Abstract
The major (Group I) allergen of rye grass pollen and two of its allergoids, adsorbed on alumina gel, were injected into three groups of non-allergic humans. In addition to inducing the anticipated blocking antibody (IgG) response, all individuals developed immediate skin hypersensitivity to the allergen and its allergoids characteristic of reaginic antibody-(IgE-)mediated reactions. At some time during the course of the study, virtually every individual's peripheral blood leucocytes were also found to release histamine when challenged in vitro with low concentrations of allergen and allergoids. Quantitatively, each person's skin and leucocyte sensitivities were not as well correlated as in naturally allergic people. Leucocyte responsiveness was generally shortlived, but could be restored by antigenic restimulation. Allergoid: allergen sensitivity ratios were greater in allergen-than allergoid-immunized individuals, but less than in naturally allergic individuals. Unexpectedly, allergoid-immunized individuals' leucocytes were more sensitive to allergen than allergoid. Despite the observed skin and leucocyte reactivities, none of the people showed clinical manifestations of hay fever following natural exposure to pollen.
The skin sensitivity of the artificially sensitized individuals could be passively transferred to non-allergic humans by intradermal injection of serum (P-K Test), thereby implicating the involvement of IgE antibody. Further proof of the role of IgE was obtained by blocking the P-K test, either by heating the serum or by adsorbing it using an anti-IgE immunosorbent.
Full text
PDF




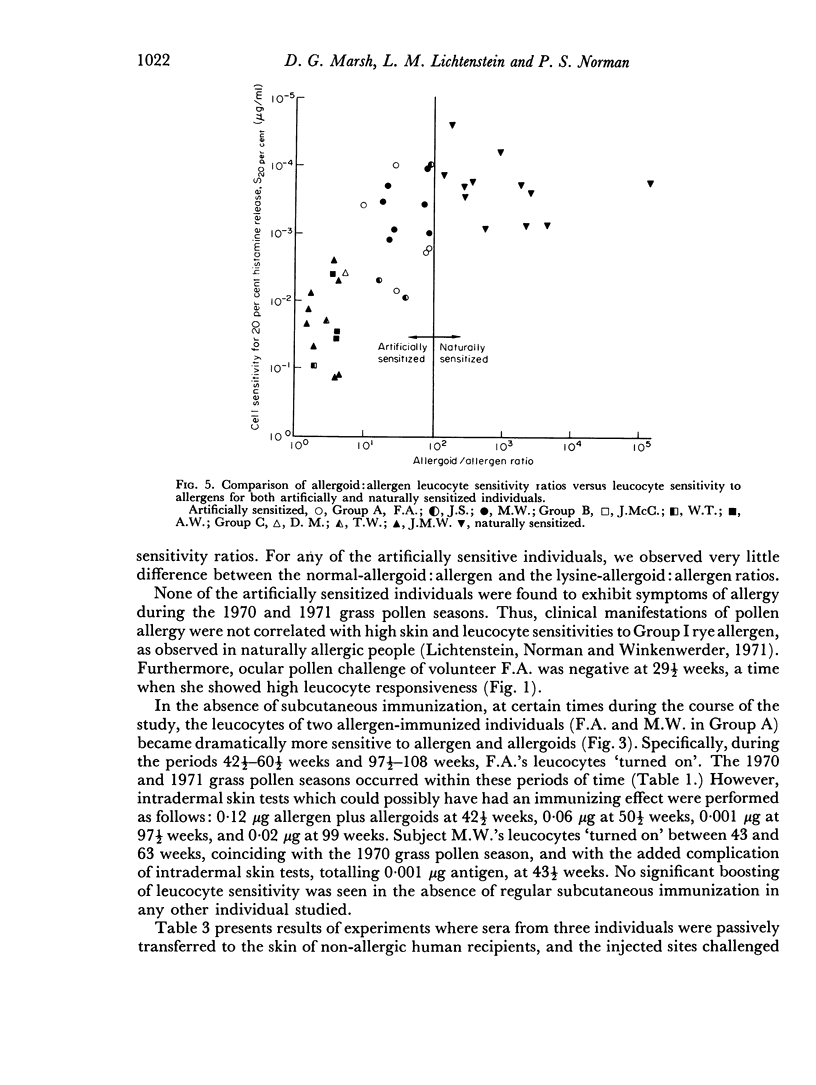

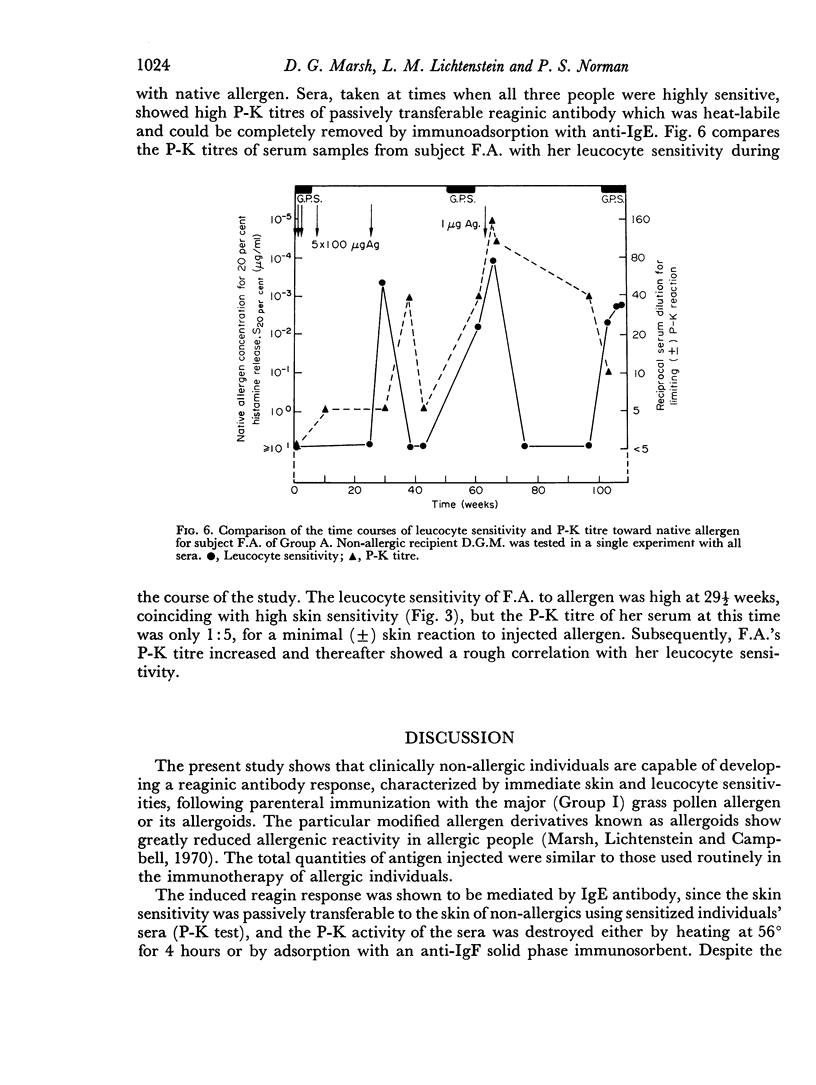




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cass R. M., Andersen B. R. The disapperance rate of skin-sensitizing antibody activity after intradermal administration. J Allergy. 1968 Jul;42(1):29–35. doi: 10.1016/0021-8707(68)90129-9. [DOI] [PubMed] [Google Scholar]
- FUCHS A. M., STRAUSS M. B. The clinical evaluation and the preparation and standardization of suspensions of a new water-insoluble whole ragweed pollen complex. J Allergy. 1959 Jan-Feb;30(1):66–82. doi: 10.1016/0021-8707(59)90060-7. [DOI] [PubMed] [Google Scholar]
- Ishizaka K., Tomioka H., Ishizaka T. Mechanisms of passive sensitization. I. Presence of IgE and IgG molecules on human leukocytes. J Immunol. 1970 Dec;105(6):1459–1467. [PubMed] [Google Scholar]
- KABAT E. A., TURINO G. M., TARROW A. B., MAURER P. H. Studies on the immunochemical basis of allergic reactions to dextran in man. J Clin Invest. 1957 Jul;36(7):1160–1170. doi: 10.1172/JCI103512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAILIN E. W., ROSSBACH E. A., WALZER M. Factors influencing reagin formation in experimental human sensitization to ascaris lumbricoides antigen; the influence of a previous sensitization on rate of sensitization. J Allergy. 1950 May;21(3):225–231. doi: 10.1016/0021-8707(50)90130-4. [DOI] [PubMed] [Google Scholar]
- LICHTENSTEIN L. M., OSLER A. G. STUDIES ON THE MECHANISMS OF HYPERSENSITIVITY PHENOMENA. IX. HISTAMINE RELEASE FROM HUMAN LEUKOCYTES BY RAGWEED POLLEN ANTIGEN. J Exp Med. 1964 Oct 1;120:507–530. doi: 10.1084/jem.120.4.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine B. B., Vaz N. M. Effect of combinations of inbred strain, antigen, and antigen dose on immune responsiveness and reagin production in the mouse. A potential mouse model for immune aspects of human atopic allergy. Int Arch Allergy Appl Immunol. 1970;39(2-3):156–171. doi: 10.1159/000230343. [DOI] [PubMed] [Google Scholar]
- Levy D. A., Lichtenstein L. M., Goldstein E. O., Ishizaka K. Immunologic and cellular changes accompanying the therapy of pollen allergy. J Clin Invest. 1971 Feb;50(2):360–369. doi: 10.1172/JCI106503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy D. A., Osler A. G. Studies on the mechanisms of hypersensitivity phenomena. XVI. In vitro assays of reaginic activity in human sera: effect of therapeutic immunization on seasonal titer changes. J Immunol. 1967 Dec;99(6):1068–1077. [PubMed] [Google Scholar]
- Lichtenstein L. M., King T. P., Osler A. G. In vitro assay of allergenic properties of ragweed pollen antigens. J Allergy. 1966 Sep;38(3):174–182. doi: 10.1016/0021-8707(66)90040-2. [DOI] [PubMed] [Google Scholar]
- Lichtenstein L. M., Norman P. S., Winkenwerder W. L., Osler A. G. In vitro studies of human ragweed allergy: changes in cellular and humoral activity associated with specific desensitization. J Clin Invest. 1966 Jul;45(7):1126–1136. doi: 10.1172/JCI105419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh D. G., Haddad Z. H., Campbell D. H. A new method for determining the distribution of allergenic fractions in biological materials: its applications to grann pollen extracts. J Allergy. 1970 Aug;46(2):107–121. doi: 10.1016/0021-8707(70)90078-x. [DOI] [PubMed] [Google Scholar]
- Marsh D. G., Lichtenstein L. M., Campbell D. H. Studies on "allergoids" prepared from naturally occurring allergens. I. Assay of allergenicity and antigenicity of formalinized rye group I component. Immunology. 1970 May;18(5):705–722. [PMC free article] [PubMed] [Google Scholar]
- Marsh D. G., Milner F. H., Johnson P. The allergenic activity and stability of purified allergens from the pollen of common rye grass (lolium perenne). Int Arch Allergy Appl Immunol. 1966;29(6):521–535. doi: 10.1159/000229739. [DOI] [PubMed] [Google Scholar]
- May C. D., Lyman M., Alberto R., Cheng J. Procedures for immunochemical study of histamine release from leukocytes with small volume of blood. J Allergy. 1970 Jul;46(1):12–20. doi: 10.1016/0021-8707(70)90056-0. [DOI] [PubMed] [Google Scholar]
- Norman P. S., Winkenwerder W. L., Lichtenstein L. M. Maintenance immunotherapy in ragweed hay fever. Booster injections at six week intervals. J Allergy. 1971 May;47(5):273–282. [PubMed] [Google Scholar]
- Ogden E. C., Raynor G. S. A new sampler for airborne pollen: the rotoslide. J Allergy. 1967 Jul;40(1):1–11. doi: 10.1016/0021-8707(67)90053-6. [DOI] [PubMed] [Google Scholar]
- Porath J., Axen R., Ernback S. Chemical coupling of proteins to agarose. Nature. 1967 Sep 30;215(5109):1491–1492. doi: 10.1038/2151491a0. [DOI] [PubMed] [Google Scholar]
- Revoltella R., Ovary Z. Preferential production of rabbit reaginic antibodies. Int Arch Allergy Appl Immunol. 1969;36(3):282–289. doi: 10.1159/000230749. [DOI] [PubMed] [Google Scholar]
- SPARKS D. B., FEINBERG S. M., BECKER R. J. Immediate skin reactivity induced in atopic and nonatopic persons following injection of emulsified pollen extracts. Clinical, immunologic, and serum studies. J Allergy. 1962 May-Jun;33:245–249. doi: 10.1016/0021-8707(62)90091-6. [DOI] [PubMed] [Google Scholar]
- SPIES J. R., BERNTON H. S. Response of nonallergic persons to injected castor bean allergen CB-IA. J Allergy. 1962 Jan-Feb;33:73–83. doi: 10.1016/0021-8707(62)90066-7. [DOI] [PubMed] [Google Scholar]
- Salvaggio J., Castro-Murillo E., Kundur V. Immunologic response of atopic and normal individuals to keyhole limpet hemocyanin. J Allergy. 1969 Dec;44(6):344–354. doi: 10.1016/0021-8707(69)90026-4. [DOI] [PubMed] [Google Scholar]
- Strejan G., Campbll D. H. Hypersensitivity toascaris anigens. V. Production of homocytotrpic antibodies in the rabbit. J Immunol. 1970 Nov;105(5):1264–1270. [PubMed] [Google Scholar]
- Waldmann T. A. Disorders of immunoglobulin metabolism. N Engl J Med. 1969 Nov 20;281(21):1170–1177. doi: 10.1056/NEJM196911202812107. [DOI] [PubMed] [Google Scholar]



