Abstract
Lytic infection of African green monkey kidney (CV-1) cells by simian virus 40 (SV40) is characterized by stimulation of DNA synthesis leading to bypass of mitosis and replication of cellular and viral DNA beyond a 4C DNA content. To define mechanisms underlying the absence of mitosis, the expression levels of upstream regulatory molecules of mitosis-promoting factor (MPF) were compared in parallel synchronized cultures of SV40-infected and uninfected CV-1 cells. The DNA replication/damage checkpoint kinase Chk1 was phosphorylated in both uninfected and SV40-infected cultures arrested at G1/S by mimosine, consistent with checkpoint activation. Following release of uninfected cultures from G1/S, Chk1 phosphorylation was lost even though Chk1 protein levels were retained. In contrast, G1/S-released SV40-infected cultures exhibited dephosphorylation of Chk1 in S phase, followed by an increase in Chk1 phosphorylation coinciding with entry of infected cells into >G2. Inhibitors of Chk1, UCN-01 and caffeine, induced mitosis and abnormal nuclear condensation and increased the protein kinase activity of MPF in SV40-infected CV-1 cells. These results demonstrate that SV40 lytic infection triggers components of a DNA damage checkpoint pathway. In addition, chemical inhibition of Chk1 activity suggests that Chk1 contributes to the absence of mitosis during SV40 lytic infection.
When a quiescent monolayer of CV-1 (African green monkey kidney) cells is infected with simian virus 40 (SV40), the cells are induced into the cell cycle. The infected cells progress through G1, S, and G2 phases but do not enter mitosis (17, 35, 58). Rather, the DNA content of the infected cells increases beyond 4C (defined as >G2 phase) because of replication of both viral and cellular DNA. By 48 h postinfection (hpi), the total DNA content per cell has increased to 10C to 12C. The majority of viral DNA is replicated in >G2 phase and accounts for 20 to 30% of the total cellular DNA content (34). Progression into >G2 involves both of the early gene products of SV40. Large-tumor antigen (large T) is essential for viral DNA replication (65) and for progression into >G2 phase (11, 12). Small-tumor antigen (small t) is not required for SV40 DNA replication, but the rate of viral DNA replication (8, 16, 60) and the rate of entry into >G2 are slower in the absence of small t (70).
One goal in understanding the altered cell cycle regulation during SV40 lytic infection is to define the mechanisms responsible for the absence of mitosis in infected cells. Mitosis in uninfected proliferating cells is controlled by mitosis-promoting factor (MPF), a heterodimer of cyclin B and the cyclin-dependent kinase Cdc2 (62, 64). The ability of MPF to induce mitosis is regulated by the nuclear-cytoplasmic localization of the cyclin B subunit and phosphorylation of Cdc2. During interphase, cyclin B is actively transported out of the nucleus by a CRM1-mediated export mechanism while the Wee1 and Myt1 kinases phosphorylate the T14 and Y15 residues of Cdc2 to inhibit catalytic activity. During mitotic initiation, cyclin B1 is phosphorylated by Plk1 and localizes in the nucleus during prophase. MPF is activated when the T14 and Y15 residues of Cdc2 are dephosphorylated by the dual-specificity phosphatases Cdc25C and Cdc25B. The phosphatase activity of Cdc25C is positively regulated by phosphorylation at a number of amino-terminal sites that can be dephosphorylated through the action of protein phosphatase 2A (PP2A) (27). At the beginning of mitosis, increased phosphorylation of Cdc25C results from increased phosphorylation by Polo-like kinase 1 (Plk1) and decreased dephosphorylation by PP2A. Active Cdc25C then dephosphorylates T14 and Y15 of Cdc2, resulting in activation of MPF. This active MPF, in turn, further phosphorylates and activates both Cdc25C and Plk1 to create a positive feedback loop. Because of this feedback loop, it is critical to maintain MPF and all of its positive regulators in their inactive states to prevent premature mitosis during interphase.
The onset of mitosis, which is regulated by MPF, is tightly controlled. Untimely initiation of mitosis may result in genomic damage, which may potentially lead to uncontrolled cell proliferation (tumorigenesis) or cell death. DNA damage checkpoint pathways hold MPF in an inactive form and prevent the distribution of damaged DNA to daughter cells (2). Maintenance of the inhibitory phosphorylation of the Cdc2 subunit is dependent on the inhibition of Cdc25C activity by the upstream protein kinases Chk1 and Chk2 (3, 14, 56, 75). Chk1 is a DNA damage checkpoint kinase conserved in yeast, Xenopus, and mammalian cells. Chk1 is phosphorylated in response to DNA damage caused by ionizing radiation (IR), UV light, or incomplete DNA replication caused by hydroxyurea (36). In mammalian cells, this activation of Chk1 requires phosphorylation of S345 by ATR (ATM [ataxia telangiectasia mutated] and Rad3 related), a member of the phosphatidylinositol-3-kinase family (77). Activated Chk1 phosphorylates the S216 residue of Cdc25C, which results in its nuclear export and preferential binding to 14-3-3 over cyclin B in the cytoplasm (43, 50). S216 is distinct from the amino-terminal phosphorylation sites critical to Cdc25C activation. The cytoplasmic localization of Cdc25C denies access to its substrate, the Cdc2 subunit, and prevents cells from going into mitosis by keeping MPF inactive, thus arresting cells at G2. Another DNA damage checkpoint kinase, Chk2/Cds1, is activated through phosphorylation of T68 by ATM, a member of the phosphatidylinositol-3-kinase family. Activated Chk2 also phosphorylates S216 of Cdc25C and inhibits MPF activity (7).
Previous studies demonstrated that the absence of mitosis in SV40 lytic infection correlates with a significant reduction in the protein kinase activity of MPF. Although the cyclin B/Cdc2 complex was present in infected cells progressing from G2 to >G2, the Cdc2 subunit was in the tyrosine-phosphorylated, inactive form (58). The capacity of SV40-infected cells to express some elements of the mitotic phenotype was demonstrated by induction of the mitotic marker MPM-2 following addition of okadaic acid, a PP2A inhibitor (13). In this study, the expression levels of upstream regulatory molecules of MPF were compared in parallel synchronized cultures of SV40-infected and uninfected CV-1 cells. The results demonstrated that SV40 lytic infection induces phosphorylation of Chk1, a component of DNA damage checkpoint pathways. In addition, chemical inhibition of Chk1 activity suggests that Chk1 contributes to the absence of mitosis during SV40 lytic infection.
MATERIALS AND METHODS
Cells and virus.
African green monkey kidney (CV-1) cells (ATCC CCL70) were grown on tissue culture dishes in minimal essential medium (MEM) containing 2× amino acids and vitamins and 5% fetal bovine serum (FBS; Gibco BRL, Grand Island, N.Y.). All cultures were maintained at 37°C in a humidified 5% CO2 incubator. SV40 wild-type (wt) strain RH911 and small t mutant strain dl888 were grown and plaque assayed on CV-1 cells as previously described (35, 70). The SV40 small t mutant dl888 was provided by Kathleen Rundell.
SV40 infection.
Confluent cultures of CV-1 cells were infected with wild-type (wt) or dl888 mutant SV40 at 100 PFU per cell and incubated with fresh MEM plus 1% FBS. In parallel, confluent cultures of uninfected cells were trypsinized and replated with fresh MEM plus 10% FBS. At 6 h after the infection (or trypsinization), cells were synchronized at the G1/S phase border with 0.2 mM mimosine (Sigma, St. Louis, Mo.) for 18 h. The cells were released from mimosine by replacing the medium with fresh MEM plus 5% FBS. The cells were harvested at the time indicated in each experiment.
Caffeine and UCN-01 treatments.
CV-1 cells were infected with SV40 and treated with mimosine, as described above, prior to addition of caffeine (Sigma) or UCN-01 (NSC 638850, the Drug Synthesis and Chemistry Branch, Developmental Therapeutics Program, Division of Cancer Treatment and Diagnosis, National Cancer Institute, National Institutes of Health). Caffeine at 6 mM was added at 3 h after release from mimosine. Addition of 300 nM UCN-01 was done at 6 h after release from mimosine. The cells were harvested at the times indicated in each experiment. The concentrations of caffeine and UCN-01 used were determined to be optimal for induction of mitotic markers in preliminary assays comparing a range of concentrations. In indicated experiments, nocodazole (Sigma) was added simultaneously with UCN-01 or caffeine to block cells in mitosis. A portion of floating cells was set aside to measure the extent of cell detachment. Attached cells were trypsinized and pooled with floating cells. Cells were then counted and used for Western blot analysis of phospho-histone H3, Ser10 (P-H3).
Antibodies.
Antibodies were obtained from the following sources: anti-Cdc25C mouse monoclonal immunoglobulin G (IgG1κ) (25C14), NeoMarkers (Fremont, Calif.); anti-Cdc25B mouse monoclonal IgG1, Transduction Laboratory (Lexington, Ky.); anti-phospho-Chk1 (Ser345) rabbit polyclonal IgG and anti-phospho-Chk2 (Thr68) rabbit IgG, Cell Signaling Technology (Beverly, Mass.); anti-MPM2 (mitotic protein monoclonal antibody 2) mouse monoclonal IgG1, anti-Cds1/Chk2 rabbit polyclonal IgG, anti-histone 3 mouse monoclonal IgG1κ, and anti-P-H3 rabbit polyclonal IgG, Upstate Biotechnology (Lake Placid, N.Y.); anti-Wee1 rabbit polyclonal IgG, Calbiochem (San Diego, Calif.); anti-Plk1 mouse monoclonal antibody cocktail of IgG1 and IgG2b, Zymed (San Francisco, Calif.); anti-Chk1 mouse monoclonal IgG2a (G-4), anti-MPP2 rabbit polyclonal IgG (C-20), anti-Cdc2 mouse monoclonal IgG2a (no. 17), and anti-Cks1 rabbit polyclonal IgG (FL-79), Santa Cruz Biotechnology (Santa Cruz, Calif.). Anti-SV40 tumor antigen monoclonal antibodies PAb101 and Pab108 were obtained from the hybridoma cell lines ATCC TIB117 and ATCC TIB230, respectively. PAb101 recognizes an epitope near the C terminus of large T, but not small t. PAb108 recognizes the N termini of both tumor antigens.
Flow cytometry.
CV-1 cells were prepared for flow cytometry as previously described (29). Briefly, for large T staining and DNA content, fixed cells were rinsed once in phosphate-buffered saline (PBS) and incubated with Pab101 in wash solution (10% goat serum, 0.002% Triton X-100, 0.1% Na azide in PBS) overnight at 4°C. The cells were rinsed twice in wash solution and incubated with a solution containing goat anti-mouse Alexa 488 secondary antibody (Molecular Probes, Eugene, Oreg.) for 2 h at 4°C. The cells were then rinsed twice in wash solution and stained with propidium iodide (50 μg/ml in PBS; Calbiochem, La Jolla, Calif.) and RNase A (500 μg/ml in PBS). Cells were analyzed by using a procedure described previously (29, 70). Analysis was carried out with a Cytofluorograph model 50 H-H flow cytometer with an argon laser at a wavelength of 488 nm and 20 mW of power. Twenty thousand cells were analyzed at each time point, and the Cyclops analysis program (Cytomation, Ft. Collins, Colo.) was used for data analysis.
Immunofluorescence assay.
Cells fixed on coverslips with acetone-methanol (7:3; −20°C) were subjected to immunofluorescence staining with antibodies against SV40 large T and P-H3. They were incubated for 1 h with a 1:25 dilution of anti-SV40 large T monoclonal antibody PAb101 and/or 20 μg of anti-P-H3 rabbit polyclonal IgG per ml diluted in the wash solution described above and washed three times with PBS for 5 min each time. Coverslips were then incubated with 20 μg of goat anti-mouse Alexa 594 per ml or 20 μg of goat anti-rabbit Alexa 488 or 594 per ml for 30 min. For nuclear staining, cells were incubated with PBS containing 4′,6′-diamidino-2-phenylindole (DAPI) (100 ng/ml) for 5 min. Anti-rabbit Alexa 594 was used as the secondary antibody when DAPI was used. Anti-rabbit Alexa 488 and anti-mouse Alexa 594 were used for P-H3 and large T, respectively. Cells were briefly washed with PBS three times and with H2O once and mounted with 90% glycerol in PBS. Images from a fluorescence microscope (magnification, ×40) (BX-50; Olympus) were captured with Scion Image software (Scion Corp., Frederick, Md.). The total number of cells was recorded from DAPI staining.
Immunoblotting.
Lysates were prepared by solubilizing the cell pellet at 104 cells per μl in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer as previously described (70). Lysates were resolved by SDS-PAGE, and proteins were transferred onto Immobilon-P (Millipore, Bedford, Mass.). The blots were then probed with antibodies as indicated.
H1 kinase assay.
All floating cells were collected and pooled with attached cells that had been released by trypsinization. They were then washed twice with PBS and counted. Cells (2 × 106) were lysed in 200 μl of the lysis buffer (50 mM Tris [pH 8.0], 250 mM NaCl, 0.5% NP-40, 50 mM NaF, 1 mM Na3VO4, 1 mM dithiothreitol [DTT], 1 mM phenylmethylsulfonyl fluoride, 25 μg of leupeptin per ml, 25 μg of aprotinin per ml, 150 μg of benzamidine per ml, and 10 μg of trypsin inhibitor per ml). The samples were centrifuged at 10,000 × g for 10 min at 4°C. The supernatant was transferred and incubated with 1 μg of anti-cyclin B1 mouse monoclonal IgG1 (GNS1) for 2 h at 4°C. The samples were further incubated with 20 μl of protein A-conjugated agarose beads for 1 h at 4°C. Immune complexes were then washed three times with lysis buffer without inhibitors and once with kinase wash buffer (50 mM Tris [pH 7.4], 10 mM MgCl2, 1 mM DTT). The kinase reaction was performed in 25 μl of assay buffer (50 mM Tris [pH 7.4], 2 μCi of [γ-32P]ATP, 50 nM ATP, 10 mM MgCl2, 1 mM DTT, 0.1 μg of histone H1 per μl). The reaction was stopped with 25 μl of 2× sample buffer. The samples were heated for 10 min at 96°C. They were then separated by SDS-10% PAGE. After the gel was dried, it was exposed to a phosphorimager screen (Molecular Dynamics, Sunnyvale, Calif.) for 4 h. The images were visualized and quantitated with Image Quant software (Molecular Dynamics).
RESULTS
Phosphorylation of Chk1 in SV40-infected cells.
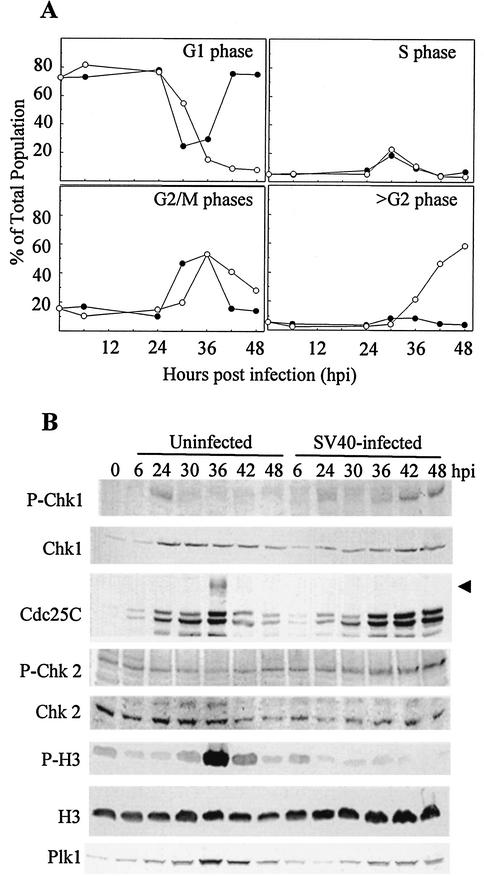
Knowing that cyclin B/Cdc2 is in the inactive, tyrosine-phosphorylated form during lytic infection, we examined upstream regulatory pathways for events unique to infected cells. Parallel confluent cultures of CV-1 cells were either infected with SV40 or trypsinized and replated without infection. The cells were then synchronized at the G1/S border with mimosine beginning at 6 hpi or trypsinization and released 18 h later (24 hpi). The cells were harvested at the time of the SV40 infection, mimosine addition, or release from mimosine and every 6 h after the release for up to 48 hpi. Flow cytometric analysis of cell cycle distribution confirmed that most of the cells were in G1 phase at times up to and including the point of release from mimosine at 24 hpi (Fig. 1A). Following release, the uninfected cells progressed through S phase, G2 phase, and mitosis. Passage through mitosis was indicated by a decrease in the G2 population and an increase in the G1 population beginning between 30 and 36 h after trypsinization. In contrast, SV40-infected cells progressed through S and G2 phases but did not pass through mitosis at any time. The exit of infected cells from G2, beginning at 36 hpi, was accompanied by an increase in the >G2 population with no evidence of cycling back into G1 phase. Western blot analysis of mitotic indicators confirmed the absence of mitosis in infected cells (Fig. 1B). The hyperphosphorylated, mitotic form of Cdc25C and strong expression of P-H3 were observed at 36 h after trypsinization in the uninfected cells but not at any time point in the infected cultures.
FIG. 1.
Cell cycle progression and expression of MPF regulatory proteins in SV40-infected and uninfected CV-1 cells. Confluent CV-1 cells were infected with SV40 at 100 PFU per cell or trypsinized and replated at 1:3. At 6 hpi or replating, mimosine was added, the cells were incubated for 18 h, and the medium was replaced with fresh medium without mimosine. Samples were harvested at the indicated times. (A) Cells were fixed and stained to determine DNA content per cell by flow cytometry. Uninfected cells, filled circles; SV40-infected cells, open circles. (B) Expression of MPF upstream regulatory molecules and detection of mitotic markers. Whole-cell lysates were resolved by SDS-PAGE and immunoblotted. Each filter was reacted with antibodies specific to each protein and then with alkaline phosphatase-conjugated goat anti-mouse or -rabbit antibody. The arrow indicates hyperphosphorylated (mitotic) Cdc25C.
To identify upstream regulatory pathways that may be responsible for the inhibition of MPF activity in SV40-infected cells, we compared the expression levels of mitotic regulatory proteins in SV40-infected and uninfected cells. Known upstream regulators of MPF were examined by Western blot analysis. The most significant difference unique to SV40-infected cells was increased phosphorylation of the checkpoint kinase Chk1. Mimosine treatment resulted in phosphorylation of Chk1 at the time of release from mimosine (24 hpi) in both infected and uninfected cells (Fig. 1B), consistent with previous descriptions of Chk1 activation by inhibitors of DNA synthesis (10, 21, 36, 77). Following release of uninfected cultures from G1/S, Chk1 phosphorylation was lost, even though Chk1 protein levels were retained. In contrast, G1/S-released infected cultures exhibited dephosphorylation of Chk1 in S phase, followed by an increase in Chk1 phosphorylation at 36 hpi coinciding with entry of infected cells into >G2 (Fig. 1B). Phospho-Chk2 is known to phosphorylate Cdc25C during stalled DNA replication and DNA damage, similar to Chk1 (3, 7). However, Chk2 phosphorylation was retained following release from mimosine in both the infected and uninfected populations (Fig. 1B). The level of Plk1, a positive regulator, increased to a peak at 36 h and then gradually decreased in uninfected cells. The expression of Plk1 in the SV40-infected cells increased to a constant level between 36 and 48 hpi (Fig. 1B). Additional upstream regulators that did not exhibit significant differences in their expression pattern included Wee1, Cdc25B, PP2A, and Cks1.
Small t-independent phosphorylation of Chk1.
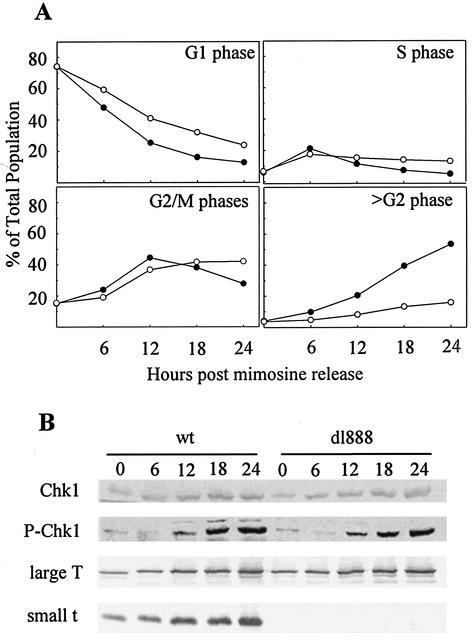
Mutant SV40 that does not express small t produces viable viral particles in CV-1 cells (8, 16, 60). However, the absence of small t reduces the rate of entry into >G2 phase compared to the wt virus (70). To test if phosphorylation of Chk1 is dependent on small t, we examined Chk1 phosphorylation following infection with mutant dl888 SV40, which does not express small t. Confluent monolayers of CV-1 cells were infected with either wt or dl888 SV40 and synchronized at G1/S with mimosine. Every 6 h from the time of release from mimosine, cells were harvested for flow cytometric and Western blot analyses. In Fig. 2A, the cell cycle distribution of wt- and dl888-infected cells is plotted against increasing time after release from the mimosine block. The rate of progression from G2 to >G2 was slower in the dl888-infected cultures than in the wt-infected cultures. Phosphorylation of Chk1 was observed in both infections beginning at 12 h after release from mimosine (36 hpi) (Fig. 2B). However, there was no significant difference in the Chk1 phosphorylation between the dl888 and wt viruses. Western blot analysis using PAb108 mouse monoclonal antibody, which recognizes both large T and small t, confirmed the absence of small t in dl888 infection (Fig. 2B). Therefore, phosphorylation of Chk1 was independent of small t expression and did not directly correlate with the rate of progression from G2 phase to >G2 phase.
FIG. 2.
Cell cycle progression of wt and dl888 mutant SV40-infected CV-1 cells. Confluent CV-1 cells were infected with either wt or dl888 mutant SV40 at 100 PFU per cell and treated with mimosine as described in the legend to Fig. 1. Samples were harvested at the indicated times postrelease from mimosine. (A) Cells were fixed and stained to determine DNA content per cell by flow cytometry. wt SV40, filled circles; dl888, open circles. (B) Expression level of phospho-Chk1 and tumor antigens (large T and small t). Whole-cell lysates were resolved by SDS-PAGE and immunoblotted. Each filter was reacted with antibodies specific to each protein and then with alkaline phosphatase-conjugated goat anti-mouse or -rabbit antibody.
Induction of mitosis by a Chk1 inhibitor, UCN-01.
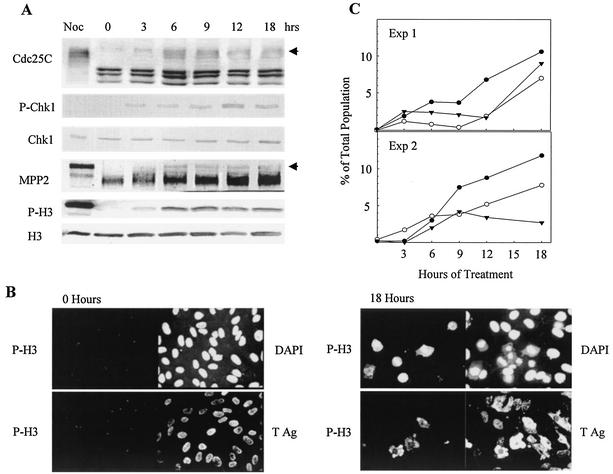
To determine whether the activity of phosphorylated Chk1 is responsible for preventing mitosis, we treated SV40-infected cells with UCN-01, a chemical inhibitor of Chk1 activity. Previous studies have shown that UCN-01 abrogates the Chk1-dependent G2 arrest checkpoint (4, 38, 74). A confluent monolayer of CV-1 cells was infected with SV40 and treated with mimosine as described above. The cells were treated with 300 nM UCN-01 beginning at 6 h after release from mimosine (30 hpi). At this time, mimosine-induced Chk1 phosphorylation was reduced and SV40-induced Chk1 phosphorylation had not yet begun. Western blot analysis demonstrated that phosphorylation of Chk1 was retained after UCN-01 addition to the infected cultures (Fig. 3A). Addition of UCN-01 resulted in the appearance of the mitotic indicators hyperphosphorylated Cdc25C, P-H3, and phosphorylated MPP2 beginning at 3 h after UCN-01 addition and persisting for up to 18 h (Fig. 3A). MPP2, also known as forkhead-related protein FKHL16, exhibits retarded electrophoretic mobility because of phosphorylation during mitosis. Attached cells were examined for signs of mitosis by immunofluorescence staining for P-H3 and for changes in nuclear morphology by staining with DAPI. At the time of UCN-01 addition, the cells displayed normal interphase morphology and there was no expression of P-H3 (Fig. 3B, 0 h). By 18 h of treatment (48 hpi), P-H3 expression and chromosome condensation (CC) were detected (Fig. 3B, 18 h). P-H3 expression was observed in prophase and metaphase cells but not in anaphase and telophase cells. P-H3 was not expressed in all cells with abnormal CC (ACC; micronuclei, apoptosis, necrosis, abnormal karyokinesis, premature CC, and others) and was not restricted to any specific chromatin morphologies. To examine possible kinetic relationships between these populations, the UCN-01-treated cells were scored for CC, ACC, and expression of P-H3. The results of two experiments (Fig. 3C) indicate that normal mitotic figures were the most frequent form of CC and increased gradually over the time course. To eliminate the possibility that cells exhibiting mitosis had escaped SV40 infection, the cells were costained for P-H3 and SV40 large T. At the time of UCN-01 addition (30 hpi), the population was large T positive with no expression of P-H3. After 18 h of treatment (48 hpi), the expression of P-H3 was observed in cells expressing large T as shown in Fig. 3B. Therefore, UCN-01 treatment resulted in the induction of apparently normal mitosis and various forms of ACC in cells expressing large T.
FIG. 3.
Induction of mitotic markers and morphology in SV40-infected cells incubated with UCN-01. Confluent CV-1 cells were infected with SV40 at 100 PFU per cell and treated with mimosine as described in the legend to Fig. 1. At 6 h after release from mimosine, the cells were incubated with 300 nM UCN-01. Samples were harvested at the indicated times postaddition. (A) Induction of mitotic markers. Whole-cell lysates were resolved by SDS-PAGE and immunoblotted. Each filter was reacted with antibodies specific to each protein and then with alkaline phosphatase-conjugated goat anti-mouse or rabbit antibody. Arrows indicate mitotic Cdc25C and mitotic MPP2. The mitotic control was prepared from uninfected CV-1 cells arrested by nocodazole (Noc). (B) Induction of P-H3 in SV40-infected cells. Cells grown on glass coverslips were fixed and stained with anti-P-H3 and anti-large T PAb101 antibodies. Nuclear morphology detail was resolved with DAPI. Cells from all time points were stained, but only samples from the time of addition and the last time point are shown. (C) Nuclear morphology and P-H3 expression. Stained samples of DAPI and P-H3 from panel B were used. DAPI staining was utilized to score the number of cells with normal or abnormal mitotic CC. The number of P-H3-positive cells was also determined. Normal mitotic condensation, filled circles; ACC, open circles; P-H3, filled triangles. Exp, experiment; Ag, antigen.
Induction of mitosis by a Chk1 activation inhibitor, caffeine.
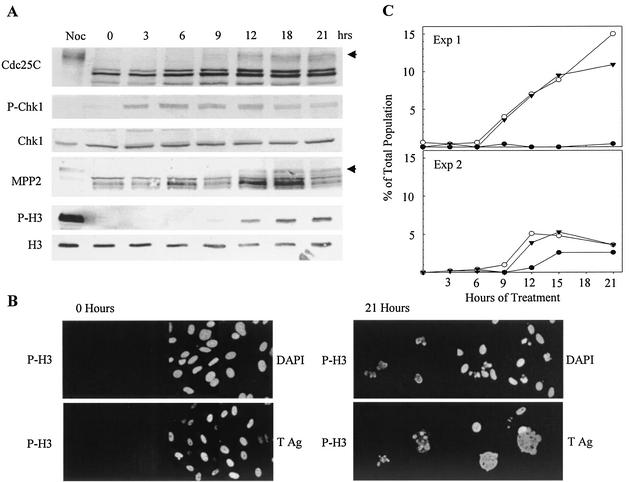
Since the inhibition of Chk1 activity by UCN-01 resulted in induction of mitosis in a subpopulation of SV40-infected cells, it might be expected that inhibition of Chk1 activation would also induce mitosis in the infected cells. To test this hypothesis, we treated infected cultures with caffeine, an inhibitor of ATR and ATM, the activators of Chk1 and Chk2, respectively. A confluent monolayer of CV-1 cells was infected with SV40 and treated with mimosine as described above. Three hours after the release from mimosine (27 hpi), the cells were treated with 6 mM caffeine, harvested at 3-h intervals, and subjected to Western blot and immunofluorescence assays. Caffeine was added at 3 h after release from mimosine to allow minimization of the mimosine-induced Chk1 phosphorylation. However, caffeine did not initially prevent phosphorylation of Chk1. Chk1 phosphorylation was observed within 3 h of treatment (30 hpi) and persisted for up to 12 h after caffeine addition (Fig. 4A). After 12 h of treatment, the level of Chk1 phosphorylation gradually decreased. The total amount of Chk1 remained relatively constant and did not correlate with the change in its degree of phosphorylation. The gradual dephosphorylation of Chk1 was accompanied by a gradual increase in the mitotic markers: hyperphosphorylated Cdc25C, P-H3, and phosphorylated MPP2. Immunofluorescence staining was performed to detect mitoses and nuclear changes in the attached cells. At the time of caffeine addition (27 hpi), the cells displayed normal interphase morphology and were P-H3 negative (Fig. 4B, 0 h). As shown in the results of two time course experiments, ACC and P-H3 were first observed between 9 and 12 h after treatment (36 to 39 hpi) (Fig. 4C). Although there was variation between experiments, ACC predominated over the appearance of normal mitotic figures. P-H3 expression was observed in cells with both normal CC and ACC, as observed in the UCN-01 treatment. The appearance of P-H3 in large T-expressing cells confirmed that mitotic markers had been induced by caffeine treatment of virus-infected cells (Fig. 4B).
FIG. 4.
Induction of mitotic markers and morphology in SV40-infected CV-1 cells incubated with caffeine. Confluent CV-1 cells were infected with wt SV40 at 100 PFU per cell and treated with mimosine as described in the legend to Fig. 1. Three hours after release from mimosine, the cells were incubated with 6 mM caffeine. Samples were harvested at the indicated times postaddition. (A) Induction of mitotic markers. Whole-cell lysates were resolved by SDS-PAGE and immunoblotted. Each filter was reacted with antibodies specific to each protein and then with alkaline phosphatase-conjugated goat anti-mouse or -rabbit antibody. Solid arrows indicate mitotic Cdc25C and mitotic MPP2. The mitotic control was prepared from uninfected CV-1 cells arrested by nocodazole (Noc). (B) Induction of P-H3 in SV40-infected cells. The attached cells were stained with anti-P-H3 and anti-large T antibodies and DAPI as described in the legend to Fig. 3. Cells from all of the time points were stained, but only samples from the time of addition and the last time point are shown. (C) Nuclear morphology change and P-H3 expression. Stained samples of DAPI and P-H3 from panel B were used. Normal mitotic condensation, filled circles; ACC, open circles; P-H3, filled triangles. Exp, experiment; Ag, antigen.
Mitotic arrest by nocodazole in UCN-01-treated cultures.
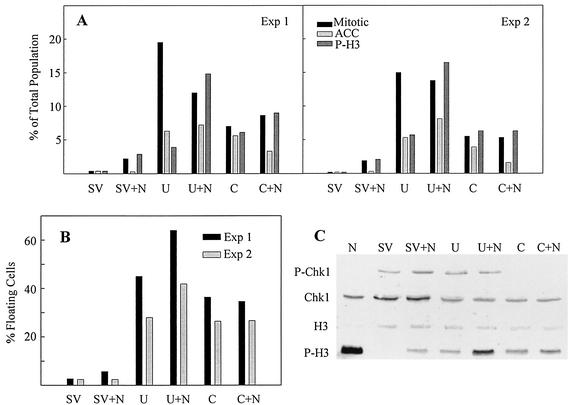
Since a significant number of cells displayed normal mitotic morphology after UCN-01 treatment, we suspected that some cells might have completed mitosis and entered the next cell cycle. To examine this possibility, nocodazole was added to SV40-infected cells treated with UCN-01 or caffeine to trap any cells passing through mitosis. Nocodazole was added to cultures in combination with either caffeine or mimosine at 3 and 6 h after release from mimosine, respectively. At 24 h after release from mimosine (48 hpi), the attached and floating cells were counted. Attached cells on coverslips were evaluated for P-H3 expression and nuclear morphology. Nocodazole effectively arrested UCN-01-treated cells in prometaphase, but cells in anaphase and telophase were absent. An increase in the number of P-H3-positive cells resulting from addition of nocodazole to UCN-01-treated cultures confirmed that UCN-01-treated cells were progressing beyond metaphase in the absence of nocodazole (Fig. 5A). As mentioned above, cells in prophase and metaphase were P-H3 positive whereas cells in anaphase and telophase were negative, which was also consistent with previous reports (22). In contrast to UCN-01-treated cells, the addition of nocodazole to caffeine-treated cells did not affect either the P-H3 expression level or the nuclear morphology of attached cells (Fig. 5A).
FIG. 5.
Combined effect of nocodazole with UCN-01 or caffeine on SV40-infected cells. Confluent CV-1 cells were infected with SV40 at 100 PFU per cell and treated with mimosine as described in the legend to Fig. 1. At 3 and 6 h after release from mimosine, the cells were incubated with 6 mM caffeine and 300 nM UCN-01, respectively, in the presence or absence of nocodazole (0.1 μg/ml). Samples were harvested at 48 hpi. (A) Expression of P-H3 and change in chromosome morphology. The number of P-H3-positive cells was also determined. Normal mitotic condensation, black; ACC, light gray; P-H3, dark gray. (B) Percentage of floating cells obtained from each of the treatments described above. (C) Whole-cell lysates were resolved by SDS-PAGE and immunoblotted. Each filter was reacted with antibodies specific to each protein and then with alkaline phosphatase-conjugated goat anti-mouse or -rabbit antibody. Exp, experiment.
Large numbers of floating cells were found in UCN-01- and caffeine-treated cultures (Fig. 5B) by 48 hpi. However, we were unable to effectively characterize these populations microscopically or by flow cytometry because of cell fragility and aggregation during the fixation process. Therefore, Western blot analysis of lysates from pooling of attached and floating cells was performed to determine if the indications from attached cells were representative of the total population (Fig. 5C). Addition of nocodazole to UCN-01-treated cultures resulted in an increase in P-H3. Western blot analysis of pooled attached and floating cells confirmed that addition of nocodazole did not result in increased P-H3 in caffeine-treated cultures. The effectiveness of the caffeine treatment was verified by the loss of Chk1 phosphorylation.
Activation of MPF kinase by UCN-01 and caffeine treatments.
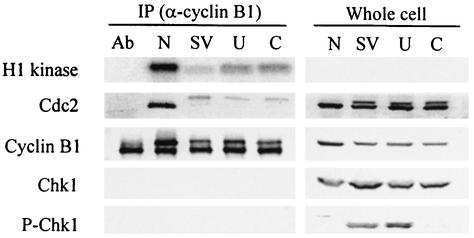
Expression of mitotic markers and induction of CC by UCN-01 and caffeine treatments of SV40-infected cells suggested that inhibition of Chk1 activity resulted in stimulation of MPF. Therefore, we measured the H1 kinase activity of MPF isolated from SV40-infected, CV-1 cells released from mimosine that were treated with either UCN-01 or caffeine. At 48 hpi, the populations were lysed, immunoprecipitated with anti-cyclin B1 antibody, and assayed for H1 kinase activity. Increased kinase activity was found in infected cells treated with UCN-01 or caffeine (Fig. 6). Western blot analysis of immunoprecipitated cyclin B/Cdc2 established that the increase in H1 kinase activity was not a consequence of an increase in cyclin B-associated Cdc2 (Fig. 6). Western blot analysis of whole-cell lysates of the same cultures demonstrated that addition of UCN-01 did not affect the phosphorylation of Chk1 whereas addition of caffeine resulted in a reduction of Chk1 phosphorylation. The dephosphorylated, high-mobility form of Cdc2 represented only a small proportion of the cyclin B-associated Cdc2 in caffeine- or UCN-01-treated SV40-infected cells, indicating that a only a small fraction of MPF was in the active form.
FIG. 6.
Increased MPF protein kinase activity after UCN-01 and caffeine treatments. Confluent CV-1 cells were infected with SV40 at 100 PFU per cell and treated with mimosine as described in the legend to Fig. 1. Caffeine (6 mM) was added to one set of cultures at 3 h after release from mimosine (lane C). UCN-01 (300 nM) was added to one set of cultures at 6 h after release from mimosine (lane U). A parallel infected culture released from mimosine was not treated with any mitotic inducers (SV). Samples were harvested at 48 hpi. A mitotic control was prepared from uninfected CV-1 cells arrested by nocodazole (lane N). Lysates were precipitated with mouse anti-cyclin B1 antibody (Ab). Precipitated samples (2 × 106 cells) were used for a kinase assay with histone H1 as the substrate. Phosphorylated H1 was resolved by SDS-PAGE and visualized by phosphorimaging. The remaining precipitated samples and whole-cell lysates were resolved by SDS-PAGE and immunoblotted. Each filter was reacted with antibodies specific to each protein and then with alkaline phosphatase-conjugated goat anti-mouse or -rabbit antibody. An anti-cyclin B1 immunoprecipitation without cell extract is shown in lane Ab.
DISCUSSION
The demonstration of Chk1 activation during SV40 lytic infection implicates components of DNA damage response pathways in the virus-host cell interaction. The results presented here suggest a role for Chk1 in the inhibition of mitosis, although additional Chk1 functions may contribute to viral replication. Previously, activation of Chk1 has been associated with cell cycle arrest resulting from either DNA damage or incomplete DNA replication (10, 36, 50, 56, 77). It is unclear whether the Chk1 phosphorylation observed in SV40 lytic infection can be categorized as resulting from stalled DNA replication or DNA damage or whether it represents a novel pathway.
When CV-1 cells were infected with the small t deletion mutant dl888, Chk1 was phosphorylated with the same kinetics as in wt infection, demonstrating that Chk1 phosphorylation was not dependent on small t. In addition, infection by dl888 established that the percentage of cells in >G2 was not directly related to the amount of Chk1 phosphorylation. SV40 dl888-infected cells exhibited a reduced rate of progression from G2 to >G2 but no alteration in the phosphorylation of Chk1 relative to that of wt-infected cells. Considering that wild-type levels of Chk1 phosphorylation are obtained without a significant number of cells in >G2, Chk1 phosphorylation appears to be a late S phase or G2 phase event that contributes to the absence of mitosis in both wt and dl888 infections.
Chk1 phosphorylation triggered by stalled replication forks could be a consequence of viral DNA replication and/or cellular DNA replication. It has been reported that phosphorylation of Chk1 in the DNA replication checkpoint response requires the primase activity, but not DNA replication activity, of DNA polymerase alpha (39). Both viral and cellular origins fulfill this prerequisite for checkpoint signaling, given that the involvement of host cell DNA polymerase alpha primase in SV40 replication is well established (68). What remain undefined in this case are the checkpoint signaling events that occur subsequent to RNA primer synthesis at the viral or cellular replication forks. The rate of viral DNA replication does not appear to be a determining factor in Chk1 phosphorylation. The rates of viral DNA synthesis in dl888 or other small t deletion mutant infections are about half of that seen in wt infections (8, 16), yet we observed no reduction of Chk1 phosphorylation in dl888 infection. However, SV40 minichromosomes, which may replicate to a copy number of 105 to 106 per cell, are found in a variety of forms, including transcriptional complexes and replicative intermediates, and in various states of maturation to virus particles. It may be that this complex environment places stresses on replication fork function that are sufficient to initiate a checkpoint response.
Cellular replication forks may also be the source of checkpoint signaling. Current examples include Chk1 activation in response to inhibitors of DNA synthesis such as hydroxyurea and aphidicolin. Both of these drugs allow synthesis of the RNA primer but inhibit elongation. Activation of Chk1 by UV light may also be a consequence of slowed progress of replication forks. In the case of lytic infection, there is no obvious inhibition of cellular DNA synthesis but the rate of cellular DNA replication may be influenced by the onset of viral DNA synthesis. We have previously shown that increased viral DNA and the late gene product VP1 can be detected at 8 h after release from mimosine, late in S phase (34). If any replication factors are limiting, the rate of synthesis at cellular DNA replication forks may be adversely affected by the onset of viral DNA replication.
DNA damage is also a stimulus for activation of the Chk1 pathway. The checkpoint response to IR primarily involves the ATM substrates Chk2, p53, and MDM2, whereas activation of Chk1 by ATR appears to play a backup role (2). During activation of the S phase checkpoint by IR, an initial rapid phase of ATM signaling is followed by an ATM-independent activation of Chk1 that is required to maintain the S phase checkpoint response (78). In contrast, the checkpoint response to UV and inhibitors of DNA replication depends primarily on ATR-dependent activation of Chk1 (2). SV40-induced chromosome damage following infection of normal human cells (41, 71) was described in the early 1960s, soon after the initial reports of SV40-induced cell transformation and tumorigenesis. The increased incidence of chromosome abnormalities within 24 h after SV40 infection of human skin fibroblasts (71) and Chinese hamster embryo cells (32) demonstrates that induction of chromosome damage following SV40 infection can occur quickly. Therefore, the phosphorylation of Chk1 observed in SV40-infected CV-1 cells may be in response to damage generated within the first 36 h of infection. Expression of cloned viral genes has established that large T alone is sufficient for the induction of chromosome instability, including chromatid exchanges, chromosome gaps, and breaks and dicentric chromosomes (54, 63). However, the mechanism of SV40-induced chromosome damage is not well defined. One possibility is that large T may generate damage indirectly by overriding cell cycle checkpoints and disallowing DNA repair. Loss of the p53-dependent G1 phase checkpoint requires the binding of large T to pRb, but the formation of complexes between large T and p53 is not essential (18, 53). Large T has also been shown to disrupt formation of DNA repair foci containing MRE11 (9). Override of the G2 phase and spindle checkpoints is found in cells expressing large T and has been detected within 5 to 10 population doublings following initiation of large T expression in human fibroblasts (6). The alteration of mitotic control within this time period following large T expression suggests that large T is directly responsible for checkpoint override rather than for generating mutations in mitotic regulators that give a selective advantage.
In this study, mitosis and/or ACC were induced in SV40-infected cells by the Chk1 inhibitor UCN-01 and by the ATM/ATR inhibitor caffeine. These inhibitors were previously used to abrogate the G2 arrest in cells with DNA damage induced by genotoxic agents such as IR and adriamycin. The viability of cells following override of G2 arrest by UCN-01 and caffeine treatments is dependent on the cell type and/or the type of genotoxic agent used (5, 38, 40, 67, 74). In SV40-infected cells, the induction of normal mitosis in caffeine-treated cells was less frequent than in UCN-01-treated cells. Although UCN-01 and caffeine are both known to inhibit Chk1 activity, the difference in their cellular effects may be explained by the additional regulators influenced by each compound. UCN-01, at concentrations of <100 nM, directly inhibits the in vitro protein kinase activity of Chk1, C-TAK1, and several isoforms of protein kinase C but does not inhibit Chk2 (5, 10, 20, 24, 59). C-TAK1 is a constitutively active cytoplasmic protein kinase that phosphorylates Ser216 of Cdc25C and thus may be responsible for maintaining Ser216 phosphorylation during interphase (46, 49). Although both Chk1 and C-TAK1 phosphorylate Cdc25C, they also each phosphorylate additional distinct substrates (44, 56, 61, 76). Downstream effects of UCN-01 have also been described. Wee1 kinase is upregulated by Chk1 in Xenopus and fission yeast cells (31, 45) and is inhibited in UCN-01-treated human and murine cells in an indirect manner (55, 74). Caffeine, as an inhibitor of ATM and ATR, inhibits the downstream activation of both Chk1 and Chk2 (57). Additional downstream kinases known to be affected by caffeine inhibition of ATM/ATR include Plk1 (67) and Plk3 (72). Both of these protein kinases phosphorylate Cdc25C but at different sites. Plk3 phosphorylates Ser216 (47), the same site phosphorylated by Chk1, Chk2, and C-TAK1. Plk1 phosphorylates Ser198, which is within the nuclear export signal, and may control nuclear localization (66). Both Plk1 (19) and Plk3 (48) are mitotic regulators, and the effect of UCN-01 on their activity has not been reported. Other ATM or ATR substrates, such as NBS1, BRCA1, and the p53 binding protein 53BP1, may also be involved in the response to caffeine (2). The complexity of the response to caffeine is indicated by the initial phosphorylation of Chk1 in SV40-infected cells exposed to caffeine for 3 h. Initial phosphorylation of Chk1 may be catalyzed by a caffeine-insensitive kinase, or additional factors may influence caffeine inhibition of ATM/ATR. In summary, the greater tendency of UCN-01 to induce normal mitosis while caffeine stimulated ACC may be explained by differences in additional pathways affected by each compound. Understanding the differences in the responses of SV40-infected CV-1 cells to UCN-01 and caffeine will require selective inhibition of Chk1 and other components of the mitotic regulatory pathways.
A related observation was the gradual increase in the normal and abnormal mitotic CC following treatment of infected cells with UCN-01 or caffeine. Unlike uninfected cells, there was not a peak of mitosis at a particular time point, as would be expected in a synchronized cell population. This failure to restore the normal mitotic program may also be explained by the loss of function of additional regulators influenced by each inhibitor, as described above. A second possibility is the involvement of other G2/M regulatory pathways, such as the p38 stress kinase (1, 4,69). DNA damage caused by IR and UV radiation stimulates different members of a group of four p38 kinase isoforms. In response to IR, the p38γ isoform, along with Chk2, is activated in an ATM-dependent manner (69). In contrast, UV radiation stimulates the kinase activity of the p38α and -β isoforms. These two isoforms of p38 phosphorylate and inactivate Cdc25B phosphatase, a mitotic initiator (4, 28, 30). A third possibility is that the presence of viral proteins prevents restoration of normal mitotic timing, even when checkpoint controls are overridden.
A number of viruses avoid entry into mitosis either by G2 phase arrest or by continued DNA synthesis, but the purpose of this block in mitotic entry is not known. There are two general categories of speculation: First, the highly complex structural and functional changes in cells during mitosis may temporarily or permanently disrupt a viral replication cycle. In this case, the virus may have evolved mechanisms by which to inhibit the initiation of mitosis. Second, the absence of mitosis may be a strictly cellular response to the stress of viral infection and a mechanism by which to limit proliferation of infected cells. The activation of Chk1 appears to be a novel mechanism for viral inhibition of mitotic initiation. However, viruses that are known to produce a G2 arrest also interact with pathways upstream from MPF. The Vpr protein of human immunodeficiency virus type 1 arrests cells in G2 phase (26). Binding of Vpr to PP2A enhances nuclear transport and consequent dephosphorylation of activating residues on Cdc25C. In the absence of active Cdc25C, MPF remains in its phosphorylated, inactive form (23). Small t of SV40 also binds to PP2A but, in contrast to Vpr, inhibits PP2A phosphatase activity (73). Overexpression of small t in normal human fibroblasts results in the accumulation of a 4C population with about 30% of the total population arrested in prometaphase. This mitotic arrest appears to result from a failure of either centrosome formation or duplication (15). However, an influence of small t on PP2A-mediated dephosphorylation of Cdc25C has not been reported. G2 arrest induced by the human parvovirus B19 is dependent on the viral regulatory protein NS-1. The G2 arrest in B19-infected UT7/Epo-S1 cells is characterized by increased protein kinase activity of MPF and cytoplasmic cyclin B. The absence of any indicators of mitosis suggests that nuclear import of MPF is blocked (42). Reovirus serotype 3 arrest of human, mouse, and canine cells in G2 phase is mediated by the σ1s protein, which is sufficient to cause G2 phase arrest when overexpressed in mouse cells (52). This G2 arrest is associated with inhibitory Y15 phosphorylation of the Cdc2 subunit of MPF, resulting in deactivation of MPF (51). G2 phase arrests of polyomavirus (33)- and human cytomegalovirus (25, 37)-infected cells have also been described.
The increased expression of mitotic markers and stimulation of MPF activity in response to inhibitors of Chk1 activity suggest that SV40 blocks MPF activation by maintaining the phosphorylation of cyclin B-associated Cdc2 at Tyr15. However, additional factors, such as protein subunits that inhibit or activate MPF, may be involved and affected by Chk1. Further characterization of Chk1 and other components of DNA damage response pathways are required to develop a better understanding of the mechanisms underlying altered cell cycle regulation during infection by SV40 and possibly other viruses exhibiting altered G2 phase control.
REFERENCES
- 1.Abbott, D. W., and J. T. Holt. 1999. Mitogen-activated protein kinase kinase 2 activation is essential for progression through the G2/M checkpoint arrest in cells exposed to ionizing radiation. J. Biol. Chem. 274:2732-2742. [DOI] [PubMed] [Google Scholar]
- 2.Abraham, R. T. 2001. Cell cycle checkpoint signaling through the ATM and ATR kinases. Genes Dev. 15:2177-2196. [DOI] [PubMed] [Google Scholar]
- 3.Blasina, A., I. V. de Weyer, M. C. Laus, W. H. Luyten, A. E. Parker, and C. H. McGowan. 1999. A human homologue of the checkpoint kinase Cds1 directly inhibits Cdc25 phosphatase. Curr. Biol. 9:1-10. [DOI] [PubMed] [Google Scholar]
- 4.Bulavin, D. V., Y. Higashimoto, I. J. Popoff, W. A. Gaarde, V. Basrur, O. Potapova, E. Appella, and A. J. Fornace, Jr. 2001. Initiation of a G2/M checkpoint after ultraviolet radiation requires p38 kinase. Nature 411:102-107. [DOI] [PubMed] [Google Scholar]
- 5.Busby, E. C., D. F. Leistritz, R. T. Abraham, L. M. Karnitz, and J. N. Sarkaria. 2000. The radiosensitizing agent 7-hydroxystaurosporine (UCN-01) inhibits the DNA damage checkpoint kinase hChk1. Cancer Res. 60:2108-2112. [PubMed] [Google Scholar]
- 6.Chang, T. H., F. A. Ray, D. A. Thompson, and R. Schlegel. 1997. Disregulation of mitotic checkpoints and regulatory proteins following acute expression of SV40 large T antigen in diploid human cells. Oncogene 14:2383-2393. [DOI] [PubMed] [Google Scholar]
- 7.Chaturvedi, P., W. K. Eng, Y. Zhu, M. R. Mattern, R. Mishra, M. R. Hurle, X. Zhang, R. S. Annan, Q. Lu, L. F. Faucette, G. F. Scott, X. Li, S. A. Carr, R. K. Johnson, J. D. Winkler, and B. B. Zhou. 1999. Mammalian Chk2 is a downstream effector of the ATM-dependent DNA damage checkpoint pathway. Oncogene 18:4047-4054. [DOI] [PubMed] [Google Scholar]
- 8.Cicala, C., M. L. Avantaggiati, A. Graessmann, K. Rundell, A. S. Levine, and M. Carbone. 1994. Simian virus 40 small-t antigen stimulates viral DNA replication in permissive monkey cells. J. Virol. 68:3138-3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Digweed, M., I. Demuth, S. Rothe, R. Scholz, A. Jordan, C. Grotzinger, D. Schindler, M. Grompe, and K. Sperling. 2002. SV40 large T-antigen disturbs the formation of nuclear DNA-repair foci containing MRE11. Oncogene 21:4873-4878. [DOI] [PubMed] [Google Scholar]
- 10.Feijoo, C., C. Hall-Jackson, R. Wu, D. Jenkins, J. Leitch, D. M. Gilbert, and C. Smythe. 2001. Activation of mammalian Chk1 during DNA replication arrest: a role for Chk1 in the intra-S phase checkpoint monitoring replication origin firing. J. Cell Biol. 154:913-923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friedrich, T. D., J. Laffin, and J. M. Lehman. 1992. Simian virus 40 large T-antigen function is required for induction of tetraploid DNA content during lytic infection. J. Virol. 66:4576-4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Friedrich, T. D., J. Laffin, and J. M. Lehman. 1994. Induction of tetraploid DNA content by simian virus 40 is dependent on T-antigen function in the G2 phase of the cell cycle. J. Virol. 68:4028-4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Friedrich, T. D., E. Okubo, J. Laffin, and J. M. Lehman. 1998. Okadaic acid induces appearance of the mitotic epitope MPM-2 in SV40-infected CV-1 cells with a >G2-phase DNA content. Cytometry 31:260-264. [PubMed] [Google Scholar]
- 14.Furnari, B., N. Rhind, and P. Russell. 1997. Cdc25 mitotic inducer targeted by chk1 DNA damage checkpoint kinase. Science 277:1495-1497. [DOI] [PubMed] [Google Scholar]
- 15.Gaillard, S., K. M. Fahrbach, R. Parkati, and K. Rundell. 2001. Overexpression of simian virus 40 small-t antigen blocks centrosome function and mitotic progression in human fibroblasts. J. Virol. 75:9799-9807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gauchat, J. F., and R. Weil. 1986. On the functional roles of simian virus 40 large and small T-antigen in the induction of a mitotic host response. Nucleic Acids Res. 14:9339-9351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gershey, E. L. 1979. Simian virus 40-host cell interaction during lytic infection. J. Virol. 30:76-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gjoerup, O., H. Chao, J. A. DeCaprio, and T. M. Roberts. 2000. pRB-dependent, J domain-independent function of simian virus 40 large T antigen in override of p53 growth suppression. J. Virol. 74:864-874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Golsteyn, R. M., K. E. Mundt, A. M. Fry, and E. A. Nigg. 1995. Cell cycle regulation of the activity and subcellular localization of PLK1, a human protein kinase implicated in mitotic spindle function. J. Cell Biol. 129:1617-1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Graves, P. R., L. Yu, J. K. Schwarz, J. Gales, E. A. Sausville, P. M. O'Connor, and H. Piwnica-Worms. 2000. The Chk1 protein kinase and the Cdc25C regulatory pathways are targets of the anticancer agent UCN-01. J. Biol. Chem. 275:5600-5605. [DOI] [PubMed] [Google Scholar]
- 21.Guo, Z., A. Kumagai, S. X. Wang, and W. G. Dunphy. 2000. Requirement for Atr in phosphorylation of Chk1 and cell cycle regulation in response to DNA replication blocks and UV-damaged DNA in Xenopus egg extracts. Genes Dev. 14:2745-2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hendzel, M. J., Y. Wei, M. A. Mancini, A. Van Hooser, T. Ranalli, B. R. Brinkley, D. P. Bazett-Jones, and C. D. Allis. 1997. Mitosis-specific phosphorylation of histone H3 initiates primarily within pericentromeric heterochromatin during G2 and spreads in an ordered fashion coincident with mitotic chromosome condensation. Chromosoma 106:348-360. [DOI] [PubMed] [Google Scholar]
- 23.Hrimech, M., X. J. Yao, P. E. Branton, and E. A. Cohen. 2000. Human immunodeficiency virus type 1 vpr-mediated G2 cell cycle arrest: vpr interferes with cell cycle signaling cascades by interacting with the B subunit of serine/threonine protein phosphatase 2A. EMBO J. 19:3956-3967. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Jackson, J. R., A. Gilmartin, C. Imburgia, J. D. Winkler, L. A. Marshall, and A. Roshak. 2000. An indolocarbazole inhibitor of human checkpoint kinase (Chk1) abrogates cell cycle arrest caused by DNA damage. Cancer Res. 60:566-572. [PubMed] [Google Scholar]
- 25.Jault, F. M., J. M. Jault, F. Ruchti, E. A. Fortunato, C. Clark, J. Corbeil, D. D. Richman, and D. H. Spector. 1995. Cytomegalovirus infection induces high levels of cyclins, phosphorylated Rb, and p53, leading to cell cycle arrest. J. Virol. 69:6697-6704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jowett, J. B. M., V. Planelles, B. Poon, N. P. Shah, M. L. Chen, and I. S. Y. Chen. 1995. The human immunodeficiency virus type 1 vpr gene arrests infected T cells in the G2+M phase of the cell cycle. J. Virol. 69:6304-6313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karaiskou, A., C. Jessus, T. Brassac, and R. Ozon. 1999. Phosphatase 2A and polo kinase, two antagonistic regulators of cdc25 activation and MPF auto-amplification. J. Cell Sci. 112:3747-3756. [DOI] [PubMed] [Google Scholar]
- 28.Karlsson, C., S. Katich, A. Hagting, I. Hoffmann, and J. Pines. 1999. Cdc25B and Cdc25C differ markedly in their properties as initiators of mitosis. J. Cell Biol. 146:573-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laffin, J., and J. M. Lehman. 1994. Detection of intracellular virus and viral products. Methods Cell Biol. 41:543-557. [DOI] [PubMed] [Google Scholar]
- 30.Lammer, C., S. Wagerer, R. Saffrich, D. Mertens, W. Ansorge, and I. Hoffmann. 1998. The cdc25B phosphatase is essential for the G2/M phase transition in human cells. J. Cell Sci. 111:2445-2453. [DOI] [PubMed] [Google Scholar]
- 31.Lee, J., A. Kumagai, and W. G. Dunphy. 2001. Positive regulation of wee1 by chk1 and 14-3-3 proteins. Mol. Biol. Cell 12:551-563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lehman, J. M. 1974. Early chromosome changes in diploid Chinese hamster cells after infection with simian virus 40. Int. J. Cancer 13:164-172. [DOI] [PubMed] [Google Scholar]
- 33.Lehman, J. M., J. Laffin, and T. D. Friedrich. 1994. DNA content distribution of mouse cells following infection with polyoma virus. Cytometry 16:138-143. [DOI] [PubMed] [Google Scholar]
- 34.Lehman, J. M., J. Laffin, and T. D. Friedrich. 2000. Simian virus 40 induces multiple S phases with the majority of viral DNA replication in the G2 and second S phase in CV-1 cells. Exp. Cell Res. 258:215-222. [DOI] [PubMed] [Google Scholar]
- 35.Lehman, J. M., J. Laffin, J. W. Jacobberger, and D. Fogleman. 1988. Analysis of simian virus 40 infection of CV-1 cells by quantitative two-color fluorescence with flow cytometry. Cytometry 9:52-59. [DOI] [PubMed] [Google Scholar]
- 36.Liu, Q., S. Guntuku, X. S. Cui, S. Matsuoka, D. Cortez, K. Tamai, G. Luo, S. Carattini-Rivera, F. DeMayo, A. Bradley, L. A. Donehower, and S. J. Elledge. 2000. Chk1 is an essential kinase that is regulated by Atr and required for the G2/M DNA damage checkpoint. Genes Dev. 14:1448-1459. [PMC free article] [PubMed] [Google Scholar]
- 37.Lu, M. S., and T. Shenk. 1996. Human cytomegalovirus infection inhibits cell cycle progression at multiple points, including the transition from G1 to S. J. Virol. 70:8850-8857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luo, Y., S. K. Rockow-Magnone, P. E. Kroeger, L. Frost, Z. Chen, E. K. Han, S. C. Ng, R. L. Simmer, and V. L. Giranda. 2001. Blocking Chk1 expression induces apoptosis and abrogates the G2 checkpoint mechanism. Neoplasia 3:411-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Michael, W. M., R. Ott, E. Fanning, and J. Newport. 2000. Activation of the DNA replication checkpoint through RNA synthesis by primase. Science 289:2133-2137. [DOI] [PubMed] [Google Scholar]
- 40.Minemoto, Y., J. Gannon, M. Masutani, H. Nakagama, T. Sasagawa, M. Inoue, Y. Masamune, and K. Yamashita. 2001. Characterization of adriamycin-induced G2 arrest and its abrogation by caffeine in FL-amnion cells with or without p53. Exp. Cell Res. 262:37-48. [DOI] [PubMed] [Google Scholar]
- 41.Moorhead, P. S., and E. Saksela. 1965. The sequence of chromosome aberrations during SV 40 transformation of a human diploid cell strain. Hereditas 52:271-284. [DOI] [PubMed] [Google Scholar]
- 42.Morita, E., K. Tada, H. Chisaka, H. Asao, H. Sato, N. Yaegashi, and K. Sugamura. 2001. Human parvovirus B19 induces cell cycle arrest at G2 phase with accumulation of mitotic cyclins. J. Virol. 75:7555-7563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morris, M. C., A. Heitz, J. Mery, F. Heitz, and G. Divita. 2000. An essential phosphorylation-site domain of human cdc25C interacts with both 14-3-3 and cyclins. J. Biol. Chem. 275:28849-28857. [DOI] [PubMed] [Google Scholar]
- 44.Muller, J., S. Ory, T. Copeland, H. Piwnica-Worms, and D. K. Morrison. 2001. C-TAK1 regulates Ras signaling by phosphorylating the MAPK scaffold, KSR1. Mol. Cell 8:983-993. [DOI] [PubMed] [Google Scholar]
- 45.O'Connell, M. J., J. M. Raleigh, H. M. Verkade, and P. Nurse. 1997. Chk1 is a wee1 kinase in the G2 DNA damage checkpoint inhibiting cdc2 by Y15 phosphorylation. EMBO J. 16:545-554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ogg, S., B. Gabrielli, and H. Piwnica-Worms. 1994. Purification of a serine kinase that associates with and phosphorylates human Cdc25C on serine 216. J. Biol. Chem. 269:30461-30469. [PubMed] [Google Scholar]
- 47.Ouyang, B., W. Li, H. Pan, J. Meadows, I. Hoffmann, and W. Dai. 1999. The physical association and phosphorylation of Cdc25C protein phosphatase by Prk. Oncogene 18:6029-6036. [DOI] [PubMed] [Google Scholar]
- 48.Ouyang, B., H. Pan, L. Lu, J. Li, P. Stambrook, B. Li, and W. Dai. 1997. Human Prk is a conserved protein serine/threonine kinase involved in regulating M phase functions. J. Biol. Chem. 272:28646-28651. [DOI] [PubMed] [Google Scholar]
- 49.Peng, C. Y., P. R. Graves, S. Ogg, R. S. Thoma, M. J. Byrnes III, Z. Wu, M. T. Stephenson, and H. Piwnica-Worms. 1998. C-TAK1 protein kinase phosphorylates human Cdc25C on serine 216 and promotes 14-3-3 protein binding. Cell Growth Differ. 9:197-208. [PubMed] [Google Scholar]
- 50.Peng, C. Y., P. R. Graves, R. S. Thoma, Z. Wu, A. S. Shaw, and H. Piwnica-Worms. 1997. Mitotic and G2 checkpoint control: regulation of 14-3-3 protein binding by phosphorylation of Cdc25C on serine-216. Science 277:1501-1505. [DOI] [PubMed] [Google Scholar]
- 51.Poggioli, G. J., T. S. Dermody, and K. L. Tyler. 2001. Reovirus-induced σ1s-dependent G2/M phase cell cycle arrest is associated with inhibition of p34(cdc2). J. Virol. 75:7429-7434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Poggioli, G. J., C. Keefer, J. L. Connolly, T. S. Dermody, and K. L. Tyler. 2000. Reovirus-induced G2/M cell cycle arrest requires σ1s and occurs in the absence of apoptosis. J. Virol. 74:9562-9570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Quartin, R. S., C. N. Cole, J. M. Pipas, and A. J. Levine. 1994. The amino-terminal functions of the simian virus 40 large T antigen are required to overcome wild-type p53-mediated growth arrest of cells. J. Virol. 68:1334-1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ray, F. A., D. S. Peabody, J. L. Cooper, L. S. Cram, and P. M. Kraemer. 1990. SV40 T antigen alone drives karyotype instability that precedes neoplastic transformation of human diploid fibroblasts. J. Cell. Biochem. 42:13-31. [DOI] [PubMed] [Google Scholar]
- 55.Rothblum-Oviatt, C. J., C. E. Ryan, and H. Piwnica-Worms. 2001. 14-3-3 binding regulates catalytic activity of human wee1 kinase. Cell Growth Differ. 12:581-589. [PubMed] [Google Scholar]
- 56.Sanchez, Y., C. Wong, R. S. Thoma, R. Richman, Z. Wu, H. Piwnica-Worms, and S. J. Elledge. 1997. Conservation of the Chk1 checkpoint pathway in mammals: linkage of DNA damage to Cdk regulation through Cdc25. Science 277:1497-1501. [DOI] [PubMed] [Google Scholar]
- 57.Sarkaria, J. N., E. C. Busby, R. S. Tibbetts, P. Roos, Y. Taya, L. M. Karnitz, and R. T. Abraham. 1999. Inhibition of ATM and ATR kinase activities by the radiosensitizing agent, caffeine. Cancer Res. 59:4375-4382. [PubMed] [Google Scholar]
- 58.Scarano, F. J., J. A. Laffin, J. M. Lehman, and T. D. Friedrich. 1994. Simian Virus 40 prevents activation of M-phase-promoting factor during lytic infection. J. Virol. 68:2355-2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Seynaeve, C. M., M. G. Kazanietz, P. M. Blumberg, E. A. Sausville, and P. J. Worland. 1994. Differential inhibition of protein kinase C isozymes by UCN-01, a staurosporine analogue. Mol. Pharmacol. 45:1207-1214. [PubMed] [Google Scholar]
- 60.Shenk, T. E., J. Carbon, and P. Berg. 1976. Construction and analysis of viable deletion mutants of simian virus 40. J. Virol. 18:664-671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shieh, S. Y., J. Ahn, K. Tamai, Y. Taya, and C. Prives. 2000. The human homologs of checkpoint kinases Chk1 and Cds1 (Chk2) phosphorylate p53 at multiple DNA damage-inducible sites. Genes Dev. 14:289-300. [PMC free article] [PubMed] [Google Scholar]
- 62.Smits, V. A., and R. H. Medema. 2001. Checking out the G2/M transition. Biochim. Biophys. Acta 1519:1-12. [DOI] [PubMed] [Google Scholar]
- 63.Stewart, N., and S. Bacchetti. 1991. Expression of SV40 large T antigen, but not small t antigen, is required for the induction of chromosomal aberrations in transformed human cells. Virology 180:49-57. [DOI] [PubMed] [Google Scholar]
- 64.Takizawa, C. G., and D. O. Morgan. 2000. Control of mitosis by changes in the subcellular location of cyclin-B1-Cdk1 and Cdc25C. Curr. Opin. Cell Biol. 12:658-665. [DOI] [PubMed] [Google Scholar]
- 65.Tegtmeyer, P. 1972. Simian virus 40 deoxyribonucleic acid synthesis: the viral replicon. J. Virol. 10:591-598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Toyoshima-Morimoto, F., E. Taniguchi, and E. Nishida. 2002. Plk1 promotes nuclear translocation of human Cdc25C during prophase. EMBO Rep. 3:341-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.van Vugt, M. A., V. A. Smits, R. Klompmaker, and R. H. Medema. 2001. Inhibition of Polo-like kinase-1 by DNA damage occurs in an ATM- or ATR-dependent fashion. J. Biol. Chem. 276:41656-41660. [DOI] [PubMed] [Google Scholar]
- 68.Waga, S., and B. Stillman. 1998. The DNA replication fork in eukaryotic cells. Annu. Rev. Biochem. 67:721-751. [DOI] [PubMed] [Google Scholar]
- 69.Wang, X., C. H. McGowan, M. Zhao, L. He, J. S. Downey, C. Fearns, Y. Wang, S. Huang, and J. Han. 2000. Involvement of the MKK6-p38γ cascade in γ-radiation-induced cell cycle arrest. Mol. Cell. Biol. 20:4543-4552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Whalen, B., J. Laffin, T. D. Friedrich, and J. M. Lehman. 1999. SV40 small T antigen enhances progression to >G2 during lytic infection. Exp. Cell Res. 251:121-127. [DOI] [PubMed] [Google Scholar]
- 71.Wolman, S. R., K. Hirschhorn, and G. J. Todaro. 1964. Chromosomal changes in SV40-infected human fibroblast cultures. Cytogenetics 3:45-61. [DOI] [PubMed] [Google Scholar]
- 72.Xie, S., H. Wu, Q. Wang, J. P. Cogswell, I. Husain, C. Conn, P. Stambrook, M. Jhanwar-Uniyal, and W. Dai. 2001. Plk3 functionally links DNA damage to cell cycle arrest and apoptosis at least in part via the p53 pathway. J. Biol. Chem. 276:43305-43312. [DOI] [PubMed] [Google Scholar]
- 73.Yang, S. I., R. L. Lickteig, R. Estes, K. Rundell, G. Walter, and M. C. Mumby. 1991. Control of protein phosphatase 2A by simian virus 40 small-t antigen. Mol. Cell. Biol. 11:1988-1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yu, L., L. Orlandi, P. Wang, M. S. Orr, A. M. Senderowicz, E. A. Sausville, R. Silvestrini, N. Watanabe, H. Piwnica-Worms, and P. M. O'Connor. 1998. UCN-01 abrogates G2 arrest through a Cdc2-dependent pathway that is associated with inactivation of the Wee1Hu kinase and activation of the Cdc25C phosphatase. J. Biol. Chem. 273:33455-33464. [DOI] [PubMed] [Google Scholar]
- 75.Zeng, Y., K. C. Forbes, Z. Wu, S. Moreno, H. Piwnica-Worms, and T. Enoch. 1998. Replication checkpoint requires phosphorylation of the phosphatase Cdc25 by Cds1 or Chk1. Nature 395:507-510. [DOI] [PubMed] [Google Scholar]
- 76.Zhang, S. H., R. Kobayashi, P. R. Graves, H. Piwnica-Worms, and N. K. Tonks. 1997. Serine phosphorylation-dependent association of the band 4.1-related protein-tyrosine phosphatase PTPH1 with 14-3-3β protein. J. Biol. Chem. 272:27281-27287. [DOI] [PubMed] [Google Scholar]
- 77.Zhao, H., and H. Piwnica-Worms. 2001. Atr-mediated checkpoint pathways regulate phosphorylation and activation of human chk1. Mol. Cell. Biol. 21:4129-4139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhou, X. Y., X. Wang, B. Hu, J. Guan, G. Iliakis, and Y. Wang. 2002. An ATM-independent S-phase checkpoint response involves CHK1 pathway. Cancer Res. 62:1598-1603. [PubMed] [Google Scholar]