Abstract
Human lymphocytes were fractionated on gradients of bovine serum albumin (BSA). Cells obtained from the more dense layers bound antigen specifically. Cells capable of interacting with antigen contained antibody which was separable with the nuclear components of the cell. Immune cells contained antibody which was specific for the immunizing antigen. Cell-bound antibody mediated and, in the presence of isologous antibody, slightly enhanced the uptake of antigen. Specific antibody passively bound to cell membranes did not promote uptake of antigen. Antigen which bound to lymphocytes in vitro was not enzymatically digested within the cell but became bound to acid-insoluble nuclear DNA.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ada G. L., Byrt P. Specific inactivation of antigen-reactive cells with 125I-labelled antigen. Nature. 1969 Jun 28;222(5200):1291–1292. doi: 10.1038/2221291a0. [DOI] [PubMed] [Google Scholar]
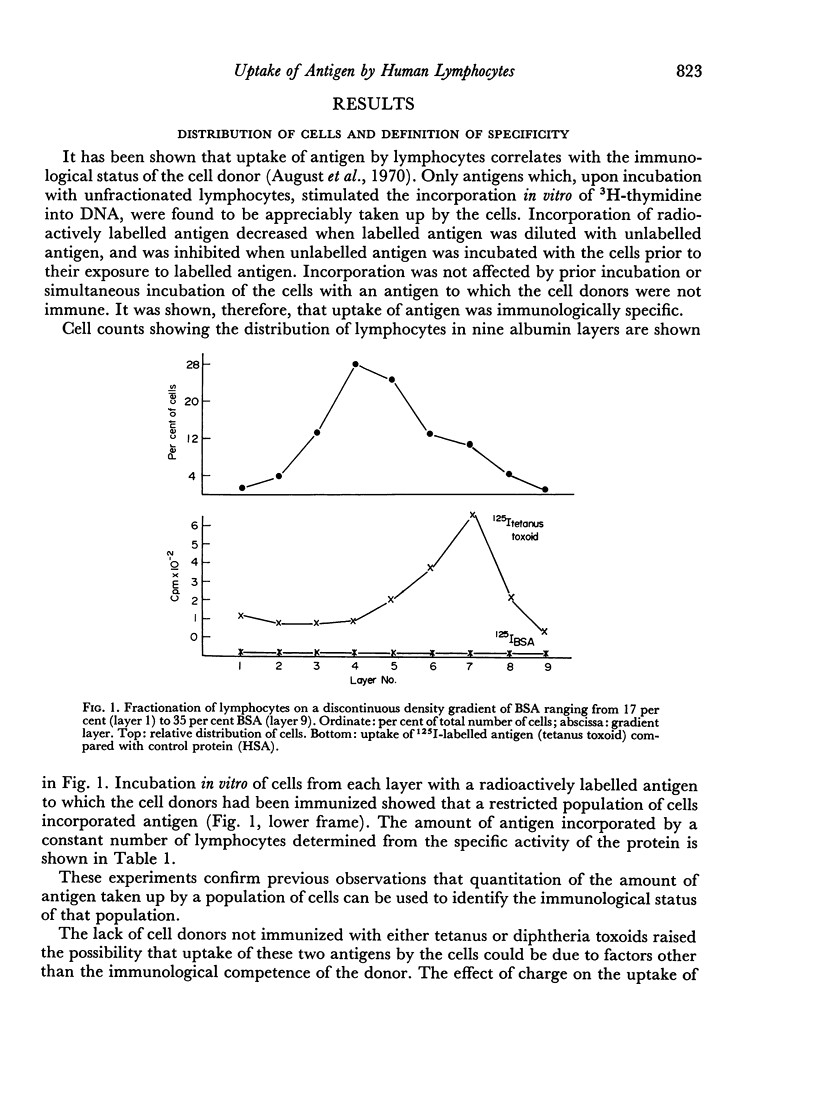
- August C. S., Merler E., Lucas D. O., Janeway C. A. The response in vitro of human lymphocytes to phytohemagglutinin and to antigens after fractionation on discontinuous density gradients of albumin. Cell Immunol. 1970 Dec;1(6):603–618. doi: 10.1016/0008-8749(70)90026-2. [DOI] [PubMed] [Google Scholar]
- Cerottini J. C., Brunner K. T. Localization of mouse isoantigens on the cell surface as revealed by immunofluorescence. Immunology. 1967 Oct;13(4):395–403. [PMC free article] [PubMed] [Google Scholar]
- Cohn Z. A. The regulation of pinocytosis in mouse macrophages. I. Metabolic requirements as defined by the use of inhibitors. J Exp Med. 1966 Oct 1;124(4):557–571. doi: 10.1084/jem.124.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy J. C., Till J. E., Siminovitch L., McCulloch E. A. The proliferative capacity of antigen-sensitive precursors of hemolytic plaque-forming cells. J Immunol. 1966 Jun;96(6):973–980. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MILLER J. J., 3rd, NOSSAL G. J. ANTIGENS IN IMMUNITY. VI. THE PHAGOCYTIC RETICULUM OF LYMPH NODE FOLLICLES. J Exp Med. 1964 Dec 1;120:1075–1086. doi: 10.1084/jem.120.6.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
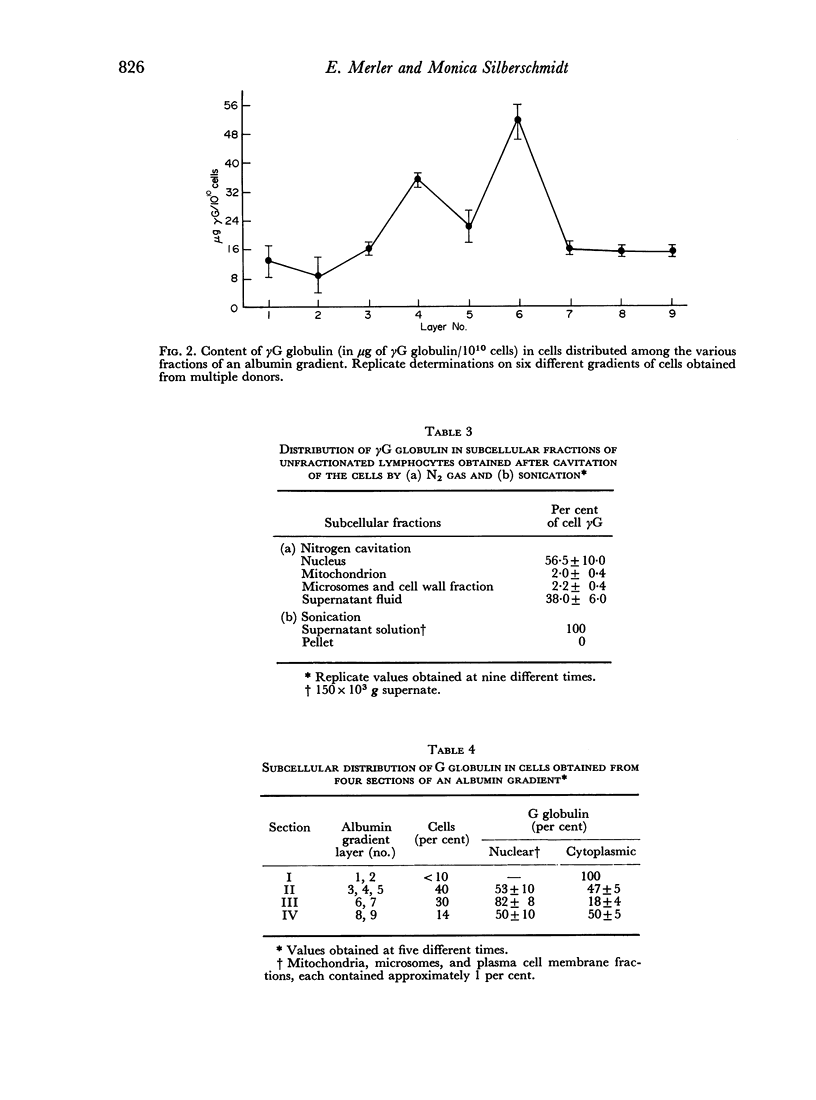
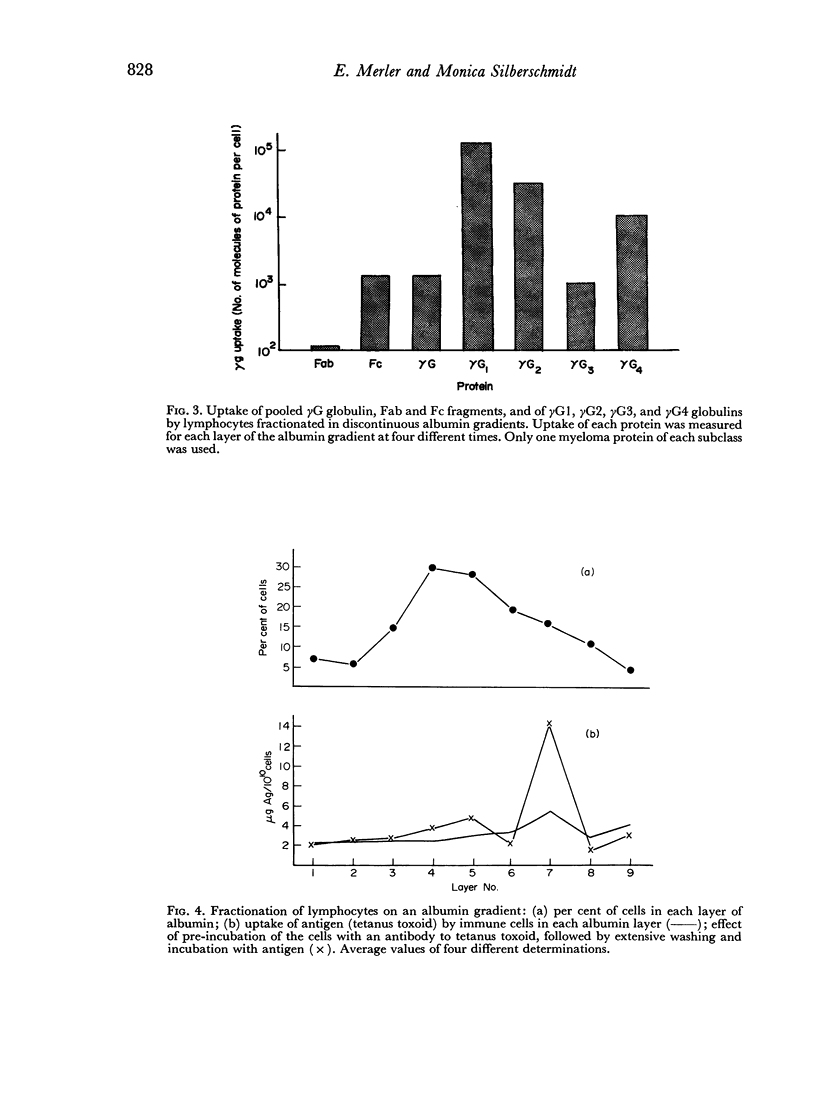
- Merler E., Silberschmidt M. Separation of antibodies from subcellular components of lymphocytes. Immunology. 1972 May;22(5):813–820. [PMC free article] [PubMed] [Google Scholar]
- Miller J. F., Mitchell G. F. Thymus and antigen-reactive cells. Transplant Rev. 1969;1:3–42. doi: 10.1111/j.1600-065x.1969.tb00135.x. [DOI] [PubMed] [Google Scholar]
- Ozer J. H., Wallace D. F. H-2 components and cellular membranes: distinctions between plasma membrane and endoplasmic reticulum governed by the H-2 region in the mouse. Transplantation. 1967 Jul;5(4):652–667. [PubMed] [Google Scholar]
- Pernis B., Forni L., Amante L. Immunoglobulin spots on the surface of rabbit lymphocytes. J Exp Med. 1970 Nov;132(5):1001–1018. doi: 10.1084/jem.132.5.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabellino E., Colon S., Grey H. M., Unanue E. R. Immunoglobulins on the surface of lymphocytes. I. Distribution and quantitation. J Exp Med. 1971 Jan 1;133(1):156–167. doi: 10.1084/jem.133.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff M. C., Sternberg M., Taylor R. B. Immunoglobulin determinants on the surface of mouse lymphoid cells. Nature. 1970 Feb 7;225(5232):553–554. doi: 10.1038/225553a0. [DOI] [PubMed] [Google Scholar]
- Wigzell H., Andersson B. Cell separation on antigen-coated columns. Elimination of high rate antibody-forming cells and immunological memory cells. J Exp Med. 1969 Jan 1;129(1):23–36. doi: 10.1084/jem.129.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigzell H., Mäkelä O. Separation of normal and immune lymphoid cells by antigen-coated coated columns. Antigen-binding characteristics of membrane antibodies as analyzed by hapten-protein antigens. J Exp Med. 1970 Jul 1;132(1):110–126. doi: 10.1084/jem.132.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dicke KA Hooft J. I., van Bekkum D. W. The selective elimination of immunologically competent cells from bone marrow and lymphatic cell mixtures. II. Mouse spleen cell fractionation on a discontinuous albumin gradient. Transplantation. 1968 Jul;6(4):562–570. doi: 10.1097/00007890-196807000-00009. [DOI] [PubMed] [Google Scholar]


