Abstract
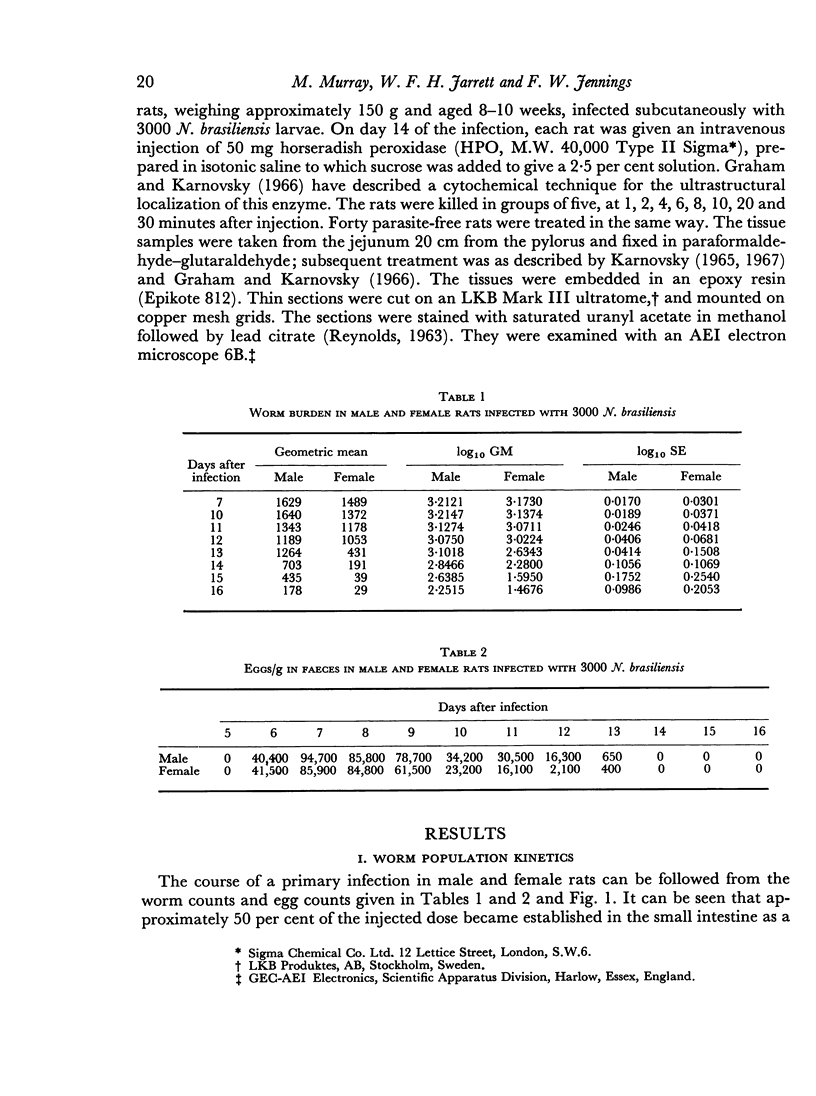
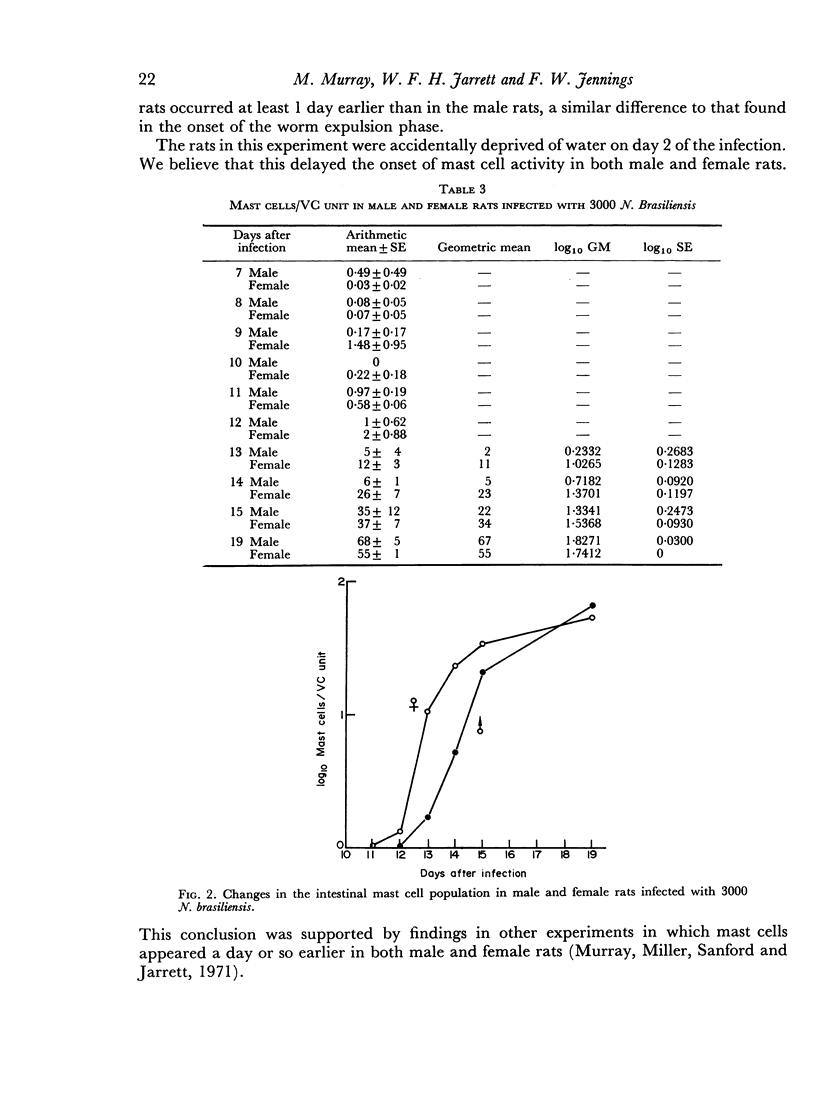
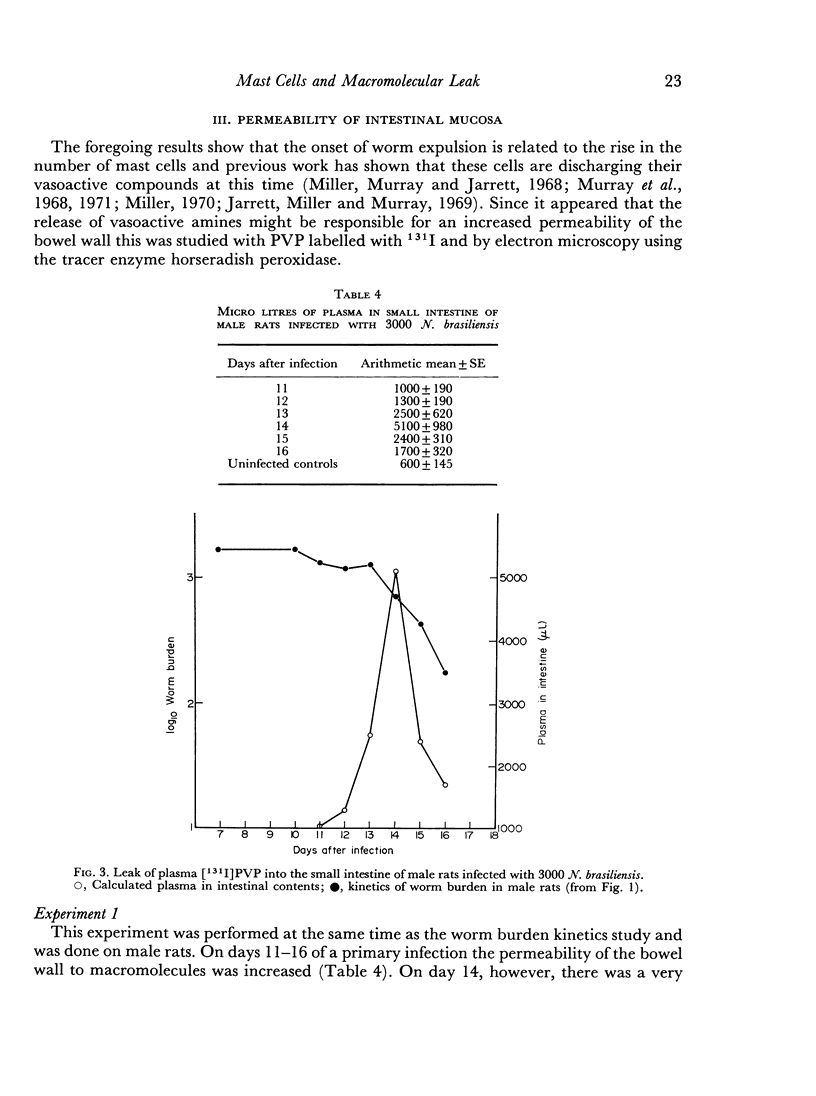
The onset of the exponential expulsion of Nippostrongylus brasiliensis worms in the rat is associated with a sharp burst of intestinal mast cell activity and increased permeability of the bowel wall. It was found that the onset of maximum velocity of worm expulsion occurred earlier in female rats than in male rats and proceeded at a faster rate. There was a corresponding difference in the timing of the mast cell rise and macromolecular leak between the sexes. This suggested that there is a relationship between these events. Cortisone, a drug known to stop worm expulsion and to suppress the mast cell response, also prevented the macromolecular leak. Electron microscopy showed that during the period of increased permeability a pathway for protein tracers had opened up between the epithelial cells and it is suggested that this is the route for enhanced antibody transfer across mucous membranes.
We suggest that a stimulus or stimuli from the parasite cause synchronous development of new populations of mast cells, IgE-producing plasma cells and plasma cells synthesizing antibodies of other classes possessing an anti-worm effect. It is also suggested that these mast cells discharge their pharmacological mediators by an allergen—reaginic antibody mediated system and that these mediators create a pathway through the intestinal mucosa for the translocation of antibody.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Enerbäck L. Mast cells in rat gastrointestinal mucosa. 2. Dye-binding and metachromatic properties. Acta Pathol Microbiol Scand. 1966;66(3):303–312. doi: 10.1111/apm.1966.66.3.303. [DOI] [PubMed] [Google Scholar]
- Ginsburg H., Lagunoff D. The in vitro differentiation of mast cells. Cultures of cells from immunized mouse lymph nodes and thoracic duct lymph on fibroblast monolayers. J Cell Biol. 1967 Dec;35(3):685–697. doi: 10.1083/jcb.35.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham R. C., Jr, Karnovsky M. J. Glomerular permeability. Ultrastructural cytochemical studies using peroxidases as protein tracers. J Exp Med. 1966 Dec 1;124(6):1123–1134. doi: 10.1084/jem.124.6.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JENNINGS F. W., MULLIGAN W., URQUHART G. M. Variables in X-ray "inactivation" of Nippostrongylus brasiliensis larvae. Exp Parasitol. 1963 Jun;13:367–373. doi: 10.1016/0014-4894(63)90087-0. [DOI] [PubMed] [Google Scholar]
- Jarrett E. E., Jarrett W. F., Urquhart G. M. Quantitative studies on the kinetics of establishment and expulsion of intestinal nematode populations in susceptible and immune hosts. Nippostrongylus brasiliensis in the rat. Parasitology. 1968 Aug;58(3):625–639. doi: 10.1017/s0031182000028924. [DOI] [PubMed] [Google Scholar]
- Jones V. E., Ogilvie B. M. Reaginic antibodies and immunity to Nippostrongylus brasiliensis in the rat. II. Some properties of the antibodies and antigens. Immunology. 1967 May;12(5):583–597. [PMC free article] [PubMed] [Google Scholar]
- Karnovsky M. J. The ultrastructural basis of capillary permeability studied with peroxidase as a tracer. J Cell Biol. 1967 Oct;35(1):213–236. doi: 10.1083/jcb.35.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MULLIGAN W., URQUHART G. M., JENNINGS F. W., NEILSON J. T. IMMUNOLOGICAL STUDIES ON NIPPOSTRONGYLUS BRASILIENSIS INFECTION IN THE RAT: "SELF-CURE" PHENOMENON. Exp Parasitol. 1965 Jun;16:341–347. doi: 10.1016/0014-4894(65)90056-1. [DOI] [PubMed] [Google Scholar]
- Miller H. R., Jarrett W. F. Immune reactions in mucous membranes. I. Intestinal mast cell response during helminth expulsion in the rat. Immunology. 1971 Mar;20(3):277–288. [PMC free article] [PubMed] [Google Scholar]
- Morse S. I., Bray K. K. The occurrence and properties of leukocytosis and lymphocytosis-stimulating material in the supernatant fluids of Bordetella pertussis cultures. J Exp Med. 1969 Mar 1;129(3):523–550. doi: 10.1084/jem.129.3.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray M., Miller H. R., Sanford J., Jarrett W. F. 5-hydroxytryptamine in intestinal immunological reactions. Its relationship to mast cell activity and worm expulsion in rats infected with Nippostrongylus brasiliensis. Int Arch Allergy Appl Immunol. 1971;40(2):236–247. [PubMed] [Google Scholar]
- OGILVIE B. M. REAGIN-LIKE ANTIBODIES IN ANIMALS IMMUNE TO HELMINTH PARASITES. Nature. 1964 Oct 3;204:91–92. doi: 10.1038/204091a0. [DOI] [PubMed] [Google Scholar]
- Ogilvie B. M. Use of cortisone derivatives to inhibit resistance to Nippostrongylus brasiliensis and to study the fate of parasites in resistant hosts. Parasitology. 1965 Nov;55(4):723–730. [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair K. B. The effect of treatment with emetine on sheep infected with immature Fasciola hepatica. Res Vet Sci. 1970 May;11(3):293–295. [PubMed] [Google Scholar]
- Urquhart G. M., Mulligan W., Eadie R. M., Jennings F. W. Immunological studies on Nippostrongylus brasiliensis infection in the rat: the role of local anaphylaxis. Exp Parasitol. 1965 Oct;17(2):210–217. doi: 10.1016/0014-4894(65)90023-8. [DOI] [PubMed] [Google Scholar]
- Uvnäs B., Wold J. K. Isolation of a mast cell degranulating polypeptide from Ascaris suis. Acta Physiol Scand. 1967 Jul-Aug;70(3):269–276. doi: 10.1111/j.1748-1716.1967.tb03625.x. [DOI] [PubMed] [Google Scholar]
- Wilson R. J., Bloch K. J. Homocytotropic antibody response in the rat infected with the nematode, Nippostrongylus brasiliensis. II. Characteristics of the immune response. J Immunol. 1968 Mar;100(3):622–628. [PubMed] [Google Scholar]
- Wilson R. J. Homocytotropic antibody response to the nematode Nippostrongylus brasiliensis in the rat--studies on the worm antigen. J Parasitol. 1967 Aug;53(4):752–762. [PubMed] [Google Scholar]





