Abstract
Flavivirus envelope proteins have been shown to play a major role in virus assembly. These proteins are anchored into cellular and viral membranes by their C-terminal domain. These domains are composed of two hydrophobic stretches separated by a short hydrophilic segment containing at least one charged residue. We investigated the role of the transmembrane domains of prM and E in the envelope formation of the flavivirus yellow fever virus (YFV). Alanine scanning insertion mutagenesis has been used to examine the role of the transmembrane domains of prM and E in YFV subviral particle formation. Most of the insertions had a dramatic effect on the release of YFV subviral particles. Some of these mutations were introduced into the viral genome. The ability of these mutant viruses to produce infectious particles was severely reduced. The alanine insertions did not affect prM-E heterodimerization. In addition, replacement of the charged residues present in the middle of the transmembrane domains had no effect on subviral particle release. Taken together, these data indicate that the transmembrane domains of prM and E play a crucial role in the biogenesis of YFV envelope. In addition, these data indicate some differences between the transmembrane domains of the hepaciviruses and the flaviviruses.
Enveloped viruses acquire a membrane by budding through one of several host cellular membranes (for a review, see reference 15). Some viruses bud at the plasma membrane, whereas others bud at the intracellular membranes. There is growing evidence that the envelope proteins play a major role in the budding process. Indeed, coexpression of the mouse hepatitis virus envelope proteins M and E in the absence of other viral components results in the release of membrane particles morphologically indistinguishable from authentic virions (3, 38). Similarly, expression of the envelope (E) and pre-membrane protein (prM) of several flaviviruses in the absence of other viral proteins results in the secretion of virus-like particles called recombinant subviral particles that have structural and functional features of the envelope of the virion (reviewed in reference 16). The budding and symmetry of these subviral particles are determined by regular, lateral interactions among the E and prM (or M) subunits (12). Interactions between envelope proteins have also been shown to play a major role in alphavirus particle assembly and symmetry (5, 13, 24). By being inserted in a cellular membrane, the transmembrane (TM) domains of these proteins also participate in the budding process. Their anchor function is necessary to isolate a fraction of a cellular membrane that becomes part of the viral envelope.
The flavivirus envelope contains two proteins: E and M. The latter is synthesized as a precursor called prM. Newly synthesized E and prM proteins associate to form heterodimers (1, 39) that are incorporated into immature virions by budding into the endoplasmic reticulum (ER) lumen (23). Interestingly, each heterodimer is associated with another heterodimer through lateral interactions between the ectodomains of E in a head-to-tail conformation (30). Additional contacts between E dimers produce a network of interactions that create an icosahedral lattice structure and likely generate the driving force, leading to budding (12, 19). The particles are transported through the secretory pathway and, shortly before release from the cell, they are converted to the active form by cleavage of prM by a cellular furin protease (35). Heterodimeric interactions between prM and E are important for proper folding of E (1, 18, 22) and probably also for the protection of the immature virion against acid inactivation during transport through acidic vesicles (16).
We investigated here the role of the TM domains of prM and E in the formation of the envelope of the flavivirus yellow fever virus (YFV). We used alanine scanning insertion mutagenesis (4, 25) in these domains to examine their functional role in the biogenesis of YFV envelope. This technique has been shown to be a powerful method for detecting dimerization of TM α-helices. Indeed, insertion of a single amino acid into a TM helix displaces the residues on the N-terminal side of the insertion by 110° relative to those on the C-terminal side of the insertion, disrupting a potential helix-helix packing interface involving residues on both sides of the insertion. Interestingly, most of the insertions had a dramatic effect on the release of YFV subviral particles. Some of these mutations were introduced into the YFV genome. The ability of these mutant viruses to produce infectious particles was severely reduced. These data point out the essential role played by the TM domains of prM and E in the biogenesis of the envelope of YFV.
MATERIALS AND METHODS
Cell culture.
The Huh7, HepG2, SW13, CV-1, and 143B (thymidine kinase-deficient) cell lines were obtained from the American Type Culture Collection, Manassas, Va. BHK21/J cells (21) were kindly provided by B. Lindenbach (Rockefeller University). Cell monolayers were grown in Dulbecco modified Eagle medium (Invitrogen) supplemented with 10% fetal bovine serum.
Plasmid constructs.
Plasmid pAP5, which contains the sequence encoding the structural proteins of the YFV 17D-204-Pasteur strain (8) was kindly provided by A. Cahour (Hôpital de la Pitié-Salpétrière, Paris, France). Plasmids expressing YFV proteins were constructed by standard methodology (32). The sequence encoding YFV prM signal sequence, followed by prME polyprotein (amino acids 101 to 788 on the YFV polyprotein), was cloned into pTM1 plasmid (26). Site-directed mutagenesis was performed by enzymatic inverse PCR as described by Stemmer and Morris (36). Plasmid pACNR-FLYF17D contains the complete cDNA sequence of the YFV17D genome (P. Bredenbeek et al., unpublished data). Mutations in the prM and E genes were constructed by fusion PCR (20) with Pfu DNA polymerase. The nucleotide sequence of all PCR derived DNA fragments was confirmed by sequencing.
Vaccinia virus recombinants.
Vaccinia virus recombinants were generated by homologous recombination essentially as described previously (17) and plaque purified twice in 143B cells under bromodeoxyuridine selection (50 mg/ml). Stocks of vTF7-3 (a vaccinia virus recombinant expressing the T7 DNA-dependent RNA polymerase) (14), the wild-type vaccinia virus strain Copenhagen, its thermosensitive derivative ts7 (9), and vaccinia virus recombinants expressing wild-type or mutated YFV proteins were grown and titrated on CV1 monolayers. The genes of YFV proteins expressed here are under the control of a T7 promoter, and expression of the proteins of interest was achieved by coinfection with vTF7-3.
In vitro synthesis and transfection of YFV full-length RNA.
For in vitro transcription of wild-type and mutant full-length infectious YFV RNA, plasmid DNA was linearized with AflII, extracted with phenol-chloroform, and ethanol precipitated. RNA was subsequently synthesized by using SP6 RNA polymerase (Invitrogen) in the presence of trace amounts of 3H-labeled UTP (31). The amount of synthesized RNA was determined by trichloroacetic acid precipitation and liquid scintillation counting. Transfection of in vitro-transcribed RNA into BHK21/J cells by electroporation was performed as described elsewhere (R. Molenkamp et al., unpublished data).
Titration of YFV recombinant virus.
Virus harvests were titrated in plaque assays, which were performed on 106 SW13 cells in 35-mm-diameter cell culture dishes. Cells were infected with dilutions (10−2 to 10−7) of virus harvest obtained at 24 h posttransfection. After 1 h of infection, a 1% agarose overlay in Dulbecco modified Eagle medium containing 1% fetal calf serum was applied to the cells. The cells were then incubated at 37°C for 6 days. Plaque assays were fixed with 10% formaldehyde in phosphate-bufferd saline (PBS) and stained with a 1% solution of crystal violet (Merck) in 50% ethanol.
Antibodies.
Monoclonal antibody (MAb) 2D12 (anti-E, ATCC CRL-1689) (34) was produced in vitro by using a MiniPerm apparatus (Heraus) as recommended by the manufacturer. The anti-YFV polyclonal antibody and MAb 1A5 (anti-NS1) were kindly provided by P. Desprès (Institut Pasteur, Paris, France) and by J. Schlesinger (University of Rochester), respectively. MAbs 1A5 and 2D12 were used at a 1:1,000 dilution for immunofluorescence as described elsewhere (Bredenbeek et al., unpublished).
Metabolic labeling and immunoprecipitation.
Cells were infected with the appropriate recombinant vaccinia viruses and metabolically labeled with 35S-protein labeling mix (530 Mbq/ml; Amersham Biosciences) as described previously (10). Cells were lysed in radioimmunoprecipitation assay (RIPA) buffer (0.05 M Tris-HCl [pH 7.2], 150 mM NaCl, 1% Triton X-100, 0.1% sodium dodecyl sulfate [SDS], 1% sodium deoxycholate) or in the same buffer devoid of SDS and deoxycholate and containing the protease inhibitor cocktail Complete (Roche Diagnostics, Mannheim, Germany). Cell lysates were passed 10 times through a syringe with a 26Gx1/2" needle to break cellular chromatin. Immunoprecipitations were carried out as described previously (11).
Sedimentation analysis of YFV subviral particles.
Supernatants containing radiolabeled subviral particles were cleared by centrifugation at 400 × g for 5 min and then treated or not treated with Igepal. Supernatants were then layered on 10-ml gradients of 20 to 50% sucrose in PBS containing or not containing 0.1% Igepal. Gradients were centrifuged overnight at 35,000 rpm at 4°C in a Beckman SW41 rotor. Fractions of 1 ml were collected and analyzed by immunoprecipitation with MAb 2D12.
Preparation of subviral particles for transmission electron microscopy.
Culture medium of cells coinfected with the vaccinia virus recombinants vprME and vTF7-3 was collected at 18 h postinfection and centrifuged for 5 min at 20,000 × g to discard the cellular debris. Supernatant was concentrated by centrifugation at 200 × g on a Biomax-100 filter with a 100K cutoff (Ultrafree; Millipore) according to the instructions of the manufacturer. Samples were then diluted in 10 ml of PBS and concentrated again by centrifugation on a new Biomax-100 filter. Culture medium of cells infected with vTF7-3 alone was used as a control. A single droplet-negative staining procedure was used for transmission electron microscopy analyses. Briefly, 20-μl droplets of subviral particle preparation or control sample were adsorbed for 3 min onto Formvar carbon-coated cooper grids (400 mesh; Electron Microscopy Science, Washington, Pa.). The excess was removed, and bound particles were washed on three droplets of distilled water and then stained with 1% uranyl acetate (pH 4.5; Merck) for 10 s. After air drying, the grids were examined under a Hitachi-7500 transmission electron microscope at 80 kV.
Sedimentation analyses of YFV proteins.
Heterodimerization of prM-E was analyzed as described previously (33). Briefly, cells expressing wild-type or mutated metabolically labeled prME polyproteins were lysed in 50 mM Tris-HCl (pH 8)-150 mM NaCl-5 mM EDTA containing 1% Triton X-100. Cell debris was discarded by a 2-min centrifugation at 20,000 × g. Samples were then analyzed by overnight centrifugation at 38,000 rpm (in a Beckman SW41 rotor) in 5 to 20% sucrose gradients. Gradients were in 50 mM triethanolamine (pH 7.5)-150 mM NaCl containing 0.1% Triton X-100. Fractions of 0.5 ml were collected and analyzed by immunoprecipitation with the anti-YFV polyclonal antibody.
RESULTS
Production of YFV subviral particles by cells expressing prME.
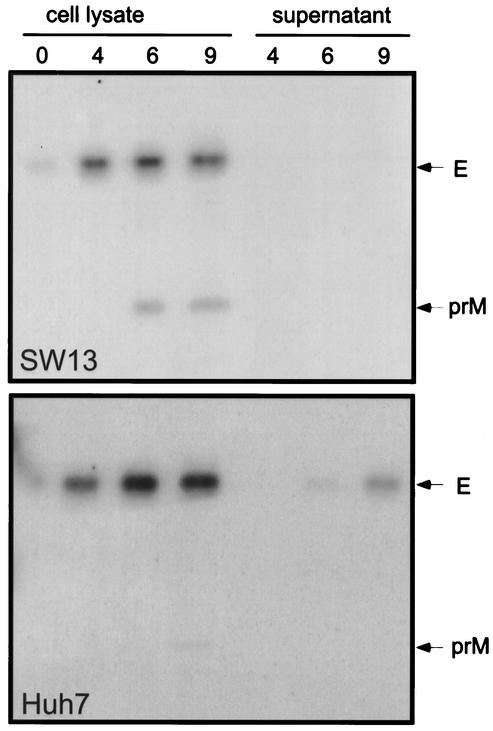
It has previously been reported that expression of a prME polyprotein of YFV leads to secretion of subviral particles (29). We therefore used a similar strategy to produce such particles in this work. A recombinant vaccinia virus (vprME) expressing prME polyprotein of YFV17-D strain was constructed. Secretion of YFV subviral particles was analyzed by immunoprecipitation of the supernatant of cell cultures expressing prME. Several cell lines were tested for their ability to produce secreted E protein. Analyses of cell lysates from Huh7 and SW13 cells showed similar levels of expression for the E protein (Fig. 1). However, differences were observed in the secretion of the E protein. This protein was almost undetectable in the supernatant of SW13 cells, whereas ca. 35% of E was secreted from Huh7 cells after a 9-h chase. The level of secretion observed in other cell lines (HepG2 and BHK21) was intermediate between that of Huh7 and SW13 (data not shown). Huh7 cells were therefore used for our functional studies. The E protein was detected in the supernatant of Huh7 cells expressing prME as soon as 6 h postlabeling, and its intensity increased until the end of the chase (9 h, Fig. 1 [Huh7]). Interestingly, prM was coprecipitated by the anti-E antibody after 6 and 9 h of chase in SW13 cells, whereas it was almost undetectable in Huh7 cells. The lower intensity of prM coprecipitated with E in Huh7 cells is likely due to cleavage of this protein during the export of the particles through the secretory pathway (35). However, since we had to use RIPA buffer in our immunoprecipitation experiments to have a better access of the epitope recognized by the anti-E antibody, we could not exclude that a fraction of prM was dissociated from E in our experimental conditions. The coprecipitation of prM with E in SW13 cells after long periods of chase, together with the low level of secretion of E, suggest that prM-E complexes, assembled or not into subviral particles, are retained in an early compartment of the secretory pathway in this cell line.
FIG. 1.
Analysis of secretion of YFV subviral particles. Huh7 or SW13 cells were coinfected with vTF7-3 and a recombinant vaccinia virus expressing prME polyprotein (vprME). At 4 h postinfection, cells were pulse-labeled for 30 min and chased for different times as indicated (in hours). Cell lysates as well as supernatants were used for immunoprecipitation with the anti-E MAb 2D12.
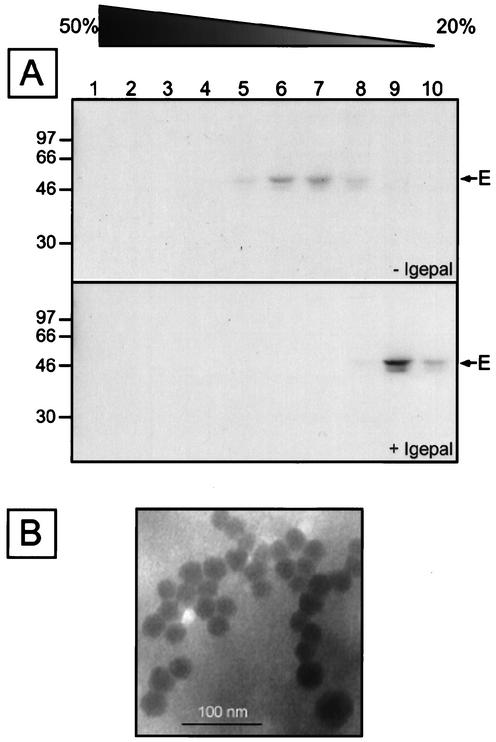
To confirm the particle nature of the secreted E protein, supernatant of Huh7 cells expressing prME were analyzed by sedimentation through sucrose gradients in the presence or absence of detergent. The E protein peaked in fractions 6 and 7 in the absence of detergent (Fig. 2A) at a density of 1.135 g/cm3, a finding consistent with results obtained with other flavivirus subviral particles (33). In addition, the E protein remained on the top of the gradient (fractions 9 and 10) when detergent was added (Fig. 2A), which is consistent with the presence of a lipid membrane. Electron microscopy analyses of YFV subviral particles stained with uracyl acetate revealed spherical particles with a diameter of 28.3 ± 2.5 nm (Fig. 2B). This is similar to what has been reported for other flaviviruses (reviewed in reference 16). Together, these data indicate that Huh7 cells expressing prME secrete subviral particles.
FIG. 2.
Analysis of YFV subviral particles by sedimentation in the presence or absence of detergent. (A) Huh7 cells were coinfected with vTF7-3 and a recombinant vaccinia virus expressing the prME polyprotein. At 4 h postinfection, cells were pulse-labeled for 30 min, followed by a 14-h chase. Supernatants were then sedimented through sucrose gradients (20 to 50%) in the presence or absence of Igepal as described in Materials and Methods. Fractions were collected from the bottom and analyzed by immunoprecipitation with an anti-E MAb. Immunoprecipitates were analyzed by SDS-polyacrylamide gel electrophoresis (PAGE; 13% polyacrylamide). Sizes of protein molecular mass markers are indicated on the left (in kilodaltons). (B) Electron micrograph of YFV subviral particles stained with uranyl acetate.
Alanine insertions in the TM domains of prM or E affect secretion of YFV subviral particles.
To investigate the role of the TM domains of the envelope proteins prM and E in the formation of YFV envelope, we analyzed the effects of mutations in these domains on the release of subviral particles. It has been shown that the TM domains of hepatitis C virus (HCV) envelope proteins are involved in E1-E2 heterodimerization (reviewed in reference 27). Since HCV and YFV belong to two different genera of the Flaviviridae family, we hypothesized that the TM domains of prM and E might also be involved in protein-protein interactions that are required for the formation of the flavivirus envelope. To analyze whether these interactions occur, we used alanine scanning insertion mutagenesis, a technique that has been shown to disrupt helix-helix interaction in a membrane environment (4, 25). This approach has been very useful for showing the role of the TM domains of HCV envelope proteins in heterodimerization (28). Like those of HCV envelope proteins, the C termini of prM and E are composed of two hydrophobic stretches separated by a short hydrophilic segment containing at least one charged residue (7) (Fig. 3). The N-terminal amino acids of these domains have not been precisely determined. It has been predicted that the minimal TM regions start at positions 248 and 739 for prM and E, respectively (7). However, another prediction suggests that, at least for E, the N terminus of the TM domain might be located at position 731 (2, 37).
FIG. 3.
Schematic representation of YFV proteins prM and E. (A) Presentation YFV polyprotein. Signal sequences and TM domains are indicated by solid and hatched boxes, respectively. The arrows indicated the sites which are cleaved by a signal peptidase. SP is the signal sequence of prM. (B) Positions of the insertion mutations in the C-terminal regions of prM and E. The putative TM domains (as predicted in reference 7) are underlined, and charged residues are indicated in boldface. The dotted line indicates the putative N terminus of the first TM sequence of E as predicted earlier (2, 37).
To analyze the role of these TM domains in subviral particle formation, a series of mutated proteins were created by introducing a single alanine residue in the TM domains of prM or E (Fig. 3). The mutated proteins were expressed by recombinant vaccinia viruses as a polyprotein composed of a mutated prM and wild-type E or wild-type prM and a mutated E. The effect of these mutations on production of subviral particles was analyzed by the detection of E protein secreted in the supernatant of Huh7 cells. Ratios of secreted versus intracellular E protein were measured for each mutant and compared to the ratio obtained with wild-type proteins. As shown in Fig. 4, insertion of an alanine residue in most positions of both hydrophobic stretches in the TM domains of prM or E dramatically reduced the production of subviral particles. Indeed, secretion of subviral particles was reduced to ≤10% for most mutants when alanine was introduced into one of the hydrophobic stretches of prM or E. Interestingly, alanine insertion immediately downstream of the Arg residue (A271′ or A755′) located between the two hydrophobic stretches had only a slight effect, with the unexpected but reproducible observation that the mutation at position 755′ increased the release of subviral particles rather than reducing it.
FIG. 4.
Effect of alanine insertions in the TM domain of prM or E on YFV subviral particles secretion. Huh7 cells were coinfected with vTF7-3 and the appropriate recombinant vaccinia virus. At 4 h postinfection, cells were pulse-labeled for 30 min, followed by a 14-h chase. Cell lysates and supernatants of cell cultures were used for immunoprecipitation with an anti-E MAb. Immunoprecipitates were analyzed by SDS-PAGE (13% polyacrylamide), and the intensities of the bands corresponding to E were measured with a phosphorimager. The data presented in this figure are from four independent experiments, and the mean percentage of subviral particle secretion was calculated as follows for each mutant: (secreted E/cell-associated E ratio from mutated polyprotein)/(secreted E/cell-associated E ratio from wild-type polyprotein). The positions of the alanine insertions in the TM domains of prM or E are indicated.
Together, these data indicate that the TM domains of prM and E are very sensitive to mutagenesis. In addition, they strongly suggest that TM domain interactions play a major role in the envelope formation of YFV.
The conserved charged residues located between the two hydrophobic stretches do not play a major role in YFV envelope formation.
Replacement of the charged residues between the two hydrophobic stretches by alanine residues has been shown to alter the ER retention and heterodimerization functions of the TM domains of HCV envelope proteins (7). We therefore expected that the Arg residue similarly located between the two stretches of hydrophobic residues of the C termini of prM and E might also be involved in similar functions in YFV and would therefore be essential for the envelope formation. To test this hypothesis, the Arg residues at positions 270 or 754 were replaced by an Ala in prME polyprotein, and we analyzed the effects of these mutations on the release of subviral particles. Surprisingly, these mutations did not reduce YFV subviral particle secretion (Fig. 5). The level of secretion was even higher for the R270A mutant. The particle nature of these mutants was confirmed by sedimentation analyses (data not shown). These data indicate that the Arg residues present in the middle of the hydrophobic C termini of prM and E do not play a major role in YFV envelope formation. However, further investigations will be necessary to determine the potential role of these charged residues in the assembly of the infectious particle. Indeed, we cannot exclude that these residues interact with the capsid protein.
FIG. 5.
Effect of charged residue mutagenesis on secretion of YFV subviral particles. Huh7 cells were coinfected with vTF7-3 and the appropriate recombinant vaccinia virus. At 4 h postinfection, cells were pulse-labeled for 30 min, followed by a 14-h chase. Cell lysates and supernatants were used for immunoprecipitation with an anti-E MAb. Immunoprecipitates were analyzed by SDS-PAGE (13% polyacrylamide), and the intensity of the band corresponding to E was measured with a phosphorimager. The mean percentage of subviral particle secretion was calculated as in Fig. 4.
Alanine insertions in the TM domains of prM or E affect YFV infectivity.
Although studies in the context of subviral particles provide interesting information on the functions of the TM domains of prM and E, we decided to verify our observations by analyzing some of the mutations in the context of an infectious YFV genome. Four mutations (261′, 275′, 749′, or 763′) were chosen for their strong effect on subviral particle secretion and to have two examples of insertion for each TM domain. An additional mutation (279′) with a milder effect on subviral particle secretion was also chosen. Full-length YFV cDNAs containing an alanine insertion at positions 261′, 275′, 279′, 749′, or 763′ were constructed, and BHK21/J cells were transfected with in vitro-transcribed YFV RNA. For all mutants and the wild-type YFV-17D control the transfection efficiencies were ca. 70%, as determined by counting the percentage of cells stained positive for YFV NS1 by immunofluorescence (Fig. 6A and data not shown). For all mutants and the YFV-17D control clear expression of the E protein was also confirmed by immunofluorescence (Fig. 6B and data not shown). No difference in expression or localization was observed.
FIG. 6.
Expression of YFV NS1 (A) and E (B) as revealed by immunofluorescence. The results for YFV-17D, mutant A279′, and A749′ are shown. All other mutants yielded essentially similar results. YFV NS1 and E were stained with MAb 1A5 and 2D12, respectively.
Virus production was analyzed by two independent plaque assays of the medium harvested from transfected cells at 24 h posttransfection. As shown in Table 1, no virus particles were detected in the medium of cells transfected by mutants A261′, A275′, A749′, and A763′. Mutant A279′ showed a 500-fold reduction in virus titer compared to wild-type YFV. In addition, the plaques produced by mutant A279′ were significantly smaller than the plaques observed for wild-type YFV (Fig. 7). Interestingly, ca. 30% of subviral particles were secreted for this mutant compared to <10% for the other mutants (Fig. 4). Metabolic labeling of wild-type and mutant YFV RNAs showed similar levels of full-length genome synthesis (data not shown), indicating that the introduced mutations did not affect genome replication. These data again confirm that the TM domains of prM and E are very sensitive to alanine insertion. In addition, they validate the studies based on mutagenesis in the context of YFV subviral particles.
TABLE 1.
Effect of alanine insertion in the TM domains of prM or E on virus replication
| Virus | Mutated protein | Titer (PFU/ml)a |
|---|---|---|
| 17D | 5 × 107 | |
| A261′ | prM | <102 |
| A275′ | prM | <102 |
| A279′ | prM | 105 |
| A749′ | E | <102 |
| A763′ | E | <102 |
The titer is the mean of two independent plaque assays.
FIG. 7.
Effect of alanine insertion mutation at position 279′ on the plaque size. Culture medium was harvested at 24 h posttransfection. Virus yields were determined by plaque assays on SW13 cells (Table 1). For the YFV-17D control and the YFVA279′ mutant, the 10−6 and the 10−3 dilution results, respectively, are shown.
Alanine insertion in the TM domains does not affect prM-E heterodimerization.
Insertion of an alanine residue in a TM domain has been shown to affect oligomerization if this alanine is inserted in the middle of a segment involved in helix-helix interactions (4, 25). Therefore, based on the data that have been accumulated on the oligomerization of E1-E2 of HCV (28) and on the observation that the insertion of alanine residues in the TM domains of prM or E affects secretion of YFV subviral particles, we expected that prM-E heterodimerization would be affected by alanine insertion.
To test this hypothesis, we analyzed prM-E heterodimerization by immunoprecipitation with the anti-E MAb. Compared to the wild-type proteins, none of the mutants showed any change in the pattern of the immunoprecipitates (data not shown). A weak band that likely corresponds to prM was observed for all of the mutants. However, since we had to use RIPA buffer in our immunoprecipitation experiments to have a better access of the epitope recognized by the anti-E antibody, we could not exclude that a fraction of prM was dissociated from E in our experimental conditions. To further investigate the effect of alanine insertion on prM-E heterodimerization, cell lysates containing wild-type or mutated prM-E complexes were placed on sucrose gradients containing 0.1% Triton X-100, and sedimentation analyses were carried out to evaluate the degree to which prM and E remain associated with each other when an alanine residue is introduced in their TM domain. This approach has already been used successfully to analyze some deletions that disrupt prM-E assembly in tick-borne encephalitis (TBE) virus (2). As shown in the top panel Fig. 8, wild-type prM and E cosedimented as a stable complex that likely corresponds to a heterodimer containing one prM molecule and one E molecule, as previously shown for TBE virus (1). An additional band detected below E was frequently observed in our immunoprecipitation studies, and it might correspond to a cleaved product of E that could have appeared during the immunoprecipitation procedure and/or the overnight centrifugation despite the presence of protease inhibitors. Alternatively, it could also be a cellular product that is nonspecifically recognized by the anti-YFV polyclonal antibody. No change in the cosedimentation of prM and E occurred for mutants A279′ and A749′ (Fig. 8). Similar results were obtained with other mutants tested (data not shown). Our data indicate that alanine insertion in the TM domains of prM or E does not interfere with the heterodimerization.
FIG. 8.
Cosedimentation analysis of prM and E. Huh7 cells were coinfected with vTF7-3 and the appropriate recombinant vaccinia virus. At 4 h postinfection, cells were pulse-labeled for 30 min, followed by a 4-h chase. Cells were lysed in triethanolamine buffer containing 0.5% Triton X-100. Cell lysates were sedimented through sucrose gradients (5 to 20%) containing 0.1% Triton X-100 as described in Materials and Methods. Fractions were collected from the bottom and immunoprecipitated with an anti-YFV polyclonal antibody. Immunoprecipitates were analyzed by SDS-PAGE (13% polyacrylamide). Fractions 1 to 6 did not contain any YFV protein, and they are not included in this figure.
DISCUSSION
Envelope proteins have been shown to play a major role in the budding process of many viruses (15). The flaviviruses represent an interesting model to study the role of the envelope proteins in viral particle assembly. Indeed, coexpression of prM and E proteins in the absence of any other viral component leads to secretion of subviral particles that have structural and functional features of the viral envelope. In this work, we studied the role played by the TM domains in the envelope formation of YFV. We showed that the TM domains of prM and E of YFV are very sensitive to mutagenesis. These mutations affected the formation of subviral particles or infectious virus but not prM-E heterodimerization. These data indicate that the TM domains of prM and E play a crucial role in a late step of the biogenesis of YFV envelope.
The impairment in secretion of YFV subviral particles after alanine scanning insertion mutagenesis strongly suggests that the TM domains of prM and E play a crucial role in envelope formation. Alanine scanning insertion mutagenesis has been shown to be very useful to identify critical segments of TM α-helices involved in helix-helix interactions (4, 25, 28). The effect of such mutations can be analyzed directly by studying protein-protein interactions (25, 28) or indirectly by analyzing the effect of mutations on the function of a protein (4). In the case of YFV proteins, we were interested in studying the role of the TM domains of prM and E in envelope formation. Assuming that an alteration in some step of the envelope formation would lead to an impairment in particle or subviral particle release, we used an assay based on the detection of subviral particle secretion to analyze the effect of the mutations. The effect of the mutations was dramatic. For most of the mutants, subviral particle secretion was reduced to <10%. Five of these mutations were introduced into the viral genome. Four of them were lethal, and the fifth one showed a severe (500-fold) reduction in infectivity. These data indicate that the TM domains of prM and E are very sensitive to alanine insertion and strongly suggest that helix-helix interactions in TM domains play a role at some step of YFV envelope formation.
Heterodimerization of prM-E is not affected by alanine insertion. By using the same technique, we have recently shown that the TM domains of HCV envelope glycoproteins are directly involved in heterodimerization (28). Based on this observation, we first suspected that the effect of mutagenesis on YFV subviral particle release could be due to an impairment in prM-E heterodimerization. However, this was not the case, indicating that the mutations affect another step in envelope formation. These data are indeed in agreement with recent studies on prM-E oligomerization in TBE virus, which mapped the sequence involved in heterodimerization in the stem region or close to it (2). Structural studies on TBE subviral particles also suggest that there is no interaction between the TM domains of M and E in the lipid environment of the envelope (12). However, the limited resolution of these studies does not allow the precise localization of this part of the envelope.
The C termini of prM and E contain two hydrophobic stretches separated by a short hydrophilic segment. The first hydrophobic stretch is supposed to be a stop transfer sequence, and the second one is a signal peptide. The C termini of prM and E likely form a hairpin structure with the hydrophilic segment between the two hydrophobic stretches facing the cytosol. The presence of one or several charged residues between two hydrophobic stretches is a motif found in the TM domains of the envelope proteins of all of the members of the Flaviviridae family (7). The data of alanine scanning insertion mutagenesis in the TM domains of prM and E are in favor of the C termini of prM and E crossing the membrane twice. Indeed, the high sensitivity of the two hydrophobic stretches to alanine insertion and the lack of effect of the mutation immediately downstream of the charged residue are consistent with a double membrane spanning topology for these sequences. In addition, the high sensitivity of the TM domains of prM and E to alanine insertion in the absence of any clear effect on prM-E heterodimerization suggests that the two TM segments within the same molecule might interact together. This is also consistent with the observation that deletion of the signal sequence at the C terminus of E has only a partial effect on TBE subviral particle secretion (2). Indeed, insertion of an alanine probably has a more global effect on the structure of the TM region, whereas the deletion of the signal sequence probably releases the constraints due to the presence of this sequence.
Transient interactions involving TM domains might also potentially explain the effect of the alanine mutations. The property of flaviviruses to produce subviral particles reflects the fact that envelope budding is driven by multimerization of the envelope proteins to create an icosahedral lattice (12). It is possible that alanine insertion interferes with multimerization of the envelope proteins. Transient contacts between the TM domains of several heterodimers might potentially occur during the budding process. Once the particle is assembled, these TM domains would dissociate due to major rearrangement in the E protein during the maturation of the particle that occurs in the trans Golgi network after cleavage of prM by a furin (35).
Differences are observed in the topology of the TM domains of the envelope proteins between the flaviviruses and the hepaciviruses. Recently, we determined the topology of the TM domains of HCV envelope proteins (6). Of utmost importance, after cleavage by a signal peptidase in the ER, the C-terminal orientation of these TM domains changes from luminal to cytosolic, and the TM domains are inserted in the ER membrane as a single membrane-spanning domain. In contrast to what we observed for HCV, replacement of the charged residue located between the two hydrophobic stretches did not affect the function(s) of the TM domains of prM and E. In addition, the hydrophobic stretches at the C termini of prM and E in the flavivirus genus are potentially longer than their counterpart in HCV (2, 7, 37). These hydrophobic stretches are potentially long enough to form α-helices that span the membrane entirely. This suggests that no change in membrane topology occurs in the C termini of prM and E, and these proteins should therefore likely be classified as polytopic proteins.
In conclusion, we have shown that the TM domains of prM and E play a crucial role in the biogenesis of the envelope of YFV. In addition, our results point out some differences between the TM domains of the hepaciviruses and the flaviviruses. It would be interesting to determine whether this reflects differences in the mechanisms of particle assembly.
Acknowledgments
We thank François Penin and Félix Rey for critical reading of the manuscript and André Pillez and Sophana Ung for excellent technical assistance. We are grateful to P. Desprès, A. Cahour, B. Lindenbach, and J. Schlesinger for providing anti-YFV polyclonal antibody, pAP5 plasmid, BHK21/J cells, and MAb 1A5, respectively.
This work was supported by the CNRS, the Institut Pasteur de Lille, the European Regional Development Fund, and EU grant QLK2-1999-00356. A.O.D.B. was successively supported by an ARC and an ANRS fellowship.
REFERENCES
- 1.Allison, S. L., K. Stadler, C. W. Mandl, C. Kunz, and F. X. Heinz. 1995. Synthesis and secretion of recombinant tick-borne encephalitis virus protein E in soluble and particulate form. J. Virol. 69:5816-5820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allison, S. L., K. Stiasny, K. Stadler, C. W. Mandl, and F. X. Heinz. 1999. Mapping of functional elements in the stem-anchor region of tick-borne encephalitis virus envelope protein E. J. Virol. 73:5605-5612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bos, E. C., W. Luytjes, H. V. van der Meulen, H. K. Koerten, and W. J. Spaan. 1996. The production of recombinant infectious DI-particles of a murine coronavirus in the absence of helper virus. Virology 218:52-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braun, P., B. Persson, R. Kaback, and G. von Heijne. 1997. Alanine insertion scanning mutagenesis of lactose permease transmembrane helices. J. Biol. Chem. 272:29566-29571. [DOI] [PubMed] [Google Scholar]
- 5.Cheng, R. H., R. J. Kuhn, N. H. Olson, M. G. Rossmann, H. K. Choi, T. J. Smith, and T. S. Baker. 1995. Nucleocapsid and glycoprotein organization in an enveloped virus. Cell 80:621-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cocquerel, L., A. Op de Beeck, M. Lambot, J. Roussel, D. Delgrange, A. Pillez, C. Wychowski, F. Penin, and J. Dubuisson. 2002. Topologic changes in the transmembrane domains of hepatitis C virus envelope glycoproteins. EMBO J. 21:2893-2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cocquerel, L., C. Wychowski, F. Minner, F. Penin, and J. Dubuisson. 2000. Charged residues in the transmembrane domains of hepatitis C virus glycoproteins play a key role in the processing, subcellular localization and assembly of these envelope proteins. J. Virol. 74:3623-3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Despres, P., A. Cahour, A. Dupuy, V. Deubel, M. Bouloy, J. P. Digoutte, and M. Girard. 1987. High genetic stability of the region coding for the structural proteins of yellow fever virus strain 17D. J. Gen. Virol. 68:2245-2247. [DOI] [PubMed] [Google Scholar]
- 9.Drillien, R., D. Spehner, and A. Kirn. 1982. Complementation and genetic linkage between vaccinia virus temperature-sensitive mutants. Virology 119:372-381. [DOI] [PubMed] [Google Scholar]
- 10.Dubuisson, J., H. H. Hsu, R. C. Cheung, H. B. Greenberg, D. G. Russell, and C. M. Rice. 1994. Formation and intracellular localization of hepatitis C virus envelope glycoprotein complexes expressed by recombinant vaccinia and Sindbis viruses. J. Virol. 68:6147-6160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dubuisson, J., and C. M. Rice. 1996. Hepatitis C virus glycoprotein folding: disulfide bond formation and association with calnexin. J. Virol. 70:778-786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferlenghi, I., M. Clarke, T. Ruttan, S. L. Allison, J. Schalich, F. X. Heinz, S. C. Harrison, F. A. Rey, and S. D. Fuller. 2001. Molecular organization of a recombinant subviral particle from tick-borne encephalitis virus. Mol. Cell 7:593-602. [DOI] [PubMed] [Google Scholar]
- 13.Forsell, K., L. Xing, T. Kozlovska, R. H. Cheng, and H. Garoff. 2000. Membrane proteins organize a symmetrical virus. EMBO J. 19:5081-5091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fuerst, T. R., E. G. Niles, F. W. Studier, and B. Moss. 1986. Eukaryotic transient-expression system based on recombinant vaccinia virus that synthesizes bacteriophage T7 RNA polymerase. Proc. Natl. Acad. Sci. USA 83:8122-8126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garoff, H., R. Hewson, and D. J. E. Opstelten. 1998. Virus maturation by budding. Microbiol. Mol. Biol. Rev. 62:1171-1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heinz, F. X., and S. L. Allison. 2000. Structures and mechanisms in flavivirus fusion. Adv. Virus Res. 55:231-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kieny, M.-P., R. Lathe, R. Drillien, D. Spehner, S. Skory, D. Schmitt, T. Wiktor, H. Koprowski, and J.-P. Lecocq. 1984. Expression of rabies virus glycoprotein from a recombinant vaccinia virus. Nature 312:163-166. [DOI] [PubMed] [Google Scholar]
- 18.Konishi, E., and P. W. Mason. 1993. Proper maturation of the Japanese encephalitis virus envelope glycoprotein requires cosynthesis with the premembrane protein. J. Virol. 67:1672-1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuhn, R. J., W. Zhang, M. G. Rossmann, S. V. Pletnev, J. Corver, E. Lenches, C. T. Jones, S. Mukhopadhyay, P. R. Chipman, E. G. Strauss, T. S. Baker, and J. H. Strauss. 2002. Structure of dengue virus: implications for flavivirus organization, maturation, and fusion. Cell 108:717-725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Landt, O., H. P. Grunert, and U. Hahn. 1990. A general method for rapid site-directed mutagenesis using the polymerase chain reaction. Gene 96:125-128. [DOI] [PubMed] [Google Scholar]
- 21.Lindenbach, B. D., and C. M. Rice. 1997. trans-Complementation of yellow fever virus NS1 reveals a role in early RNA replication. J. Virol. 71:9608-9617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lorenz, I. C., S. L. Allison, F. X. Heinz, and A. Helenius. 2002. Folding and dimerization of tick-borne encephalitis virus envelope proteins prM and E in the endoplasmic reticulum. J. Virol. 76:5480-5491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mackenzie, J. M., and E. G. Westaway. 2001. Assembly and maturation of the flavivirus Kunjin virus appear to occur in the rough endoplasmic reticulum and along the secretory pathway, respectively. J. Virol. 75:10787-10799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mancini, E. J., M. Clarke, B. E. Gowen, T. Rutten, and S. D. Fuller. 2000. Cryo-electron microscopy reveals the functional organization of an enveloped virus, Semliki Forest virus. Mol. Cell 5:255-266. [DOI] [PubMed] [Google Scholar]
- 25.Mingarro, I., P. Whitley, M. Lemmon, and G. von Heijne. 1996. Ala-insertion scanning mutagenesis of the glycophorin A transmembrane helix: a rapid way to map helix-helix interactions in integral membrane protein. Protein Sci. 5:1339-1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moss, B., O. Elroy-Stein, T. Mizukami, W. A. Alexander, and T. R. Fuerst. 1990. New mammalian expression vectors. Nature 348:91-92. [DOI] [PubMed] [Google Scholar]
- 27.Op De Beeck, A., L. Cocquerel, and J. Dubuisson. 2001. Biogenesis of hepatitis C virus envelope glycoproteins. J. Gen. Virol. 82:2589-2595. [DOI] [PubMed] [Google Scholar]
- 28.Op De Beeck, A., R. Montserret, S. Duvet, L. Cocquerel, R. Cacan, B. Barberot, M. Le Maire, F. Penin, and J. Dubuisson. 2000. Role of the transmembrane domains of hepatitis C virus envelope proteins E1 and E2 in the assembly of the noncovalent E1E2 heterodimer. J. Biol. Chem. 275:31428-31437. [DOI] [PubMed] [Google Scholar]
- 29.Pincus, S., P. W. Mason, E. Konishi, B. A. Fonseca, R. E. Shope, C. M. Rice, and E. Paoletti. 1992. Recombinant vaccinia virus producing the prM and E proteins of yellow fever virus protects mice from lethal yellow fever encephalitis. Virology 187:290-297. [DOI] [PubMed] [Google Scholar]
- 30.Rey, F. A., F. X. Heinz, C. Mandl, C. Kunz, and S. C. Harrison. 1995. The envelope glycoprotein from tick-borne encephalitis virus at 2 Å resolution. Nature 375:291-298. [DOI] [PubMed] [Google Scholar]
- 31.Rice, C. M., R. Lewis, J. H. Strauss, and H. V. Huang. 1987. Production of infectious RNA transcripts from Sindbis virus cDNA clones: mapping of lethal mutations, rescue of a temperature-sensitive marker, and in vitro mutagenesis to generate defined mutants. J. Virol. 61:3809-3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 33.Schalich, J., S. L. Allison, K. Stiasny, C. W. Mandl, C. Kunz, and F. X. Heinz. 1996. Recombinant subviral particles from tick-borne encephalitis virus are fusogenic and provide a model system for studying flavivirus envelope glycoprotein functions. J. Virol. 70:4549-4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schlesinger, J. J., M. W. Brandriss, and T. P. Monath. 1983. Monoclonal antibodies distinguish between wild and vaccine strains of yellow fever virus by neutralization, hemagglutination inhibition, and immune precipitation of the virus envelope protein. Virology 125:8-17. [DOI] [PubMed] [Google Scholar]
- 35.Stadler, K., S. L. Allison, J. Schalich, and F. X. Heinz. 1997. Proteolytic activation of tick-borne encephalitis virus by furin. J. Virol. 71:8475-8481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stemmer, W. P. C., and S. K. Morris. 1992. Enzymatic inverse PCR: a restriction site independent, single-fragment method for high-efficiency, site directed mutagenesis. BioTechniques 13:215-220. [PubMed] [Google Scholar]
- 37.Stiasny, K., S. L. Allison, A. Marchler-Bauer, C. Kunz, and F. X. Heinz. 1996. Structural requirements for low-pH-induced rearrangements in the envelope glycoprotein of tick-borne encephalitis virus. J. Virol. 70:8142-8147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vennema, H., G. J. Godeke, J. W. Rossen, W. F. Voorhout, M. C. Horzinek, D. J. Opstelten, and P. J. Rottier. 1996. Nucleocapsid-independent assembly of coronavirus-like particles by co-expression of viral envelope protein genes. EMBO J. 15:2020-2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wengler, G. 1989. Cell-associated West Nile flavivirus is covered with E+pre-M protein heterodimers which are destroyed and reorganized by proteolytic cleavage during virus release. J. Virol. 63:2521-2526. [DOI] [PMC free article] [PubMed] [Google Scholar]