Abstract
In the previous paper we showed that the immune response to sheep red cells (SRBC) was degenerate; serum antibodies showed increasing loss of specificity, signalled by cross-reactions with horse RBC (HRBC), with time after immunization and with hyperimmunization. In the present report we have analysed the role T cells may play in this process.
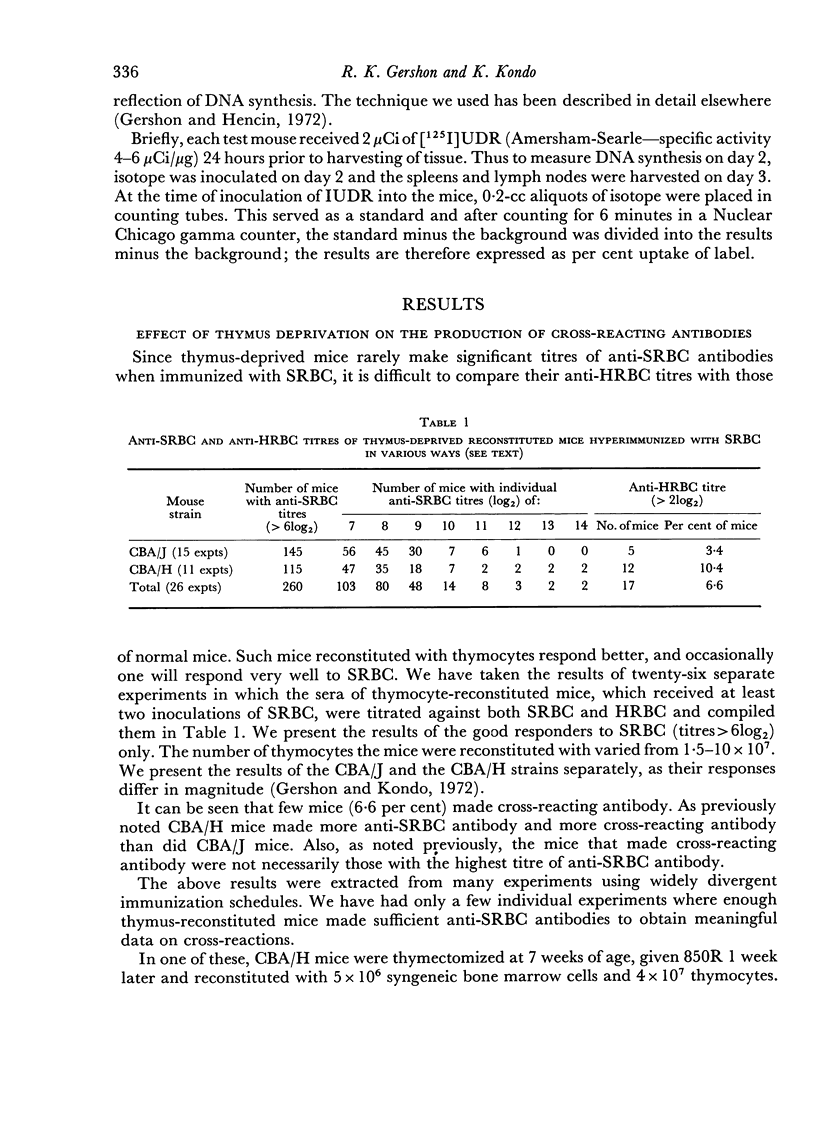
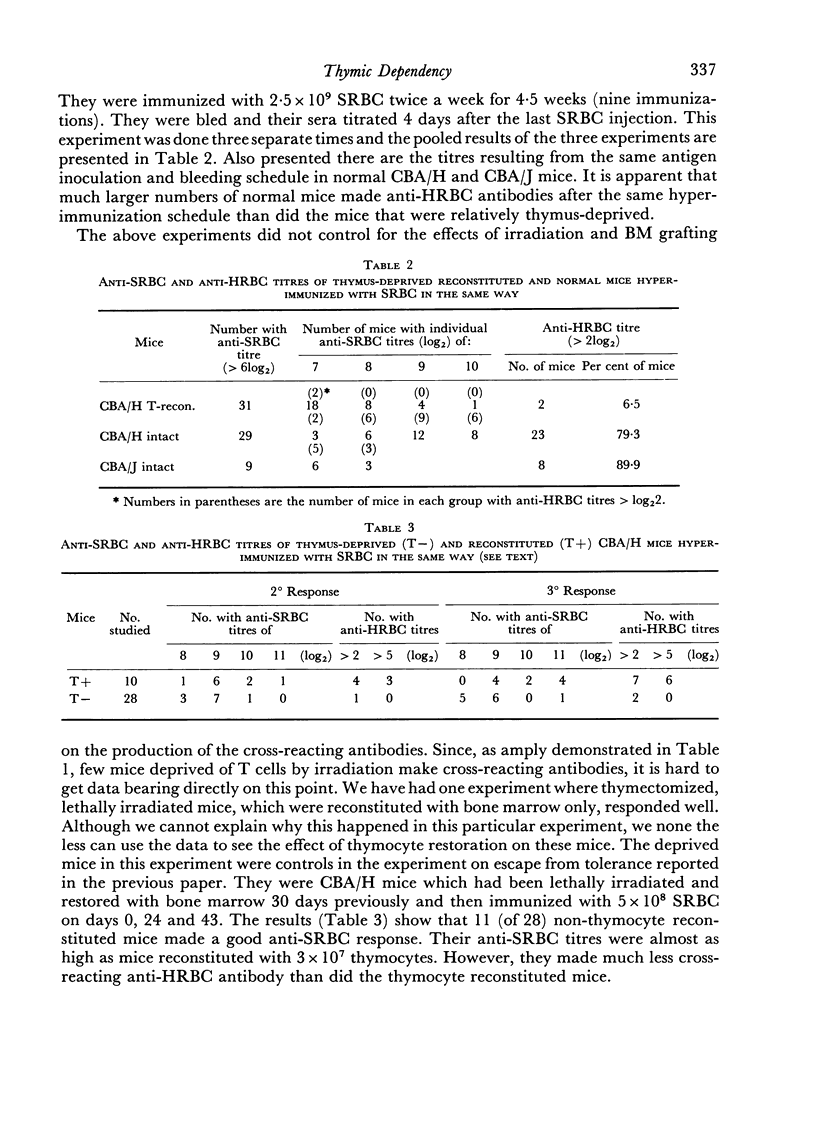
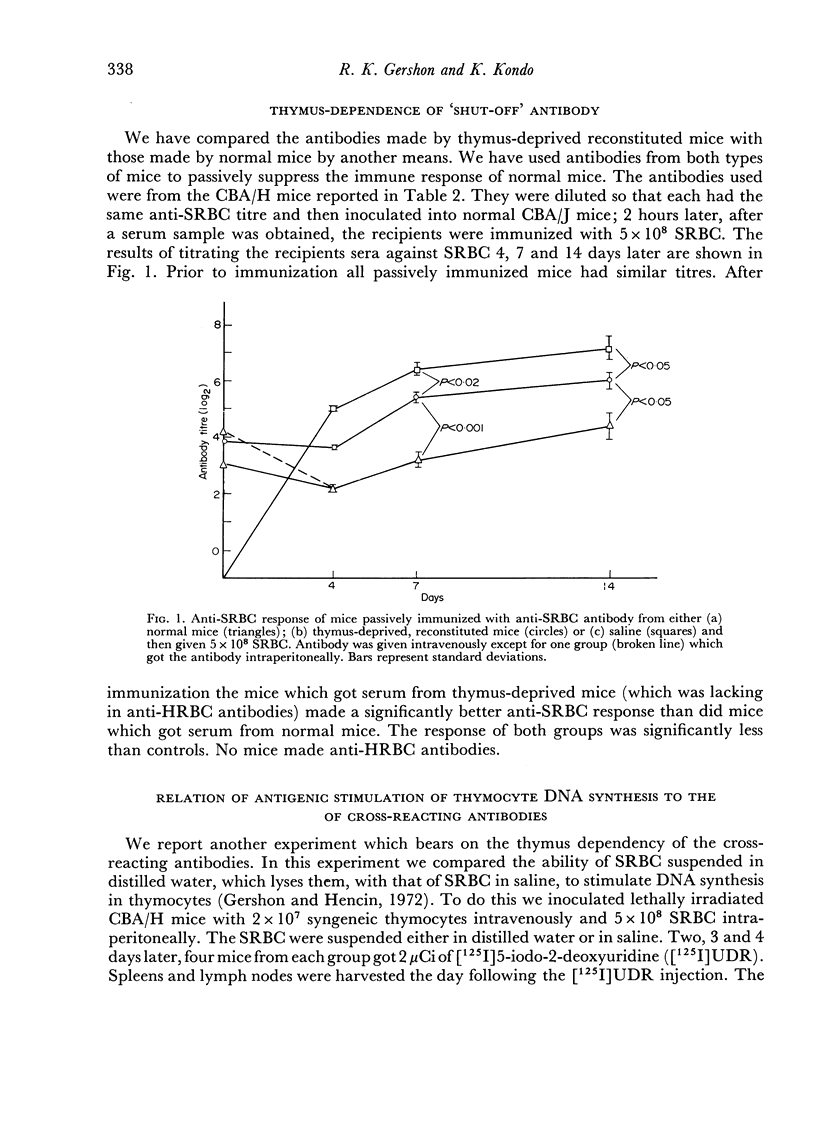
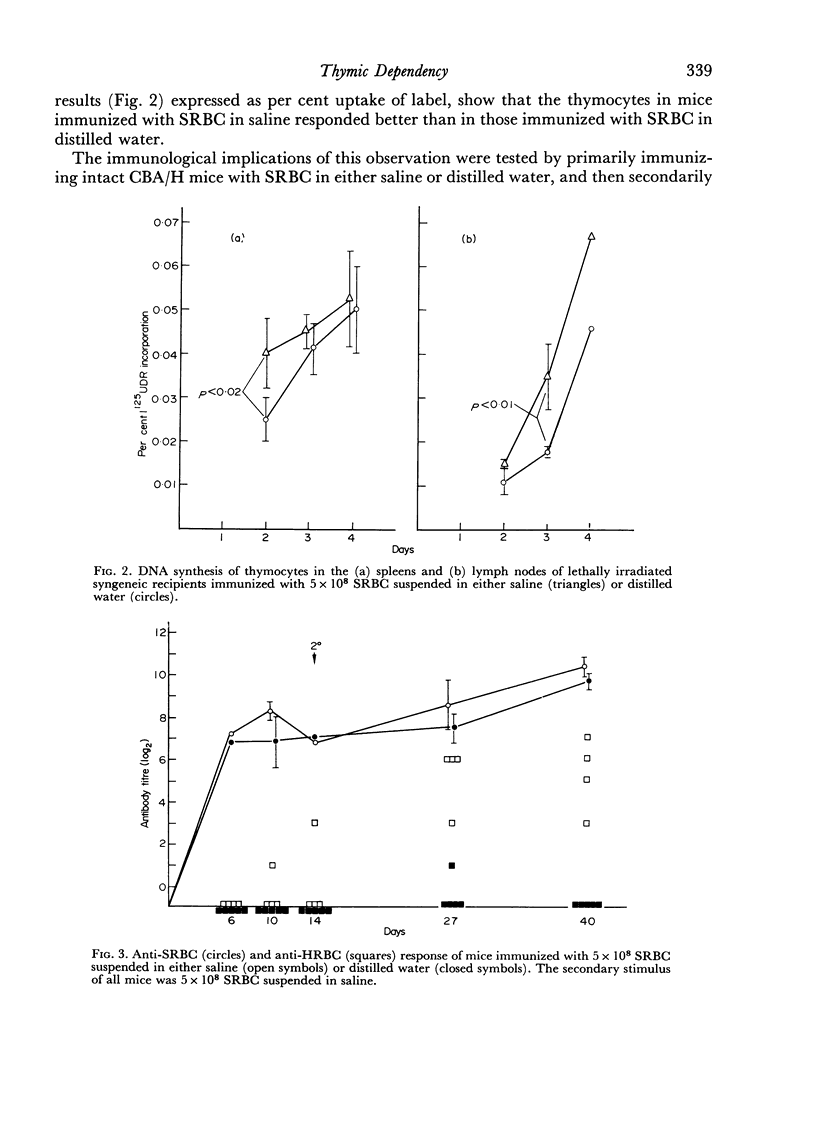
To do this we studied the antibodies made by mice deprived of T cells by irradiation as well as those made by normal mice immunized with SRBC in distilled water, which was shown to depress the DNA synthetic response of T cells. In both instances the mice with fewer responding T cells made less antibody which could agglutinate HRBC than did the appropriate controls. This was true even when no difference in anti-SRBC titre was apparent. In addition we showed that the antibody made by mice with reduced numbers of T cells was less effective at passive suppression of the immune response to SRBC than was similarly titred (against SRBC) antibody made by normal mice.
Thus, certain sub-populations of antibodies, normally made in response to SRBC immunization, are particularly thymus dependent. We have discussed why we think they are those of particularly high affinity.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson B., Blomgren H. Evidence for thymus-independent humoral antibody production in mice against polyvinylpyrrolidone and E. coli lipopolysaccharide. Cell Immunol. 1971 Oct;2(5):411–424. doi: 10.1016/0008-8749(71)90052-9. [DOI] [PubMed] [Google Scholar]
- BIRO C. E., GARCIA G. THE ANTIGENICITY OF AGGREGATED AND AGGREGATE-FREE HUMAN GAMMA-GLOBULIN FOR RABBITS. Immunology. 1965 Apr;8:411–419. [PMC free article] [PubMed] [Google Scholar]
- Baker P. H., Stashak P. W. Quantitative and qualitative studies on the primary antibody response to pneumococcal polysaccharides at ehe cellular level. J Immunol. 1969 Dec;103(6):1342–1348. [PubMed] [Google Scholar]
- Basch R. S. Immunologic competence after thymectomy. Int Arch Allergy Appl Immunol. 1966;30(2):105–119. doi: 10.1159/000229800. [DOI] [PubMed] [Google Scholar]
- DRESSER D. W. Specific inhibition of antibody production. II. Paralysis induced in adult mice by small quantities of protein antigen. Immunology. 1962 May;5:378–388. [PMC free article] [PubMed] [Google Scholar]
- FREI P. C., BENACERRAF B., THORBECKE G. J. PHAGOCYTOSIS OF THE ANTIGEN, A CRUCIAL STEP IN THE INDUCTION OF THE PRIMARY RESPONSE. Proc Natl Acad Sci U S A. 1965 Jan;53:20–23. doi: 10.1073/pnas.53.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon R. K., Hencin R. S. The DNA synthetic response of adoptively transferred thymocytes in the spleens of lethally irradiated mice. I. Effects of varying antigen and thymocyte doses. J Immunol. 1971 Dec;107(6):1723–1728. [PubMed] [Google Scholar]
- Gershon R. K., Kondo K. Cell interactions in the induction of tolerance: the role of thymic lymphocytes. Immunology. 1970 May;18(5):723–737. [PMC free article] [PubMed] [Google Scholar]
- Gershon R. K., Kondo K. Degeneracy of the immune response to sheep red cells. Immunology. 1972 Sep;23(3):321–334. [PMC free article] [PubMed] [Google Scholar]
- Gershon R. K., Paul W. E. Effect of thymus-derived lymphocytes on amount and affinity of anti-hapten antibody. J Immunol. 1971 Mar;106(3):872–874. [PubMed] [Google Scholar]
- Gery I., Gershon R. K., Waksman B. H. Potentiation of cultured mouse thymocyte responses by factors released by peripheral leucocytes. J Immunol. 1971 Dec;107(6):1778–1780. [PubMed] [Google Scholar]
- Isaković K., Smith S. B., Waksman B. H. Role of the thymus in tolerance. I. Tolerance to bovine gamma globulin in thymectomized, irradiated rats grafted with thymus from tolerant donors. J Exp Med. 1965 Dec 1;122(6):1103–1123. doi: 10.1084/jem.122.6.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerbel R. S., Eidinger D. Variable effects of anti-lymphocyte serum on humoral antibody formation: role of thymus dependency of antigen. J Immunol. 1971 Apr;106(4):917–926. [PubMed] [Google Scholar]
- Miller J. F., Dukor P., Grant G., Sinclair N. R., Sacquet E. The immunological responsiveness of germ-free mice thymectomized at birth. I. Antibody production and skin homograft rejection. Clin Exp Immunol. 1967 Sep;2(5):531–542. [PMC free article] [PubMed] [Google Scholar]
- Sinclair N. R. Delayed development of primary immunological responsiveness in neonatally thymectomized swiss albino mice. Clin Exp Immunol. 1967 Nov;2(6):701–704. [PMC free article] [PubMed] [Google Scholar]
- Siskind G. W., Dunn P., Walker J. G. Studies on the control of antibody synthesis. II. Effect of antigen dose and of suppression by passive antibody on the affinity of antibody synthesized. J Exp Med. 1968 Jan 1;127(1):55–66. doi: 10.1084/jem.127.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor R. B., Wortis H. H. Thymus dependence of antibody response: variation with dose of antigen and class of antibody. Nature. 1968 Nov 30;220(5170):927–928. doi: 10.1038/220927a0. [DOI] [PubMed] [Google Scholar]
- Walker J. G., Siskind G. W. Studies on the control of antibody synthesis. Effect of antibody affinity upon its ability to suppress antibody formation. Immunology. 1968 Jan;14(1):21–28. [PMC free article] [PubMed] [Google Scholar]



