Abstract
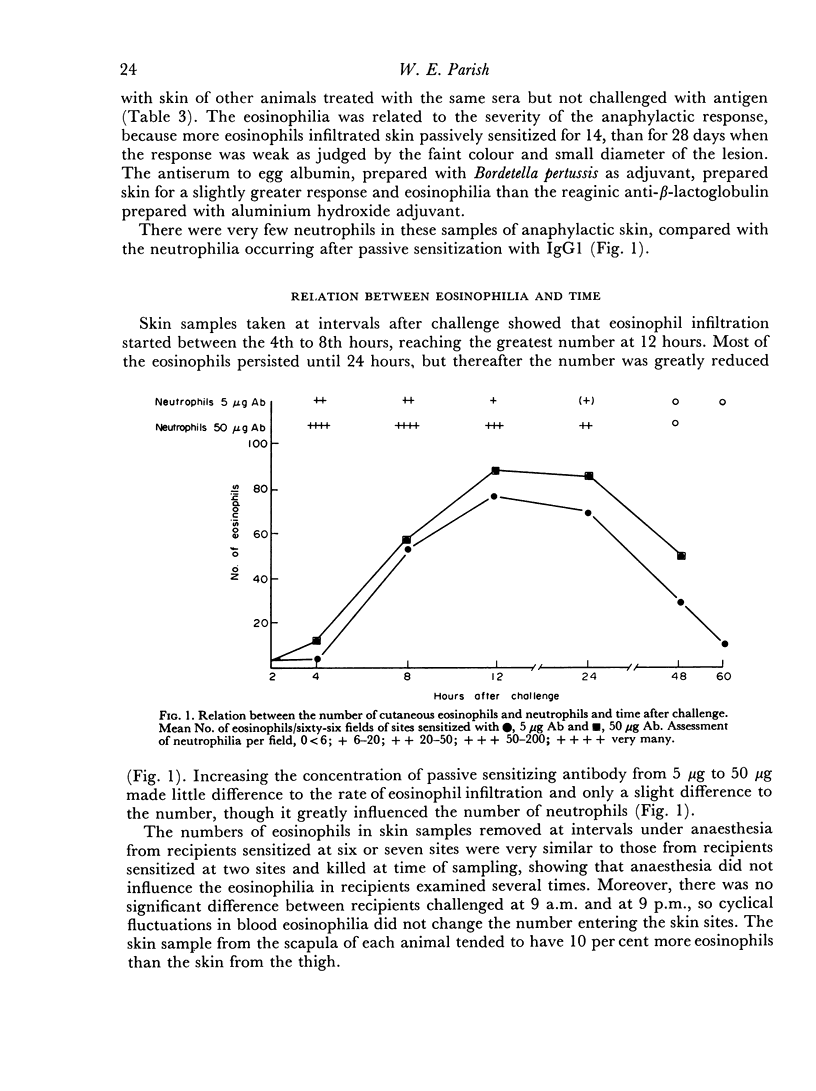
More eosinophils accumulate in sites of cutaneous anaphylaxis mediated by homologous IgG1 or reagin, than in skin treated with IgG2, 18 hours after intravenous challenge. The increased number of eosinophils becomes apparent at 4 hours and reaches a maximum at 12–24 hours, whereas neutrophils infiltrate skin sensitized with IgG1 within 15 minutes of challenge and are most numerous at 4–8 hours. There is a much higher ratio of eosinophils to neutrophils in skin passively sensitized with reagin and challenged after 14 or 28 days.
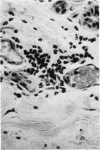
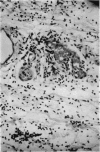
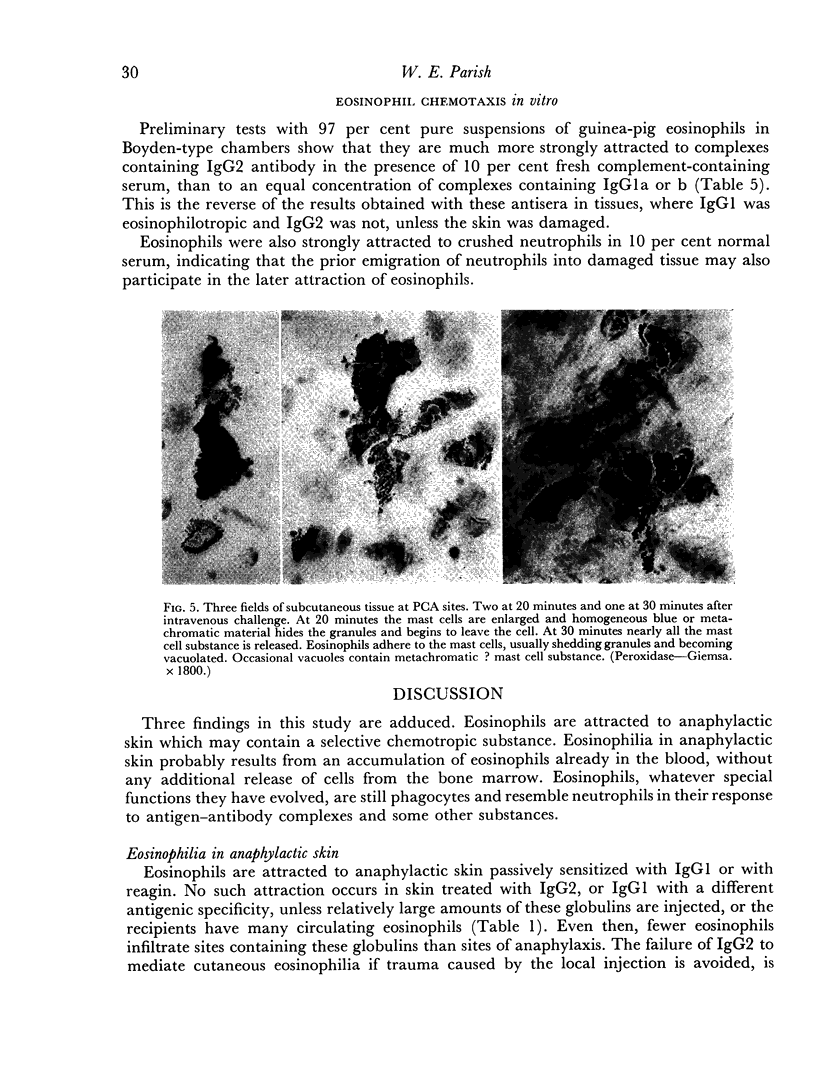
In anaphylactic skin, eosinophils accumulate round changed mast cells.
The numbers of eosinophils in anaphylactic skin reflect the numbers in the blood when challenged, and no increase in the number of haematogenous eosinophils occurs between the time of challenge and sampling.
The behaviour of eosinophils in vitro appears to differ from that in vivo, in that they, like neutrophils, are attracted more strongly to complexes containing IgG2 than IgG1 antibody. They are also attracted to damaged neutrophils.
It is suggested that eosinophils are not selectively attracted to sites of cutaneous anaphylaxis, but enter them with the neutrophils in the relative proportions in which they are present in the blood. They are however selectively retained in anaphylactic, or anaphylactoid tissue, while the neutrophils continue to emigrate.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLOCH K. J., KOURILSKY F. M., OVARY Z., BENACERRAF B. Properties of guinea pig 7S antibodies. III. Identification of antibodies involved in complement fixation and hemolysis. J Exp Med. 1963 Jun 1;117:965–981. doi: 10.1084/jem.117.6.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOYDEN S. The chemotactic effect of mixtures of antibody and antigen on polymorphonuclear leucocytes. J Exp Med. 1962 Mar 1;115:453–466. doi: 10.1084/jem.115.3.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day R. P. Eosinophil cell separation from human peripheral blood. Immunology. 1970 Jun;18(6):955–959. [PMC free article] [PubMed] [Google Scholar]
- EIDINGER D., WILKINSON R., ROSE B. A STUDY OF CELLULAR RESPONSES IN IMMUNE REACTIONS UTILIZING THE SKIN WINDOW TECHNIQUE. I. IMMEDIATE HYPERSENSITIVITY REACTIONS. J Allergy. 1964 Jan-Feb;35:77–85. doi: 10.1016/0021-8707(64)90052-8. [DOI] [PubMed] [Google Scholar]
- Fowler J. W., 3rd, Lowell V. C. The accumulation of eosinophils as an allergic response to allergen applied to the denuded skin surface. J Allergy. 1966 Jan;37(1):19–28. doi: 10.1016/0021-8707(66)90106-7. [DOI] [PubMed] [Google Scholar]
- Kay A. B. Studies on eosinophil leucocyte migration. I. Eosinophil and neutrophil accumulation following antigen-antibody reactions in guinea-pig skin. Clin Exp Immunol. 1970 Jan;6(1):75–86. [PMC free article] [PubMed] [Google Scholar]
- Keller H. U., Nussenzweig V., Sorkin E. Studies on chemotaxis. 8. The role of 7S-gamma-1 and 7S-gamma-2 guinea-pig antibodies for chemotaxis in granulocytes. Immunochemistry. 1968 May;5(3):293–295. doi: 10.1016/0019-2791(68)90073-6. [DOI] [PubMed] [Google Scholar]
- Lachmann P. J., Kay A. B., Thompson R. A. The chemotactic activity for neutrophil and eosinophil leucocytes of the trimolecular complex of the fifth, sixth and seventh components of human complement (C567) prepared in free solution by the 'reactive lysis' procedure. Immunology. 1970 Dec;19(6):895–899. [PMC free article] [PubMed] [Google Scholar]
- Lieberman M. K., Ovary Z. Histology of PCA reactions in guinea pigs. A comparison of reactions produced by guinea pig gamma-G1 and rabbit antibodies. J Immunol. 1968 Jan;100(1):159–168. [PubMed] [Google Scholar]
- MOTA I., VUGMAN I. Effects of anaphylactic shock and compound 48/80 on the mast cells of the guinea pig lung. Nature. 1956 Mar 3;177(4505):427–429. doi: 10.1038/177427a0. [DOI] [PubMed] [Google Scholar]
- Mann P. R. An electron-microscope study of the relations between mast cells and eosinophil leucocytes. J Pathol. 1969 Jul;98(3):183–186. doi: 10.1002/path.1710980304. [DOI] [PubMed] [Google Scholar]
- Mota I., Perini A. A heat labile mercaptoethanol susceptible homocytotropic antibody in the guinea pig. Life Sci II. 1970 Aug 22;9(16):923–930. doi: 10.1016/0024-3205(70)90063-9. [DOI] [PubMed] [Google Scholar]
- Parish W. E. Eosinophilia. I. Eosinophilia in guinea-pigs mediated by passive anaphylaxis and by antigen-antibody complexes containing homologous IgG1a and IgG1b. Immunology. 1972 Jun;22(6):1087–1098. [PMC free article] [PubMed] [Google Scholar]
- Parish W. E. Homologous serum passive cutaneous anaphylaxis in guinea pigs mediated by two gamma-1 or gamma-1-type heat-stable globulins and a non-gamma-1 heat-labile reagin. J Immunol. 1970 Nov;105(5):1296–1298. [PubMed] [Google Scholar]
- WELSH R. A., GEER J. C. Phagocytosis of mast cell granule by the eosinophilic leukocyte in the rat. Am J Pathol. 1959 Jan-Feb;35(1):103–111. [PMC free article] [PubMed] [Google Scholar]
- Ward P. A. Chemotaxis of human eosinophils. Am J Pathol. 1969 Jan;54(1):121–128. [PMC free article] [PubMed] [Google Scholar]
- Zolov D. M., Levine B. B. Correlation of blood eosinophilia with antibody classes. Studies with the penicillin hypersensitivity system. Int Arch Allergy Appl Immunol. 1969;35(2):179–193. doi: 10.1159/000230170. [DOI] [PubMed] [Google Scholar]