Abstract
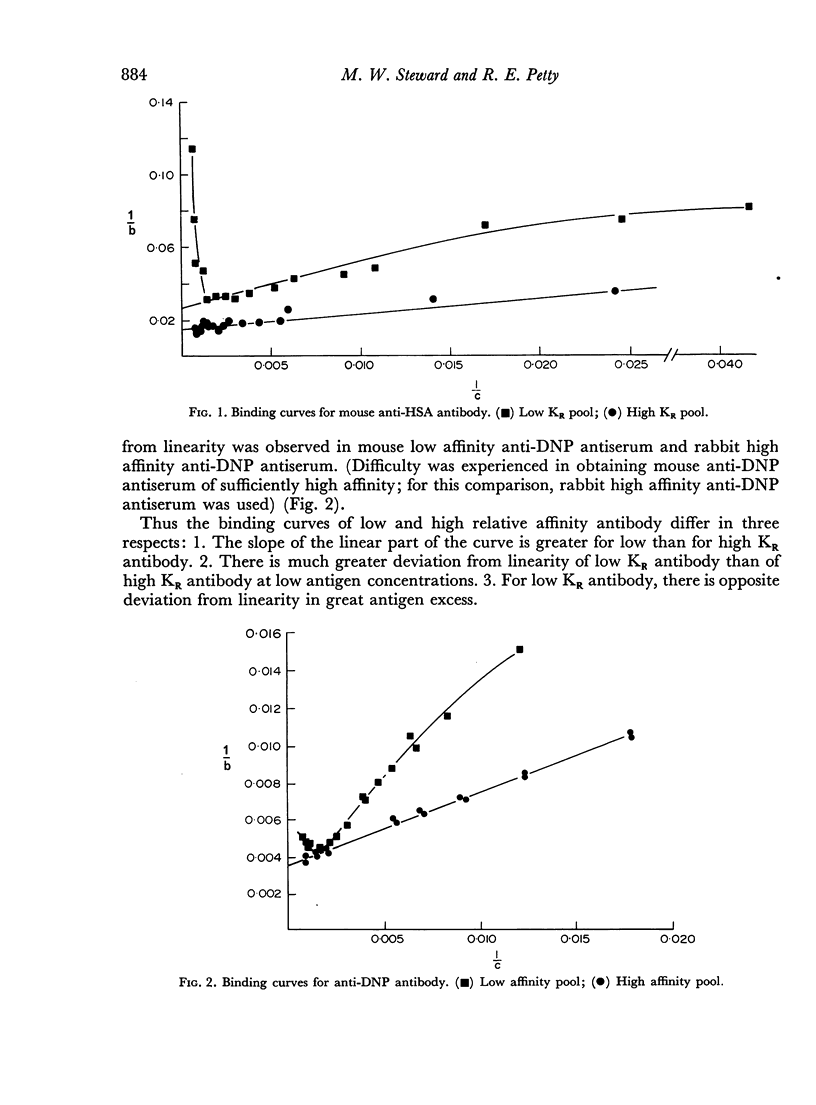
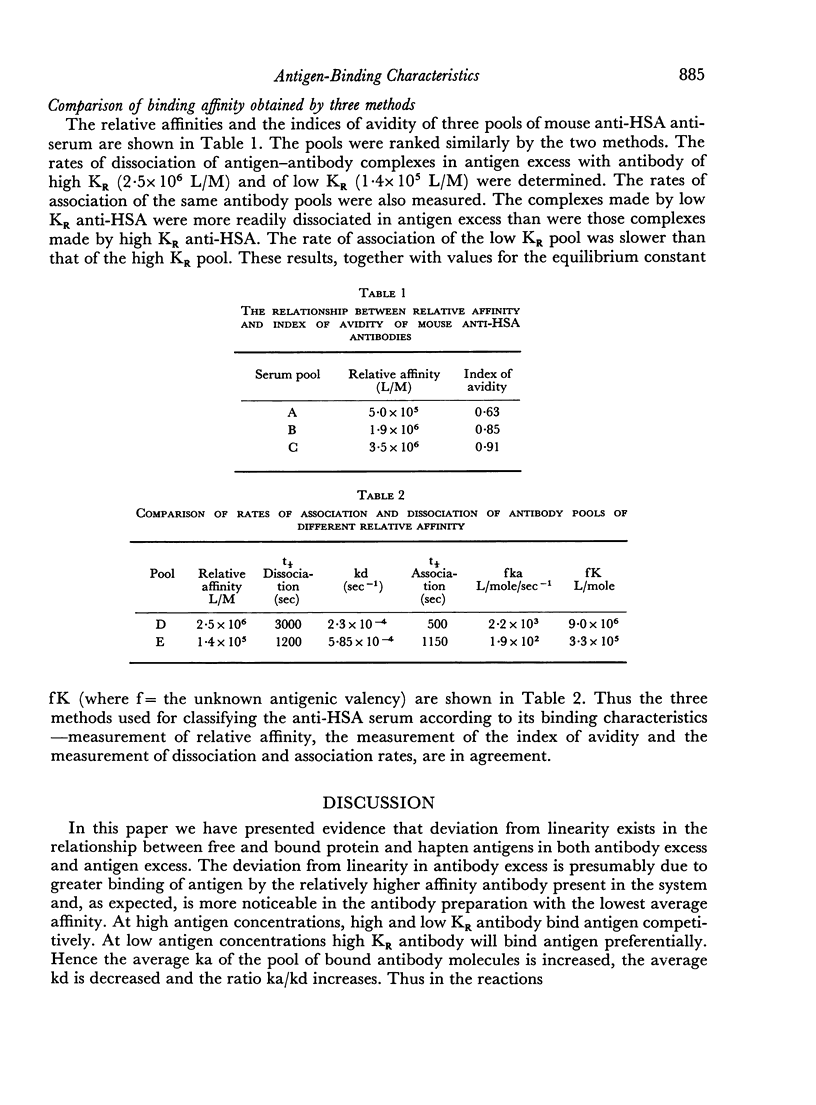
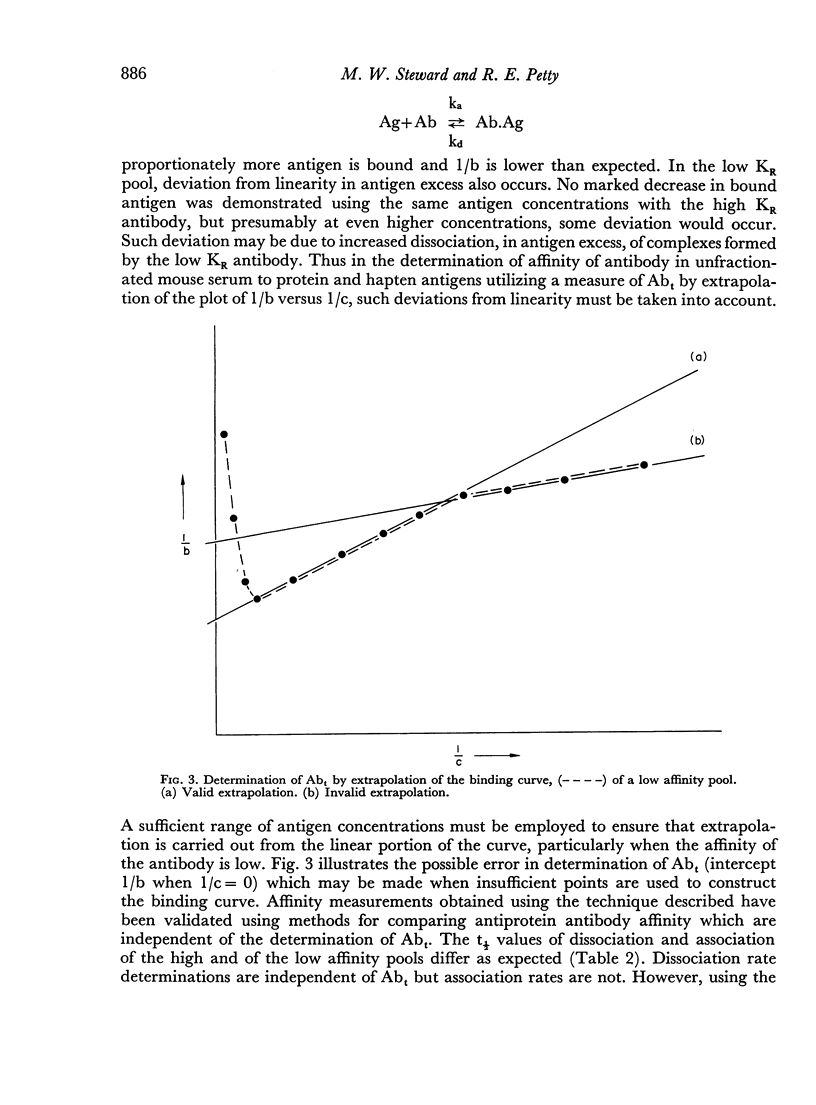
The interstrain differences in relative affinity of mouse anti-HSA antibody previously shown by studies at equilibrium are also observed when rates of association and dissociation and avidity indices are measured. Marked differences are demonstrated in the binding characteristics of high and low relative affinity antibody to both protein and hapten antigens. These results are explained on the basis of heterogeneity of antibody-binding affinity.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Celada F., Schmidt D., Strom R. Determination of avidity of anti-albumin antibodies in the mouse. Influence of the number of cells transferred on the quality of the secondary adoptive response. Immunology. 1969 Aug;17(2):189–198. [PMC free article] [PubMed] [Google Scholar]
- Eisen H. N., Simms E. S., Potter M. Mouse myeloma proteins with antihapten antibody acitivity. The protein produced by plasma cell tumor MOPC-315. Biochemistry. 1968 Nov;7(11):4126–4134. doi: 10.1021/bi00851a048. [DOI] [PubMed] [Google Scholar]
- GREY H. M. STUDIES ON CHANGES IN THE QUALITY OF RABBIT-BOVINE SERUM ALBUMIN ANTIBODY FOLLOWING IMMUNIZATION. Immunology. 1964 Jan;7:82–90. [PMC free article] [PubMed] [Google Scholar]
- McFARLANE A. S. Efficient trace-labelling of proteins with iodine. Nature. 1958 Jul 5;182(4627):53–53. doi: 10.1038/182053a0. [DOI] [PubMed] [Google Scholar]
- Steward M. W., Petty R. E. The use of ammonium sulphate globulin precipitation for determination of affinity of anti-protein antibodies in mouse serum. Immunology. 1972 May;22(5):747–756. [PMC free article] [PubMed] [Google Scholar]
- TALMAGE D. W. The kinetics of the reaction between antibody and bovine serum albumin using the Farr method. J Infect Dis. 1960 Jul-Aug;107:115–132. doi: 10.1093/infdis/107.1.115. [DOI] [PubMed] [Google Scholar]


