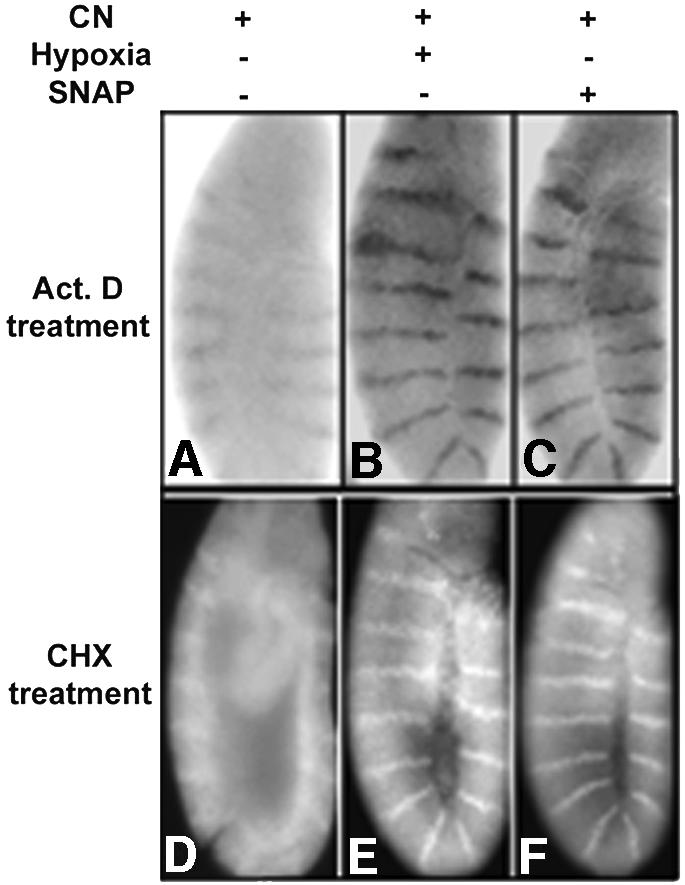
Fig. 5. Cyanide (CN) treatment does not mimic hypoxia. en RNA and protein were detected by in situ hybridization (A–C) or antibody staining (D–F) after different treatments. Incubation of wild-type embryos for 1 h with 0.2% NaCN, followed by a 4 h treatment with actinomycin D (A) or cycloheximide (D), did not induce stabilization of en RNA or protein. Pre-treatment of wild-type embryos for 30 min in hypoxia, followed by incubation for 1 h in 0.2% NaCN and 4 h treatment with actinomycin D (B) or cycloheximide (E), showed the characteristic en RNA and protein stripes. Similarly, embryos pre-treated with 10 mM SNAP for 30 min, incubated with 0.2% NaCN in the presence of SNAP and then incubated with actinomycin D or cycloheximide maintained en RNA (C) and protein (F) patterns.
An NO scavenger inhibits responses to hypoxia
Given that ectopic NO phenocopies hypoxic responses, we asked whether NO is required for the responses to hypoxia. PTIO is an NO scavenger that reacts stoichiometrically with NO (Akaike et al., 1993). In the presence of PTIO, treatment of embryos with actinomycin D or cycloheximide caused a decline in en RNA or protein irrespective of hypoxia (Figure 6B, D, G and I), suggesting that PTIO prevented the stabilization of en RNA and protein caused by hypoxia (Figures 2, 6A and F). Treatment with PTIO affected neither normal en levels in normoxia (Figure 6E and J) nor its decay upon either actinomycin D or cycloheximide treatment during normoxia (Figure 6D and I). We conclude that the hypoxia-induced stabilization of en RNA and protein is prevented by the NO scavenger PTIO.
Fig. 6. A scavenger of NO inhibits the hypoxic responses. We tested whether NO is necessary to stabilize en RNA or protein in hypoxia as detected by en in situ hybridization (A–E) and En antibody staining (F–J). Hypoxia induces stabilization of en RNA (A) and protein (F). In contrast, en RNA (B) and protein (G) were unstable when embryos were incubated for 2 h in 20 mM PTIO, made hypoxic and treated with actinomycin D (ActD) or cycloheximide (CHX) in the presence of PTIO. The characteristic en striped pattern persisted in embryos made hypoxic in the presence of PTIO without addition of inhibitors of gene expression (C and H), suggesting that continued expression was occurring during hypoxia. Treatment with PTIO did not affect the en pattern in normoxia (E and J) or its decay upon cycloheximide treatment (D and I). (K) To test whether PTIO can bypass the hypoxia-imposed block to de novo protein synthesis, we incubated hs-lacZ transgenic embryos in 20 mM PTIO for 2 h (lanes 4–6) or without PTIO (lanes 1–3) and induced hs-lacZ for 15 min, at 37°C, in normoxia in both sets. Each set of embryos was split into normoxic and hypoxic batches, and both were incubated for 75 min and analyzed by western blotting. β-galactosidase was detected in both normoxic samples (lane 1 and 4); however, there is a block on β-galactosidase accumulation in hypoxia (lane 2) that is overridden by PTIO (lane 5). Given that the bypass of the block to β-galactosidase accumulation by hypoxia is partial, we show two different exposures of the gel: 1 min exposure (first row) and 9 min exposure (second row), which show that the induction of β-galactosidase during hypoxia in the presence of PTIO is clearly different from the control.
Interestingly, even though PTIO blocked the stabilization of en RNA and protein by hypoxia, the striped en pattern nonetheless persisted in embryos made hypoxic in the presence of PTIO (Figure 6C and H). This might be explained by continued en gene expression during hypoxia when embryos are treated with PTIO.
To test NO involvement in the hypoxic block to gene expression, we examined β-galactosidase induction in parallel embryo collections, one of which was incubated in the presence of PTIO for 2 h prior to, as well as during, treatments. As before, we induced hs-lacZ at 37°C for 15 min in normoxia and then incubated for 75 min in either normoxia or hypoxia. Western blots revealed similar levels of induced β-galactosidase in normoxic samples regardless of the presence of PTIO (Figure 6K, lanes 1 and 4); however, hypoxia effectively blocked β-galactosidase induction only in the embryos lacking PTIO (Figure 6K, lanes 2 and 5). While hypoxia still diminished the induction in the PTIO-treated embryos (Figure 6K, lanes 4 and 5), comparison of different exposures (Figure 6K, upper and middle panels) shows that β-galactosidase expression in hypoxic PTIO-treated embryos was significantly higher than in hypoxic control embryos. While the residual effect of hypoxia might suggest NO-independent routes of response, the diminished expression in hypoxia could equally well be attributed to the fact that β-galactosidase synthesis occurred in the face of metabolic limitations during severe hypoxia. The result shows that the absence of oxygen does not inevitably block gene expression, and suppression of the block by PTIO argues that NO is an essential mediator of a stringent block to gene expression.
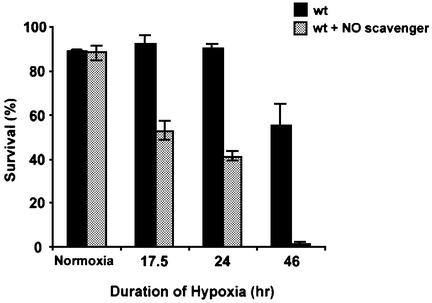
The above findings suggest the unlikely possibility that PTIO somehow alleviates the consequences of oxygen deprivation. To test this, we scored survival after different periods of hypoxia in the presence or absence of PTIO. Embryos (4 h old) were incubated for 4 h with or without 20 mM PTIO, made hypoxic, and survival assessed by restoring oxygen after different durations of hypoxia (Figure 7). More than half of the control embryos survived 46 h of hypoxia, while only 1% of the PTIO-treated embryos survived. PTIO did not affect the survival of normoxic embryos (data not shown). PTIO, therefore, compromises survival of hypoxia, presumably by blocking NO-dependent adaptive responses.
Fig. 7. Reduced embryonic survival in hypoxia after NO scavenging. Eight- to 10-h wild-type embryos were made hypoxic and assayed for survival (black bars). In parallel, embryos were pre-incubated with the NO scavenger PTIO, made hypoxic, and assayed for survival (gray bars). Samples were incubated in normoxic or hypoxic conditions for 0, 17.5, 24 and 46 h, and then placed on an agar plate in normoxia, and scored for the number of embryos that hatched into larvae. The percentage of embryos that survived was 88.7 ± 1, 92.0 ± 4, 90.0 ± 2 and 55.3 ± 10% after 0 (normoxia), 17.5, 24 and 46 h of hypoxia, respectively. In contrast, 88.0 ± 3, 52.7 ± 4, 41.3 ± 2 and 1.3 ± 1% of PTIO-incubated embryos survived after equivalent periods of hypoxia.
Discussion
Like other hypoxia-tolerant organisms, Drosophila can be induced to enter quiescence (Foe and Alberts, 1985; Wingrove and O’Farrell, 1999; Padilla and Roth, 2001). The extraordinarily rapid events of Drosophila embryogenesis halt abruptly when oxygen is suddenly and severely reduced, and can be successfully reinitiated hours, or even days, later upon restoration of oxygen (Foe and Alberts, 1985; Wingrove and O’Farrell, 1999). This reversible arrest of embryogenesis is particularly remarkable because a myriad of events, such as cell proliferation, tissue movements, patterning and differentiation, must synchronously shut down if their coordination is to be maintained and development successfully renewed upon restoration of oxygen. In contrast to some other well-studied adaptations to hypoxia that are induced by HIF-dependent changes in gene expression, quiescence appears to be largely independent of new transcription. We provide evidence that the embryo rapidly shuts down ongoing gene expression and turnover of RNA and protein products. In addition to energy conservation, this suspension of gene expression appears to provide a way of stalling the dynamic cascade of transcriptional events that govern embryonic patterning. Importantly, our analyses suggest that NO is a mediator of the downregulation in gene expression and entry into quiescence.
Preserving pattern information
Successful transient arrest of development must surmount many difficulties. Among these, the preservation of the detailed map of localized gene expression that guides development seems problematic. At the stages of development studied here, localized expression of patterning genes, such as en, is not fixed (Heemskerk et al., 1991). The signals that promote expression of the pattern regulators are themselves not constant, and this drives the refinements in the evolving pattern of gene expression. Consequently, if the transcriptional cascade were to continue during hypoxia, the evolving spatial patterns of gene expression and associated regulatory signals would get ahead of the arrested embryo. On the other hand, blocking transcription would lead to decay of the regulators and loss of pattern information (DiNardo et al., 1994). Nonetheless, we found that when hypoxia arrested the germ band extended embryo, the pattern of en RNA and protein remained as it was at the time of imposition of hypoxia, except for a slow decay in levels over a period of days. Analyses of a second important patterning gene, the homeotic gene Ubx, showed that its spatial patterns of accumulation of RNA also persisted.
We explored this stasis in the transcriptional cascade, and demonstrated that hypoxia greatly stabilizes en RNA and protein (Figure 2; data not shown). Since inhibition of decay was not accompanied by increases in the level of en gene products, we inferred that synthesis is largely blocked during hypoxia, and confirmed a block to gene expression by showing that induction of a hs-lacZ transgene was blocked by hypoxia. We concluded that the ‘frozen’ en pattern results from a coordinated block to both synthesis and degradation of en RNA and protein. Based on similar results for Ubx RNA and the requirement for persistence of numerous pattern regulators, we suggest that there is a general block to the cascade of patterning gene expression and that the stabilization of the RNA and protein products preserves pattern information during the arrest.
The failure to induce lacZ products during hypoxia suggests that the block to expression is not unique to the patterning genes. Additionally, mitotic cyclin degradation is blocked during hypoxia (P.G.DiGregorio, unpublished), a factor that might be either the cause or the consequence of the metaphase block induced by hypoxia (DiGregorio et al., 2001). These observations are consistent with a widespread block to synthesis and degradation. Such a widespread block could contribute importantly to the embryonic diapause and to the conservation of energy reserves. This proposal is consistent with analyses in other hypoxia-tolerant organisms, suggesting a decline in protein synthesis (Kwast and Hand, 1996; Hochachka and Lutz, 2001). It will be interesting to test whether endogenous sequences under the control of HIF might escape this inhibition. However, analysis of a reporter construct revealed that HIF-induced expression was maximal between 3 and 5% oxygen (the minimum required for growth) and fell dramatically at 1% oxygen, an oxygen concentration at which embryos arrest (Lavista-Llanos et al., 2002). This suggests that the utility of the HIF response will be confined to conditions of less severe hypoxia that do not induce a strong arrest.
Transcriptional inputs
Several observations in previous reports suggest that Drosophila embryos have responses to hypoxia that do not require an input from new transcription, suggesting operation of mechanisms independent of the HIF transcription factor. First, some responses are extremely fast, with arrest of the embryonic cell cycle occurring within minutes (Foe and Alberts, 1985; DiGregorio et al., 2001) and changes in larval behavior occurring within seconds (Wingrove and O’Farrell, 1999; J.A.Wingrove and P.H.O’Farrell, unpublished data), and are likely to be too fast to be governed by changes in gene expression. Secondly, hypoxia arrests stages of early embryogenesis that occur independently of transcription (Zalokar and Erk, 1976; Foe and Alberts, 1985; DiGregorio et al., 2001). Thirdly, even when hypoxia is imposed during mitosis, when there is no productive transcription (Shermoen and O’Farrell, 1991), cells respond by arresting at metaphase (DiGregorio et al., 2001). Presumably, mechanisms other than HIF-induced changes in expression contribute to these biological responses. The arrest of gene expression that we have examined is also fast and not easily attributable to accumulation of new gene product. Indeed, our studies suggest that there is little opportunity for accumulation of new gene products during severe hypoxia.
Passive versus active response
Since oxygen is so central to metabolism, the simplest explanation for a cellular shutdown in response to hypoxia is that there is a passive depletion of energy reserves and that numerous processes respond independently and passively to this diminished energy supply. However, several previous observations suggest that the hypoxic response is active. Direct measurement of ATP levels following hypoxia shows that embryos retain substantial ATP levels in the face of severe hypoxia: cellularized embryos such as those studied here maintained 73% of their ATP (DiGregorio et al., 2001). Additionally, while a passive response is not expected to require specialized mediators, studies of larval behavior and cell cycle arrest identified NO as a likely mediator of the response. Similarly, we provide evidence that NO mediates the hypoxia-induced shutdown of gene expression and turnover of RNA and protein. Furthermore, inhibition of respiration by CN, although it induces an embryonic arrest and reduces ATP levels to a greater extent than hypoxia (DiGregorio et al., 2001), fails to stabilize en RNA and protein as occurs during hypoxia. Hence, it appears that hypoxia produces a signal that actively induces this change. Drosophila embryos provide a powerful system in which to investigate the nature of this signal.
NO is a candidate mediator of the arrest of gene expression in response to hypoxia
NO is a signaling molecule with a wide range of functions in animal physiology. It promotes vasodilation in mammals and its levels are thought to rise at sites that are hypoxic due to increased respiratory demands or poor perfusion (Stamler et al., 1997). NO has also been implicated in signaling hypoxia in Drosophila (Wingrove and O’Farrell, 1999; DiGregorio et al., 2001). We have shown that, like hypoxia, NO donors can induce a reversible embryonic stasis. Furthermore, treatment with an agent that quenches NO overrode hypoxic suppression of gene expression and product turnover (Figure 6), and dramatically compromised the ability of the embryos to survive hypoxia (Figure 7). We suggest that NO mediates adaptive responses that contribute to survival of hypoxic stress. To further test this proposal, we hope to develop genetic tools to eliminate NO or block its action, and biochemical tools to reliably assess the endogenous NO levels in an embryo.
One well-characterized mode of action of NO is through its known ability to activate soluble guanylate cyclase (sGC), raising cGMP levels and activating, among others, protein kinase G (PKG). To test the possible involvement of sGC/PKG in the hypoxia-induced stabilization of RNA and protein, we examined the responses of embryos carrying either a hypomorph of sCG (sGC738) (Gibbs et al., 2001) or a naturally occurring allele of PKG (fors), which has a slightly diminished activity (Osborne et al., 1997). Additionally, we examined embryos treated with the hydrolysis-resistant cGMP analog 8-Br-cGMP. We saw no obvious difference between these embryos and wild-type embryos in their ability to stabilize en gene products after hypoxia.
A variety of hypoxia-tolerant organisms rapidly enter reversible quiescence during severe hypoxia. During this quiescence, there is an almost complete suppression of protein turnover and active transport of ions, the major energy-consuming reactions of a cell. Drosophila embryos survive severe hypoxia for a few days (Foe and Alberts, 1985; Wingrove and O’Farrell, 1999), retain substantial ATP levels during at least the early phases of the arrest (DiGregorio et al., 2001), and shut down gene expression and turnover of RNA and proteins (this report). We suggest that the shutdown of gene expression and product turnover is important to energy conservation responses that contribute to survival of hypoxia. We also suggest that this shutdown is mediated by an NO-dependent signal. The conserved involvement of NO in responses to hypoxia and the widespread use of an energy conservation strategy suggest that mechanisms characterized in Drosophila might be widely relevant to surviving hypoxic stress.
Materials and methods
Fly strains, fixation and permeabilization
Wild-type Drosophila embryos were of the Sevelen strain. All embryos were dechorionated in 50% bleach for 2 min. For in situ hybridization and antibody staining, embryos were fixed for 5 min in a two-phase mixture of heptane and 37% formaldehyde, and devitellinized with methanol. When appropriate, embryos were permeabilized for 4 min in octane and resuspended in Schneider’s media supplemented with 0.03% Triton X-100. For western blot analysis, embryos were rapidly fixed by immersion in methanol prior to transfer to sample buffer.
Hypoxia, SNAP, NaCN and PTIO treatments
For all hypoxia treatments, Drosophila embryos were dechorionated and placed into degassed Schneider’s media in 0.75 ml Eppendorf tubes. Tubes were filled with the degassed solution, closed immediately, wrapped tightly in parafilm and put in an N2-sparged glass jar. Normoxic embryos were exposed to room air. For NO treatment, 10 mM SNAP in PBS/0.03% Triton X-100 was used. For NaCN or PTIO treatments, embryos were pre-incubated for 1 h in a 0.2% solution of NaCN or 20 mM PTIO for 2 h and then processed as indicated.
Stabilization of transcripts and gene products by hypoxia
Dechorionated and octane-permeabilized wild-type embryos were incubated in normoxia, 30 min of hypoxia or 30 min in a 10 mM SNAP solution. Embryos were then incubated for an additional 4 h in either 100 µg/ml actinomycin D or 1 mg/ml cycloheximide. After 4 h, all embryos were fixed and processed as appropriate. For CN treatments, embryos where pre-treated for 1 h in 0.2% NaCN, after which actinomycin D or cycloheximide was added and embryos incubated an additional 4 h. When appropriate, hypoxia or 10 mM SNAP pre-treatments were carried out 30 min prior to the addition of 0.2% NaCN. For experiments with the NO scavenger PTIO, embryos were treated with 20 mM PTIO in Schneider’s medium for 2 h under normoxic conditions. Embryos were then split into normoxic and hypoxic batches. For each batch, embryos were incubated for an additional 4 h in PTIO plus either actinomycin D or cycloheximide, or without addition of drugs. At the end of 4 h, all embryos were fixed and analyzed as indicated. To test the viability of embryos after the SNAP treatment, permeabilized embryos were washed in PBS/0.03% Triton X-100 and allowed to recover for 1 h following incubation in a solution of 1 mg/ml BrdU for 20 min, to assess S-phase entry. Also, 100 embryos (three times) were washed from the SNAP solution after 4 h treatment, deposited in standard fly food bottles, incubated at 25°C and the number of adults that eclosed counted.
Heat shock procedures
We collected hs-lacZ embryos and let them age 180 min. To assess whether abrupt hypoxia blocked transcription, we made embryos hypoxic for 30 min, heat shocked them for 45 min, at 37°C, in normoxia or in hypoxia. For NO donor treatments, we pre-incubated embryos with 10 mM SNAP for 30 min and then induced heat shock for 45 min, at 37°C, in the presence of SNAP and room air. After the heat shock, RNA was immediately extracted and analyzed by northern blotting. To determine whether hypoxia or NO blocked accumulation of β-galactosidase, we aged a collection of hs-lacZ embryos for 180 min and heat shocked them for 15 min at 37°C in normoxia, after which embryos were split and subjected to normoxia, hypoxia or 10 mM SNAP. Each sample was incubated for 75 min and fixed immediately afterwards. Where indicated, the hypoxic and SNAP samples were split prior to fixation, and some embryos were released from hypoxia or washed from SNAP solution for another 75 min prior to fixation.
In situ hybridization and antibody staining
Whole-mount in situ hybridization was carried out in fixed embryos essentially as described previously (Tautz and Pfeifle, 1989), but without the proteinase K treatment. Digoxigenin-labeled DNA probes corresponding to the en cDNA were used. Antibody staining was performed according to standard protocols. The rabbit anti-engrailed antibody (C.H.Girdham and P.H.O’Farrell, unpublished data) was diluted 1:150.
RNA preparation and northern blot analysis
Total RNA was isolated from embryos that were aged to 180 min. We used Trizol reagent (Gibco-BRL) to extract total RNA according to the manufacturer’s specifications. Equivalent volumes of total RNA were electrophoresed in a formaldehyde–agarose gel, blotted and UV cross-linked to a Hybond-N+ membrane (Amersham), and incubated with probe overnight at 65°C. Digoxigenin-labeled DNA probes against lacZ and rp49 cDNA were produced and the signal was developed using an anti-DIG–alkaline phosphatase-conjugated immunodetection system (NBT/BCIP precipitate).
Protein preparation and western blot analysis
Protein extracts were prepared by homogenizing 40 embryos in 40 µl of 1% SDS and 0.1% β-mercaptoethanol, and 5 µl of the homogenate were resolved by SDS–PAGE, followed by western blot analysis using a rabbit anti-β-galactosidase antibody (1:15 000; Sigma) and a mouse anti-α-tubulin (1:2000; Sigma) antibody. The blot was developed using ECL reagent (Amersham).
Hatching assay
Dechorionated embryos were washed in PBS/0.03% Triton X-100. To scavenge NO, dechorionated embryos were incubated in 20 mM PTIO for 4 h prior to the hypoxic treatment. Both PTIO-treated and non-treated embryos were subjected to various periods of hypoxia, after which 100 embryos were plated in three different agar plates, incubated for 24–30 h at 25°C and scored for the number of eggs that hatched into larvae. Each measure represents an average of three independent experiments.
Microscopy and software image processing
All images were acquired using an upright fluorescent microscope (Leica DMRD) and recorded using a CCD camera. Images were processed using Adobe Photoshop Version 6.0 and Adobe Illustrator Version 9.0. The graph was plotted using Microsoft Excel.
Acknowledgments
Acknowledgements
We would like to thank Devin Parry, Renny Feldman, Pascale Dijkers and Tin Tin Su for critical reading of the manuscript. R.O.T. is supported by the Fundação para a Ciência e Tecnologia under the Gulbenkian PhD program-Portugal, and the work was supported by NIH GM 37193 and GM 60988.
References
- Akaike T., Yoshida,M., Miyamoto,Y., Sato,K., Kohno,M., Sasamoto,K., Miyazaki,K., Ueda,S. and Maeda,H. (1993) Antagonistic action of imidazolineoxyl N-oxides against endothelium-derived relaxing factor/.NO through a radical reaction. Biochemistry, 32, 827–832. [DOI] [PubMed] [Google Scholar]
- Bruick R.K. and McKnight,S.L. (2001) A conserved family of prolyl-4-hydroxylases that modify HIF. Science, 294, 1337–1340. [DOI] [PubMed] [Google Scholar]
- Cooper C.E. (2002) Nitric oxide and cytochrome oxidase: substrate, inhibitor or effector? Trends Biochem. Sci., 27, 33–39. [DOI] [PubMed] [Google Scholar]
- DiGregorio P.J., Ubersax,J.A. and O’Farrell,P.H. (2001) Hypoxia and nitric oxide induce a rapid, reversible cell cycle arrest of the Drosophila syncytial divisions. J. Biol. Chem., 276, 1930–1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiNardo S., Kuner,J.M., Theis,J. and O’Farrell,P.H. (1985) Development of embryonic pattern in D.melanogaster as revealed by accumulation of the nuclear engrailed protein. Cell, 43, 59–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiNardo S., Heemskerk,J., Dougan,S. and O’Farrell,P.H. (1994) The making of a maggot: patterning the Drosophila embryonic epidermis. Curr. Opin. Genet. Dev., 4, 529–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohoe P.H. and Boutilier,R.G. (1998) The protective effects of metabolic rate depression in hypoxic cold submerged frogs. Respir. Physiol., 111, 325–336. [DOI] [PubMed] [Google Scholar]
- Foe V.E. and Alberts,B.M. (1985) Reversible chromosome condensation induced in Drosophila embryos by anoxia: visualization of interphase nuclear organization. J. Cell Biol., 100, 1623–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs S.M., Becker,A., Hardy,R.W. and Truman,J.W. (2001) Soluble guanylate cyclase is required during development for visual system function in Drosophila. J. Neurosci., 21, 7705–7714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillemin K. and Krasnow,M.A. (1997) The hypoxic response: huffing and HIFing. Cell, 89, 9–12. [DOI] [PubMed] [Google Scholar]
- Heemskerk J., DiNardo,S., Kostriken,R. and O’Farrell,P.H. (1991) Multiple modes of engrailed regulation in the progression towards cell fate determination. Nature, 352, 404–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochachka P.W. (1986) Defense strategies against hypoxia and hypothermia. Science, 231, 234–241. [DOI] [PubMed] [Google Scholar]
- Hochachka P.W. and Lutz,P.L. (2001) Mechanism, origin and evolution of anoxia tolerance in animals. Comp. Biochem. Physiol. B Biochem. Mol. Biol., 130, 435–459. [DOI] [PubMed] [Google Scholar]
- Hochachka P.W., Buck,L.T., Doll,C.J. and Land,S.C. (1996) Unifying theory of hypoxia tolerance: molecular/metabolic defense and rescue mechanisms for surviving oxygen lack. Proc. Natl Acad. Sci. USA, 93, 9493–9498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignarro L.J., Buga,G.M., Wood,K.S., Byrns,R.E. and Chaudhuri,G. (1987) Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc. Natl Acad. Sci. USA, 84, 9265–9269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivan M. et al. (2001) HIFα targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science, 292, 464–468. [DOI] [PubMed] [Google Scholar]
- Kwast K.E. and Hand,S.C. (1996) Acute depression of mitochondrial protein synthesis during anoxia: contributions of oxygen sensing, matrix acidification and redox state. J. Biol. Chem., 271, 7313–7319. [DOI] [PubMed] [Google Scholar]
- Lavista-Llanos S., Centanin,L., Irisarri,M., Russo,D.M., Gleadle,J.M., Bocca,S.N., Muzzopappa,M., Ratcliffe,P.J. and Wappner,P. (2002) Control of the hypoxic response in Drosophila melanogaster by the basic helix–loop–helix PAS protein Similar. Mol. Cell. Biol., 22, 6842–6853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne K.A., Robichon,A., Burgess,E., Butland,S., Shaw,R.A., Coulthard,A., Pereira,H.S., Greenspan,R.J. and Sokolowski,M.B. (1997) Natural behavior polymorphism due to a cGMP-dependent protein kinase of Drosophila. Science, 277, 834–836. [DOI] [PubMed] [Google Scholar]
- Padilla P.A. and Roth,M.B. (2001) Oxygen deprivation causes suspended animation in the zebrafish embryo. Proc. Natl Acad. Sci. USA, 98, 7331–7335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer R.M., Ferrige,A.G. and Moncada,S. (1987) Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature, 327, 524–526. [DOI] [PubMed] [Google Scholar]
- Semenza G.L. (2001a) HIF-1 and mechanisms of hypoxia sensing. Curr. Opin. Cell Biol., 13, 167–171. [DOI] [PubMed] [Google Scholar]
- Semenza G.L. (2001b) HIF-1, O2 and the 3 PHDs: how animal cells signal hypoxia to the nucleus. Cell, 107, 1–3. [DOI] [PubMed] [Google Scholar]
- Shermoen A.W. and O’Farrell,P.H. (1991) Progression of the cell cycle through mitosis leads to abortion of nascent transcripts. Cell, 67, 303–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamler J.S., Jia,L., Eu,J.P., McMahon,T.J., Demchenko,I.T., Bonaventura,J., Gernert,K. and Piantadosi,C.A. (1997) Blood flow regulation by S-nitrosohemoglobin in the physiological oxygen gradient. Science, 276, 2034–2037. [DOI] [PubMed] [Google Scholar]
- Tautz D. and Pfeifle,C. (1989) A non-radioactive in situ hybridization method for the localization of specific RNAs in Drosophila embryos reveals translational control of the segmentation gene hunchback. Chromosoma, 98, 81–85. [DOI] [PubMed] [Google Scholar]
- Vincent J.P. and O’Farrell,P.H. (1992) The state of engrailed expression is not clonally transmitted during early Drosophila development. Cell, 68, 923–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingrove J.A. and O’Farrell,P.H. (1999) Nitric oxide contributes to behavioral, cellular and developmental responses to low oxygen in Drosophila. Cell, 98, 105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalokar M. and Erk,I. (1976) Division and migration of nuclei during early embryogenesis of Drosophila melanogaster. J. Microsc. Biol. Cell., 25, 97–107. [Google Scholar]