Abstract
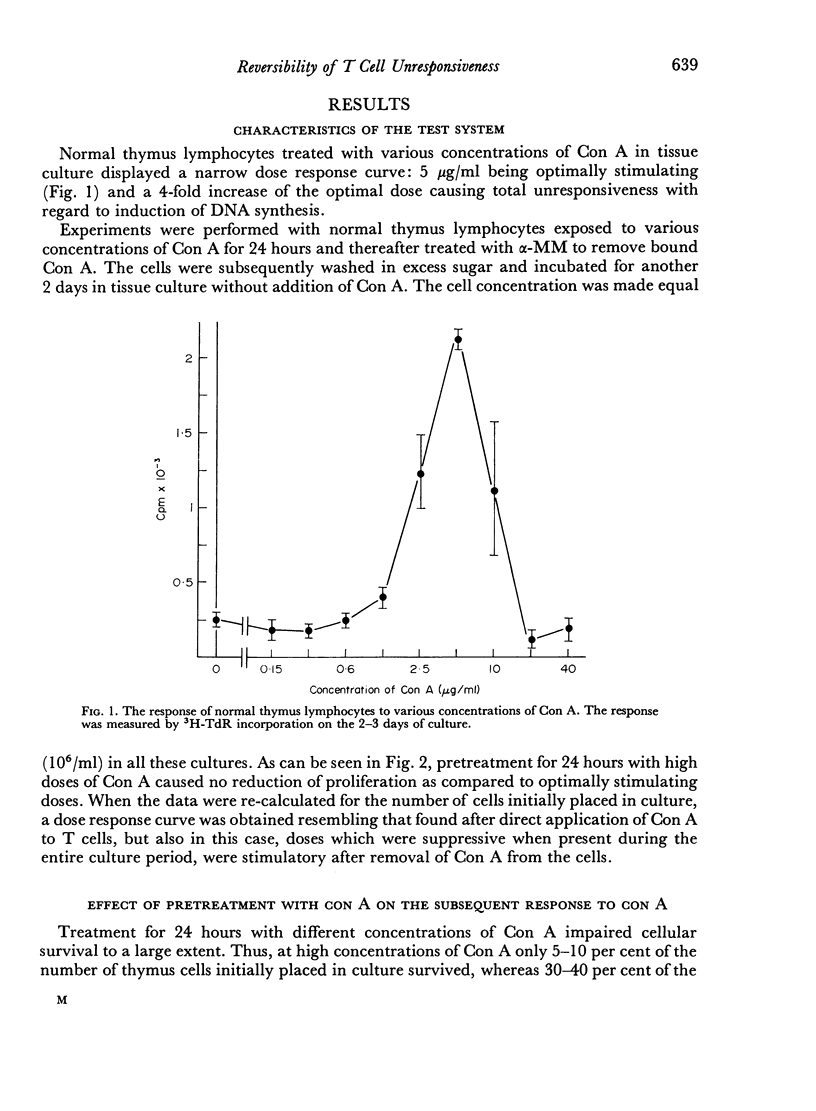
Mouse thymus lymphocytes stimulated by Concanavalin A (Con A) displayed a typical dose curve with regard to induction of DNA synthesis. An optimal dose induced cellular proliferation, whereas both higher and lower Con A concentrations were inactive.
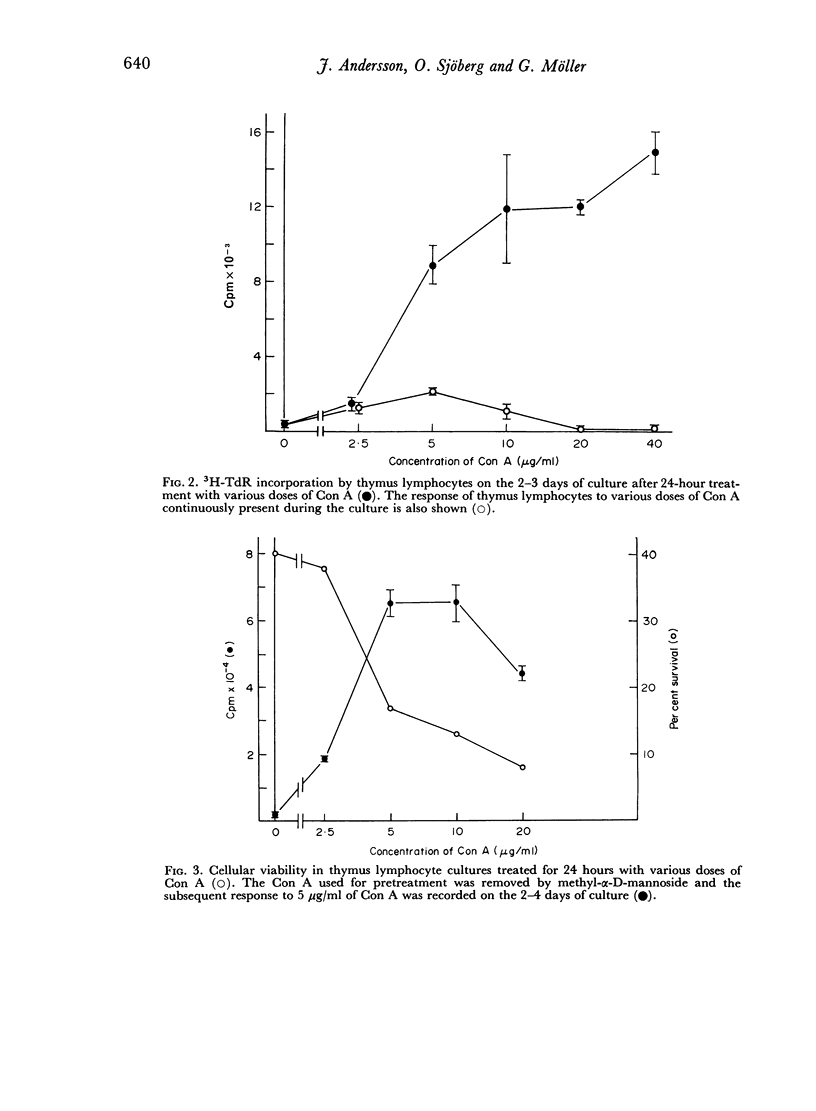
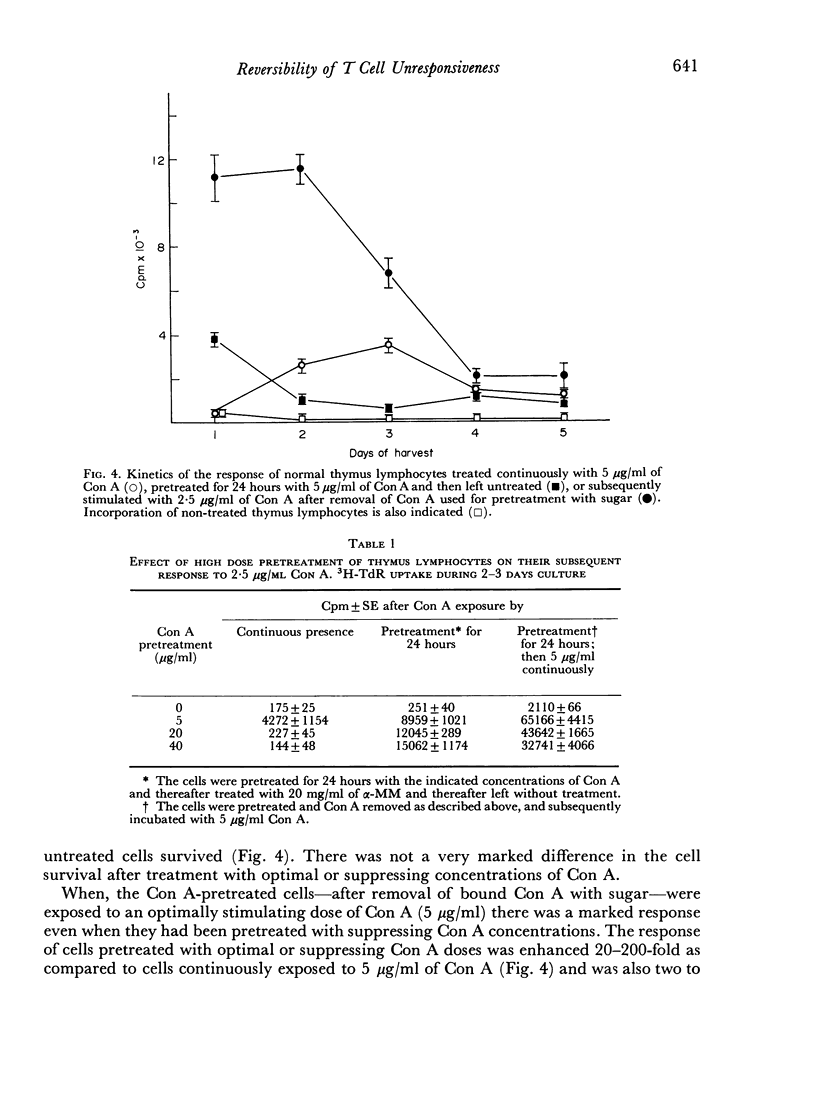
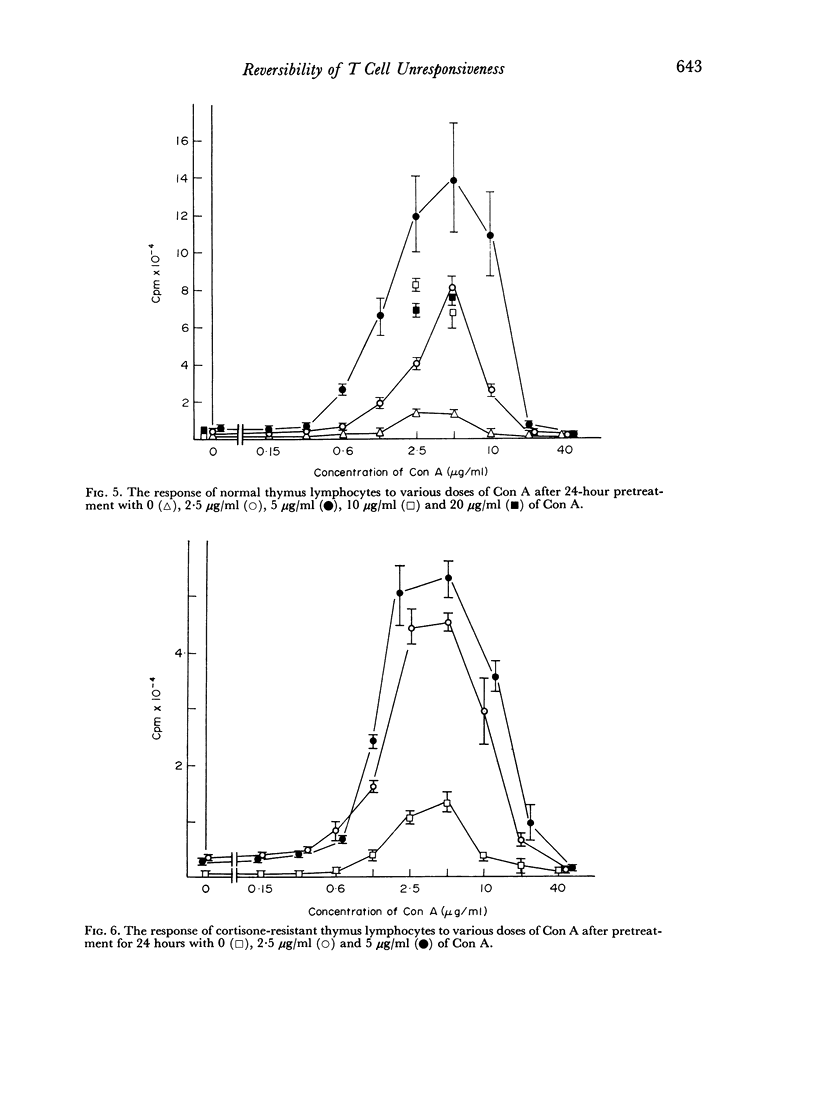
When thymus lymphocytes were treated with Con A for 24 hours followed by removal of Con A by the addition of competing sugar and subsequently stimulated with Con A, the response was markedly enhanced as compared to cells continuously treated with Con A, but there was no change in the dose required for optimal induction of proliferation. The most enhancing effect was found after pretreatment with optimally stimulating doses of Con A. Cells pretreated with high doses of Con A, which were totally suppressive when present during the entire culture period, also exhibited a markedly enhanced response to an optimal dose of Con A. Similar results were obtained with cortisone-resistant thymocytes.
The results show that the unresponsiveness caused by high concentrations of Con A requires the continuous presence of this substance. Furthermore, the unresponsive state was reversible and could be changed to a high response by removing the high concentration and subsequently challenging the cells with an optimally stimulating dose of Con A.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blomgren H., Andersson B. Evidence for a small pool of immunocompetent cells in the mouse thymus. Exp Cell Res. 1969 Oct;57(2):185–192. doi: 10.1016/0014-4827(69)90140-2. [DOI] [PubMed] [Google Scholar]
- Byers V. S., Sercarz E. E. Induction and reversal of immune paralysis in vitro. J Exp Med. 1970 Nov;132(5):845–857. doi: 10.1084/jem.132.5.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLAMAN H. N., TALMAGE D. W. THYMECTOMY: PROLONGATION OF IMMUNOLOGICAL TOLERANCE IN THE ADULT MOUSE. Science. 1963 Sep 20;141(3586):1193–1194. doi: 10.1126/science.141.3586.1193. [DOI] [PubMed] [Google Scholar]
- Cohen I. R., Globerson A., Feldman M. Autosensitization in vitro. J Exp Med. 1971 Apr 1;133(4):834–845. doi: 10.1084/jem.133.4.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dresser D. W., Mitchison N. A. The mechanism of immunological paralysis. Adv Immunol. 1968;8:129–181. doi: 10.1016/s0065-2776(08)60466-6. [DOI] [PubMed] [Google Scholar]
- Gershon R. K., Kondo K. Infectious immunological tolerance. Immunology. 1971 Dec;21(6):903–914. [PMC free article] [PubMed] [Google Scholar]
- Howard J. G. Cellular events in the induction and loss of tolerance to pneumococcal polysaccharides. Transplant Rev. 1972;8:50–75. doi: 10.1111/j.1600-065x.1972.tb01564.x. [DOI] [PubMed] [Google Scholar]
- Jacobson E. B., Herzenberg L. A. Active suppression of immunoglobulin allotype synthesis. I. Chronic suppression after perinatal exposure to maternal antibody to paternal allotype in (SJL x BALB-c)F 1 mice. J Exp Med. 1972 May 1;135(5):1151–1162. doi: 10.1084/jem.135.5.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janossy G., Greaves M. F. Lymphocyte activation. I. Response of T and B lymphocytes to phytomitogens. Clin Exp Immunol. 1971 Oct;9(4):483–498. [PMC free article] [PubMed] [Google Scholar]
- McCullagh P. J. The abrogation of sheep erythrocyte tolerance in rats by means of the transfer of allogeneic lymphocytes. J Exp Med. 1970 Nov;132(5):916–925. doi: 10.1084/jem.132.5.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison N. A. Recovery from immunological paralysis in relation to age and residual antigen. Immunology. 1965 Aug;9(2):129–138. [PMC free article] [PubMed] [Google Scholar]
- Möller G. Immunocyte triggering. Cell Immunol. 1970 Dec;1(6):573–582. doi: 10.1016/0008-8749(70)90023-7. [DOI] [PubMed] [Google Scholar]
- Nilsson B. S. Differential effect of medium change in lymphocyte cultures stimulated by PPD tuberculin or PHA. Cell Immunol. 1972 Feb;3(2):333–337. doi: 10.1016/0008-8749(72)90172-4. [DOI] [PubMed] [Google Scholar]
- Peavy D. L., Adler W. H., Smith R. T. The mitogenic effects of endotoxin and staphylococcal enterotoxin B on mouse spleen cells and human peripheral lymphocytes. J Immunol. 1970 Dec;105(6):1453–1458. [PubMed] [Google Scholar]
- Sjöberg O. Rapid breaking of tolerance against Escherichia coli lipopolysaccharide in vivo and in vitro. J Exp Med. 1972 Apr 1;135(4):850–859. doi: 10.1084/jem.135.4.850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAYLOR R. B. AN EFFECT OF THYMECTOMY ON RECOVERY FROM IMMUNOLOGICAL PARALYSIS. Immunology. 1964 Sep;7:595–602. [PMC free article] [PubMed] [Google Scholar]



