Abstract
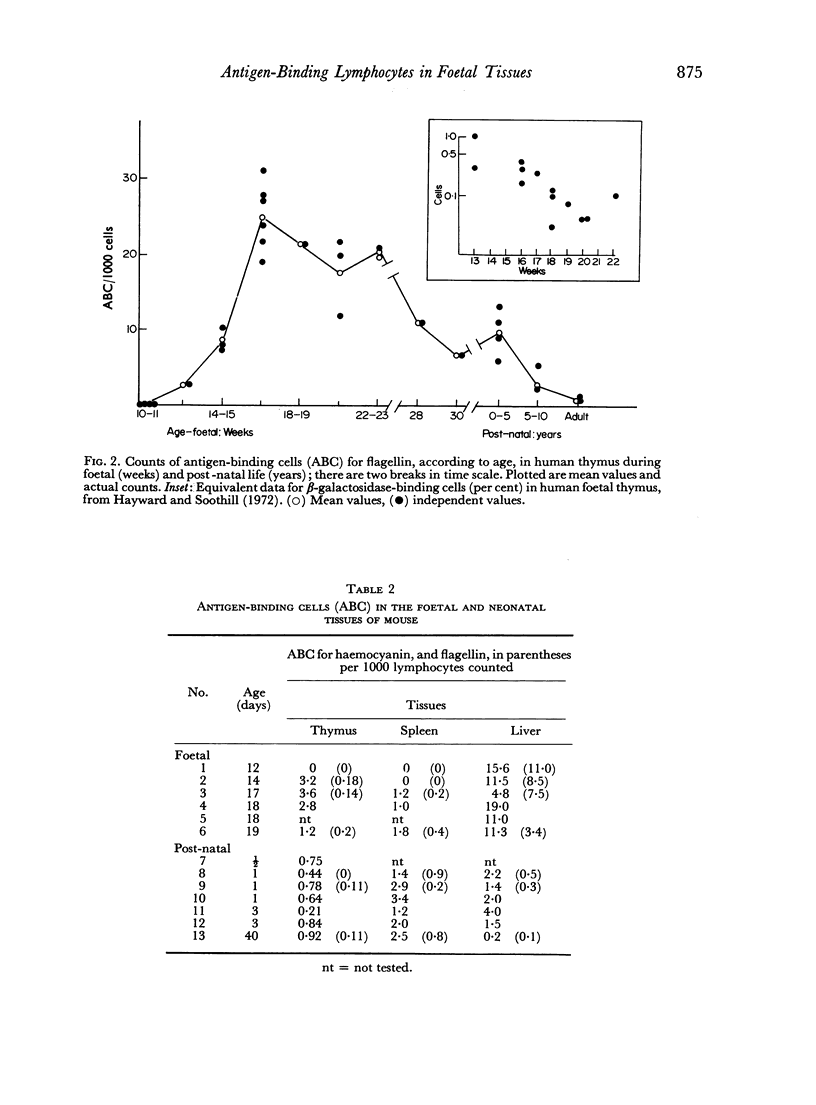
The time of appearance and counts of antigen-binding cells, using radioiodine-labelled flagellin and haemocyanin, was studied in the human and mouse foetus at different gestational ages: the capacity for binding radioiodine-labelled antigens was equated with acquisition of immunological funciton. Cells resembling lymphocytes from human and mouse liver and bone marrow showed antigen binding at early gestational ages, but this binding could not be prevented with species-specific antisera to immunoglobulins. Specific antigen binding to lymphocytes was detected first with thymic lymphocytes, at gestational ages of 12 weeks in humans and 14 days in mice, then with splenic lymphocytes, at 16 weeks in humans and 17 days in mice, and still later with gut lymphocytes. Relative counts of antigen-binding cells in human foetal thymus were maximal at 16–22 weeks and decreased thereafter. Lymphocyte immunocompetence, as judged by the capacity specifically to bind antigen, develops rapidly after the appearance in thymus of cells with the morphology of lymphocytes; this seemed to occur at the equivalent foetal stage in the species studied.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ada G. L., Byrt P. Specific inactivation of antigen-reactive cells with 125I-labelled antigen. Nature. 1969 Jun 28;222(5200):1291–1292. doi: 10.1038/2221291a0. [DOI] [PubMed] [Google Scholar]
- Byrt P., Ada G. L. An in vitro reaction between labelled flagellin or haemocyanin and lymphocyte-like cells from normal animals. Immunology. 1969 Oct;17(4):503–516. [PMC free article] [PubMed] [Google Scholar]
- Cooper M. D., Perey D. Y., McKneally M. F., Gabrielsen A. E., Sutherland D. E., Good R. A. A mammalian equivalent of the avian bursa of Fabricius. Lancet. 1966 Jun 25;1(7452):1388–1391. doi: 10.1016/s0140-6736(66)90300-x. [DOI] [PubMed] [Google Scholar]
- Dwyer J. M., Mackay I. R. Antigen-binding lymphocytes in human blood. Lancet. 1970 Jan 24;1(7639):164–167. doi: 10.1016/s0140-6736(70)90406-x. [DOI] [PubMed] [Google Scholar]
- Dwyer J. M., Mackay I. R. Antigen-binding lymphocytes in human fetal thymus. Lancet. 1970 Jun 6;1(7658):1199–1202. doi: 10.1016/s0140-6736(70)91787-3. [DOI] [PubMed] [Google Scholar]
- Dwyer J. M., Mackay I. R. Validation of autoradiography for recognition of antigen-binding lymphocytes in blood and lymphoid tissues. Quantitation and specificity of binding. Clin Exp Immunol. 1972 Apr;10(4):581–597. [PMC free article] [PubMed] [Google Scholar]
- Dwyer J. M., Warner N. L. Antigen binding cells in embryonic chicken bursa and thymus. Nat New Biol. 1971 Feb 17;229(7):210–211. doi: 10.1038/newbio229210a0. [DOI] [PubMed] [Google Scholar]
- Dwyer J. M., Warner N. L., Mackay I. R. Specificity and nature of the antigen-combining sites on fetal and mature thymus lymphocytes. J Immunol. 1972 May;108(5):1439–1446. [PubMed] [Google Scholar]
- Fichtelius K. E. The mammalian equivalent to bursa Fabricii of birds. Exp Cell Res. 1967 Apr;46(1):231–234. doi: 10.1016/0014-4827(67)90427-2. [DOI] [PubMed] [Google Scholar]
- Kennedy J. C., Treadwell P. E., Lennox E. S. Antigen-specific synergism in the immune response of irradiated mice given marrow cells and peritoneal cavity cells or extracts. J Exp Med. 1970 Aug 1;132(2):353–367. doi: 10.1084/jem.132.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriazis A. A., Esterly J. R. Fetal and neonatal development of lymphoid tissues. Arch Pathol. 1971 May;91(5):444–451. [PubMed] [Google Scholar]
- Miller A., DeLuca D., Decker J., Ezzell R., Sercarz E. E. Specific binding of antigen to lymphocytes. Evidence for lack of unispecificity in antigen-binding cells. Am J Pathol. 1971 Nov;65(2):451–465. [PMC free article] [PubMed] [Google Scholar]
- Miller H. C., Cudkowicz G. Antigen-specific cells in mouse bone marrow. I. Lasting effects of priming on immunocyte production by transferred marrow. J Exp Med. 1970 Dec 1;132(6):1122–1137. doi: 10.1084/jem.132.6.1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller H. C., Cudkowicz G. Antigen-specific cells in mouse bone marrow. II. Fluctuation of the number and potential of immunocyte precursors after immunization. J Exp Med. 1971 May 1;133(5):973–986. doi: 10.1084/jem.133.5.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PLAYFAIR J. H., WOLFENDALE M. R., KAY H. E. The leucocytes of peripheral blood in the human foetus. Br J Haematol. 1963 Jul;9:336–344. doi: 10.1111/j.1365-2141.1963.tb06558.x. [DOI] [PubMed] [Google Scholar]
- Taylor R. B. Pluripotential stem cells in mouse embryo liver. Br J Exp Pathol. 1965 Aug;46(4):376–383. [PMC free article] [PubMed] [Google Scholar]
- Tyan M. L. Studies on the ontogeny of the mouse immune system. I. Cell-bond immunity. J Immunol. 1968 Mar;100(3):535–542. [PubMed] [Google Scholar]
- VENZKE W. G. Morphogenesis of the thymus of chicken embryos. Am J Vet Res. 1952 Jul;13(48):395–404. [PubMed] [Google Scholar]
- van Furth R., Schuit H. R., Hijmans W. The immunological development of the human fetus. J Exp Med. 1965 Dec 1;122(6):1173–1188. doi: 10.1084/jem.122.6.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]



