Abstract
Unlike most adenovirus (Ad) serotypes, the species B Ads do not use the coxsackie-adenovirus receptor as an attachment receptor. The species B attachment receptor(s) has not yet been identified and is also poorly characterized. Species B Ads can be further divided into species B1 and B2 Ads, and these display different organ tropisms, suggesting a difference in receptor usage. We have studied the receptor interactions of the species B1 serotypes 3p and 7p and the species B2 serotypes 11p and 35 and characterized the properties of the species B receptor(s). Reciprocal blocking experiments using unlabeled Ad11p or Ad3p virions to block the binding to A549 cells of 35S-labeled 3p, 7p, 11p, and 35 showed that only Ad11p virions efficiently blocked the binding of all the species B Ads studied (≥70%). Thus, there is apparently a common species B Ad receptor (sBAR). However, Ad3p virions only partially (≤30%) blocked the binding of Ad11p and Ad35 to A549 cells. Binding experiments after trypsin treatment of the cells confirmed that the species B2 serotypes address at least two different receptors on A549 and J82 cells, since sBAR is trypsin sensitive but the species B2 Ad receptor (sB2AR) is not. Both receptors are proteins or glycoproteins, since binding of all species B serotypes was abolished after proteinase K or subtilisin treatment of A549 or J82 cells. Furthermore, binding of the species B serotypes to sBAR was abolished with EDTA and restored with Ca2+, whereas the binding of Ad11p and Ad35 to SB2AR was independent of divalent cations.
Adenoviruses (Ads) are nonenveloped, double-stranded DNA viruses with icosahedral symmetry. A characteristic antenna-like projection, the fiber, protrudes from each of the twelve vertices of the virion. The distal knob domain of the fiber protein mediates the initial binding of the virion to host cells (17, 21), and recombinant fibers have been shown to block the binding of virions to cells at levels of up to 90% (15).
Ad entry into host cells has so far been studied in detail only for Ad2 and Ad5p (13, 26), which belong to Ad species C, and has been found to involve interactions with two separate receptors. After the initial binding of the fiber knob to the coxsackie-adenovirus receptor (CAR) (5, 22), subsequent interactions between the penton base RGD motif and αv integrins (second-step receptors) lead to endocytosis of the virus in clathrin-coated vesicles (26). Ads from all species, with the exception of Ad40 and Ad41, which constitute the enteric Ads (species F), have an RGD motif in their penton bases (2), suggesting that most Ads can use RGD-interacting integrins for their internalization. However, uptake of species B2 Ads has been reported to be independent of the presence of αv integrins (20).
At present, the set of human Ads comprises 51 members (10) and has been subdivided further into six species (A to F) on the basis of genome size and organization, DNA homology, hemagglutination properties, and oncogenicity in newborn hamsters. In general, Ad tropism for different organs differs among species (23), although Ads from different species can cause the same symptoms. Typically, Ads infect the respiratory tract (species B1, C, and E), the kidney and urinary tract (species B2), the intestines (species A and F), and the eye (species D and E) (23). One of the key determinants of tropism is believed to be the initial high-affinity attachment of the virion to host cell fiber receptors.
The species C Ads, and probably also some or most of the species A, C, D, E, and F Ad serotypes, can use CAR as a fiber attachment receptor, since soluble CAR has been shown to bind to representatives of all of the different species with the exception of species B Ads (18). Species B Ads have been known for a long time to use a different, as yet unknown fiber attachment receptor (9, 12, 14, 21). Not all of the species D Ad serotypes use CAR as a fiber attachment receptor, however. The epidemic keratoconjunctivitis (EKC)-causing serotypes (viz., Ad8, Ad19, and Ad37) use sialic acid rather than CAR (3, 4). In this case, differences in tropism clearly correlate with receptor usage.
The species B Ads have been further subdivided into species B1 and B2 on the basis of restriction enzyme cleavage patterns (25), and these two subspecies also show different tropisms (24). In this study, we set out to investigate whether the species B1 and B2 Ads use the same attachment receptor as well as to characterize the properties of the receptor(s) and to study the virus-receptor interaction. For this purpose we have used two different cell lines, A549 and J82 cells, representing respectively the two tissue types preferentially infected by species B Ads, the respiratory tract and the urinary tract.
MATERIALS AND METHODS
Cells and viruses.
The continuous cell line A549, originating from a human alveolar cell carcinoma of the lung, and the continuous cell line J82, originating from a human urinary bladder carcinoma, were used in the study. The cells were maintained in Dulbecco's modified Eagle medium (DMEM) (Sigma) supplemented with 20 mM HEPES-20 U of penicillin/ml-20 μg of streptomycin/ml-5 or 10% fetal bovine serum (FBS) (Gibco) for A549 or J82 cells, respectively.
The Ad serotypes used in this study, 3p (GB), 5p (Ad75), 7p (Gomen), 11p (Slobitski), 35 (strain S-761, prototype like), and 37 (strain 1477, prototype like), were all confirmed with respect to type by examination of their restriction patterns (1). All serotypes were grown in A549 cells and purified on CsCl gradients as described previously (16).
35S-labeling of Ads.
The virus-infected A549 cultures (∼10,000 million cells/175-cm2 bottle) were labeled at 22 h postinfection. Before addition of the isotope, the Ad-infected cultures were starved for 2 h in 8 ml of methionine- and cysteine-free DMEM. Thereafter, 1.4 mCi of [35S]methionine and [35S]cysteine (specific activity of l-cysteine and l-methionine, >1,000 Ci/mmol; Redivue Promix, Amersham Pharmacia) was added to each cell culture bottle.
After labeling for 1 h, 1 mM l-cysteine was added, and after labeling for another 4.5 h, 1 mM l-methionine was added. After labeling for 24 h, the concentrations of both l-cysteine and l-methionine were adjusted to 2 mM. The virus particles were harvested and purified at 72 h postinfection.
Binding experiments.
The binding experiments were performed in 96-well microtiter plates, using 50,000 or 100,000 cells in suspension/well, in an interaction volume of 50 or 100 μl, respectively. Phosphate-buffered saline (PBS) supplemented with 1% bovine serum albumin and 0.01% NaN3 (PBS binding buffer) was used in all experiments, except in the Mg2+ and Ca2+ titration binding experiments, in which Tris-buffered saline (TBS) (pH 7.4) supplemented in the same way (TBS binding buffer) was used. A549 or J82 cells not treated with proteases were detached from culture bottles by using PBS supplemented with 0.05% EDTA. To allow recovery from the EDTA treatment, the cells were incubated thereafter in DMEM containing 5 or 10% FBS for 1 h at 37°C on a rocker platform. The exception to this was in the Mg2+ and Ca2+ titration binding experiments, where no recovery of the cells was allowed before the start of the experiment. The cells were then applied to a microtiter plate and incubated with 10 pg/cell of 35S-labeled Ad3p, Ad5p, Ad11p, or Ad35 in PBS binding buffer containing 0.1 or 10 mM EDTA or TBS binding buffer containing 0, 0.5, 2, or 4 mM CaCl2 or MgCl2. To allow binding of the viruses, the cells were incubated with the virions for 30 min at +4°C on a rocking platform. (Binding kinetics experiments showed that the species B Ads do not reach their binding equilibrium within 6 h of incubation. With the species B Ads, after 30 min of incubation we had an easily detectable signal, and we used this incubation time throughout the present study.) Thereafter, the cells were washed once with 150 μl of PBS or TBS binding buffer and then resuspended in 100 μl of the same buffer and transferred to scintillation vials. A total of 2 ml of a scintillation liquid, Optiphase Hi Safe 3 (Wallac), was added, and counts per minute were measured in a liquid scintillation counter (Wallac 1409).
Binding experiments on A549 or J82 cells were also performed as described above after treatment with proteinase K (Amersham Pharmacia) or subtilisin (Sigma) (200 μg/ml) in 10 ml of DMEM or trypsin (Gibco) (200 μg/ml) in 10 ml of PBS for 1 h at 37°C. At the end of the incubation with the different serine proteases, 1 mM Pefabloc C (Roche) or, in the case of trypsin treatment, 10 ml of DMEM containing 5% FBS was added to the culture bottles. The detached cells were pelleted and washed once with 5 ml of PBS binding buffer before the application of virions. No recovery of the cells was allowed before the start of the experiment. The viability of the cells after protease treatment was controlled with Trypan Blue and was always found to be > 90%.
Finally, binding experiments were also performed on A549 cells after treatment with 20 mU of neuraminidase (from Vibrio cholerae; Sigma) per 200,000 cells in 100 μl of PBS-2% FBS for 90 min at 37°C on a rocking platform and on A549 cells treated with both trypsin and neuraminidase as described above.
Blocking experiments.
After detachment with PBS-EDTA and recovery, A549 cells were incubated with 10, 20, 40, or 80 pg/cell of unlabeled Ad3p or Ad11p in PBS binding buffer for 30 min on ice. Then 10 pg/cell of 35S-labeled Ad3p, Ad5p, Ad7p, Ad11p, or Ad35 was added to the cells, and they were incubated on ice for another 30 min. Thereafter, the cells were washed in 150 μl of PBS binding buffer and then resuspended in 100 μl of the same buffer and transferred to scintillation vials. Counts per minute were measured as described above.
RESULTS
Ad11p virions block the binding of both species B1 and species B2 virions to A549 cells, but Ad3p virions only partially block the binding of species B2 Ads to A549 cells.
To evaluate whether the species B1 and species B2 Ads share receptors for attachment, reciprocal blocking experiments were performed. The binding of 35S-labeled Ad3p and Ad7p (species B1) and Ad11p and Ad35 (species B2) virions to A549 cells was measured after preincubation of the cells with unlabeled Ad3p or Ad11p. The binding of 35S-labeled Ad5p (species C) was also measured as a reference.
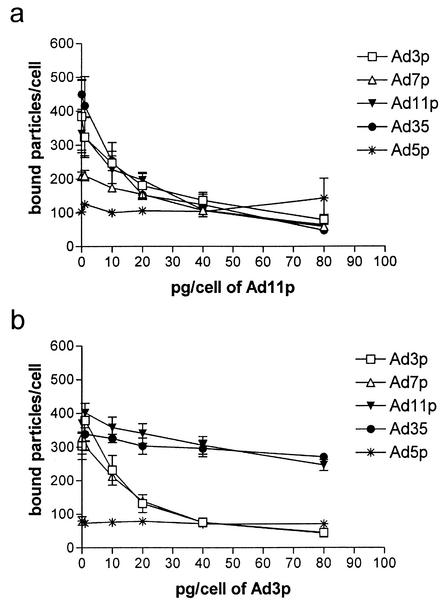
Ad11p blocked the binding of all the other species B Ads to A549 cells as efficiently as it blocked its own binding (by approximately 80%) to the same cells (Fig. 1a). There is apparently a common species B Ad receptor (sBAR). Ad3p blocked the binding of Ad7p to A549 cells with efficiency similar to that of the blocking of its own binding, but interestingly, Ad3p could only partially block the binding of Ad11p and Ad35 to A549 cells (by 30 and 20%, respectively) (Fig. 1b). Thus, the blocking was not reciprocal, indicating that apart from sBAR, there is also a species B2-specific Ad receptor, sB2AR. Neither Ad3p nor Ad11p affected the binding of Ad5p to A549 cells.
FIG. 1.
(a and b) Reciprocal blocking of Ad species B1 and B2 binding to A549 cells. A549 cells were preincubated for 30 min at +4°C with 0, 1, 10, 20, 40, or 80 pg/cell of unlabeled Ad11p (species B2) (a) or Ad3p (species B1) (b), and thereafter, 1 pg/cell of 35S-labeled Ad3p, Ad7p, Ad11p, Ad35, and Ad5p was added and the cells were incubated for another 30 min. The results are means ± standard errors of the means (SEM) of duplicate data from two independent experiments.
The common sBAR and sB2AR are proteins, but only sBAR is trypsin sensitive.
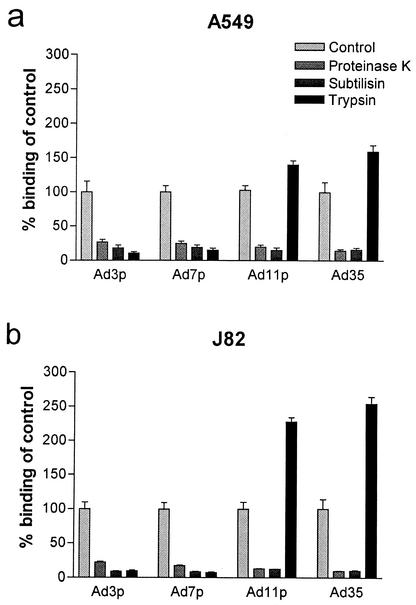
To investigate whether the species B receptor(s) is a protein, binding experiments with 35S-labeled Ad3p, Ad7p, Ad11p, and Ad35 were done after treatment of A549 or J82 cells with subtilisin, proteinase K, or trypsin (Fig. 2). Proteinase K has no particular cleavage specificity. Subtilisin has broad cleavage specificity but cleaves preferably after neutral or acidic amino acids, whereas trypsin only cleaves after arginine or lysine.
FIG. 2.
Binding of species B1 and B2 Ads after protease treatment of A549 or J82 cells. A549 cells (a) or J82 cells (b) pretreated with subtilisin, proteinase K, or trypsin (200 μg/ml) for 1 h at 37°C were incubated with 10 pg/cell of 35S-labeled Ad3p, Ad7p, Ad11p, and Ad35 for 30 min at +4°C. Binding to A549 or J82 cells detached with PBS-EDTA and recovered in medium was used as a reference. The results are means ± SEM of duplicate data from two to three independent experiments.
The binding of all species B Ads studied decreased by 80 to 90% after cleavage of the A549 or J82 cell surfaces with subtilisin and by 75 to 90% after treatment with proteinase K (Fig. 2). The binding of species B1 Ad3p and species B1 Ad7p to A549 or J82 cells decreased by ≥85% after trypsin treatment of the cells (Fig. 2). However, the binding of species B2 Ad11p and species B2 Ad35 to A549 or J82 cells increased after trypsin treatment (Fig. 2) of the cells. The binding of Ad11p and Ad35 to A549 cells increased by 40 and 60%, respectively. The increases in binding of the species B2 serotypes to J82 cells were markedly higher; the increases for Ad11p and Ad35 were 130 and 155%, respectively. Thus, there are at least two different species B adenovirus receptors. Apart from sBAR there is also sB2AR, exclusively used by the species B2 Ads tested. Both species B adenovirus rceptors are proteins, but only sBAR was trypsin sensitive.
Residual protease activity may affect binding of virions. To ascertain whether this was the case, virions incubated with protease-treated cells were also analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis in parallel with equal amounts of purified virions and virions incubated with each of the proteases. The autoradiograms showed no signs of proteolysis of virions that had been incubated with protease-treated cells (data not shown).
Binding of the species B Ads to sBAR increases and binding of the species B2 Ads to sB2AR decreases after neuraminidase treatment of A549 cells.
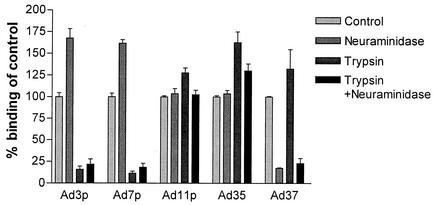
Sialic acid has been proposed to be an attachment receptor for the EKC-causing species D Ads. The species B Ads are known to be non-CAR binding, and to evaluate whether sialic acid might be of importance in the receptor interaction with species B Ads, binding experiments were done on A549 cells after neuraminidase treatment. As a control for the neuraminidase activity, Ad37 was also included in the experiment. The binding of Ad37 has been shown to decrease by 80 to 90% when sialic acid is effectively removed (3).
The binding of Ad11p and Ad35 to neuraminidase-treated A549 cells was unaffected. However, the binding of Ad3p and Ad7p to A549 cells increased by >60% after neuraminidase cleavage. To evaluate whether this increased binding of the species B1 Ads to neuraminidase-treated A549 cells was due to increased binding to sBAR or was rather a consequence of acquired binding capacity to sB2AR, binding experiments were also performed on A549 cells that had been treated with both trypsin and neuraminidase, since sBAR is trypsin sensitive but sB2AR is not.
The binding of Ad3p and Ad7p to A549 cells decreased almost as much (by approximately 75%) after combined trypsin and neuraminidase treatment as after trypsin treatment alone (Fig. 3). Thus, the increased binding of the species B1 serotypes to neuraminidase-treated A549 cells could not be explained by acquired binding capacity to sB2AR. The binding of Ad11p and Ad35 increased significantly after trypsin treatment of A549 cells. However, the binding of Ad11p and Ad35 after combined trypsin and neuraminidase treatment did not increase as much as after trypsin treatment of A549 cells. Thus, the increased binding to sB2AR after trypsin treatment was lowered by the neuraminidase treatment. Taken together, the results indicate that neuraminidase treatment increases the binding of the species B serotypes to sBAR but decreases the binding of the species B2 serotypes to sB2AR.
FIG. 3.
Binding of species B1 and B2 Ads after neuraminidase treatment or combined neuraminidase and trypsin treatment of A549 cells. A549 cells pretreated with 20 mU/100 μl of neuraminidase or 200 μg/ml of trypsin or 20 mU/100 μl of neuraminidase and 200 μg/ml of trypsin were incubated with 10 pg/cell of 35S-labeled Ad3p, Ad7p, Ad11p, Ad35, and Ad37 for 30 min at +4°C. Binding to A549 cells detached with PBS-EDTA and recovered in medium was used as reference. The results are means ± SEM of duplicate data from three or four independent experiments.
Binding of the species B Ads to sBAR is dependent of Ca2+ ions.
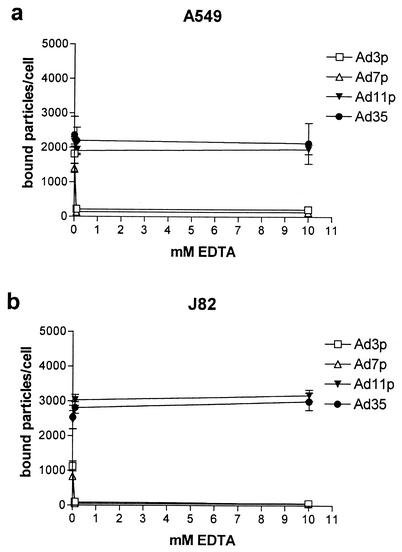
To determine whether the binding of the species B Ads to sBAR and sB2AR on A549 and J82 cells was dependent on divalent cations, binding experiments were carried out in the presence of 0.1 or 10 mM EDTA (Fig. 4).
FIG. 4.
Binding of species B1 and B2 Ads to A549 or J82 cells in the presence of EDTA. A total of 10 pg/cell of 35S-labeled Ad3p, Ad7p, Ad11p, and Ad35 was incubated with A549 cells (a) or J82 cells (b) (detached with PBS-EDTA and recovered in medium) for 30 min at +4°C in PBS binding buffer supplemented with 0.1, or 10 mM EDTA. The results are means ± SEM of duplicate data from two or three independent experiments.
The binding of 35S-labeled Ad3p and Ad7p was totally disrupted after the addition of 0.1 mM EDTA, whereas the binding of 35S-labeled Ad11p and Ad35 was essentially unchanged in the presence of EDTA (Fig. 4). Thus, binding of the species B Ads to sBAR was dependent on divalent cations. The binding of the species B2 Ads to sB2AR was not affected by EDTA, however.
To elucidate which divalent cation was of importance for binding of the species B Ads to sBAR, binding experiments were performed in the presence of 0, 0.5, 1, 2, and 4 mM Ca2+ or Mg2+ ions on A549 or J82 cells previously treated with PBS-EDTA.
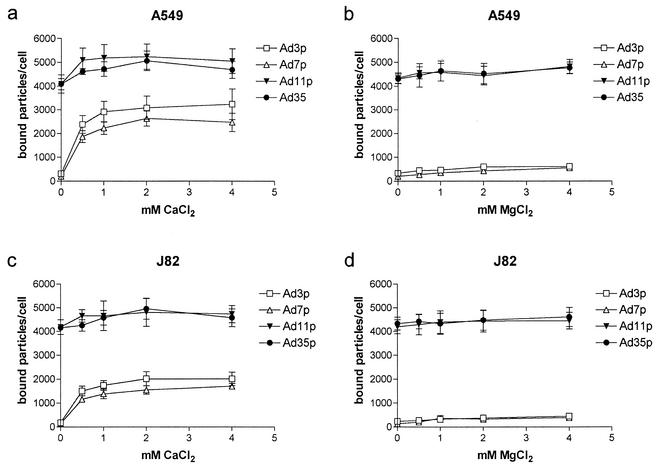
Ca2+ ions totally restored the A549 or J82 cell binding of all species B Ads studied, whereas Mg2+ ions only marginally increased the binding of Ad3p and Ad7p to A549 or J82 cells (Fig. 5). The binding of Ad11p or Ad35 to A549 or J82 cells was essentially unaffected after the addition of Mg2+. Taken together, these results indicate that Ca2+ ions are necessary for the binding of the species B serotypes to sBAR.
FIG. 5.
Binding of species B1 and B2 Ads to A549 or J82 cells in the presence of Mg2+ or Ca2+ ions. A total of 10 pg/cell of 35S-labeled Ad3p, Ad7p, Ad11p, and Ad35 was incubated with A549 cells (a and b) or J82 cells (c and d) (detached with PBS-EDTA and not recovered) for 30 min at +4°C in TBS binding buffer supplemented with 0, 0.5, 1, 2, or 4 mM of CaCl2 (a and c) or MgCl2 (b and d). The results are means ± SEM of duplicate data from two independent experiments.
Another observation is that the species B1 serotypes showed 1.5 to 1.7 times higher binding to A549 cells than to J82 cells whereas the species B2 serotypes bound equally well to J82 and A549 cells (Fig. 5).
DISCUSSION
Currently, CAR is the only well-characterized Ad fiber binding or attachment receptor (5, 6, 8, 22). Previous studies have also indicated that most human Ads, with the exception of species B, use CAR as an attachment receptor (18). The species B adenovirus receptor(s) has not yet been identified and is also poorly characterized. Species B Ads group into two clusters on the basis of DNA homology and of the display of different tissue tropisms, the latter suggesting a difference in receptor usage. In this study, we have shown for the first time that there is indeed a difference in receptor usage within the species B group which also correlates well with the differences in tropism between species B1 and B2 serotypes.
Reciprocal blocking experiments have shown that there is a common sBAR, since Ad11p (B2) virions blocked the binding of all the other species B serotypes studied. However, Ad3p (B1) only partially blocked the binding of Ad11p and Ad35, strongly indicating that the species B2 serotypes have yet another receptor. Binding experiments after trypsin treatment of A549 or J82 cells confirmed the existence of sB2AR, since sBAR appears to be trypsin sensitive but sB2AR is not. Thus, binding of the species B1 serotypes to A549 or J82 cells was abolished after trypsin treatment of the cells whereas the binding of Ad11p and Ad35 increased after trypsin treatment of the cells. The increase in binding after trypsin treatment of A549 or J82 cells seems to be higher for Ad35 than for Ad11p, indicating that Ad35 has higher affinity than Ad11p for sB2AR. Binding experiments on A549 or J82 cells treated with a protease with broad specificity, such as subtilisin and proteinase K, indicated that both receptors are proteins (or glycoproteins), since such treatment disrupted the binding of all of the species B Ads to A549 and J82 cells. Our virus binding data have thus clearly shown that the species B2 serotypes, Ad11p and Ad35, bind to at least one more receptor on A549 and J82 cells, namely, sB2AR. This appears to be a species B2-specific Ad receptor, since sB2AR could not be addressed by the species B1 serotypes Ad3p and Ad7p. Our binding results also indicated that sB2AR is expressed to a greater extent on J82 cells than on A549 cells, since the species B2 serotypes showed higher binding to J82 cells than to A549 cells after trypsin cleavage, which removes sBAR. Furthermore, the species B2 serotypes bound equally well to J82 and A549 cells but the species B1 serotypes bound better to A549 cells than to J82 cells. This also indicates a higher expression of sB2AR as well as a lower expression of sBAR on J82 cells than on A549 cells. Our data thus indicate that a tissue expression pattern of receptors exists that fits well with the different tropisms described for species B1 and B2 serotypes. These data and recent data showing that Ad5p can also use VCAM (which shares homologous regions with CAR) as an attachment receptor (7) might indicate that other Ad serotypes also use more than one attachment receptor that is species specific. If this is so, it would probably explain the differences in tropism observed among the CAR binding species A, C, D, E, and F (23).
Sialic acid serves as the attachment receptor for the EKC-causing Ads Ad8, Ad19a, and Ad37 of species D, since binding of these Ads to A549 cells can be disrupted by neuraminidase treatment of the cells. Furthermore, Ad37 has been shown not to bind to CAR (3). Species B Ads are also non-CAR binding Ads, although having different tropisms from those of the EKC-causing Ads. Our results show that after removal of A549 cell surface sialic acid, the binding of the species B1 serotypes increases by about 60%. This increase in binding might be explained by increased affinity for sBAR after removal of sialic acid from sBAR or increased accessibility of sBAR after removal of adjacent disturbing sialylated structures. Ad5p manifests an analogous enhanced binding to A549 cells after neuraminidase treatment (3). The increase in binding of the species B1 serotypes after neuraminidase treatment cannot be explained by acquired binding capacity to sB2AR, however, since neuraminidase treatment of trypsin-treated A549 cells did not restore binding of the species B1 Ads. (sBAR is trypsin sensitive, whereas sB2AR is not.).
The binding of the species B2 serotypes to A549 cells did not change significantly after neuraminidase treatment of the cells. However, the increase in binding of the species B2 serotypes after trypsin treatment diminished when trypsin treatment was combined with neuraminidase treatment, indicating that the affinity for sB2AR of the species B2 serotypes diminished after sialic acid removal. Decreased affinity for sB2AR would also explain why the net effect on binding of the species B2 serotypes after neuraminidase treatment alone was around zero, since the binding to sBAR seems to increase after neuraminidase treatment. These results indicate that sB2AR is sialylated and that sialic acid might be part of the binding interaction. However, sialic acid does not seem to be of crucial importance for the binding of the species B serotypes to either sBAR or sB2AR. Thus, identification of the species B receptors would add two as yet unidentified proteins or glycoproteins to the family of Ad receptors.
Ad3 virions, as well as Ad3 recombinant fiber (species B1), have earlier been shown by virus overlay protein binding assay to bind to a membrane protein of an apparent size of 130 kDa, and the binding was also shown to be dependent on divalent cations (11). In our experiments, we confirmed that all receptor interactions for the species B1 serotypes Ad3p and Ad7p were dependent on divalent cations, since binding of Ad3p and Ad7p was abolished in the presence of EDTA. We also showed that Ca2+ ions restored the binding of Ad3p and Ad7p. Addition of Mg2+ ions after EDTA treatment marginally increased the binding of Ad3p and Ad7p but could not restore the binding to control levels, indicating that the tertiary structure of sBAR is dependent on Ca2+. Binding of the species B2 serotypes Ad11p and Ad35 to A549 or J82 cells was essentially unchanged in the presence of EDTA, showing that divalent cations are not important for binding to sB2AR.
It appears that sBAR and sB2AR have rather different properties and might be two nonrelated proteins. The different tissue tropisms of species B1 and species B2 Ads might indicate differences in the tissue expression of sBAR and sB2AR.
Ad vectors based on species B Ads are promising alternatives when dealing with cells with low or no expression of CAR, such as hematopoietic cells (19, 20). This paper clearly shows that there are differences in receptor usage within the species B Ads which should be considered when creating a species B-based vector. Our findings indicate, however, that an Ad3- or Ad7-based vector would have a narrow tropism compared to that of an Ad35- or Ad11-based vector and that it is of importance to consider whether a species B1 or a species B2 vector (depending on the target) would be the more suitable.
Acknowledgments
This project was financed with grants from the Swedish Science Council (VR), grant no. K2001-06X-05688-22B.
REFERENCES
- 1.Adrian, T., G. Wadell, J. C. Hierholzer, and R. Wigand. 1986. DNA restriction analysis of adenovirus prototypes 1 to 41. Arch. Virol. 91:277-290. [DOI] [PubMed] [Google Scholar]
- 2.Albinsson, B., and A. H. Kidd. 1999. Adenovirus type 41 lacks an RGD α(v)-integrin binding motif on the penton base and undergoes delayed uptake in A549 cells. Virus Res. 64:125-136. [DOI] [PubMed] [Google Scholar]
- 3.Arnberg, N., K. Edlund, A. H. Kidd, and G. Wadell. 2000. Adenovirus type 37 uses sialic acid as a cellular receptor. J. Virol. 74:42-48. [PMC free article] [PubMed] [Google Scholar]
- 4.Arnberg, N., A. H. Kidd, K. Edlund, F. Olfat, and G. Wadell. 2000. Initial interactions of subgenus D adenoviruses with A549 cellular receptors: sialic acid versus αv integrins. J. Virol. 74:7691-7693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergelson, J. M., J. A. Cunningham, G. Droguett, E. A. Kurt-Jones, A. Krithivas, J. S. Hong, M. S. Horwitz, R. L. Crowell, and R. W. Finberg. 1997. Isolation of a common receptor for coxsackie B viruses and adenoviruses 2 and 5. Science 275:1320-1323. [DOI] [PubMed] [Google Scholar]
- 6.Bewley, M. C., K. Springer, Y. B. Zhang, P. Freimuth, and J. M. Flanagan. 1999. Structural analysis of the mechanism of adenovirus binding to its human cellular receptor, CAR. Science 286:1579-1583. [DOI] [PubMed] [Google Scholar]
- 7.Chu, Y., D. Heistad, M. I. Cybulsky, and B. L. Davidson. 2001. Vascular cell adhesion molecule-1 augments adenovirus-mediated gene transfer. Arterioscler. Thromb. Vasc. Biol. 21:238-242. [DOI] [PubMed] [Google Scholar]
- 8.Cohen, C. J., J. T. Shieh, R. J. Pickles, T. Okegawa, J. T. Hsieh, and J. M. Bergelson. 2001. The coxsackievirus and adenovirus receptor is a transmembrane component of the tight junction. Proc. Natl. Acad. Sci. USA 98:15191-15196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Defer, C., M.-T. Belin, M. L. Caillet-Boudin, and P. Boulanger. 1990. Human adenovirus-host cell interactions: comparative study with members of subgroups B and C. J. Virol. 64:3661-3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Jong, J. C., A. G. Wermenbol, M. W. Verweij-Uijterwaal, K. W. Slaterus, P. Wertheim-Van Dillen, G. J. J. Van Doornum, S. H. Khoo, and J. C. Hierholzer. 1999. Adenoviruses from human immunodeficiency virus-infected individuals, including two strains that represent new candidate serotypes Ad50 and Ad51 of species B1 and D, respectively. J. Clin. Microbiol. 37:3940-3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Di Guilmi, A. M., A. Barge, P. Kitts, E. Gout, and J. Chroboczek. 1995. Human adenovirus serotype 3 (Ad3) and the Ad3 fiber protein bind to a 130-kDa membrane protein on HeLa cells. Virus Res. 38:71-81. [DOI] [PubMed] [Google Scholar]
- 12.Gall, J., A. Kass-Eisler, L. Leinwand, and E. Falck-Pedersen. 1996. Adenovirus type 5 and 7 capsid chimera: fiber replacement alters receptor tropism without affecting primary immune neutralization epitopes. J. Virol. 70:2116-2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greber, U. F., M. Willetts, P. Webster, and A. Helenius. 1993. Stepwise dismantling of adenovirus 2 during entry into cells. Cell 75:477-486. [DOI] [PubMed] [Google Scholar]
- 14.Krasnykh, V. N., G. V. Mikheeva, J. T. Douglas, and D. T. Curiel. 1996. Generation of recombinant adenovirus vectors with modified fibers for altering viral tropism. J. Virol. 70:6839-6846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mei, Y. F., K. Lindman, and G. Wadell. 2002. Human adenoviruses of subgenera B, C, and E with various tropisms differ in both binding to and replication in the epithelial A549 and 293 cells. Virology 295:30-43. [DOI] [PubMed] [Google Scholar]
- 16.Mei, Y. F., K. Lindman, and G. Wadell. 1998. Two closely related adenovirus genome types with kidney or respiratory tract tropism differ in their binding to epithelial cells of various origins. Virology 240:254-266. [DOI] [PubMed] [Google Scholar]
- 17.Philipson, L., K. Lonberg-Holm, and U. Pettersson. 1968. Virus-receptor interaction in an adenovirus system. J. Virol. 2:1064-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roelvink, P. W., A. Lizonova, J. G. Lee, Y. Li, J. M. Bergelson, R. W. Finberg, D. E. Brough, I. Kovesdi, and T. J. Wickham. 1998. The coxsackievirus-adenovirus receptor protein can function as a cellular attachment protein for adenovirus serotypes from subgroups A, C, D, E, and F. J. Virol. 72:7909-7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Segerman, A., Y. F. Mei, and G. Wadell. 2000. Adenovirus types 11p and 35p show high binding efficiencies for committed hematopoietic cell lines and are infective to these cell lines. J. Virol. 74:1457-1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shayakhmetov, D. M., T. Papayannopoulou, G. Stamatoyannopoulos, and A. Lieber. 2000. Efficient gene transfer into human CD34+ cells by a retargeted adenovirus vector. J. Virol. 74:2567-2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stevenson, S. C., M. Rollence, J. Marshall-Neff, and A. McClelland. 1997. Selective targeting of human cells by a chimeric adenovirus vector containing a modified fiber protein. J. Virol. 71:4782-4790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tomko, R. P., R. Xu, and L. Philipson. 1997. HCAR and MCAR: the human and mouse cellular receptors for subgroup C adenoviruses and group B coxsackieviruses. Proc. Natl. Acad. Sci. USA 94:3352-3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wadell, G. 1994. Adenoviruses, p. 1-7. In Encyclopedia of virology, vol. 1. Academic Press, Inc., New York, N.Y.
- 24.Wadell, G. 1984. Molecular epidemiology of human adenoviruses. Curr. Top. Microbiol. Immunol. 110:191-220. [DOI] [PubMed] [Google Scholar]
- 25.Wadell, G., M. L. Hammarskjold, G. Winberg, T. M. Varsanyi, and G. Sundell. 1980. Genetic variability of adenoviruses. Ann. N. Y. Acad. Sci. 354:16-42. [DOI] [PubMed] [Google Scholar]
- 26.Wickham, T. J., P. Mathias, D. A. Cheresh, and G. R. Nemerow. 1993. Integrins alpha v beta 3 and alpha v beta 5 promote adenovirus internalization but not virus attachment. Cell 73:309-319. [DOI] [PubMed] [Google Scholar]