Abstract
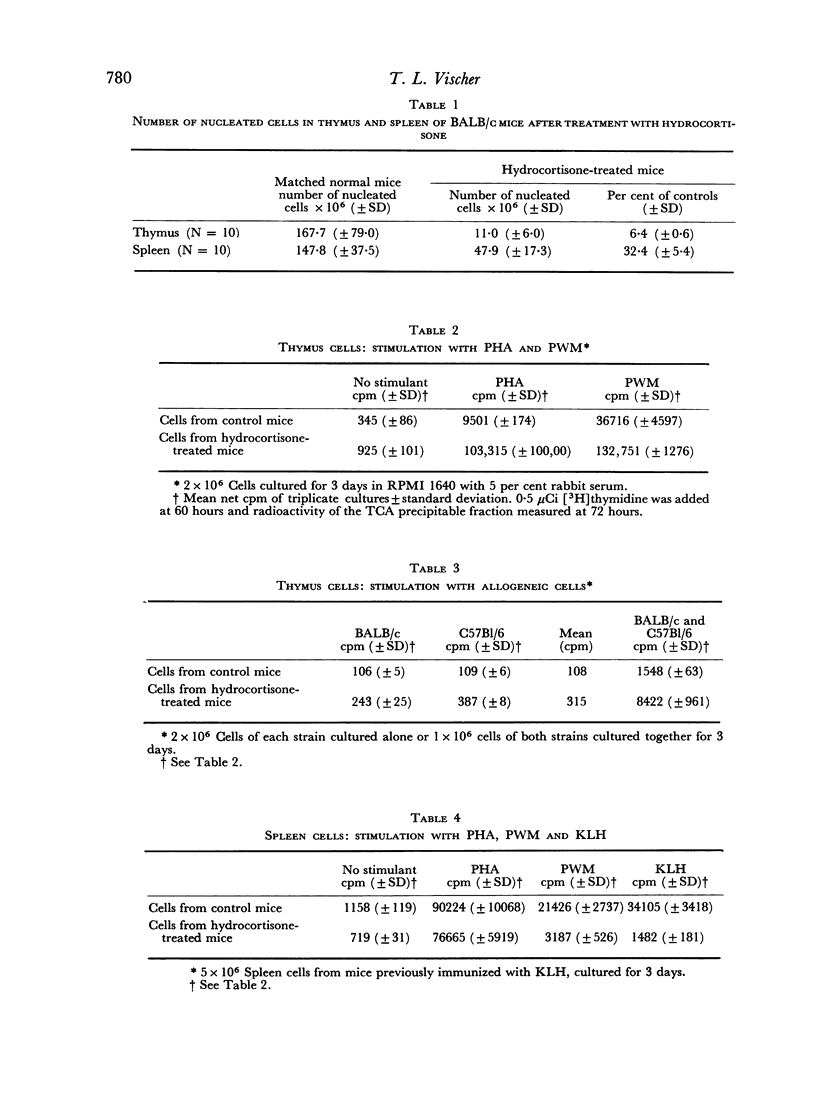
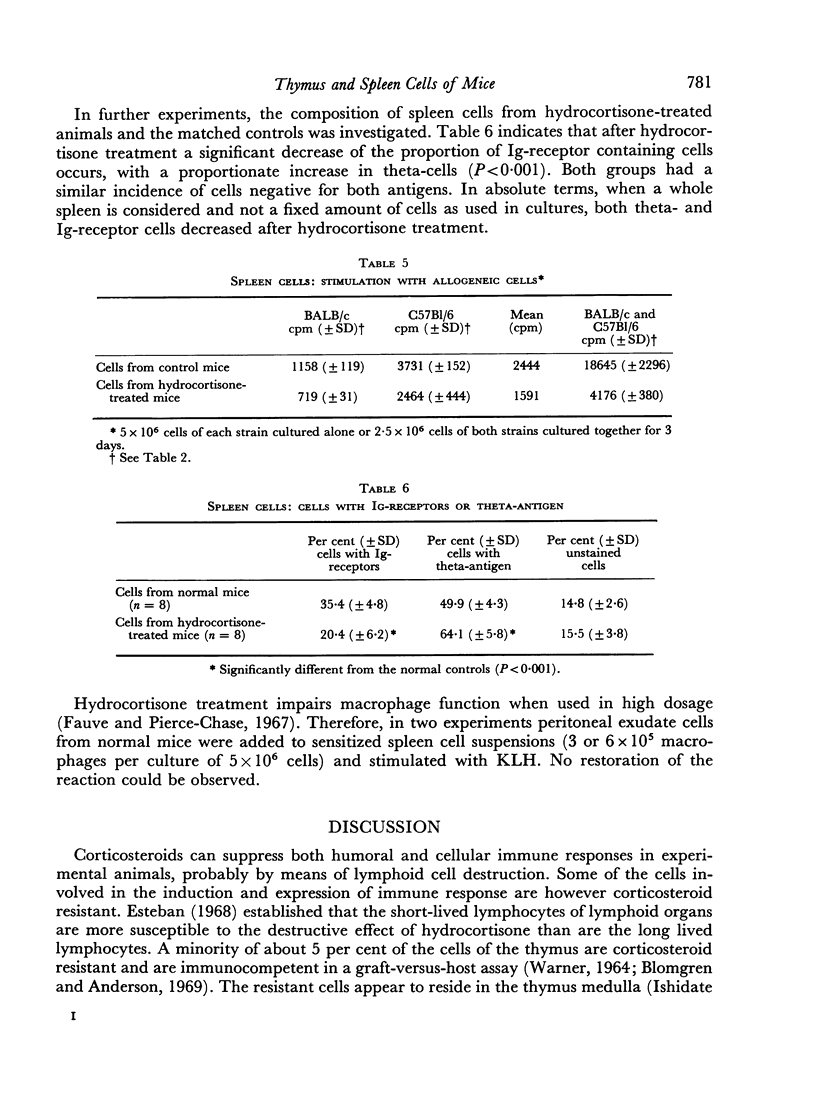
Treatment of mice with hydrocortisone reduced the number of thymus-cells to 6 per cent and the number of spleen-cells to 32 per cent of matched controls. Per spleen, an absolute decrease of cells carrying immunoglobulin receptors on the surface as determined by immunofluorescence was found and a smaller decrease in cells with theta-antigen. Equal numbers of thymus and spleen cells from hydrocortisone-treated mice and matched controls were cultured and stimulated with phytohaemagglutinin (PHA), pokeweed mitogen (PWM), allogeneic cells, and, following immunization, with keyhole limpet haemocyanin (KLH). Stimulation was assessed by incorporation of [3H]thymidine into the acid precipitable fraction of the cultured cells. With thymus cells, hydrocortisone treatment increased the reaction to PHA and allogeneic cells. Thymus cells from untreated animals already gave a good response to PWM, further increased by treatment with hydrocortisone. With spleen cells, hydrocortisone treatment reduced the reaction to KLH, PWM, allogeneic cells and PHA in decreasing order. The results are discussed with reference to cells affected by hydrocortisone treatment and to the mechanism of in vitro lymphocyte stimulation.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blau J. N., Gaugas J. M. Parathymic lymph nodes in rats and mice. Immunology. 1968 May;14(5):763–765. [PMC free article] [PubMed] [Google Scholar]
- Blomgren H., Andersson B. Evidence for a small pool of immunocompetent cells in the mouse thymus. Exp Cell Res. 1969 Oct;57(2):185–192. doi: 10.1016/0014-4827(69)90140-2. [DOI] [PubMed] [Google Scholar]
- Blomgren H., Svedmyr E. In vitro stimulation of mouse thymus cells by PHA and allogeneic cells. Cell Immunol. 1971 Aug;2(4):285–299. doi: 10.1016/0008-8749(71)90063-3. [DOI] [PubMed] [Google Scholar]
- Cohen J. J., Claman H. N. Thymus-marrow immunocompetence. V. Hydrocortisone-resistant cells and processes in the hemolytic antibody response of mice. J Exp Med. 1971 May 1;133(5):1026–1034. doi: 10.1084/jem.133.5.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. J., Fschbach M., Claman H. N. Hydrocortisne resistance of graft vs host activity in mouse thymus, spleen and bone marrow. J Immunol. 1970 Nov;105(5):1146–1150. [PubMed] [Google Scholar]
- Colley D. G., Wu A. Y., Waksman B. H. Cellular differentiation in the thymus. 3. Surface properties of rat thymus and lymph node cells separated on density gradients. J Exp Med. 1970 Dec 1;132(6):1107–1121. doi: 10.1084/jem.132.6.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteban J. N. The differential effect of hydrocortisone on the short-lived small lymphocyte. Anat Rec. 1968 Nov;162(3):349–356. doi: 10.1002/ar.1091620309. [DOI] [PubMed] [Google Scholar]
- Fauve R. M., Pierce-Chase C. H. Comparative effects of corticosteroids on host resistance to infection in relation to chemical structure. J Exp Med. 1967 May 1;125(5):807–821. doi: 10.1084/jem.125.5.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajl-Peczalska K., Meuwissen H. J., Good R. A. Pokeweed mitogen induced blastoid transformation in purified and non-purified leukocyte cultures. Int Arch Allergy Appl Immunol. 1969;36(6):546–553. doi: 10.1159/000230775. [DOI] [PubMed] [Google Scholar]
- Gallily R., Garvey J. S. Primary stimulation of rats and mice with hemocyanin in soluton and adsorbed on bentonite. J Immunol. 1968 Nov;101(5):924–929. [PubMed] [Google Scholar]
- ISHIDATE M., METCALF D. THE PATTERN OF LYMPHOPOIESIS IN THE MOUSE THYMUS AFTER CORTISONE ADMINISTRATION OR ADRENALECTOMY. Aust J Exp Biol Med Sci. 1963 Dec;41:637–649. doi: 10.1038/icb.1963.53. [DOI] [PubMed] [Google Scholar]
- Janis M., Back F. H. Potentiation of in vitro lymphocyte reactivity. Nature. 1970 Jan 17;225(5229):238–239. doi: 10.1038/225238a0. [DOI] [PubMed] [Google Scholar]
- Janossy G., Greaves M. F. Lymphocyte activation. I. Response of T and B lymphocytes to phytomitogens. Clin Exp Immunol. 1971 Oct;9(4):483–498. [PMC free article] [PubMed] [Google Scholar]
- Leckband E., Boyse E. A. Immunocompetent cells among mouse thymocytes: a minor population. Science. 1971 Jun 18;172(3989):1258–1260. doi: 10.1126/science.172.3989.1258. [DOI] [PubMed] [Google Scholar]
- Oppenheim J. J., Leventhal B. G., Hersh E. M. The transformation of column-purified lymphocytes with nonspecific and specific antigenic stimuli. J Immunol. 1968 Aug;101(2):262–267. [PubMed] [Google Scholar]
- Playfair J. H. Cell cooperation in the immune response. Clin Exp Immunol. 1971 Jun;8(6):839–856. [PMC free article] [PubMed] [Google Scholar]
- Raff M. Evidence for subpopulation of mature lymphocytes within mouse thymus. Nat New Biol. 1971 Feb 10;229(6):182–184. doi: 10.1038/newbio229182a0. [DOI] [PubMed] [Google Scholar]
- Reif A. E., Allen J. M. Mouse thymic iso-antigens. Nature. 1966 Jan 29;209(5022):521–523. doi: 10.1038/209521b0. [DOI] [PubMed] [Google Scholar]
- Seeger R. C., Oppenheim J. J. Synergistic interaction of macrophages and lymphocytes in antigen-induced transformation of lymphocytes. J Exp Med. 1970 Jul 1;132(1):44–65. doi: 10.1084/jem.132.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockman G. D., Gallagher M. T., Heim L. R., South M. A., Trentin J. J. Differential stimulation of mouse lymphoid cells by phytohemagglutinin and pokeweed mitogen. Proc Soc Exp Biol Med. 1971 Mar;136(3):980–982. doi: 10.3181/00379727-136-35410. [DOI] [PubMed] [Google Scholar]
- Takiguchi T., Adler W. H., Smith R. T. Cellular recognition in vitro by mouse lymphocytes. Effects of neonatal thymectomy and thymus graft restoration on alloantigen and PHA stimulation of whole and gradient-separated subpopulations of spleen cells. J Exp Med. 1971 Jan 1;133(1):63–80. doi: 10.1084/jem.133.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unanue E. R., Grey H. M., Rabellino E., Campbell P., Schmidtke J. Immunoglobulins on the surface of lymphocytes. II. The bone marrow as the main source of lymphocytes with detectable surface-bound immunoglobulin. J Exp Med. 1971 Jun 1;133(6):1188–1198. doi: 10.1084/jem.133.6.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vischer T. L., Jaquet C. Effect of antibodies against immunoglobulins and the theta antigen on the specific and non-specific stimulation of mouse spleen cells in vitro. Immunology. 1972 Feb;22(2):259–266. [PMC free article] [PubMed] [Google Scholar]
- WARNER N. L. THE IMMUNOLOGICAL ROLE OF DIFFERENT LYMPHOID ORGANS IN THE CHICKEN. II. THE IMMUNOLOGICAL COMPETENCE OF THYMIC CELL SUSPENSIONS. Aust J Exp Biol Med Sci. 1964 Jun;42:401–416. doi: 10.1038/icb.1964.38. [DOI] [PubMed] [Google Scholar]


