Abstract
Productive infection and replication of herpesviruses usually occurs in growth-arrested cells, but there has been no direct evidence in the case of Epstein-Barr virus (EBV), since an efficient lytic replication system without external stimuli does not exist for the virus. Expression of the EBV lytic-switch transactivator BZLF1 protein in EBV-negative epithelial tumor cell lines, however, is known to arrest the cell cycle in G0/G1 by induction of the tumor suppressor protein p53 and the cyclin-dependent kinase (CDK) inhibitors p21WAF-1/CIP-1 and p27KIP-1, followed by the accumulation of a hypophosphorylated form of the Rb protein. In order to determine the effect of the onset of lytic viral replication on cellular events in latently EBV-infected B LCLs, a tightly controlled induction system of the EBV lytic-replication program by inducible BZLF1 protein expression was established in B95-8 cells. The induction of lytic replication completely arrested cell cycle progression and cellular DNA replication. Surprisingly, the levels of p53, p21WAF-1/CIP-1, and p27KIP-1 were constant before and after induction of the lytic program, indicating that the cell cycle arrest induced by the lytic program is not mediated through p53 and the CDK inhibitors. Furthermore, although cellular DNA replication was blocked, elevation of cyclin E/A expression and accumulation of hyperphosphorylated forms of Rb protein were observed, a post-G1/S phase characteristic of cells. Thus, while the EBV lytic program promoted specific cell cycle-associated activities involved in the progression from G1 to S phase, it inhibited cellular DNA synthesis. Such cellular conditions appear to especially favor viral lytic replication.
Infection by the Epstein-Barr virus (EBV) occurs in most individuals. The EBV is a B lymphotropic gammaherpesvirus which is a causative agent of infectious mononucleosis and is known to be closely associated with several human cancers, including Burkitt's lymphoma, nasopharyngeal carcinoma, and lymphoproliferative disorder (11). The life cycle of EBV is quite distinct from those of other herpesviruses, such as herpes simplex virus type 1 (HSV-1) or cytomegalovirus (CMV). In the cases of HSV-1 and CMV, full lytic replication can be accomplished by infection of certain cell types with virus. Such an efficient lytic-replication system, however, does not exist for EBV.
The EBV genome in the virus particle is a linear double-stranded DNA which is 172 kbp in length, encoding >80 open reading frames (3). EBV specifically infects resting B lymphocytes via CD21 and HLA class II molecules on the cell surface (23), inducing the continuous proliferation of the B cells (11). The resulting lymphoblastoid cell lines (LCLs) express a limited number of EBV gene products, including six nuclear proteins (EBNA-1, EBNA-2, EBNA-3A, EBNA-3B, EBNA-3C, and EBNA-LP), three membrane proteins (LMP-1, LMP-2A, and LMP-2B), EBV-encoded small RNAs (EBER1 and EBER2), and transcripts from the BamHI A region. Together, they activate quiescent B cells to enter the cell cycle, maintain continuous proliferation, and prevent cells from undergoing apoptosis. The viral genome is maintained as covalently closed circular plasmids forming nucleosomal structures with histone proteins in the nuclei of the LCLs. The number of copies is maintained at 10 to 50 per cell, to be duplicated once during each cell division cycle by the host cellular DNA replication machinery (43).
In most asymptomatic carriers of EBV, the virus is periodically replicated, and infectious virions can be recovered from oral secretions. This replication, which results in the production of infectious virus, is known as EBV lytic replication. Activation of the lytic program appears to occur in memory B cells recirculating through the lymphoid tissue associated with the oropharyngeal mucosa (10). Also, host immunosuppression may trigger reactivation of the virus in latently infected B cells, which leads to productive infection. However, the mechanism underlying this viral reactivation in vivo is not clearly understood.
Lytic replication differs from the latent amplification state in that multiple rounds of replication are initiated within oriLyt (14), and the replication process has a greater dependence on EBV-encoded replication proteins (12). Upon reactivation, the two key EBV immediate-early (IE) lytic genes, BZLF1 and BRLF1, are expressed. These genes encode transactivators that activate viral and certain cellular promoters and lead to an ordered cascade of viral gene expression: activation of early gene expression, followed by the lytic cascade of viral genome replication and late gene expression (11). In the viral productive cycle, the EBV genome is amplified approximately 100-fold. Intermediates of viral DNA replication are found as large head-to-tail concatemeric molecules, probably resulting from rolling-circle DNA replication (14), which are subsequently cleaved into unit length genomes and packaged into virions in nuclei.
To understand the molecular basis for the reactivation and progression of lytic EBV replication, chemical agents, such as phorbol esters, sodium n-butyrate, and calcium ionophores or anti-immunoglobulin G (IgG) antibodies (39), have often been utilized as BZLF1 inducers in latently infected cells. However, these approaches are somewhat problematic for specifically analyzing the cellular events altered by the EBV lytic program alone, since it is difficult to distinguish between effects of the virus and those that are induced by the treatment itself. For instance, treatment of latently infected cells with most lytic-cycle-inducing agents causes a G0/G1 arrest prior to detectable expression of IE genes (13, 18, 31). As another and more favorable approach, introduction of the BZLF1 expression vector, which alone is sufficient to activate the EBV lytic cascade (14), has been applied. It has been demonstrated that the BZLF1 protein inhibits host cell proliferation by causing cell cycle arrest in G0/G1 in several epithelial tumor cell lines lacking the EBV genome (6, 31). Such G0/G1 arrest was found to result from induction of the tumor suppressor protein p53 and the cyclin-dependent kinase (CDK) inhibitors p21WAF-1/CIP-1 and p27KIP-1, followed by accumulation of a hypophosphorylated form of the Rb protein (6). However, the studies have thus far been limited to the effects of BZLF1 expression in cells derived from an epithelial origin and lacking EBV. On the other hand, it was demonstrated that the other IE transactivator, the BRLF1 protein, can induce contact-inhibited, quiescent human fibroblasts to enter S phase and dramatically increase the level of E2F1 (38). E2F1 activates the transcription of many proteins involved in cellular DNA synthesis and cell cycle progression (1) and probably transcription of the EBV DNA polymerase gene as well (24), suggesting that E2F activity is required for lytic viral DNA replication. The molecular and cellular biological characterization of the BZLF1 protein-induced lytic replication in LCLs latently infected with EBV remains to be done.
In this study, therefore, we established a tetracycline-inducible expression system of the BZLF1 protein in the B95-8 B LCL, which is latently infected with EBV and possesses normal p53 (9). It was thus clearly demonstrated that induction of the BZLF1 protein results in arrest of cell cycle progression and inhibition of cellular DNA replication simultaneously with induction of viral DNA replication. Unexpectedly, the levels of p53 and the CDK inhibitors p21WAF-1/CIP-1, p27KIP-1, and p16INK4a were constant before and after lytic program induction. Rather, activation of S-phase-promoting CDK (S-CDK), that is, cyclin E/A-CDK2, was observed. Thus, while the EBV lytic program promoted an environment conductive to DNA replication, it inhibited cellular DNA synthesis. Such cellular conditions seem to especially favor viral lytic replication.
MATERIALS AND METHODS
Construction of recombinant plasmids.
The tetracycline-inducible retrovirus vector pCMSCV-RevTRE(hyg) consists of an AflIII-BstEII segment of pCLXSN (IMGENEX) (27) containing the CMV-5′ long terminal repeat (LTR) fusion promoter, a BstEII-BanIII segment of pRev-TRE (Clontech) containing a hygromycin B resistance gene and a tetracycline-responsive promoter, and a BanIII-AflIII segment of pMSCV-neo (Clontech) containing the murine stem cell virus (MSCV) 3′ LTR. A BamHI-SalI segment of the BZLF1 gene was inserted between BamHI and SalI sites of pCMSCV-RevTRE(hyg) to construct pCMSCV-RevTRE(hyg)-BZLF1. The BZLF1 segment containing BamHI and SalI cohesive ends and a leader sequence, ACC, for efficient translation flanking the initiation codon, was obtained by PCR with the oligonucleotides 5′-CGGATCCACCATGATGGACCCAAACTCGACTT-3′ (sense) and 5′-GCGTCGACTTAGAAATTTAAGAGATCCTCGTG-3′ (antisense) as primers and pBlcZ containing an EBV BZLF1 open reading frame derived from an EBV-producing epithelial hybrid cell line in the cloning plasmid pBluescript (33) as a template.
The retroviral vector pCLMSCV-puro-IRES2-EGFP consists of an XbaI-EcoRI segment of pCLXSN containing the CMV-5′ LTR fusion promoter, an EcoRI-BglII segment of IRES2-EGFP containing the internal ribosomal entry site (IRES) of the encephalomyocarditis virus followed by the enhanced green fluorescent protein (EGFP) coding region, a BglII-BanIII segment of pMSCVpuro[RI-Bg] containing the PGK promoter followed by the puromycin resistance gene, and a BanIII-XbaI segment of pMSCVneo (Clontech) containing the MSCV 3′ LTR. pMSCVpuro[RIBg] was constructed by inserting double-stranded oligonucleotides, 5′-GATCGAATTCGTTAACCTCGAGAGATCT-3′ and 5′-AATTAGATCTCTCGAGGTTAACGAATTC-3′, between BglII and EcoRI sites in the multicloning site of pMSCVpuro (Clontech). The IRES2-EGFP segment was obtained by PCR with 5′-TCGAATTCTGCAGTCGACGGTACCGCGGG-3′ (sense) and 5′-GAAGATCTTACTTGTACAGCTCGTCCATGCCG-3′ (antisense) primers and pIRES2-EGFP (Clontech) as a template, followed by digestion with EcoRI and BglII. A retroviral vector expressing the reverse tetracycline-controlled transactivator (rtTA) was constructed by inserting an EcoRI-BamHI segment of the rtTA between EcoRI and BamHI sites of pCLMSCV-puro-IRES2-EGFP. The rtTA segment containing EcoRI and BamHI cohesive ends and a leader sequence, CACC, for efficient translation flanking the initiation codon, was obtained by PCR with the oligonucleotides 5′-GGAATTCCACCATGTCTAGATTAGATAAAAG-3′ (sense) and 5′-CGGATCCTCGCGCCCCCTACCCACCGTA-3′ (antisense) as primers and pRevTet-On (Clontech) as a template. After the PCR product was cloned into pCR4Blunt-TOPO (Invitrogen), the rtTA segment was obtained by digesting the resultant plasmid with EcoRI and BamHI.
Generation of recombinant retroviruses.
The 293 cells were seeded at a total of 106 cells in 6-cm-diameter tissue culture plates, incubated at 37°C overnight, and then transfected with TransIt293 (panVera Corp.) with pHCMV-VSV-G (a VSV-G protein expression vector provided by T. Matsubara) (26), pCL-Gag/Pol (a Gag-Pol expression vector provided by T. Kiyono), and either pCMSCV-RevTRE(hyg)-BZLF1 or pCLMSCV-puro-rtTA-IRES2-EGFP to produce the pseudotyped recombinant retrovirus rv-BZLF1 or rv-rtTA, respectively. Virus-containing supernatants were harvested 48 to 96 h posttransfection, filtered (0.45-μm-pore-size filter), and stored at −70°C.
Cloning of Tet-BZLF1/B95-8 cells.
B95-8 cells, an EBV-infected marmoset B-cell line, were seeded at 106/well in a 24-well plate, and the stocks of the recombinant retroviruses, rv-BZLF1 and rv-rtTA, were added to each well in the presence of 8 μg of Polybrene/ml. The plate was centrifuged at 1,000 × g for 90 min at 32°C and incubated at 32°C overnight. The next day, the medium in the wells was replaced with fresh medium containing 1 μg of puromycin/ml, and the plate was incubated at 37°C for 2 days. On day 4, the medium was replaced with fresh medium containing 100 μg of hygromycin-B/ml, and the plate was incubated at 37°C for 5 days. Clones resistant to puromycin and hygromycin B were isolated by limiting dilution and checked for expression of the BZLF1 and BALF2 proteins with doxycycline by Western blot analysis.
Cell culture.
B95-8 cells were maintained in RPMI medium containing 10% fetal calf serum at 37°C in a humidified atmosphere containing 5% CO2. Tet-BZLF1/B95-8 cells were maintained in RPMI medium supplemented with 1 μg of puromycin/ml, 200 μg of hygromycin B/ml, and 10% tetracycline-free fetal calf serum. To induce lytic EBV replication in Tet-BZLF1/B95-8 cells, a tetracycline derivative, doxycycline, was added to the culture medium at a final concentration of 1 μg/ml.
Treatment of the Tet-BZLF1/B95-8 cells with gamma irradiation was performed as follows. The cells were irradiated with gamma radiation at a dose of 10 Gy and then incubated for 6 h. The cells were harvested and subjected to Western blot analysis.
Quantification of viral DNA synthesis during lytic replication.
Tet-BZLF1/B95-8 cells were incubated with or without 1 μg of doxycycline/ml and harvested at appropriate times. Total DNAs were purified with a DNeasy tissue kit (Qiagen) and quantified. Diluted amounts of DNA samples were dot blotted on a Hybond-N membrane (Amersham) and hybridized with a 32P-labeled DNA fragment consisting of a portion of the EBV EBNA1 coding region (EBV nucleotides 109110 to 109379). The signal intensity was quantified with an Image Guider (BAS2500; Fuji film). A standard curve for viral DNA quantification was obtained from serial dilutions of plasmid p500 DNA, which harbors an EBNA1 coding region. The copy numbers of the viral genome per cell were determined from the standard curve and serial dilutions of DNA from Raji cells, in which about 45 copies of the viral genome per cell are present (7). DNA from BJAB cells was also used as a negative control.
Immunofluorescence.
Cells were harvested at appropriate times postinduction (p.i.) with doxycycline (1 μg/ml), washed with phosphate-buffered saline (PBS), and resuspended in cold PBS containing 0.04% EDTA, followed by fixation with 70% ice-cold methanol at −20°C overnight. The fixed cells were resuspended in PBS containing 0.05% Triton X-100, incubated at room temperature for 15 min, and then washed with PBS containing 0.05% goat serum. The cells were incubated in 1 ml of 0.5% goat serum for 20 min and then stained with anti-BZLF1 protein polyclonal antibody or anti-BMRF1 protein-specific monoclonal antibody in PBS containing 0.05% goat serum for 60 min at room temperature. The cells were washed with PBS containing 0.05% Tween 20, suspended in PBS containing 0.05% goat serum, and reacted with biotin-conjugated goat anti-rabbit or anti-mouse immunoglobulin antibody (1:200 dilution) for 30 min at room temperature. The cells were washed three times with 1 ml of PBS containing 0.05% Tween 20 and suspended in 50 μl of PBS containing 0.05% goat serum with 5 μg of phycoerythrin-conjugated streptavidin. Samples were incubated for 30 min in the dark on ice and then washed and suspended in PBS and subjected to analysis by a fluorescence-activated cell sorter (FACS) system (Becton Dickinson).
Cell cycle analysis.
Cell cycle analysis of the Tet-BZLF1/B95-8 cells with the EBV lytic program was carried out as follows. The untreated cells and the cells treated with 1 μg of doxycycline/ml were collected at appropriate times, washed once with PBS, suspended in cold PBS containing 0.04% EDTA, and then fixed with 70% ice-cold methanol at −20°C overnight. The cells were resuspended in PBS containing 0.05% Triton X-100, incubated at room temperature for 15 min, and washed with PBS containing 0.05% goat serum. The cells were suspended in 1 ml of 0.5% goat serum and incubated for 20 min. The cells were then suspended in 30 μl of the first antibody solution containing anti-BMRF1 monoclonal antibody (Chemicon), 0.05% goat serum, and 0.05% Tween 20 in PBS. The samples were incubated for 1 h at room temperature, washed three times with 1 ml of PBS containing 0.05% Tween 20, and then suspended in 100 μl of the second antibody solution containing a 1:200 dilution of biotin-conjugated goat anti-mouse immunoglobulin antibody, 0.05% goat serum, and 0.05% Tween 20 in PBS. Samples were then incubated for 30 min at room temperature and washed three times with 1 ml of PBS containing 0.05% Tween 20. The cells were suspended in 50 μl of PBS containing 0.05% goat serum and 5 μg of dichlorotriazinyl aminofluorescein-conjugated streptavidin. Samples were then incubated for 30 min on ice in the dark. The cells were washed once with PBS containing 0.05% Tween 20 and suspended in 500 μl of PBS. Then, RNase A was added to a final concentration of 0.5%. Samples were incubated for 30 min at room temperature, and then 25 μg of propidium iodide (PI)/ml was added. Samples were incubated for 20 min at room temperature and then analyzed by a FACS system (Becton Dickinson).
Analysis of DNA synthesis by BrdU staining.
The Tet-BZLF1/B95-8 cells were incubated with 10 μM bromodeoxyuridine (BrdU; Sigma) at 48 h p.i. with or without doxycycline for 60 min. The cells were collected, washed once with PBS, suspended in cold PBS containing 0.04% EDTA, and fixed with 70% ice-cold methanol at −20°C overnight. The methanol-fixed cells were washed with PBS, followed by resuspension in 750 μl of 2 N HCl containing 0.5% Triton X-100 for 30 min at room temperature to denature the labeled, double-stranded DNA. Acid was neutralized by resuspending the cells in 750 μl of 0.1 M sodium tetraborate (pH 9.0), followed by incubation at room temperature for 5 min. The neutralized cells were centrifuged and resuspended in 20 μl of fluorescein isothiocyanate-conjugated anti-BrdU monoclonal antibody (Becton Dickinson), which was then further diluted with 50 μl of PBS containing 0.05% goat serum and 0.05% Tween 20. After incubation in the dark at room temperature for 30 min, the cells were washed twice in PBS containing 0.05% Tween 20 and resuspended in 500 μl of PBS. The suspensions were treated with RNase A to a final concentration of 0.5% for 30 min at room temperature, and the DNA was stained with 25 μg of PI/ml. Samples were incubated for 20 min at room temperature, and fluorescence was measured with a FACS system.
Protein preparation.
Tet-BZLF1/B95-8 cells were harvested at appropriate times p.i. with or without doxycycline, washed with PBS, and treated with lysis buffer (0.02% sodium dodecyl sulfate [SDS], 0.5% Triton X-100, 300 mM NaCl, 20 mM Tris-HCl [pH 7.6], 1 mM EDTA, 1 mM dithiothreitol, 10 μg of leupeptin/ml, 5 μg of Pepstatin A/ml) for 20 min on ice. Samples were centrifuged at 15,000 × g for 10 min at 4°C, and the protein concentrations of the clarified cell extracts were measured by Bradford's method.
Immunoblot analysis.
Two micrograms of protein was loaded in each lane of the SDS-10% polyacrylamide (acrylamide, 29.2; bisacrylamide, 0.8) gel electrophoresis (PAGE) gel. To separate phosphorylated forms of RB proteins, the proteins were subjected to SDS-7.5% PAGE (acrylamide, 60; bisacrylamide, 1). The separated proteins were transferred to polyvinylidene difluoride membranes. The membranes were washed with blotting buffer (1× PBS containing 0.1% Tween 20) and blocked for 60 min in blotting buffer containing 10% low-fat powdered milk. Then they were washed once with blotting buffer and incubated at room temperature for 60 min with each primary antibody in blotting buffer. The membranes were then washed in blotting buffer and incubated with horseradish peroxidase-conjugated secondary antibody at room temperature for 60 min, and protein-antibody complexes were detected by the enhanced-chemiluminescence detection system (Amersham). The images were processed by LumiVision PRO (Aisin/Taitec Inc.) with a cooled charge-coupled device (CCD) camera and assembled in an Apple G4 computer using Adobe Photoshop 5.0. The signal intensity was quantified with a LumiVision image analyzer. The system used in this study mounts the cooled CCD camera, which has 16-bit (65,535) gray-scale wide dynamic range. It enhances the accuracy of the quantitative analysis up to 100 times compared with ordinary quantitative analysis, scanning an X-ray film into the personal computer after exposing the signal to the film.
Antibodies.
The preparation and specificities of the anti-BZLF1 protein-specific, anti-BALF2 protein-specific, anti-BALF5 protein-specific, and anti-BBLF2/3 protein-specific polyclonal antibodies were described previously (40, 41, 44). An anti-BMRF1 protein-specific monoclonal antibody, clone R3, was purchased from Chemicon. Anti-p21WAF-1/CIP-1-specific and anti-p53-specific monoclonal antibodies were purchased from Calbiochem Corp. Anti-p27KIP1-specific monoclonal antibody and anti-pRB-specific monoclonal antibody were purchased from Becton Dickinson Transduction Laboratories. Anti-CDK1-specific and anti-CDK2-specific monoclonal antibodies and anti-phospho-Rb (ser 780)-specific polyclonal antibodies were purchased from MBL Inc. Anti-cyclin A-specific rabbit polyclonal antibodies and anti-cyclin E-specific mouse monoclonal antibodies were purchased from Santa Cruz Biotechnology Inc. An anti-phospho-Rb (ser 612)-specific monoclonal antibody was a gift of Katsuyuki Tamai (MBL).
RESULTS
Conditional expression of BZLF1 protein induces the EBV lytic program.
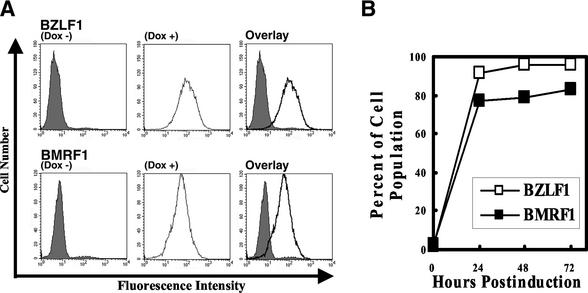
In order to investigate the effects of the EBV lytic program on the cell cycle of B lymphocytes latently infected with EBV (latency III) in detail, Tet-BZLF1/B95-8 cells in which the exogenous BZLF1 protein is conditionally expressed under the control of a tetracycline-regulated promoter were isolated. Flow cytometry analysis revealed that >90% of the Tet-BZLF1/B95-8 cells were positive for the BZLF1 protein 24 h p.i. with doxycycline (Fig. 1). To confirm expression of the functional BZLF1 protein, the cells were assayed for expression of an early EBV protein, the BMRF1 protein, used as an index of lytic infection. Approximately 80% of the cells were positive for the BMRF1 protein 48 h p.i. in the presence of doxycycline. These results confirm that treatment with doxycycline produces functional BZLF1 proteins in a majority of the cells.
FIG. 1.
(A) Populations of BZLF1 or BMRF1 protein-positive Tet-BZLF1/B95-8 cells after 72 h p.i. with doxycycline. Tet-BZLF1/B95-8 cells were cultured for 72 h in the presence (Dox +) or absence (Dox −) of doxycycline (1 μg/ml) and analyzed for expression of the BZLF1 (top) or BMRF1 (bottom) protein by immunofluorescence staining and FACS analysis with anti-BZLF1 or anti-BMRF1 protein-specific antibody. The right-hand histograms represent overlays of the Dox − and Dox + histograms. Isotype control antibodies and EBV-negative BJAB cells were also used as controls in the experiments described above to confirm which cells are the BZLF1- or BMRF1-expressing cells (data not shown). (B) Percentages of populations of BZLF1 or BMRF1 protein-positive Tet-BZLF1/B95-8 cells after the indicated times p.i. with doxycycline.
Western blot analyses revealed that expression of the exogenous BZLF1 protein was almost undetectable in the Tet-BZLF1/B95-8 cells in the absence of doxycycline but became detectable 4 h after the addition of doxycycline to the growth media (Fig. 2A). The migration of the BZLF1 protein on SDS-PAGE is known to vary among EBV strains (28, 45). The exogenously expressed BZLF1 protein derived from an EBV-producing epithelial hybrid cell line (33) migrates more slowly than the endogenous protein on SDS-PAGE. The expression level of the exogenous BZLF1 protein reached a plateau 24 h p.i. The endogenous BZLF1 protein appeared after 8 h p.i. and reached almost the same expression level as the exogenous BZLF1 protein at 24 h p.i. The other IE protein, the BRLF1 protein, also appeared 8 h p.i., and the expression level reached a plateau 24 h p.i. The BZLF1 protein is known to activate the promoter PR, at which bicistronic mRNAs containing the BRLF1 and BZLF1 open reading frames are initiated (22). Induction of other early lytic EBV proteins, the BALF2, BALF5, BMRF1, and BBLF2/3 proteins, was also examined by immunoblot analysis. The BMRF1 Pol accessory protein became clearly detectable 8 h p.i. and reached a plateau 24 h p.i. The BALF5 Pol catalytic protein, the BALF2 single-stranded DNA binding protein, and the BBLF2/3 putative helicase-primase-associated protein appeared 16 h p.i. and reached a plateau 24 h p.i.
FIG. 2.
(A) Expression of lytic-phase EBV proteins after induction of lytic phase. Tet-BZLF1/B95-8 cells were cultured in the presence (+) of 1 μg of doxycycline/ml and harvested at the indicated times. Cell lysates were separated by SDS-10% PAGE, transferred to polyvinylidene difluoride membranes, and subjected to Western blot analysis with antibodies against the indicated proteins. The molecular weight of the induced exogenous BZLF1 protein is 43,000 (43K), whereas that of the endogenous B95-8 BZLF1 protein is 40K, as judged by comparison with the BZLF1 protein expressed in B95-8 cells induced by chemical agents. (B) Kinetics of viral DNA synthesis in Tet-BZLF1/B95-8 cells after induction of the lytic phase with doxycycline. To confirm the induction of viral DNA synthesis, slot blot assays for viral DNA were performed as described in Materials and Methods. The signal intensity was quantified with an Image Guider (Fujifilm).
Slot blot assays were performed to confirm the induction of lytic viral DNA replication. As shown in Fig. 2B, the copy number of the EBV genome in the Tet-BZLF1/B95-8 cells was calculated to be ∼40 copies per cell before induction. The copy number of the viral DNA was amplified to >2,600 copies per cell 72 h p.i. Taken together, the data show that the conditional expression system induced lytic viral replication efficiently and is sufficient to allow us to examine the effect of the lytic program on the cell cycle progression.
Induction of the EBV lytic program inhibits cell proliferation.
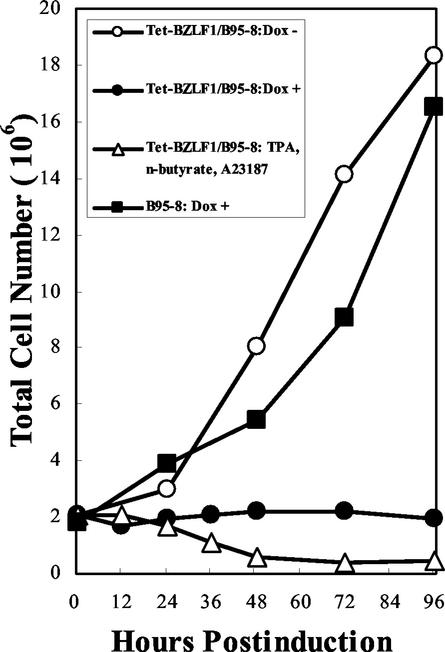
To determine whether the induction of the EBV lytic program inhibits cell proliferation, Tet-BZLF1/B95-8 cells were seeded at 2 × 105 per ml, and the cells were counted 24, 48, and 72 h after being cultured in the presence or absence of doxycycline (Fig. 3). The Tet-BZLF1/B95-8 cells ceased to divide following doxycycline annexation. In the absence of the drug, the cells continued to proliferate normally. The parental B95-8 cells continued to proliferate even in the presence of the same concentration of doxycycline, indicating that the growth arrest observed in Tet-BZLF1/B95-8 cells was not due to nonspecific effects of doxycycline on cell growth but rather to the induction of lytic replication. Cell growth arrest was also observed after the treatment of the B95-8/BZLF1 cells with drugs, such as tetradecanoyl phorbol acetate, sodium-n-butyrate, and calcium ionophore, under conditions inducing full lytic replication. Thus, it was clearly demonstrated that the conditional expression of the BZLF1 protein inhibits the proliferation of Tet-BZLF1/B95-8 cells.
FIG. 3.
Lytic-cycle induction leads to cell growth arrest. Tet-BZLF1/B95-8 cells were seeded at a density of 2 × 105 per ml in 10-cm-diameter dishes on day zero and maintained in the presence (+) or absence (−) of doxycycline (1 μg/ml). B95-8 cells were also seeded at the same density and maintained in the presence of doxycycline. At the indicated times, the cells were harvested, and the total cell numbers were determined with a hemocytometer. In a parallel experiment, Tet-BZLF1/B95-8 cells were treated with tetradecanoyl phorbol acetate (TPA), sodium n-butyrate, and calcium ionophore A23187, and cell growth was examined.
EBV lytic program arrests cells mainly with 2 N DNA content, in which viral but not cellular DNA replication is activated.
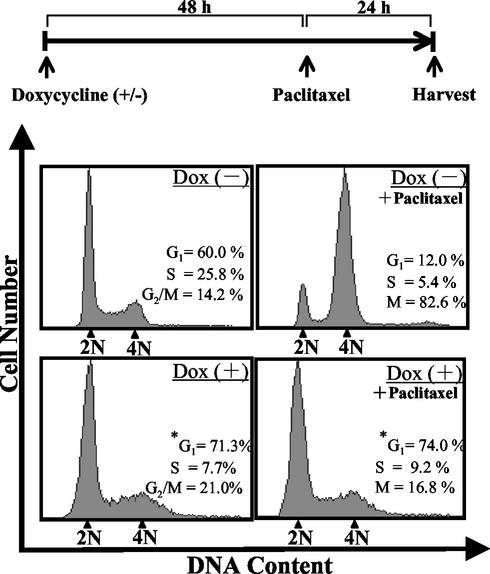
To examine the cell cycle profile of the arrested Tet-BZLF1/B95-8 cells, they were treated for 72 h with doxycycline, double stained with PI to monitor DNA content and with anti-BMRF1 antibodies as an index of lytic infection, and analyzed by flow cytometry. As shown in Fig. 4, the cell cycle profile of untreated Tet-BZLF1/B95-8 cells showed that 60, 25.8, and 14.2% of the cells were in G1, S, and G2/M phases, respectively. The cell cycle profiles of the uninduced cells were almost unchanged for 72 h (data not shown). Induction of the lytic program did not change the apparent cell cycle distribution pattern significantly, except for a slight increase in the population with 2 N DNA content. At 72 h p.i., the population of the cells with 2 N DNA content reached 71.3% of the BMRF1 protein-positive cells (Fig. 4, bottom left). The data led us to conclude that the cells are arrested mainly with 2 N DNA content, although somewhat within S or G2/M phase. To further confirm this, paclitaxel was added as an M-phase inhibitor at 48 h p.i.. The cells were further cultured for 24 h and then analyzed (Fig. 4, upper and lower right). Paclitaxel binds to microtubules and blocks the cell's ability to break down the mitotic spindle during mitosis. In the absence of doxycycline, cells in G2/M phase accumulated significantly, whereas the population of cells in G1 and S phases drastically declined, clearly indicating cell cycle progression through G1 and S phase and arrest at G2/M phase. In contrast, in BMRF1 protein-expressing cells, such accumulation was not observed, further supporting the conclusion.
FIG. 4.
Lytic program arrests cell cycle progression of Tet-BZLF1/B95-8 cells. Tet-BZLF1/B95-8 cells were cultured in the presence [Dox (+)] or absence [Dox (−)] of 1 μg of doxycycline/ml for 72 h. If necessary, paclitaxel (20 μM) was added at 48 h p.i. to arrest the cell cycle at M phase, and the cells were incubated for another 24 h (top). After fixation with methanol, the cells were doubly stained with anti-BMRF1 IgG and PI and analyzed by FACS. The cell cycle distribution was analyzed in the total cell population [Dox (−)] and the BMRF1-positive population [Dox (+)] by using ModFit LT software (Becton Dickinson).
In general, the inhibition of cell proliferation ought to result from one of two sources: cell cycle arrest or induction of programmed cell death. No PI-stained population with an apparently sub-G1 (<2 N) DNA content was detected until 96 h p.i. (data not shown), showing that EBV lytic replication blocks the increase in cell number by halting cell cycle progression, not by apoptosis.
To investigate the cellular and viral DNA replication profiles, lytic-program-induced or uninduced Tet-BZLF1/B95-8 cells were double stained by PI and anti-BrdU antibody after BrdU pulse-labeling for 1 h at 47 h p.i. in the presence or absence of phosphonoacetic acid (PAA), a specific inhibitor of the herpesvirus DNA polymerase. As shown in Fig. 5A, in the absence of doxycycline, it was demonstrated that 54.9% of the cells were in G1 and 39% were in S phase, incorporating BrdU. Addition of PAA to the culture medium did not significantly affect BrdU incorporation, demonstrating that PAA does not affect cellular DNA replication at all (Fig. 5B). In lytic-program-induced cells, 65% of the cells incorporated BrdU, two-thirds of which showed ∼2 N DNA content (Fig. 5C). Addition of PAA to the culture drastically reduced BrdU incorporation, indicating that BrdU incorporation in lytic-program-induced cells reflects mainly viral DNA synthesis (compare Fig. 5C with D). Therefore, lytic viral DNA replication is likely to be induced mainly in cells arrested with 2 N DNA content. However, from these analyses, we could not distinguish whether the cell cycle-arrested cells containing 2 N DNA were in G1 or early S phase.
FIG. 5.
BrdU incorporation and DNA content analyses in the presence (+) or absence (−) of PAA. (A and C) Tet-BZLF1/B95-8 cells were cultured in the presence (Dox +) or absence (Dox −) of 1 μg of doxycycline/ml, pulsed with BrdU for 1 h at 47 h p.i., and then harvested. (B and D) The same experiments were performed in the presence of 400 μg of PAA/ml. The harvested cells were doubly stained with fluorescein isothiocyanate-labeled anti-BrdU antibody and PI for analysis by FACS. The cell cycle distribution was analyzed in the total cell population assessed by FACS. On the left are schematic diagrams of cell cycle distributions in the non-doxycycline-treated (top) and doxycycline-treated (bottom) Tet-BZLF1/B95-8 cells.
It should be noted that when the lytic-program-induced cells were treated with PAA, a significant proportion of cells were distributed in the sub-G1 apoptotic compartment (Fig. 5D), suggesting that EBV late gene products expressed after viral DNA synthesis are an absolute requirement for the effective protection of host cells from apoptotic cell death. In accord with this notion, Inman et al. have recently reported that EBV can modulate the regulation of programmed cell death in cells treated with lytic-cycle-inducing agents (16).
Cell cycle arrest by lytic infection is not mediated by induction of p53 and CDK inhibitors.
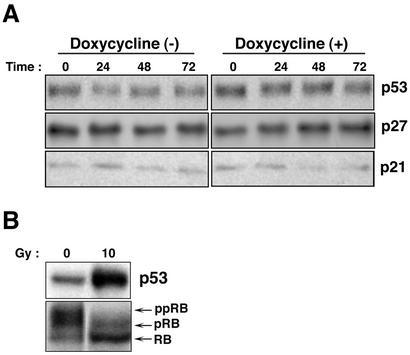
It has been reported that expression of the EBV lytic switch transactivator protein BZLF1 strongly induces the expression level of the tumor suppressor protein p53 and the CDK inhibitors p21WAF-1/CIP-1 and p27KIP-1 and results in the accumulation of a hypophosphorylated form of the Rb protein, thereby leading to G0/G1 arrest in epithelial cell lines (6). We therefore analyzed the expression levels of p53 and three CDK inhibitors, p21WAF-1/CIP-1, p27KIP-1, and p16INK4A, in Tet-BZLF1/B95-8 cells following lytic-program induction (Fig. 6). Surprisingly, the levels of p53, p21WAF-1/CIP-1, p27KIP-1 (Fig. 6A), and p16INK4A (data not shown) were constant before and after induction of the BZLF1 protein in Tet-BZLF1/B95-8 cells. Also, in uninduced cells, the levels of p53 and the CDK inhibitors were unchanged for 72 h.
FIG. 6.
Expression levels of p53 and CDK inhibitors, p21WAF-1/CIP-1 and p27KIP-1, after induction of the lytic program in Tet-BZLF1/B95-8 cells. Tet-BZLF1/B95-8 cells were cultured in the presence (+) or absence (−) of 1 μg of doxycycline/ml and harvested at the indicated times (in hours). Clarified cell lysates were prepared, separated by SDS-10% PAGE (p53 and CDK inhibitors) or by SDS-7.5% PAGE (pRB), and subjected to Western blot analysis with each specific antibody. Corresponding proteins were detected with enhanced chemiluminescence reagents. The images were processed by LumiVisionPRO (Aisin/Taitec Inc.) with a cooled CCD camera and assembled in an Apple G4 computer using Adobe Photoshop version 5.0. The signal intensity was quantified with a LumiVision image analyzer. (A) Expression levels of p53, p21WAF-1/CIP-1, and p27KIP-1 in Tet-BZLF1/B95-8 cells after doxycycline annexation. Tet-BZLF1/B95-8 cells were untreated or treated with doxycycline (1 μg/ml) and harvested at the indicated times. The expression level of p16INK4A was low and was not affected by the addition of doxycycline (data not shown). (B) Levels of p53 and phosphorylation status of Rb protein by gamma irradiation. Tet-BZLF1/B95-8 cells were irradiated with gamma radiation at a dose of 10 Gy and harvested 6 h postirradiation. The slower-migrating bands are hyperphosphorylated forms of the Rb protein (pRB and ppRB). The faster-migrating band is the hypophosphorylated form of the Rb protein (RB).
LCLs expressing the full component of latent viral genes are very sensitive to gamma irradiation or DNA-damaging agents, such as cisplatin. The response includes a rapid accumulation of p53 and p21WAF-1/CIP-1, unlike many Burkitt's lymphoma cell lines, in which mutated p53 is overproduced and inactivated (2, 9). The level of p53 in B95-8 cells was the same as that in human LCLs (data not shown) and was up-regulated by cisplatin (data not shown) or gamma irradiation. When the cells were gamma irradiated at a dose of 10 Gy, the level of p53 was elevated over fourfold and a hypophosphorylated form of the Rb protein accumulated (Fig. 6B), clearly indicating that Tet-BZLF1/B95-8 cells harbor functional and responsive p53 and retain the ability to activate checkpoint pathways. These observations indicate that lack of induction of p53 and the CDK inhibitors in Tet-BZLF1/B95-8 after lytic program induction is not due to dysfunction of the proteins.
Activation of S-phase-promoting CDK in cells arrested by lytic infection.
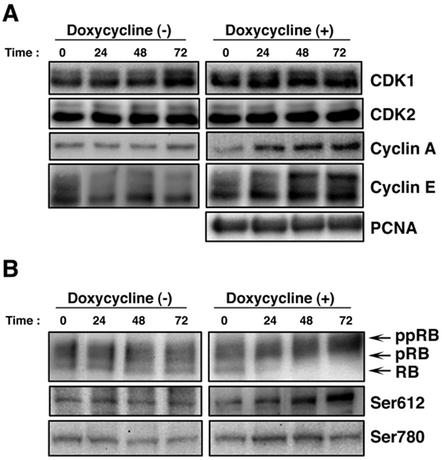
We next examined the expression kinetics of cell cycle regulatory molecules, CDK1, CDK2, cyclin A, and cyclin E, with PCNA as a control, after induction of the lytic replication program. The levels of CDK1, CDK2, and PCNA were not changed during lytic replication in non-lytic-program-induced and lytic-program-induced cells, as shown in Fig. 7A. Interestingly, after induction of the lytic phase, the level of cyclin E was increased twofold compared with the protein level at time zero; in particular, the thickness of the upper band of cyclin E was elevated. Also, the levels of cyclin A were elevated from 24 to 72 h p.i. In contrast, the levels of cyclin A and E in uninduced cells were not changed. These data suggest that S-phase-promoting CDK, namely, cyclin A/E-Cdk2, was activated during lytic infection. To test whether this is indeed the case, we examined the phosphorylation status of the Rb protein (Fig. 7B). The current concept of phosphorylation regulation of the Rb protein is as follows (5, 15, 19, 21). During early G1 phase, Rb is phosphorylated by cyclin D-CDK4/6, releasing cyclin E expression from Rb-mediated inhibition. Near the end of G1 phase, the Rb protein becomes maximally hyperphosphorylated by cyclin E/Cdk2, allowing cells to enter S phase, and the hyperphosphorylated state is maintained by cyclin A/E-CDK2 during S phase. We found that slowly migrating hyperphosphorylated Rb proteins were accumulated as lytic EBV infection proceeded. To test more precisely whether hyperphosphorylated Rb is indeed phosphorylated by CDK2 kinase, we utilized antibodies that recognize two specific phosphorylated residues of Rb, i.e., Ser780 is preferentially phosphorylated by cyclin D-CDK4/6 (20) and Ser612 is preferentially phosphorylated by cyclin E/A-CDK2 (37). As shown in Fig. 7B, CDK2 phosphorylation of Ser612 in Rb continued to increase threefold up to 72 h p.i. while CDK4-specific phosphorylation of Ser780 increased slightly from 24 to 48 h p.i. Taken together, these data clearly indicate that cell cycle arrest by lytic replication occurs around the G1/S boundary with high S-CDK activity.
FIG. 7.
Accumulation of phosphorylated Rb proteins after induction of the lytic program in Tet-BZLF1/B95-8 cells. Tet-BZLF1/B95-8 cells were cultured in the presence (+) or absence (−) of 1 μg of doxycycline/ml and harvested at the indicated times (in hours). Clarified cell lysates were prepared, separated by SDS-10% PAGE (cyclin proteins and CDKs) or by SDS-7.5% PAGE (pRB), and subjected to Western blot analysis with each specific antibody. Corresponding proteins were detected with enhanced chemiluminescence reagents. The images were processed by LumiVisionPRO (Aisin/Taitec Inc.) with a cooled CCD camera and assembled in an Apple G4 computer using Adobe Photoshop version 5.0. The signal intensity was quantified with a LumiVision image analyzer. (A) Expression levels of CDKs, cyclin proteins, and PCNA at indicated times after lytic program induction. (B) Expression levels of Rb proteins at indicated times after lytic program induction. (Top) The slower-migrating bands are hyperphosphorylated forms of Rb (pRB and ppRB). The faster-migrating band is the hypophosphorylated form of the Rb protein (RB). (Middle and bottom) Analyses of phosphospecific RB protein. Ser612 in the RB protein is phosphorylated by CDK2/cyclin E or A, whereas Ser780 is specifically phosphorylated by CDK4/cyclin D.
DISCUSSION
We succeeded in the establishment of a cell line, Tet-BZLF1/B95-8, that is simple and highly efficient for conditional induction of the lytic replication program in the absence of any other external stimuli. More than 80% of Tet-BZLF1/B95-8 cells became positive for the BZLF1 and BMRF1 proteins after doxycycline annexation, allowing us to analyze cellular events occurring in the EBV lytic-cycle-induced cells.
After activation of the lytic program by BZLF1 induction, Tet-BZLF1/B95-8 cells arrest mainly around the G1/S boundary, in which expression of p53 and CDK inhibitors is not induced and S-phase CDK activity is increased. Zacny et al. showed that induction of the lytic phase in AKATA cells, an EBV-positive Burrkitt's cell line (latency I), by IgG cross-linking resulted in accumulation of a hyperphosphorylated form of the Rb protein (46). Although the result is in agreement with our present findings, it remains possible that their result was modulated by signal transduction from surface IgG and does not reflect the natural lytic program. On the other hand, our results appear to disagree with the findings of Cayrol and Flemington that expression of the BZLF1 protein in an EBV-negative epithelial cell system induced G0/G1 cell cycle arrest with upregulation of p53 and CDK inhibitor expression (6). Also, in other reports, it has been demonstrated that BZLF1 can induce expression of p53 protein and/or CDK inhibitors (6, 30, 31), and the BZLF1 protein has been reported to possess the role of blocking progression through the restriction point in G1 phase and inhibiting DNA replication. There are several possible explanations for the discrepancy. One is a difference in the cell types used; B95-8 is derived from B cells, natural hosts of EBV infection, while EBV-negative epithelial-cell lines were used in the above-described studies. A more favorable explanation is that in B95-8 cells, many other viral proteins are expressed after induction of lytic replication, affecting cellular conditions. The BRLF1 protein may be a good candidate for such a protein. Swenson et al. showed that, using an adenoviral vector, another IE protein, BRLF1, can induce the expression of E2F1 (38). The BRLF1 protein can also activate c-myc, a cellular gene promoting cell cycle progression (29, 36). The BRLF1 protein, therefore, is likely to counteract BZLF1 and to elevate S-phase CDK activity. This point should be more deeply examined in future.
The mechanism by which the EBV lytic replication program blocks cellular DNA replication in cells with high S-phase-promoting CDK activity is also unclear. Cellular DNA synthesis is normally promoted by high S-CDK activity. The EBV lytic program may therefore have evolved specific mechanisms to inhibit cellular DNA replication, possibly by directly interfering with the process of cellular DNA synthesis. One possibility is that an EBV lytic protein(s) might interfere with the assembly or activation of prereplicative complexes. Another is that an EBV lytic protein(s) interferes with functions at chromosomal replication forks. To investigate these possibilities, further studies are needed.
Is a cellular environment produced by activated S-CDK, in other words, S-phase-like conditions, favorable for EBV lytic replication? Since herpesviruses, including EBV, encode many viral proteins for their own DNA replication, as well as nucleotide metabolism enzymes, it has often been suggested that lytic replication takes place in G0/G1 phase (4, 8, 25, 32). However, EBV DNA replication is not completely independent of cellular replication proteins, e.g., DNA ligase I and FEN 1 may be required for viral DNA replication. It is conceivable that the activities of these cellular replication proteins are promoted under S-phase-like conditions. Also, there may be more significant energy generation and greater production of other resources under S-phase-like conditions, which may support viral replication. On the other hand, it is known that transcription of the EBV DNA polymerase is activated by E2F (24). Thus, it is presumed that a cellular environment produced by activated S-CDK is favorable for EBV lytic replication. Assuming that this is indeed the case, observed specific inhibition of cellular DNA replication by lytic replication also seems reasonable; it may facilitate viral replication machinery, plundering the cellular replication proteins.
A setting similar to the cellular environment evoked by EBV lytic replication has been observed in CMV infection. Whereas CMV arrests cell cycle progression around the G1/S boundary in several different cell systems (4, 8, 25, 32), such cell cycle arrest takes place in the presence of up-regulated Cdk2/cyclin E activity and a hyperphosphorylated Rb protein (4, 17). In addition, human CMV induces the expression of proliferation markers, such as topoisomerase II and PCNA (8). The IE2 protein of human CMV has been identified as a regulatory factor that mediates cell cycle arrest. Wiebusch and Hagemeier have recently demonstrated that IE2 is a rather unique protein in that it allows cells to pass the restriction point with elevated CDK2 activity but then interferes with S-phase progression, thereby stopping cells from replicating their genomes (42). Unfortunately, the molecular basis for such an interesting phenotype evoked by CMV, as well as that for EB viral lytic replication, remains unclear.
HSV DNA replication has been thought to occur in cells at G0/G1 phase and to inhibit cellular DNA replication. However, it has recently been proposed, using several specific CDK2 inhibitors, that CDK2 kinase activity enhances viral DNA replication (34, 35). The accumulating data for CMV, HSV, and EBV suggest that herpesviruses may have evolved mechanisms by which S-phase-promoting CDK is activated while cellular DNA synthesis is inhibited to support high-level viral DNA replication.
Acknowledgments
We thank Y. Nishikawa and T. Yoshida for technical assistance. We also thank K. Tamai for providing the antibody specific for the Ser612 phosphorylation site of the Rb protein.
This work was supported by grants-in-aid for Scientific Research on Priority Areas from the Ministry of Education, Science, Sports and Culture of Japan (14021138 and 12470073 to T.T.).
REFERENCES
- 1.Adams, P. D., and W. G. Kaelin, Jr. 1995. Transcriptional control by E2F. Semin. Cancer. Biol. 6:99-108. [DOI] [PubMed] [Google Scholar]
- 2.Allday, M. J., A. Sinclair, G. Parker, D. H. Crawford, and P. J. Farrell. 1995. Epstein-Barr virus efficiently immortalizes human B cells without neutralizing the function of p53. EMBO J. 14:1382-1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baer, R., A. T. Bankier, M. D. Biggin, P. L. Deininger, P. J. Farrell, T. J. Gibson, G. Hatfull, G. S. Hudson, S. C. Satchwell, C. Seguin, et al. 1984. DNA sequence and expression of the B95-8 Epstein-Barr virus genome. Nature 310:207-211. [DOI] [PubMed] [Google Scholar]
- 4.Bresnahan, W. A., I. Boldogh, E. A. Thompson, and T. Albrecht. 1996. Human cytomegalovirus inhibits cellular DNA synthesis and arrests productively infected cells in late G1. Virology 224:150-160. [DOI] [PubMed] [Google Scholar]
- 5.Cardoso, M. C., H. Leonhardt, and B. Nadal-Ginard. 1993. Reversal of terminal differentiation and control of DNA replication: cyclin A and Cdk2 specifically localize at subnuclear sites of DNA replication. Cell 74:979-992. [DOI] [PubMed] [Google Scholar]
- 6.Cayrol, C., and E. K. Flemington. 1996. The Epstein-Barr virus bZIP transcription factor Zta causes G0/G1 cell cycle arrest through induction of cyclin-dependent kinase inhibitors. EMBO J. 15:2748-2759. [PMC free article] [PubMed] [Google Scholar]
- 7.Decaussin, G., V. Leclerc, and T. Ooka. 1995. The lytic cycle of Epstein-Barr virus in the nonproducer Raji line can be rescued by the expression of a 135-kilodalton protein encoded by the BALF2 open reading frame. J. Virol. 69:7309-7314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dittmer, D., and E. S. Mocarski. 1997. Human cytomegalovirus infection inhibits G1/S transition. J. Virol. 71:1629-1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farrell, P. J., G. J. Allan, F. Shanahan, K. H. Vousden, and T. Crook. 1991. p53 is frequently mutated in Burkitt's lymphoma cell lines. EMBO J. 10:2879-2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Faulkner, G. C., A. S. Krajewski, and D. H. Crawford. 2000. The ins and outs of EBV infection. Trends Microbiol. 8:185-189. [DOI] [PubMed] [Google Scholar]
- 11.Fields, B. N., D. M. Knipe, and P. M. Howley. 2002. Fields virology, 3rd ed. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 12.Fixman, E. D., G. S. Hayward, and S. D. Hayward. 1995. Replication of Epstein-Barr virus oriLyt: lack of a dedicated virally encoded origin-binding protein and dependence on Zta in cotransfection assays. J. Virol. 69:2998-3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fukuda, M., T. A. Satoh, M. Takanashi, K. Hirai, E. Ohnishi, and T. Sairenji. 2000. Inhibition of cell growth and Epstein-Barr virus reactivation by CD40 stimulation in Epstein-Barr virus-transformed B cells. Viral Immunol. 13:215-229. [DOI] [PubMed] [Google Scholar]
- 14.Hammerschmidt, W., and B. Sugden. 1988. Identification and characterization of oriLyt, a lytic origin of DNA replication of Epstein-Barr virus. Cell 55:427-433. [DOI] [PubMed] [Google Scholar]
- 15.Harbour, J. W., and D. C. Dean. 2000. The Rb/E2F pathway: expanding roles and emerging paradigms. Genes Dev. 14:2393-2409. [DOI] [PubMed] [Google Scholar]
- 16.Inman, G. J., U. K. Binne, G. A. Parker, P. J. Farrell, and M. J. Allday. 2001. Activators of the Epstein-Barr virus lytic program concomitantly induce apoptosis, but lytic gene expression protects from cell death. J. Virol. 75:2400-2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jault, F. M., J. M. Jault, F. Ruchti, E. A. Fortunato, C. Clark, J. Corbeil, D. D. Richman, and D. H. Spector. 1995. Cytomegalovirus infection induces high levels of cyclins, phosphorylated Rb, and p53, leading to cell cycle arrest. J. Virol. 69:6697-6704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kanamori, M., M. Tajima, Y. Satoh, Y. Hoshikawa, Y. Miyazawa, K. Okinaga, T. Kurata, and T. Sairenji. 2000. Differential effect of TPA on cell growth and Epstein-Barr virus reactivation in epithelial cell lines derived from gastric tissues and B cell line Raji. Virus Genes 20:117-125. [DOI] [PubMed] [Google Scholar]
- 19.Kato, J. 1999. Induction of S phase by G1 regulatory factors. Front. Biosci. 4:D787-D792. [DOI] [PubMed] [Google Scholar]
- 20.Kitagawa, M., H. Higashi, H. K. Jung, I. Suzuki-Takahashi, M. Ikeda, K. Tamai, J. Kato, K. Segawa, E. Yoshida, S. Nishimura, and Y. Taya. 1996. The consensus motif for phosphorylation by cyclin D1-Cdk4 is different from that for phosphorylation by cyclin A/E-Cdk2. EMBO J. 15:7060-7069. [PMC free article] [PubMed] [Google Scholar]
- 21.Krude, T., M. Jackman, J. Pines, and R. A. Laskey. 1997. Cyclin/Cdk-dependent initiation of DNA replication in a human cell-free system. Cell 88:109-119. [DOI] [PubMed] [Google Scholar]
- 22.Le Roux, F., A. Sergeant, and L. Corbo. 1996. Epstein-Barr virus (EBV) EB1/Zta protein provided in trans and competent for the activation of productive cycle genes does not activate the BZLF1 gene in the EBV genome. J. Gen. Virol. 77:501-509. [DOI] [PubMed] [Google Scholar]
- 23.Li, Q., M. K. Spriggs, S. Kovats, S. M. Turk, M. R. Comeau, B. Nepom, and L. M. Hutt-Fletcher. 1997. Epstein-Barr virus uses HLA class II as a cofactor for infection of B lymphocytes. J. Virol. 71:4657-4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu, C., N. D. Sista, and J. S. Pagano. 1996. Activation of the Epstein-Barr virus DNA polymerase promoter by the BRLF1 immediate-early protein is mediated through USF and E2F. J. Virol. 70:2545-2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu, M., and T. Shenk. 1996. Human cytomegalovirus infection inhibits cell cycle progression at multiple points, including the transition from G1 to S. J. Virol. 70:8850-8857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsubara, T., R. W. Beeman, H. Shike, N. J. Besansky, O. Mukabayire, S. Higgs, A. A. James, and J. C. Burns. 1996. Pantropic retroviral vectors integrate and express in cells of the malaria mosquito, Anopheles gambiae. Proc. Natl. Acad. Sci. USA 93:6181-6185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Naviaux, R. K., E. Costanzi, M. Haas, and I. M. Verma. 1996. The pCL vector system: rapid production of helper-free, high-titer, recombinant retroviruses. J. Virol. 70:5701-5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Packham, G., M. Brimmell, D. Cook, A. J. Sinclair, and P. J. Farrell. 1993. Strain variation in Epstein-Barr virus immediate early genes. Virology 192:541-550. [DOI] [PubMed] [Google Scholar]
- 29.Polack, A., K. Hortnagel, A. Pajic, B. Christoph, B. Baier, M. Falk, J. Mautner, C. Geltinger, G. W. Bornkamm, and B. Kempkes. 1996. c-myc activation renders proliferation of Epstein-Barr virus (EBV)-transformed cells independent of EBV nuclear antigen 2 and latent membrane protein 1. Proc. Natl. Acad. Sci. USA 93:10411-10416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodriguez, A., M. Armstrong, D. Dwyer, and E. Flemington. 1999. Genetic dissection of cell growth arrest functions mediated by the Epstein-Barr virus lytic gene product, Zta. J. Virol. 73:9029-9038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rodriguez, A., E. J. Jung, Q. Yin, C. Cayrol, and E. K. Flemington. 2001. Role of c-myc regulation in Zta-mediated induction of the cyclin-dependent kinase inhibitors p21 and p27 and cell growth arrest. Virology 284:159-169. [DOI] [PubMed] [Google Scholar]
- 32.Salvant, B. S., E. A. Fortunato, and D. H. Spector. 1998. Cell cycle dysregulation by human cytomegalovirus: influence of the cell cycle phase at the time of infection and effects on cyclin transcription. J. Virol. 72:3729-3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sato, H., H. Takeshita, M. Furukawa, and M. Seiki. 1992. Epstein-Barr virus BZLF1 transactivator is a negative regulator of Jun. J. Virol. 66:4732-4736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schang, L. M., J. Phillips, and P. A. Schaffer. 1998. Requirement for cellular cyclin-dependent kinases in herpes simplex virus replication and transcription. J. Virol. 72:5626-5637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schang, L. M., A. Rosenberg, and P. A. Schaffer. 2000. Roscovitine, a specific inhibitor of cellular cyclin-dependent kinases, inhibits herpes simplex virus DNA synthesis in the presence of viral early proteins. J. Virol. 74:2107-2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schuhmacher, M., F. Kohlhuber, M. Holzel, C. Kaiser, H. Burtscher, M. Jarsch, G. W. Bornkamm, G. Laux, A. Polack, U. H. Weidle, and D. Eick. 2001. The transcriptional program of a human B cell line in response to Myc. Nucleic Acids Res. 29:397-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suzuki, S., K. Tamai, and S. Yoshida. 2002. Enzyme-linked immunosorbent assay for distinct cyclin-dependent kinase activities using phosphorylation-site-specific anti-pRB monoclonal antibodies. Anal. Biochem. 301:65-74. [DOI] [PubMed] [Google Scholar]
- 38.Swenson, J. J., A. E. Mauser, W. K. Kaufmann, and S. C. Kenney. 1999. The Epstein-Barr virus protein BRLF1 activates S phase entry through E2F1 induction. J. Virol. 73:6540-6550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takada, K. 1984. Cross-linking of cell surface immunoglobulins induces Epstein-Barr virus in Burkitt lymphoma lines. Int. J. Cancer 33:27-32. [DOI] [PubMed] [Google Scholar]
- 40.Tsurumi, T., A. Kobayashi, K. Tamai, T. Daikoku, R. Kurachi, and Y. Nishiyama. 1993. Functional expression and characterization of the Epstein-Barr virus DNA polymerase catalytic subunit. J. Virol. 67:4651-4658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsurumi, T., A. Kobayashi, K. Tamai, H. Yamada, T. Daikoku, Y. Yamashita, and Y. Nishiyama. 1996. Epstein-Barr virus single-stranded DNA-binding protein: purification, characterization, and action on DNA synthesis by the viral DNA polymerase. Virology 222:352-364. [DOI] [PubMed] [Google Scholar]
- 42.Wiebusch, L., and C. Hagemeier. 2001. The human cytomegalovirus immediate early 2 protein dissociates cellular DNA synthesis from cyclin-dependent kinase activation. EMBO J. 20:1086-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yates, J. L., and N. Guan. 1991. Epstein-Barr virus-derived plasmids replicate only once per cell cycle and are not amplified after entry into cells. J. Virol. 65:483-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yokoyama, N., K. Fujii, M. Hirata, K. Tamai, T. Kiyono, K. Kuzushima, Y. Nishiyama, M. Fujita, and T. Tsurumi. 1999. Assembly of the Epstein-Barr virus BBLF4, BSLF1 and BBLF2/3 proteins and their interactive properties. J. Gen. Virol. 80:2879-2887. [DOI] [PubMed] [Google Scholar]
- 45.Young, L. S., R. Lau, M. Rowe, G. Niedobitek, G. Packham, F. Shanahan, D. T. Rowe, D. Greenspan, J. S. Greenspan, A. B. Rickinson, et al. 1991. Differentiation-associated expression of the Epstein-Barr virus BZLF1 transactivator protein in oral hairy leukoplakia. J. Virol. 65:2868-2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zacny, V. L., J. Wilson, and J. S. Pagano. 1998. The Epstein-Barr virus IE gene product, BRLF1, interacts with the retinoblastoma protein during the viral lytic cycle. J. Virol. 72:8043-8051. [DOI] [PMC free article] [PubMed] [Google Scholar]