Abstract
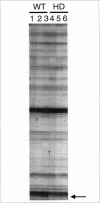
Huntington's disease (HD) is caused by the inheritance of the huntingtin gene with an expanded CAG repeat. The function of the normal or mutant form of the huntingtin protein remains to be determined. We used differential display to determine differences in steady-state mRNA levels between wild-type and the R6/2 transgenic mouse model of HD. Using this method, we determined that the steady-state mRNA levels of protein kinase C beta II (PKC beta II) subunit are decreased in symptomatic HD mice compared with age-matched wild-type controls. The decrease in PKC beta II mRNA levels occurred in both the striatum and cortex. Previously, it had been demonstrated that PKC beta II immunoreactivity is decreased in the caudate-putamen of patients with Huntington's disease. PKC has been implicated in the long-term potentiation model of brain plasticity and learning, and the loss of PKC may affect information storage in HD. The expression of htt-HD throughout the brain affects the transcription of specific genes in regions not associated with widespread neurodegeneration.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arzberger T., Krampfl K., Leimgruber S., Weindl A. Changes of NMDA receptor subunit (NR1, NR2B) and glutamate transporter (GLT1) mRNA expression in Huntington's disease--an in situ hybridization study. J Neuropathol Exp Neurol. 1997 Apr;56(4):440–454. doi: 10.1097/00005072-199704000-00013. [DOI] [PubMed] [Google Scholar]
- Augood S. J., Faull R. L., Emson P. C. Dopamine D1 and D2 receptor gene expression in the striatum in Huntington's disease. Ann Neurol. 1997 Aug;42(2):215–221. doi: 10.1002/ana.410420213. [DOI] [PubMed] [Google Scholar]
- Bank B., DeWeer A., Kuzirian A. M., Rasmussen H., Alkon D. L. Classical conditioning induces long-term translocation of protein kinase C in rabbit hippocampal CA1 cells. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1988–1992. doi: 10.1073/pnas.85.6.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhide P. G., Day M., Sapp E., Schwarz C., Sheth A., Kim J., Young A. B., Penney J., Golden J., Aronin N. Expression of normal and mutant huntingtin in the developing brain. J Neurosci. 1996 Sep 1;16(17):5523–5535. doi: 10.1523/JNEUROSCI.16-17-05523.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibb J. A., Yan Z., Svenningsson P., Snyder G. L., Pieribone V. A., Horiuchi A., Nairn A. C., Messer A., Greengard P. Severe deficiencies in dopamine signaling in presymptomatic Huntington's disease mice. Proc Natl Acad Sci U S A. 2000 Jun 6;97(12):6809–6814. doi: 10.1073/pnas.120166397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caine E. D., Ebert M. H., Weingartner H. An outline for the analysis of dementia. The memory disorder of Huntingtons disease. Neurology. 1977 Nov;27(11):1087–1092. doi: 10.1212/wnl.27.11.1087. [DOI] [PubMed] [Google Scholar]
- Caine E. D., Shoulson I. Psychiatric syndromes in Huntington's disease. Am J Psychiatry. 1983 Jun;140(6):728–733. doi: 10.1176/ajp.140.6.728. [DOI] [PubMed] [Google Scholar]
- Carter R. J., Lione L. A., Humby T., Mangiarini L., Mahal A., Bates G. P., Dunnett S. B., Morton A. J. Characterization of progressive motor deficits in mice transgenic for the human Huntington's disease mutation. J Neurosci. 1999 Apr 15;19(8):3248–3257. doi: 10.1523/JNEUROSCI.19-08-03248.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha J. H., Kosinski C. M., Kerner J. A., Alsdorf S. A., Mangiarini L., Davies S. W., Penney J. B., Bates G. P., Young A. B. Altered brain neurotransmitter receptors in transgenic mice expressing a portion of an abnormal human huntington disease gene. Proc Natl Acad Sci U S A. 1998 May 26;95(11):6480–6485. doi: 10.1073/pnas.95.11.6480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denovan-Wright E. M., Robertson H. A. Cannabinoid receptor messenger RNA levels decrease in a subset of neurons of the lateral striatum, cortex and hippocampus of transgenic Huntington's disease mice. Neuroscience. 2000;98(4):705–713. doi: 10.1016/s0306-4522(00)00157-3. [DOI] [PubMed] [Google Scholar]
- Glass M., Faull R. L., Dragunow M. Loss of cannabinoid receptors in the substantia nigra in Huntington's disease. Neuroscience. 1993 Oct;56(3):523–527. doi: 10.1016/0306-4522(93)90352-g. [DOI] [PubMed] [Google Scholar]
- Hashimoto T., Kitamura N., Saito N., Komure O., Nishino N., Tanaka C. The loss of beta II-protein kinase C in the striatum from patients with Huntington's disease. Brain Res. 1992 Jul 10;585(1-2):303–306. doi: 10.1016/0006-8993(92)91224-3. [DOI] [PubMed] [Google Scholar]
- Ludlow C. L., Connor N. P., Bassich C. J. Speech timing in Parkinson's and Huntington's disease. Brain Lang. 1987 Nov;32(2):195–214. doi: 10.1016/0093-934x(87)90124-6. [DOI] [PubMed] [Google Scholar]
- Mangiarini L., Sathasivam K., Seller M., Cozens B., Harper A., Hetherington C., Lawton M., Trottier Y., Lehrach H., Davies S. W. Exon 1 of the HD gene with an expanded CAG repeat is sufficient to cause a progressive neurological phenotype in transgenic mice. Cell. 1996 Nov 1;87(3):493–506. doi: 10.1016/s0092-8674(00)81369-0. [DOI] [PubMed] [Google Scholar]
- Metzler M., Chen N., Helgason C. D., Graham R. K., Nichol K., McCutcheon K., Nasir J., Humphries R. K., Raymond L. A., Hayden M. R. Life without huntingtin: normal differentiation into functional neurons. J Neurochem. 1999 Mar;72(3):1009–1018. doi: 10.1046/j.1471-4159.1999.0721009.x. [DOI] [PubMed] [Google Scholar]
- Penney J. B., Jr, Young A. B. Quantitative autoradiography of neurotransmitter receptors in Huntington disease. Neurology. 1982 Dec;32(12):1391–1395. doi: 10.1212/wnl.32.12.1391. [DOI] [PubMed] [Google Scholar]
- Perutz M. F. Glutamine repeats and inherited neurodegenerative diseases: molecular aspects. Curr Opin Struct Biol. 1996 Dec;6(6):848–858. doi: 10.1016/s0959-440x(96)80016-9. [DOI] [PubMed] [Google Scholar]
- Perutz M. F. Glutamine repeats as polar zippers: their role in inherited neurodegenerative disease. Mol Med. 1995 Nov;1(7):718–721. [PMC free article] [PubMed] [Google Scholar]
- Perutz M. F., Johnson T., Suzuki M., Finch J. T. Glutamine repeats as polar zippers: their possible role in inherited neurodegenerative diseases. Proc Natl Acad Sci U S A. 1994 Jun 7;91(12):5355–5358. doi: 10.1073/pnas.91.12.5355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perutz M. F. Polar zippers: their role in human disease. Pharm Acta Helv. 1995 Mar;69(4):213–224. doi: 10.1016/0031-6865(95)00003-r. [DOI] [PubMed] [Google Scholar]
- Richfield E. K., Herkenham M. Selective vulnerability in Huntington's disease: preferential loss of cannabinoid receptors in lateral globus pallidus. Ann Neurol. 1994 Oct;36(4):577–584. doi: 10.1002/ana.410360406. [DOI] [PubMed] [Google Scholar]
- Richfield E. K., O'Brien C. F., Eskin T., Shoulson I. Heterogeneous dopamine receptor changes in early and late Huntington's disease. Neurosci Lett. 1991 Oct 28;132(1):121–126. doi: 10.1016/0304-3940(91)90448-3. [DOI] [PubMed] [Google Scholar]
- Ross C. A. When more is less: pathogenesis of glutamine repeat neurodegenerative diseases. Neuron. 1995 Sep;15(3):493–496. doi: 10.1016/0896-6273(95)90138-8. [DOI] [PubMed] [Google Scholar]
- Sharp A. H., Loev S. J., Schilling G., Li S. H., Li X. J., Bao J., Wagster M. V., Kotzuk J. A., Steiner J. P., Lo A. Widespread expression of Huntington's disease gene (IT15) protein product. Neuron. 1995 May;14(5):1065–1074. doi: 10.1016/0896-6273(95)90345-3. [DOI] [PubMed] [Google Scholar]
- Stott K., Blackburn J. M., Butler P. J., Perutz M. Incorporation of glutamine repeats makes protein oligomerize: implications for neurodegenerative diseases. Proc Natl Acad Sci U S A. 1995 Jul 3;92(14):6509–6513. doi: 10.1073/pnas.92.14.6509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trottier Y., Devys D., Imbert G., Saudou F., An I., Lutz Y., Weber C., Agid Y., Hirsch E. C., Mandel J. L. Cellular localization of the Huntington's disease protein and discrimination of the normal and mutated form. Nat Genet. 1995 May;10(1):104–110. doi: 10.1038/ng0595-104. [DOI] [PubMed] [Google Scholar]
- Van der Zee E. A., Luiten P. G., Disterhoft J. F. Learning-induced alterations in hippocampal PKC-immunoreactivity: a review and hypothesis of its functional significance. Prog Neuropsychopharmacol Biol Psychiatry. 1997 Apr;21(3):531–572. doi: 10.1016/s0278-5846(97)00017-1. [DOI] [PubMed] [Google Scholar]
- White J. K., Auerbach W., Duyao M. P., Vonsattel J. P., Gusella J. F., Joyner A. L., MacDonald M. E. Huntingtin is required for neurogenesis and is not impaired by the Huntington's disease CAG expansion. Nat Genet. 1997 Dec;17(4):404–410. doi: 10.1038/ng1297-404. [DOI] [PubMed] [Google Scholar]
- Young A. B., Penney J. B., Starosta-Rubinstein S., Markel D. S., Berent S., Giordani B., Ehrenkaufer R., Jewett D., Hichwa R. PET scan investigations of Huntington's disease: cerebral metabolic correlates of neurological features and functional decline. Ann Neurol. 1986 Sep;20(3):296–303. doi: 10.1002/ana.410200305. [DOI] [PubMed] [Google Scholar]