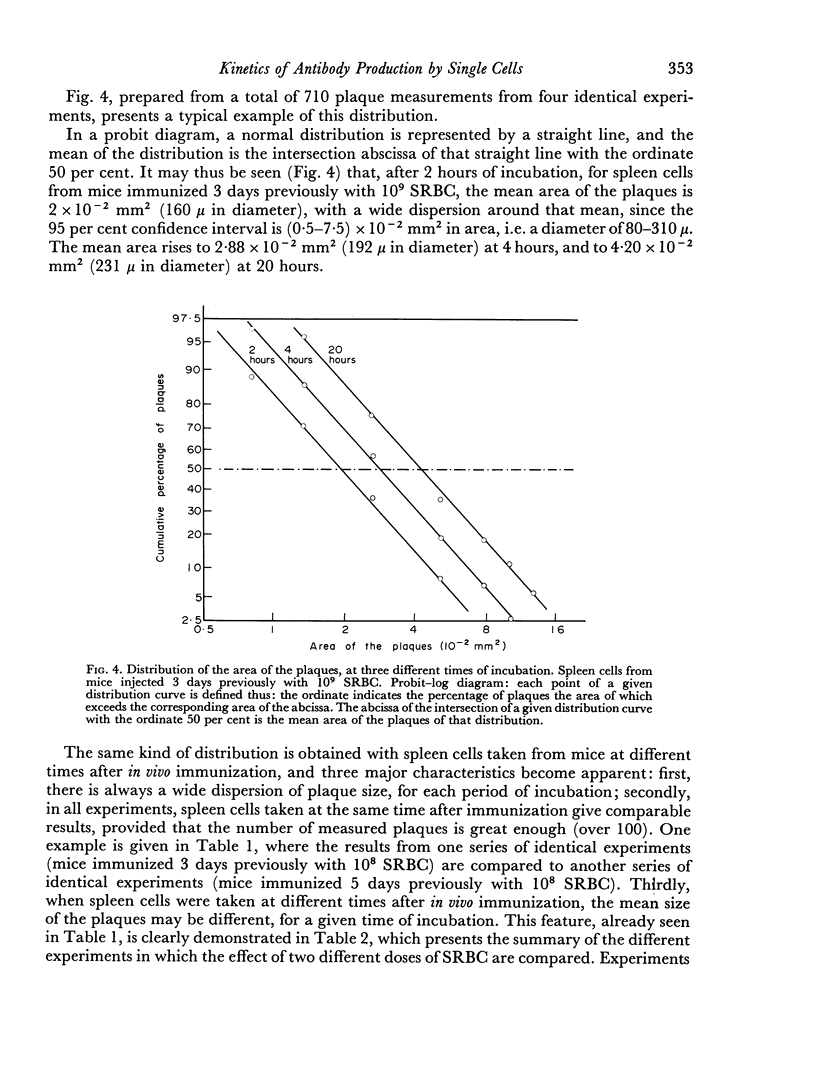
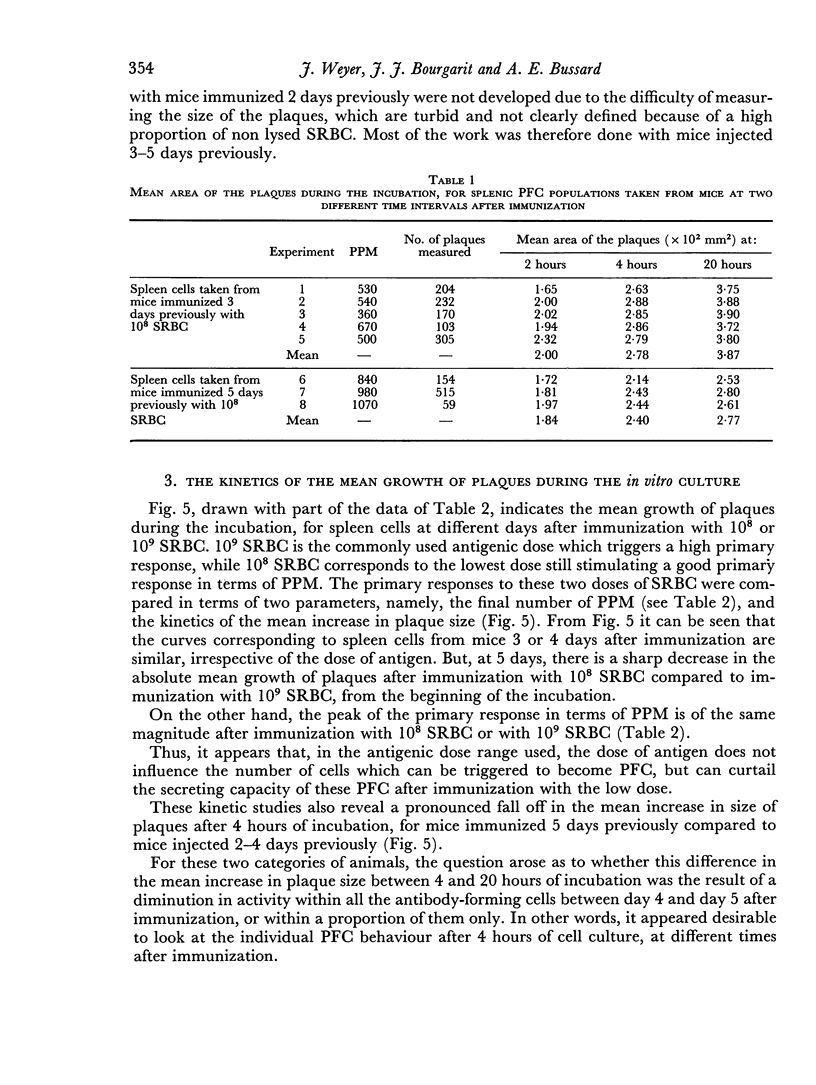
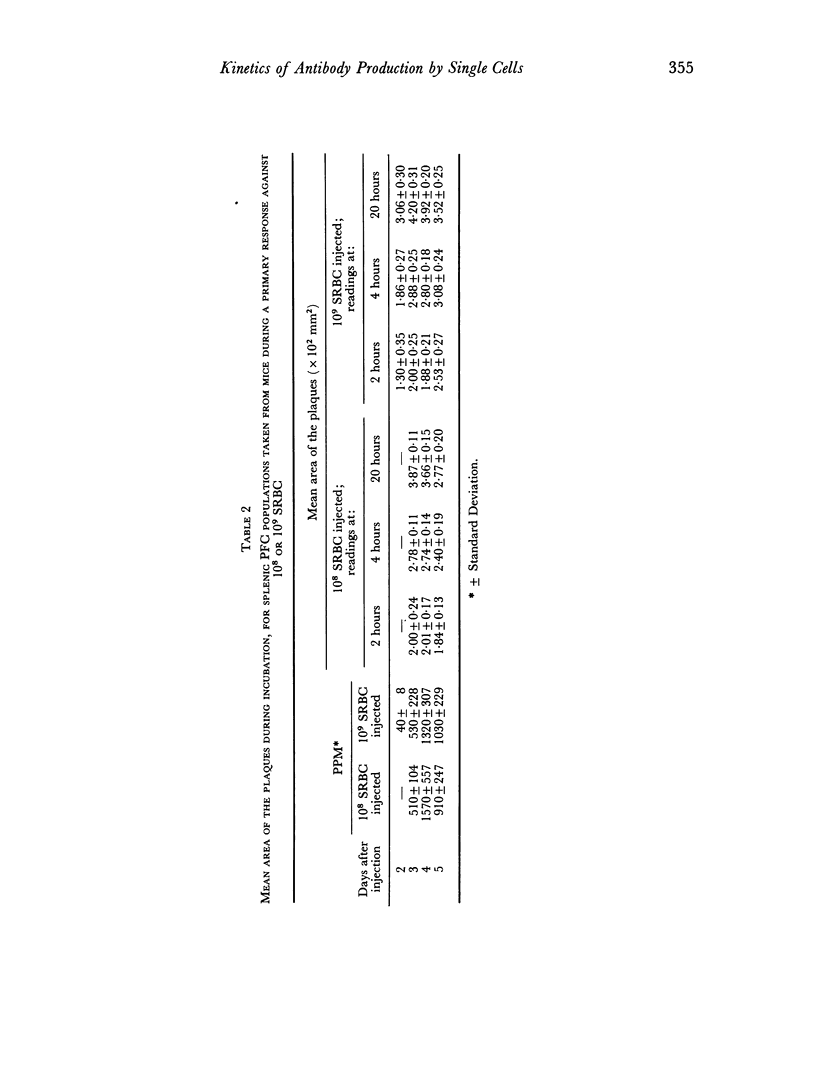
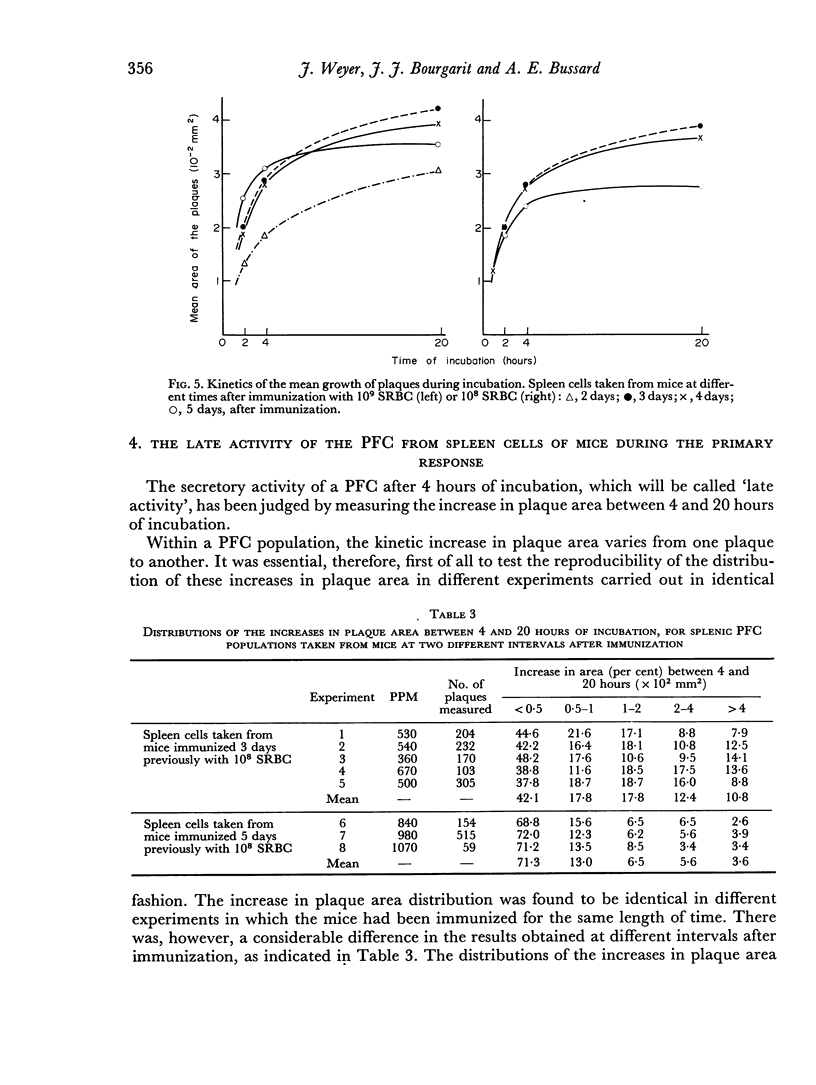
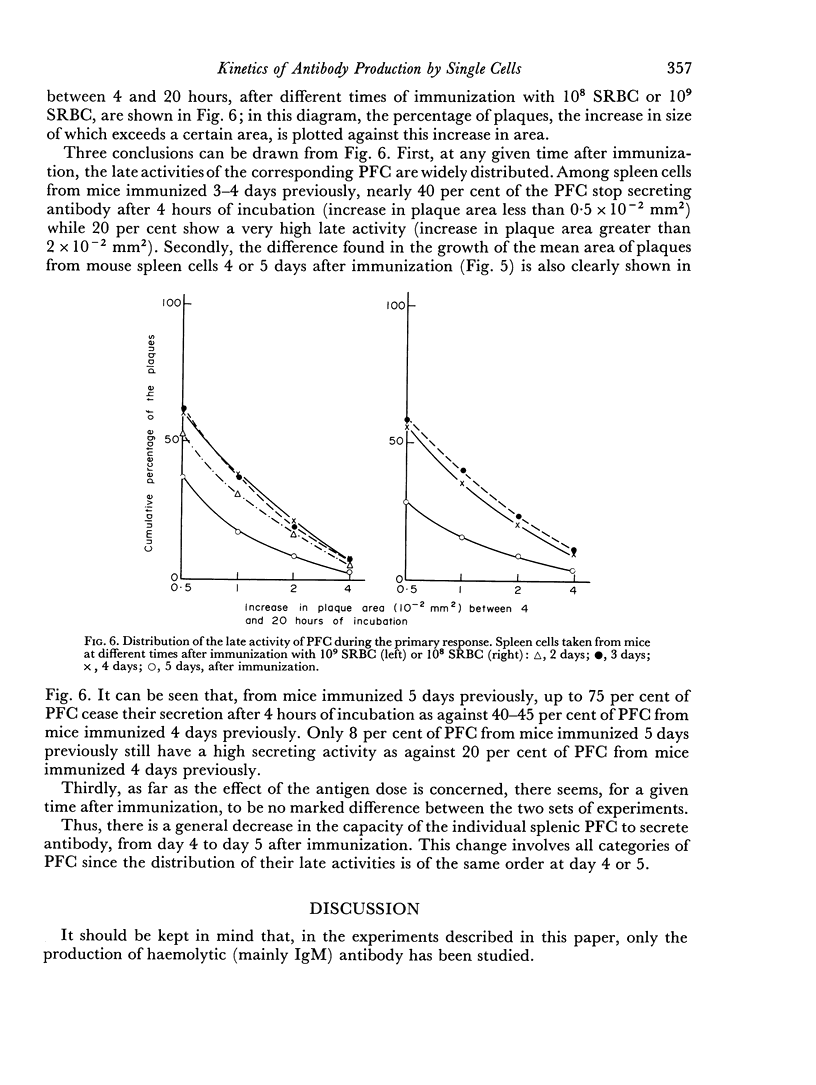
Abstract
The primary antibody response of the IgM type against sheep red blood cells, by spleen cells obtained from mice immunized in vivo has been studied at the cellular level. The modified plaque assay technique which was used allows the quantitative recording of plaque formation and growth in a carboxymethylcellulose gel. The distribution of plaque size at different times of incubation indicates that the secretion rate and the final amount of antibody released differ considerably between individual PFC of the same splenic population. However, the mean sizes of plaques from splenic populations at the same time after immunization are similar from mouse to mouse. During in vitro incubation, the kinetics of plaque growth display characteristic features, thus differentiating PFC populations from mice examined at different times after in vivo immunization. Most notably, there is a decrease in the mean size of the plaques between day 4 and day 5 following immunization. These results are discussed in terms of antibody secretion by PFC populations during the primary response.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boris S., Bussard A. E., Deutsch S., Nossal G. J. In vitro stimulation of antibody formation by peritoneal cells. 3. Effect of active immunization on the subsequent in vitro performance of peritoneal and spleen cells. Immunology. 1970 Nov;19(5):743–757. [PMC free article] [PubMed] [Google Scholar]
- Bussard A. E., Nossal G. J., Mazie J. C., Lewis H. In vitro stimulation of antibody formation by peritoneal cells. II. Cell interactions and effects of immunochemical or metabolic inhibitors. J Exp Med. 1970 May 1;131(5):917–935. doi: 10.1084/jem.131.5.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EISEN H. N., SISKIND G. W. VARIATIONS IN AFFINITIES OF ANTIBODIES DURING THE IMMUNE RESPONSE. Biochemistry. 1964 Jul;3:996–1008. doi: 10.1021/bi00895a027. [DOI] [PubMed] [Google Scholar]
- Humphrey J. H., Dourmashkin R. R. The lesions in cell membranes caused by complement. Adv Immunol. 1969;11:75–115. doi: 10.1016/s0065-2776(08)60478-2. [DOI] [PubMed] [Google Scholar]
- INGRAHAM J. S., BUSSARD A. APPLICATION OF A LOCALIZED HEMOLYSIN REACTION FOR SPECIFIC DETECTION OF INDIVIDUAL ANTIBODY-FORMING CELLS. J Exp Med. 1964 Apr 1;119:667–684. doi: 10.1084/jem.119.4.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- INGRAHAM J. [Individual identification of antibody-producing cells by a local hemolytic reaction]. C R Hebd Seances Acad Sci. 1963 Jun 5;256:5005–5008. [PubMed] [Google Scholar]
- JERNE N. K., NORDIN A. A. Plaque formation in agar by single antibody-producing cells. Science. 1963 Apr 26;140(3565):405–405. [PubMed] [Google Scholar]
- Merchant B., Petersen B. Comparative sensitivities of the hemolytic plaque-in-agar and microchamber assays for detection of rabbit immune cells. J Immunol. 1968 Nov;101(5):860–867. [PubMed] [Google Scholar]
- Nossal G. J., Bussard A. E., Lewis H., Mazie J. C. In vitro stimulation of antibody formation by peritoneal cells. I. Plaque technique of high sensitivity enabling access to the cells. J Exp Med. 1970 May 1;131(5):894–916. doi: 10.1084/jem.131.5.894. [DOI] [PMC free article] [PubMed] [Google Scholar]