ABSTRACT
Objective: To demonstrate the flexibility, adaptability, and efficacy of endoscopic endonasal removal of the inferior half of the middle turbinate in a cadaveric study and in surgery for the treatment of different sphenoid sinus and skull base lesions. Methods: Anatomic Cadaveric Study: Five adult cadaveric heads were studied. Six nostrils of 3 cadavers were studied endoscopically after the lower half of the middle turbinate was removed. Two adult cadaveric heads underwent bilateral paraseptal sagittal sectioning and were studied after the lower half of the middle turbinate was removed. Sixty-five patients with different sphenoid sinus and skull base-related lesions were treated through this surgical approach. Results: This approach increased surgical exposure, decreased tubular vision, and offered wider anatomic panoramic orientation with 0-degree and angled endoscopes. In the surgical group, there were no major intra- or postoperative complications. The approach improved exposure, accessibility to the lesion, and permitted good hemostasis, tumor resection, and repair of the skull base defect. Conclusion: The current approach provides a wide surgical field without increasing morbidity. It avoids unnecessary trauma to the other nostril as occurs in a binostril approach. The harvested piece of turbinate tissue is an excellent source of donor material for successful reconstruction of the sellar floor without inducing side effects or complications.
Keywords: Middle turbinate, endoscopic, endonasal
During the past decade, the popularity of the endoscopic endonasal approach for the treatment of different sphenoid sinus and related skull base lesions has increased because its minimal invasiveness improved patient comfort.1,2 The procedure offered a panoramic view, allowing observation of all anatomic structures along the surgical route as well as those in the skull base.2
Cappabianca et al3 addressed three problems with the endoscopic endonasal approach: (1) less room to work, (2) conflicts between the surgeon's hands and the instruments, and (3) possible damage to intranasal structures during introduction of instruments into the nose.
In endoscopic endonasal surgery, the middle turbinate is a significant landmark of the lateral nasal wall.4 Removal of the lower half of the middle turbinate did not affect, to a great extent, its physiological function and stability.5
To determine the efficacy of excision of the lower half of the middle turbinate in endoscopic endonasal surgery for the treatment of sphenoid sinus and skull base lesions, a preliminary endonasal endoscopic anatomic study with excision of the lower half of the middle turbinate in cadavers was conducted. Subsequently, partial inferior middle turbinectomy became a standard step in our endoscopic endonasal surgery for the treatment of sphenoid sinus and skull base lesions.
RELEVANT ANATOMY
Middle Turbinate Surgical Anatomy
The middle turbinate is a part of the ethmoid bone. It is covered by mucus membrane with a ciliated columnar epithelium and overhangs the middle meatus (Fig. 1A). It lies medial to several important sinus structures: the anterior ethmoid air cells, the maxillary sinus ostium, the nasofrontal duct, and the uncinate process. The mean length of the middle turbinate is 40 mm, and its mean height is 14.5 mm anteriorly and 7 mm posteriorly.6 The line of attachment on the anterior end of the middle turbinate runs almost vertically upward to join the remainder of the turbinate at the angle of the genu. Beneath this genu lies the frontal recess. The anterior third of the middle turbinate inserts at the base of the skull at the lateral edge of the cribrifrom plate (lamina cribrosa). The middle third of the middle turbinate is fixed to the lamina papyracea by its ground lamella, which runs in an almost frontal plane. The posterior third of the middle turbinate (horizontal ground lamella) is attached to the lamina papyracea, the lateral wall of the nasal cavity, or both.6
Figure 1.
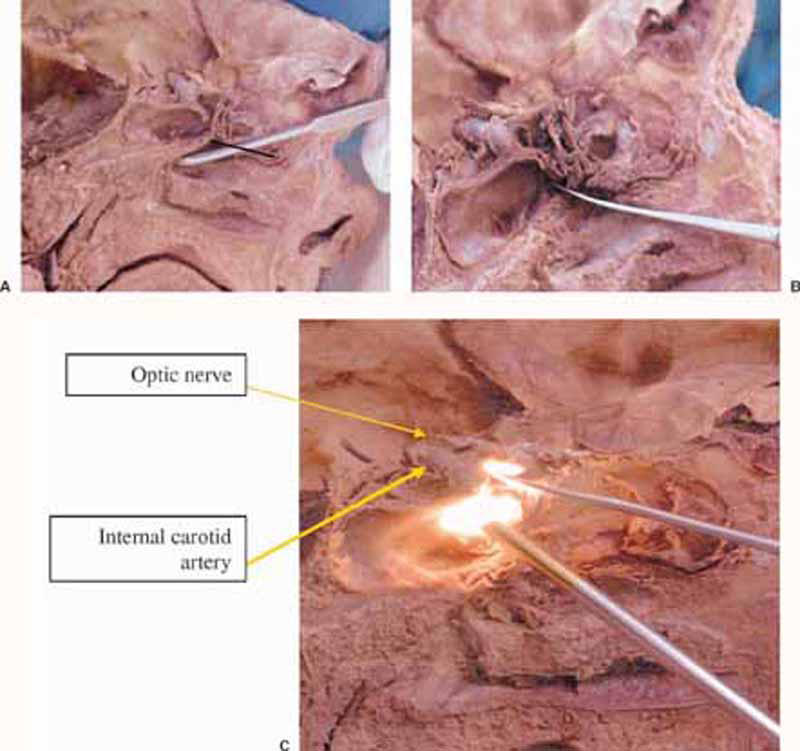
(A) Sagittal view of cadaver specimen at the level of attachment of the left middle turbinate to the anterior skull base and lateral wall of the nose. The black line is at the site of desired incision. Freer elevator placed at the anterior wall of the sphenoid sinus. (B) The same view after removal of the lower half of the middle turbinate and anterior sphenoidotomy. (C) The same view with a 30-degree endoscope introduced to view the upper part of the sellar compartment.
Anatomical variations of the middle turbinate include an L-shaped middle turbinate, a paradoxically bent middle turbinate, a concha bullosa, and a sagittal groove formation of the inferior aspect of the middle turbinate. Hyperpneumatization of the middle turbinate is known as concha bullosa and usually occurs bilaterally. It can cause a significant obstruction of the middle meatus, the hiatus semilunaris, and the ethmoid infindibulum. In such cases, surgical treatment (partial resection of the concha bullosa) may be considered.5
The following points about endoscopic nasal surgery should be remembered:
First, the vertical anterior portion of the middle turbinate is attached to the cribrifrom plate. Hence, medial displacement of the middle turbinate, when necessary, should be done with extreme care to avoid fracturing its superior attachment. Second, the middle turbinate converges toward the superior turbinate posteriorly. Its posterior end becomes an important landmark when the sphenoid ostium in the sphenoethmoidal recess is sought endoscopically. Third, the main sphenoid sinus cavity is usually located above and medial to the posterior attachment of the middle turbinate. Finally, during endoscopic nasal surgery, it is important to recognize the superior attachment of the middle turbinate to avoid injury to the cribrifrom plate.6
MATERIAL AND METHODS
Anatomic Cadaveric Study
Five adult cadaveric heads were studied. All specimens had well-pneumatized sphenoid sinuses. Six nostrils of three cadavers were studied endoscopically. After the lower halves of the middle turbinate of these cadavers were removed endoscopically, the anatomy was examined endoscopically. Nasal endoscopy is usually performed by introducing the endoscope between the nasal turbinates and nasal septum. Therefore, in the other two adult cadaveric heads bilateral paraseptal sagittal sections were made between the nasal septum and the middle turbinate toward the rostrum of the sphenoidal sinus (Fig. 1). The approach was studied after the lower half of the middle turbinate was removed.
Rigid endoscopes, 4 mm wide and 18 cm long, with 0-, 30-, and 70-degree lenses were used. An integrated imaging system with a high-resolution digital still camera and a three-chip digitally enhanced video camera enabled still and video images of the endoscopic anatomy to be captured during the study. Digital pictures were reproduced by coupling the video images with a computer video capture system.
Surgical Study Group
Between June 1997 and December 2002, 65 consecutive patients with different sphenoid sinus and skull base-related lesions underwent endoscopic endonasal surgery with a lower-half middle turbinectomy approach. They were managed at otolaryngological and neurosurgical departments at El-Menoufyia University Hospital, El-Menoufyia, El-Hikmah Hospital for Neurosurgery, and El-Mansoura International Hospital, Dakahlia, Egypt. All patients underwent clinical assessments, including preoperative nasal endoscopy before surgery. When indicated, ophthalmic, endocrine, and radiological evaluations (plain radiography, plain CT before surgery when indicated, CT cisternography, MRI, and MRI with contrast) were done before surgery.
PATIENTS WITH PITUITARY ADENOMAS
The group consisted of 45 patients (25 males and 20 females) between 20 and 67 years of age. The histological diagnoses were microadenomas in 20 patients, intrasellar macroadenomas in 21 patients, and macroadenomas with suprasellar extension in 4 patients. Four patients had recurrent pituitary adenomas. Hormone-secreting pituitary adenomas were found in 21 patients (18 prolactinomas and 3 were acromegalic). Twenty-four patients had nonsecreting pituitary adenomas (21 patients presented with a visual disorder and 3 presented with symptoms of pituitary apoplexy). Previously, patients with prolactinomas had undergone bromocriptine therapy with little improvement.
PATIENTS WITH ISOLATED SPHENOID SINUS INFLAMMATORY DISEASE
This group consisted of 10 patients (6 males and 4 females) between 28 and 75 years of age. All patients showed evidence of isolated sphenoid sinus inflammatory disease, which did not resolve with medical treatment. The surgical findings from these cases were inspissated secretions (7 patients), fungal debris (2 patients), and mucopyoceles (1 patient).
PATIENTS WITH MENINGOENCEPHALOCELE
This group consisted of three patients (two males and one female) between 8 and 34 years of age. Their presenting symptoms were spontaneous intermittent cerebrospinal fluid (CSF) rhinorrhea, nasal obstruction from an intranasal cavity mass, or both. CT and MRI showed the lesions in all cases.
PATIENTS WITH CSF RHINORRHEA FROM SPHENOID SINUS
They were three male patients in this category. A 65-year-old man had headache and intermittent binostril rhinorrhea for 6 months after undergoing his fifth functional endoscopic sinus surgery for recurrent sinonasal polyposis involving the sphenoid sinus. CT cisternography and MRI showed evidence of a CSF leak inside the sphenoid sinus associated with nasal polyposis. The second patient, a 45-year-old man, had a parasellar pituitary macroadenoma removed through a sublabial transnasal trans-septal trans-sphenoidal microsurgical approach followed by radiotherapy at age 32. He developed intermittent bilateral CSF rhinorrhea 6 months before surgery. CT cisternography and MRI showed evidence of a CSF leak inside the left sphenoid sinus compartment associated with a large intrasellar arachnoid cyst. The third patient was 35 years old and had experienced spontaneous intermittent unilateral rhinorrhea for 5 months. CT cisternography and MRI showed a cranial base defect in the floor of the sella turcica.
PATIENTS WITH CLIVAL CHORDOMA
Two men, aged 34 and 48 years, respectively, had a clival chordoma. Headache was the presenting symptom in the first case and headache with diplopia related to oculomotor and abducent nerve palsy were the presenting symptoms in the second case. CT and MRI showed the lesions in both cases.
PATIENTS WITH SKULL BASE MENINGIOMAS
A 40-year-old man had an extensive greater wing meningioma that reached the nasal cavity. It was treated through a combined approach (transcranial pterional approach for the cranial portion and the current approach for the transnasal portion). Surgery was followed by radiotherapy to the residual tumor in the lateral sellar compartment. A second patient, a 45-year-old man, had a recurrent olfactory groove meningioma that also was treated through a combined approach (oblique subfrontal approach for the cranial portion and the current approach for the transnasal portion).
Surgical Technique
The procedure was performed by an otolaryngologist or by both an otolaryngologist and a neurosurgeon. The operation was performed under hypotensive general anesthesia to minimize blood loss and to obtain a bloodless field to facilitate the work with the endoscope. The nose was packed with 1:100,000 of epinephrine cottonoids. The patient was placed in a reversed Trendelenburg position about 20 degrees above the horizontal. The head was tilted to the right, extended about 10 degrees, and secured with Mayfield head pins.
The entire procedure was approached via a nostril under fluoroscopic monitoring. In all patients, the C-arm was positioned so that a cross-table lateral radiograph of the sphenoid sinus and sella could be obtained. The face and right lower quadrant of the abdomen were prepared sterilely and draped. Prophylactic antibiotics were administered.
A septoplasty was performed to correct a markedly deviated septum in 10 cases (to facilitate the surgical approach in 6 and to improve the blocked nasal airway in 4 patients). The septoplasty incision was made on the contralateral side of the approached nostril. Initially, a 0-degree endoscope was used. The middle turbinate was injected with 1% lidocaine with 1:100,000 of epinephrine and the lower half was excised (Figs. 2A,B). Bleeding from the inferior surface of the remaining part of the middle turbinate was controlled by bipolar diathermy. The upper half, which is directly attached to the anterior skull base, was not violated to avoid CSF leakage.5 Because the lower half of the middle turbinate was removed, the full length of the ipsilateral nasal cavity, including the related part of the anterior skull base, was widened and exposed.
Figure 2.
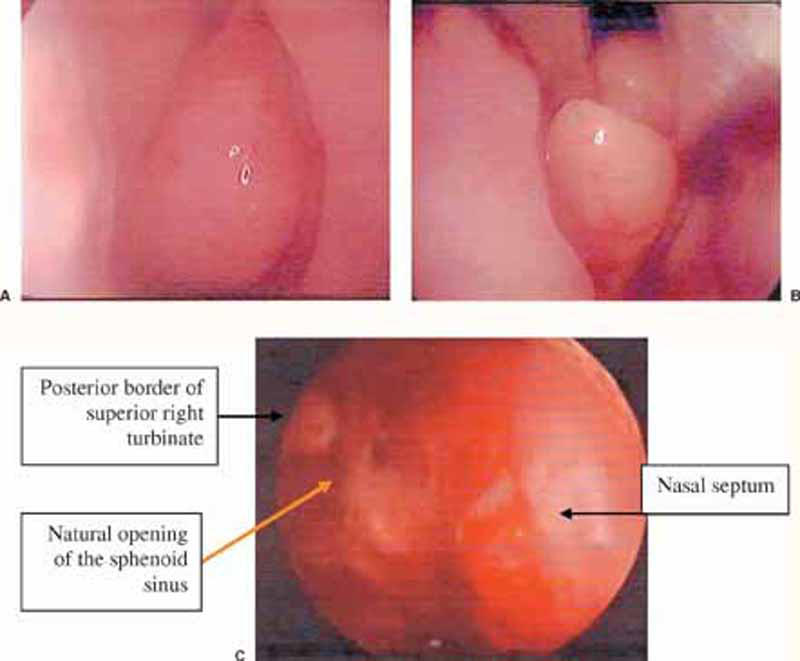
(A) Endoscopic (0-degree) view of the left nasal cavity showing the attachment of the left middle turbinate to the anterior skull base and lateral wall of the nose. (B) The same view showing the site of removal of the lower half of the left middle turbinate. (C) A surgical field obtained after excision of the lower half of the right middle turbinate.
The procedure used depended on the nature of the lesions. Patients with pituitary adenomas were treated as prescribed by Jho et al.7 Isolated sphenoid sinus inflammatory diseases were treated as prescribed by Stammberger et al.8 Patients with meningoencephalocele and CSF rhinorrhea were treated as prescribed by Mattox and Kennedy.9 Chordomas and meningiomas were treated as prescribed by Jho10 and colleagues.11
In cases that required reconstruction of a skull base defect, the harvested turbinate tissue was used to repair the defect so that the turbinate bone and mucoperiostium lay superiorly toward the surgical site of the lesion and the covering turbinate mucosa lay inferiorly toward the nasal cavity.
At the end of the procedure, bilateral anterior nasal packs with petroleum jelly-impregnated gauze and gentamicin ointment were inserted into the nasal cavities to control bleeding from the operated nasal tissue, to provide temporary support for the inserted grafts, and to prevent early formation of intranasal synechia. Patients were kept in the hospital to receive systemic intravenous antibiotics, to be observed for the onset of diabetes insipidus (DI), to have the nasal pack removed 48 hours after surgery, and to exclude CSF leakage or other complications. Patient follow-up ranged from 7 to 48 months.
RESULTS
Anatomic Cadaveric Study
The presence of the nasal septum medially and the middle turbinates laterally restricted the size and shape of the field of vision. Removal of the inferior half of the middle turbinate offered more surgical space, less restricted tubular vision, and avoided, to a great extent, coaxial work of the microinstruments inside the nasal cavity (Fig. 1B). Removal of the lower half of the middle turbinate exposed the full length of the ipsilateral anterior wall of the sphenoid sinus. This maneuver facilitated identification of the ipsilateral natural opening of the sinus and removal of its anterior wall (Fig. 1B).
With 0- and 30-degree endoscopes, the approach offered a wide anatomic panoramic orientation of the surgical field in the ipsilateral nasal cavity and sphenoid sinus-related structures. The 0-degree endoscopes provided the least distortion in the surgical anatomical images. The angled endoscopes with 30- and 70-degree lenses provided an extended lateral view that clarified remote anatomic corners (Fig. 1). They also enhanced distortion of the same anatomic structures.
Surgical Study Group
PATIENTS WITH PITUITARY ADENOMAS
There were no neural or vascular injuries. Intraoperative CSF leakage was encountered in 14 patients (12 macroadenomas and 2 microadenomas). The mean operative time was 2.1 hours (standard deviation: 0.7), and the average intraoperative blood loss was 130 mL. No postoperative CSF leaks or other complications could be related to the partial middle turbinectomy or the closure technique.
Temporary DI occurred in 3 cases. Hospital stays were overnight in 21 patients, 2 nights in 18 patients, and 3 nights in 6 patients. Postoperative intranasal synechia were found in 5 patients during the first 2 weeks after surgery. They were divided under local anesthesia. Of the 18 patients with prolactinomas, 14 were cured clinically and their serum prolactin levels returned to normal. Four improved clinically with mildly elevated serum prolactin levels, and they received bromocriptine therapy. Acromegalic patients were cured clinically, and their serum growth hormone returned to normal levels. Of the 25 patients with nonsecreting pituitary macroadenomas who presented with a visual disorder, all exhibited improvement in their visual acuity and visual fields. All patients with pituitary apoplexy had their symptoms resolved after surgery.
PATIENTS WITH ISOLATED SPHENOID SINUS INFLAMMATORY DISEASE
There were no major operative or postoperative complications. Partial middle turbinectomy facilitated the approach (Figs. 3A,B), postoperative cleaning, and surveillance. All patients were free of symptoms 12 weeks after surgery and have remained so during the follow-up (mean follow-up, 23.1 months).
Figure 3.
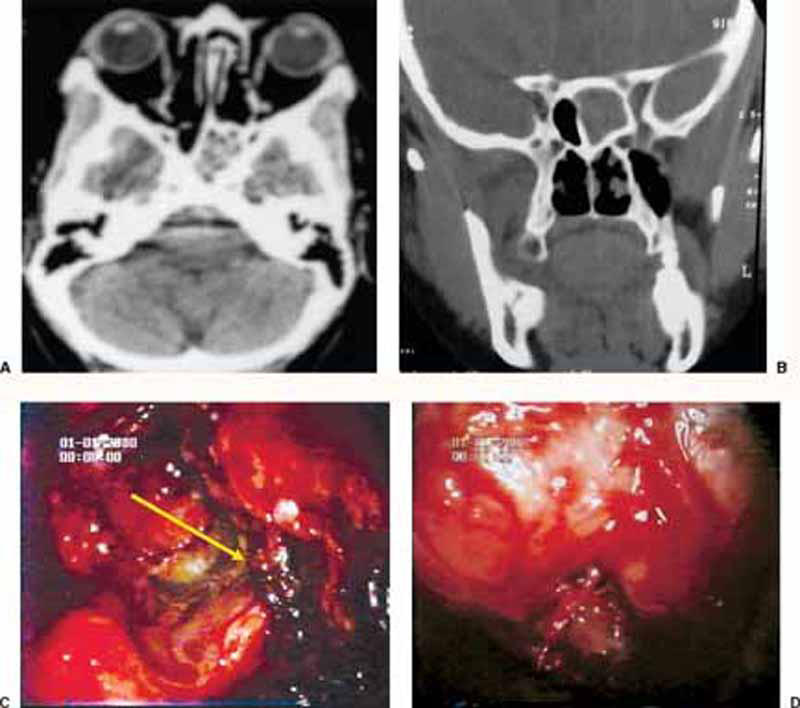
(A) Preoperative plain CT (axial and coronal views) of a case with isolated sphenoid fungal sinusitis. (C) Intraoperative endoscopic (30-degree) view of the same case showing the fungal debris filling the lower compartment of the sphenoid sinus (arrow). (D) The same endoscopic view after removal of the fungal debris and appearance of the inferior compartment of the sphenoid sinus.
PATIENTS WITH CSF RHINORRHEA FROM SPHENOID SINUS
The defects in the sellar floor were 2 mm in two cases and 4 mm in a patient with an intrasellar arachnoid cyst (Fig. 4). There were no major operative or postoperative complications and the leaks were closed completely. There were no recurrences during follow-up.
Figure 4.
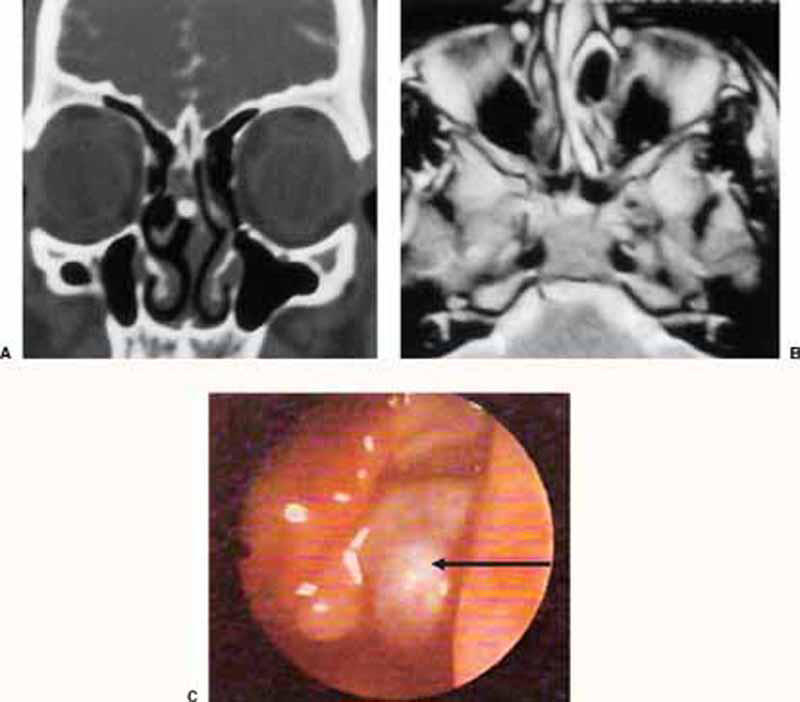
(A) Preoperative plain CT (coronal view) showing herniated intranasal meningoencephalocele in the left nasal cavity. (B) Preoperative noncontrast MRI (axial T2 WI) of the same case. (C) Intraoperative endoscopic (30-degree) view of the same case showing the meningoencephalocele (arrow) protruding between left middle turbinate and lateral nasal wall.
PATIENTS WITH MENINGOENCEPHALOCELE
These lesions originated from the ethmoid complex in two patients (Figs. 5A,B) and from the junction between the nasal septum and cribriform plate in the third. Complete excision of the herniated part and closure of the defect were achieved in all patients. No complications were seen and no patients developed meningitis or postoperative anosmia.
Figure 5.
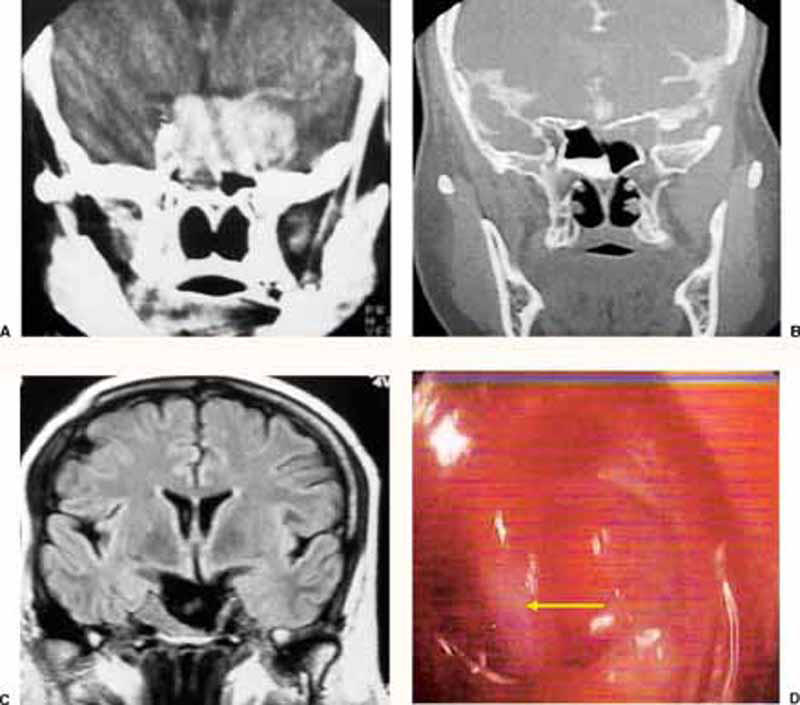
(A) Preoperative plain CT (coronal view) of a case of sellar and parasellar pituitary macroadenoma. (B) Postoperative plain CT (coronal view) of the same case. The patient presented with delayed CSF rhinorrhea 12 years after surgical removal of the lesion via sublabial microsurgical approach followed by postoperative radiation. (C) Preoperative noncontrast MRI (coronal T2 WI) of the same lesion showing a large CSF collection, mostly inside arachnoid pouch, filling most of the sellar cavity. (D) Intraoperative endoscopic (30-degree) view of the same case showing the internal carotid artery protruding through the medial wall of right cavernous sinus (arrow).
PATIENTS WITH CLIVAL CHORDOMA
There were no major intra- or postoperative complications (e.g., CSF leaks or cranial nerve paralysis). Subtotal resection (at least 90% of tumor removed, estimated from postoperative MRIs 6 months after surgery) was achieved in one case (Fig. 6A). A partial resection (less than 90% of tumor removed as estimated from postoperative MRI 6 months after surgery) was achieved in the second patient 3 months after surgery. This patient underwent an endoscopically assisted transoral-transpharyngeal approach to the craniovertebral junction prescribed by Frempong-Boadu and associates.12
Figure 6.
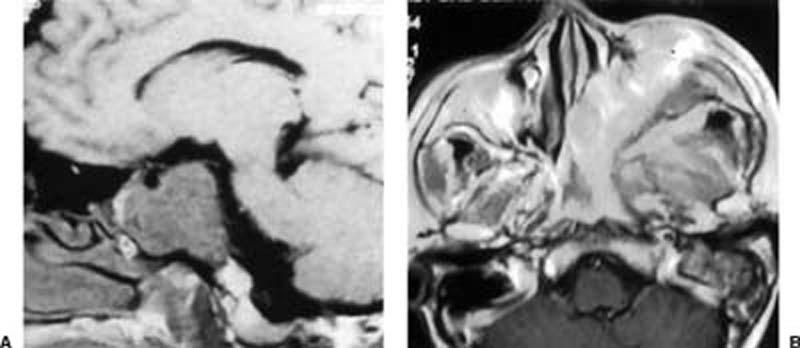
(A) Preoperative noncontrast MRI (axial T1 WI) of a 48-year-old male with a clival chordoma. Partial resection was achieved (less than 90% of the tumor was removed) as noted in the second 3 months after surgery. This patient had undergone an endoscopically assisted transoral-transpharyngeal approach to the craniovertebral junction prescribed by Frempong-Boadu and associates.12 (B) Preoperative noncontrast MRI (axial T2 WI) of the case with the extensive greater wing meningioma reaching the nasal cavity. It was treated by a combined approach (transcranial pterional approach for the cranial portion and endonasal endoscopic with a lower-half middle turbinectomy for the transnasal portion) and followed by radiotherapy.
PATIENTS WITH SKULL BASE MENINGIOMAS
Endoscopic exposure for the meningiomas would not be possible without excision of the lower half of the middle turbinate, which also facilitated the performance of ethmoidectomy, sphenoidectomy, tumor visualization, and resection near the palatine bone and medial pterygoid process (Fig. 6B). No major operative or postoperative complications were seen, and skull reconstruction was successful with no CSF leakage.
DISCUSSION
In 1987, Griffith and Veerapen13 introduced a direct endonasal endoscopic approach to the pituitary. Since then, several endonasal endoscopic techniques have been reported for the treatment of different sphenoid sinus and related skull base lesions. Increasing experience and confidence with this approach have allowed modifications tailored to individual nasal, paranasal sinus, and skull base-related lesions.
Endoscopic endonasal anatomic features could be distorted because of deviation of the nasal septum, hypertrophy of both turbinates, concha bullosa of the middle turbinates, or other unexpected anatomic variations. These variations can produce difficulties and reduce the safety of accessing the nasal cavity and skull base. Trimming the middle turbinate was considered a standard step in treating chronic sinusitis via the Wigand technique14 and in some situations via the Messerklinger technique.5 It did not affect the mucociliary's clearance of the nasal or paranasal sinus cavity,5 and there was no risk of CSF leak as long as its superior attachment to the skull base was not violated.8
In the cadaveric study with removal of the lower half of the middle turbinate, the endonasal use of the 0-degree and angled endoscopes provided an extended ipsilateral view of the full length of the nasal cavity. With the 0-degree endoscope, the entire anterior wall of the sphenoid sinus could be visualized (Fig. 3B), which facilitated anterior sphenoidotomy. With the angle of the 30-degree endoscope directed medially, it extended the ipsilateral view of the mucoperichondrium covering the nasal septum and related cribriform plate. When the endoscope was directed laterally, the entire lateral wall of the nose was visualized (Fig. 2C).
Through an anterior sphenoidotomy with the use of a 0-degree endoscope, the posterior wall of the sphenoidal sinus was visualized with a wide panoramic view. To expose the lateral recess at the posterior wall of the sphenoidal sinus, 30- and 70-degree angled-lens endoscopes were used. Rostral extension of the anterior sphenoidotomy could be adjusted to reach the area of the planum sphenoidale superiorly, the floor of the sphenoid sinus inferiorly, the lateral wall of the nasal cavity and lateral wall of the sphenoid sinus (from anterior to posterior) laterally, the entire length of the nasal septum, and the remainder of the sphenoid cavity medially.
During the surgery for pituitary adenomas, a 0-degree endoscope provided a wide exposure, and the ipsilateral paramedian compartment of the posterior wall of the sphenoid sinus was at the center of the surgical exposure. Once the sella was entered and dura mater was diathermized and opened, the tumor was removed under direct endoscopic visualization while the normal pituitary gland tissue was preserved. The wider surgical field created by this approach enhanced visualization of the suprasellar region, especially with the 30-degree endoscope (Fig. 4D). The previously harvested piece of turbinate tissue obtained by the current approach was used to repair the sellar floor defect so that there was no need to use any other reconstructive materials—neither autogenous materials such as cartilaginous or bony septum, which in some instances were unavailable,7 nor artificial materials such as titanium plates15 or ceramic substances.16 These materials are limited by their rigidity, availability, compatibility with MRI, and cost. With the current approach to pituitary surgery, the operating time and amount of intraoperative bleeding were less than those reported in the literature.2,3,4,5,6,7
Endoscopic endonasal approaches to inflammatory diseases of the sphenoid sinus can be performed through the nasal side or from posterior ethmoid cells.8,9,10,11,12,13,14,15,16,17,18 The latter approach is longer, difficult, and involves unnecessary surgery in cases of isolated sphenoid sinus inflammatory disease.18 An endoscopic endonasal approach to the sphenoid sinus through the rostrum of the sphenoid or through the natural os17 can be difficult because the anatomy varies or when polypoid disease is present. With the current approach the instruments were less crowded in the working nostril and more bulky instruments could be used (e.g., a sidebiting rongeur and an antral backbiting rongeur). Anterior sphenoidotomy through the rostrum of the sphenoid or through the natural os became easier, safer, and quicker. It also facilitated postoperative cleaning of the surgical field, thereby avoiding the risk of stenosis of the sphenoid sinus opening or reinfection as reported by others.18
In the patients with a clival chordoma, the approach created a wide, hollow surgical space large enough to expose the clival region. The optics and illumination of the endoscope were in the resection field at the level of the abnormality, which increased the safety of extensive resection of the lesion. By rotating the 30-degree angled endoscope, we were able to look laterally and inferiorly to obtain a larger panoramic view that allowed maximum clearance of the lesion. The current technique reduces the overall morbidity that is associated with other approaches.10,11,12,13,14,15,16,17,18,19
Wide exposure of the meningoencephalocele and meticulous hemostasis are essential for surgical success.9 In the present work, adequate access of the herniated portion of the lesion was facilitated by removal of the lower half of the middle turbinate. This maneuver allowed easy manipulation of the bipolar coagulation forceps to obtain good hemostasis and gradual fulguration of the lesion to avoid intracranial hemorrhage. To improve accessibility by the current approach, the 0- or 30-degree endoscopes were used to expose the skull base for several millimeters around the defect. This maneuver allowed the harvested piece of turbinate tissue to reconstruct the defects with other grafting material (e.g., Gelfoam®). The maneuver was easy to perform, and a watertight seal was achieved without restriction related to the working space when compared with other similar approaches.9,10,11,12,13,14,15,16,17,18,19,20
Kato and associates21 described the difficulties encountered during endonasal resection of anterior skull base lesions including meningiomas. These difficulties were partially associated with the restrictions imposed by the deep narrow working channel intrinsic to this approach. Furthermore, significant limitations in exposing tumors with lateral extensions made it extremely difficult to control the source of hemorrhage because the offending vessel was positioned laterally. With the middle turbinectomy approach, the anterior sphenoidotomy can be extended laterally to the pterygoid bone, thus widening access to the lateral sphenoidal sinus structures. This maneuver improves exposure of the intranasal part of the meningiomas and thereby enables good hemostasis and tumor resection.
CONCLUSION
The current approach widened the surgical field without increasing morbidity. It avoids unnecessary trauma of the other nostril as occurs in a binostril approach. The harvested turbinate tissue is an excellent source of donor material for successful reconstruction of the sellar floor without inducing side effects or complications.
REFERENCES
- Gibbons M D, Sillers M J. Minimally invasive approaches to the sphenoid sinus. Otolaryngol Head Neck Surg. 2002;126:635–641. doi: 10.1067/mhn.2002.125759. [DOI] [PubMed] [Google Scholar]
- Nasseri S S, Kasperbauer J L, Strome S E, McCaffrey T V, Atkinson J L, Meyer F B. Endoscopic transnasal pituitary surgery: report on 180 cases. Am J Rhinol. 2001:281–287. [PubMed] [Google Scholar]
- Cappabianca P, Alfieri A, de Divitiis E. Endoscopic endonasal transsphenoidal approach to the sella: towards functional endoscopic pituitary surgery (FEPS) Minim Invasive Neurosurg. 1998;41:66–73. doi: 10.1055/s-2008-1052019. [DOI] [PubMed] [Google Scholar]
- Becker S P. Applied anatomy of the paranasal sinuses with emphasis on endoscopic surgery. Ann Otol Rhinol Laryngol Suppl. 1994;162:3–32. doi: 10.1177/00034894941030s401. [DOI] [PubMed] [Google Scholar]
- Liu Z, Wang C, Gao Q, Cui Y. The effect of managements of middle turbinate on nasal airway resistance in endoscopic sinus surgery [in Chinese] Lin Chuang Er Bi Yan Hou Ke Za Zhi. 2002;16:201–203. [PubMed] [Google Scholar]
- Yanagisawa E. Endoscopic view of the middle turbinate. Ear Nose Throat J. 1993;72:725–727. [PubMed] [Google Scholar]
- Jho H D, Carrau R L, Ko Y. In: Rengachary SS, Wilkins RH, editor. Neurosurgical Operative Atlas. Park Ridge, IL: 1996. Endoscopic pituitary surgery. p. 112. American Association of Neurological Surgeons.
- Stammberger H. Endoscopic endonasal surgery—concepts in treatment of recurring rhinosinusitis. Part II. Surgical technique. Otolaryngol Head Neck Surg. 1986;94:147–156. doi: 10.1177/019459988609400203. [DOI] [PubMed] [Google Scholar]
- Mattox D E, Kennedy D W. Endoscopic management of cerebrospinal fluid leaks and cephalocele. Laryngoscope. 1990;100:857–862. doi: 10.1288/00005537-199008000-00012. [DOI] [PubMed] [Google Scholar]
- Jho H D, Carrau R L, Mc Laughlin M L, Somaza S C. Endoscopic transsphenoidal resection of a large chordoma in the posterior fossa. Case report. Neurosurg Focus. 1996;1:e3, discussion 1p following e3. [PubMed] [Google Scholar]
- Jho H D. Endoscopic transsphenoidal surgery. J Neurooncol. 2001;54:187–195. doi: 10.1023/a:1012969719503. [DOI] [PubMed] [Google Scholar]
- Frempong-Boadu A K, Faunce W A, Fessler R G. Endoscopically assisted transoral-transpharyngeal approach to the craniovertebral junction. Neurosurgery. 2002;51(5 Suppl):S60–S66. [PubMed] [Google Scholar]
- Griffith H B, Veerapen R. A direct transnasal approach to the sphenoid sinus [technical note] J Neurosurg. 1987;66:140–142. doi: 10.3171/jns.1987.66.1.0140. [DOI] [PubMed] [Google Scholar]
- Wigand M. Endoscopic Surgery of the Paranasal Sinuses and Anterior Skull Base. New York, NY: 1990. Thieme. [PubMed]
- Arita K, Kurisu K, Tominaga A, et al. Size-adjustable titanium plate for reconstruction of the sella turcica: technical note. J Neurosurg. 1999;91:1055–1057. doi: 10.3171/jns.1999.91.6.1055. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Sugita K, Matsuo K, Inoue T. Reconstruction of the sellar floor during transsphenoidal operations using alumina ceramic. Surg Neurol. 1981:196–197. doi: 10.1016/0090-3019(81)90142-7. [DOI] [PubMed] [Google Scholar]
- Alfieri A. Endoscopic endonasal transsphenoidal approach to the sellar region: technical evolution of the methodology and refinement of a dedicated instrumentation. J Neurosurg Sci. 1999;43:85–92. [PubMed] [Google Scholar]
- Martin T J, Smith T L, Smith M M, Loehrl T A. Evaluation and surgical management of isolated sphenoid sinus disease. Arch Otolaryngol Head Neck Surg. 2002;128:1413–1419. doi: 10.1001/archotol.128.12.1413. [DOI] [PubMed] [Google Scholar]
- Gay E, Sekhar L N, Rubinstein E, et al. Chordomas and chondrosarcomas of the cranial base: results and follow-up of 60 patients. Neurosurgery. 1995;36:887–896. doi: 10.1227/00006123-199505000-00001. [DOI] [PubMed] [Google Scholar]
- Marshall A H, Jones N S, Robertson I J. Endoscopic management of basal encephaloceles. J Laryngol Otol. 2001;115:545–547. doi: 10.1258/0022215011908450. [DOI] [PubMed] [Google Scholar]
- Kato T, Sawamura Y, Abe H, Nagashima M. Transsphenoidal-transtuberculum sellae approach for supradiaphragmatic tumours: technical note. Acta Neurochir (Wien) 1998;140:715–718. discussion 719. doi: 10.1007/s007010050167. [DOI] [PubMed] [Google Scholar]


