Abstract
Altering adenovirus vector (Ad vector) targeting is an important goal for a variety of gene therapy applications and involves eliminating or reducing the normal tropism of a vector and retargeting through a distinct receptor-ligand pathway. The first step of Ad vector infection is high-affinity binding to a target cellular receptor. For the majority of adenoviruses and Ad vectors, the fiber capsid protein serves this purpose, binding to the coxsackievirus and adenovirus receptor (CAR) present on a variety of cell types. In this study we have explored a novel approach to altering Ad type 5 (Ad5) vector targeting based on serotypic differences in fiber function. The subgroup B viruses bind to an unidentified receptor that is distinct from CAR. The subgroup F viruses are the only adenoviruses that express two distinct terminal exons encoding fiber open reading frames. We have constructed chimeric fiber adenoviruses that utilize the tandem fiber arrangement of the subgroup F genome configuration. By taking advantage of serotypic differences in fiber expression, fiber shaft length, and fiber binding efficiency, we have developed a tandem fiber vector that has low binding efficiency for the known fiber binding sites, does not rely on an Ad5-based fiber, and can be grown to high titer using conventional cell lines. Importantly, when characterizing these vectors in vivo, we find the subgroup B system and our optimal tandem fiber system demonstrate reduced liver transduction by over 2 logs compared to an Ad5 fiber vector. These attributes make the tandem fiber vector a useful alternative to conventional strategies for fiber manipulation of adenovirus vectors.
The efficiency of adenovirus transduction into a variety of cell lines has contributed to its popularity as a gene transfer vector. However, to increase its usefulness as a vector, it is important to develop ligand-specific targeted adenovirus vectors (Ad vectors). There are two initial objectives to address in a retargeting strategy for Ad vectors: elimination of the native binding of adenovirus and generation of a virus that has high affinity for a novel cellular receptor. The multistep process of adenovirus infection of a target cell has been well characterized in permissive tissue culture cell lines, revealing a significant role for the major capsid proteins in the successful delivery of viral DNA into the nucleus (13). The first step in virus entry is high-affinity binding of the virus to the target cell. The Ad fiber homotrimer is primarily responsible for high-affinity binding, and the majority of adenoviruses bind to the coxsackievirus and adenovirus receptor protein (CAR) present on a variety of cell and tissue types (2, 30, 31).
Characterization of fiber-CAR complexes has resulted in considerable insight into the mechanism of adenovirus attachment. Fiber-CAR binding involves a complex interaction of several faces of the fiber homotrimer with the extracellular domain of CAR. Specific point mutations in the AB, CD, and DG loops of fiber abolish CAR binding (16, 17, 26) and support model structures predicted by X-ray diffraction of CAR binding fibers complexed with the D1 domain of CAR (3, 32). In addition to CAR, other cellular receptors are known to bind Ad subgroup C viruses. Major histocompatibility complex class I antigens (14), heparan sulfate glycosaminoglycans (8), and αv integrins (18, 19, 34) can contribute to the binding of this group of viruses. Integrin binding to the RGD motif present in penton not only serves as a secondary attachment pathway for adenovirus but also contributes to virus entry by stimulating endocytosis (34).
Virus attachment to target cells is further complicated when moving from an in vitro tissue culture system to an animal model of gene transduction. Systemic administration of Ad5-based vectors results in a primary infection of hepatic tissue with low-level gene transduction in secondary sites such as spleen and kidney. Studies using mutant Ad5-based vectors defective in CAR binding, which are clearly compromised in vitro, are only modestly reduced in their ability to transduce liver in vivo (1, 10, 17, 21, 28). Similarly, mutations in the integrin-binding motif of Ad5 penton are still primarily localized to the liver (10, 21, 28). These studies serve to illustrate the difficulty in abolishing native binding to the Ad5-based vector in vivo. Although CAR binding serotypes of adenovirus are the best studied to date, there are Ad serotypes that contain fiber homotrimers that do not bind CAR. The subgroup B adenoviruses are the primary exception to the fiber-CAR adenovirus binding interaction (9, 12, 29). They bind to an unknown cellular receptor and show no affinity for CAR (24). A CAR binding serotype can be converted to a subgroup B receptor-targeted vector simply by genetically switching the fiber terminal exons (12). Subgroup B viruses can bind to many of the same cell types as CAR binding viruses, but there are also cell types that demonstrate differential affinity for the subgroup B fiber versus the CAR binding serotypes (20, 23-25, 33). Another distinct class of fiber-containing adenoviruses is the enteric subgroup F viruses (serotypes 40 and 41). These viruses are different from other adenoviruses in that they contain two distinct fiber terminal exons (15, 35). The first fiber terminal exon, L5-1, codes for a 41.4-kDa fiber (fiber 41 short, or F41S), which is shorter (fewer shaft repeat units) than the downstream 60.5-kDa fiber exon L5-2 (fiber 41 long, or F41L). Baculovirus-expressed knob domains of F41L bind to CAR, while recombinant F41S knobs do not bind CAR (25).
This study was initiated to determine if the subgroup F viruses or their fiber moieties could be useful in transducing cell types that were low in CAR expression (6), with a particular interest in the function of the F41S fiber. Because of the difficulty in growing subgroup F viruses, we chose to construct chimeric Ad5 vectors by using cloned fiber terminal exons from Ad41 viral DNA. We constructed two chimeric Ad5 viruses containing either the tandem F41S-41L terminal exons or the F41L terminal exon alone, but we were unable to isolate a virus containing only F41S. In characterizing these new constructs, we were not able to reveal distinct new binding functions attributable to the F41 fibers. However, we discovered that although the F41S fiber was not contributing to an identifiable binding function, it was expressed as the dominant fiber in purified chimeric viruses. We have extended the concept of using the nonbinding F41S fiber as a dominant fiber by generating a chimera that expresses F41S and F7. This virus demonstrates an exceptionally low level of gene transduction in vitro, can be grown in traditional 293 cell lines, and when used in vivo demonstrates an over 2-log reduced transduction of hepatocytes. By coexpressing subgroup B (F7) and F (F41S) fibers from the Ad5 major late transcription unit (MLTU), we have developed a vector system that provides distinct advantages over vectors based on subgroup C fiber.
MATERIALS AND METHODS
Adenovirus stocks and cell lines.
Wild-type (wt) adenoviruses (reference strain, adenoid 6) 7a and 41 (Tak) were obtained from the American Type Culture Collection (ATCC) and grown in HeLa or HEK-293 suspension cells. Ad41 could not be passaged more than three times without a nearly complete loss of infectivity. HeLa and HEK-293 suspension cell cultures were grown in S-minimal essential medium (S-MEM; Joklik modified) plus 5% horse serum. A549 monolayer cultures were maintained in either F12K plus 10% calf serum (or fetal bovine serum [FBS]) or Dulbecco's modified Eagle's medium (DMEM) plus 10% calf serum, while HEK-293 monolayer cultures were kept in DMEM plus 5 to 10% calf serum. IM-9 B lymphocytes (ATCC CCL-159) were grown in RPMI plus 10% FBS. The intestinal epithelial cell line T84 was obtained from the ATCC (CCL-248), maintained in 1:1 DMEM-Ham's F12 plus 5% FBS, and induced to form polarized epithelial monolayers by plating at confluency (106 cells/cm2) on collagen-coated transwell membranes (Transwell-COL; Corning-Costar). Caco-2 cells (ATCC HTB-37), another intestinal epithelial cell line, were generously provided by Enrique Rodriquez-Boulan and grown in Eagle's-MEM with Earle's balanced salt solution plus 20% FBS as recommended by the ATCC. Caco-2 cells were induced to grow as a polarized monolayer by plating at confluency (106/cm2) on tissue culture-treated wells or membranes (Transwell; Corning-Costar).
Virus infections.
Small-scale infections of monolayer cells (106 to 107 cells) were performed in culture medium without serum in a minimal volume for 1 or 2 h, with fresh medium plus serum usually added on top, or the virus plus medium was replaced with fresh medium plus serum. The infections were harvested as crude lysates (two freeze-thaw cycles to release intracellular virus) between 3 and 14 days, depending on the input concentration of virus and the presence of cytopathic effect. For large-scale preparations of virus, 4 × 108 cells, either as a suspension or monolayer, were infected with 100 to 500 particles per cell and harvested 65 to 72 h postinfection. Infected cells were washed with phosphate-buffered saline (PBS), resuspended in 10 mM sodium phosphate, pH 7.2, lysed with 0.6% deoxycholic acid (37°C; 0.5 h), and treated with 35 mM MgCl2 plus 5 mg of DNase I/ml (37°C; 0.5 h). The lysate was cleared by centrifugation at 3,000 × g for 0.5 h and three extractions with trichlorotrifluoroethane. The cleared lysate was then purified over two cesium chloride gradients (1.2 to 1.46 g/ml). Glycerol was added to 20% and the virus was dialyzed against two 1-liter volumes of dialysis buffer (50 mM Tris [pH 7.5], 0.15 M NaCl, 20% glycerol). Virus was diluted 1:2 in storage buffer (0.1 M Tris [pH 7.5], 0.25 M NaCl, 1 mg of bovine serum albumin/ml, 50% glycerol) for storage at −20°C.
pAd70-100dlE3-F41L, -F41S, -F41T, -F7F5, and -F7F41S plasmids.
The starting plasmid used in construction of the chimeric viruses was pAd70-100dlE3 or pAd70-100dlE3-Fiber7 (12). These plasmids contain the right-end 70 to 100 map units (MU) of Ad5 viral DNA, deleted from 78.6 to 85.9 MU with a new PacI restriction site at the deletion junction site and a new BamHI site at MU 91. The added restriction sites are unique and flank the L5 terminal exon of Ad5 or Ad7 in pAd70-100dlE3 or pAd70-100dlE3.F7, respectively. The Ad41 fiber genes were subcloned into pAd70-100dlE3 by PacI/BamHI replacements with the PCR amplicons of either both fiber genes (pAd70-100dlE3.F41Tandem, primers 3 [5′ CCGGATCCTTAATTAAGTTCACCTTTTTACCCTGAC] and 4 [5′ TTCGAAGGATCCAATTCTGTAAAATGATC]), just the long fiber gene (FLong, primers 5 [5′ TTCGAATTAATTAAGCATTTCTTTTTTTCC] and 4), or just the short fiber gene (FShort, primers 6 [5′ TTCGAATTAATTAACCTTTTTACCCTGACCC] and 7 [5′ TTCGAAGGATCCGATCAATAAAGTTATAAAAAAAGAAATGCGAAG]). As with subcloning of the Ad7a fiber gene into pAd70-100dlE3, the Ad41 5′ and 3′ RNA processing signals for each fiber gene were included in the PCR-amplified fragments. The pAd70-100dlE3.F41Tandem plasmid was engineered to express only the short fiber protein by XbaI digestion, fill in with DNA polymerase I (Klenow fragment), and recircularization to disrupt the long fiber protein open reading frame (ORF). The Ad7a fiber gene was subcloned into pAd70-100dlE3 without replacing the Ad5 fiber gene, by the insertion of a smart linker containing a PacI site into pAd70-100dlE3.Fiber7 at the unique BamHI site (pAd70-100dlE3.F7 plus 2Xba). The Ad7 fiber gene PacI-PacI fragment from pAd70-100dlE3.F7 plus 2Xba was ligated to PacI-linearized pAd70-100dlE3 to generate pAd70-100dlE3.F7F5. The pAd70-100dlE3.F7F41S was generated by subcloning the F41S exon into the F5 site of pAd70-100dlE3.F7F5.
Radiolabeling of adenovirus. (i) [3H]thymidine labeling of adenovirus DNA.
Suspension HEK-293 cells (4 × 108) infected with adenovirus (multiplicity of infection [MOI] = 5) were concentrated to 8 × 107 cells/ml in a 1:1 mix of 2× MEM-[3H]thymidine (5 mCi) at 8 to 16 h postinfection and incubated at 37°C for 1 h with constant mixing. The cells were diluted to 1.6 × 106 cells/ml with S-MEM plus 2.5% horse serum and harvested at 60 h postinfection, and the virus was purified from the cells as described above for a large-scale preparation of virus.
(ii) [35S]methionine labeling of adenovirus proteins.
Monolayers of HEK-293 cells (∼4 × 107) in a T-150 flask were infected with 10 MOI of adenovirus vectors in DMEM for 2 h, the medium was replaced with DMEM plus 5% FBS, and 16 h later the medium was changed to DMEM without l-methionine plus 5% FBS. The infected cells were labeled with 1 mCi of [35S]methionine at 100 μCi/ml for 8 h at 37°C and 5% CO2 in DMEM plus 5% FBS and harvested at 65 h postinfection, and the virus was purified as for a large-scale preparation. Aliquots of the 35S-labeled virus were precipitated with 15% trichloroacetic acid (0.5 h, on ice, with a brief wash with 90% acetone plus a 10% solution of 0.1 N HCl) to concentrate the virus and remove the cesium chloride before loading on the sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel.
Metabolic labeling of infected cells.
Aliquots of 5 × 105 HEK-293 cells grown in a 24-well dish were infected with 10 MOI of adenovirus vectors as described above. At the stated times, the culture medium was replaced with 250 μl of DMEM without l-methionine plus 2.5% FBS with 10 μCi of [35S]methionine/ml, and the cells were harvested 2 or 18 h later. Cells were harvested by rinsing with PBS, scraping after 10 min at room temperature in TEN scrape buffer (40 mM Tris [pH 7.4], 1 mM EDTA, 150 mM NaCl), and resuspension in the cell pellet in 100 μl of Laemmli sample loading buffer.
RNA isolation and Northern analysis.
HEK-293 cells seeded into 10-cm2 plates were infected with 1,000 particles of the indicated virus/cell (or 5,000 particles of Ad5.F7F41S/cell), and RNA was isolated 24 h postinfection with TRIzol reagent (Gibco BRL) according to the manufacturer's instructions. Ten micrograms of total RNA was separated under denaturing conditions on a 1% agarose-formaldehyde gel and transferred to a positively charged nylon membrane. Blots were probed with radiolabeled DNA fragments corresponding to various full-length fiber exons and visualized by autoradiography.
In vitro transduction assays.
Cell lines used in this study were seeded into 6- or 24-well plates and infected with the indicated dose of virus in medium without serum for 30 min at 37°C. After infection, virus was aspirated and fresh medium was added back. Infected cells were harvested 24 h postinfection in TEN scrape buffer and resuspended in 0.25 M Tris (pH 7.8). Lysates were subjected to three freeze-thaw cycles followed by a 10-min incubation at 65°C to inactivate cellular deacetylases. Chloramphenicol acetyltransferase (CAT) activity assays were performed as described previously (12).
In vivo transduction assays.
Six-week-old female B129 mice were obtained from Taconic or Jackson Laboratories. Mice were infected retroorbitally with 1010 virus particles or PBS vehicle alone in a total volume of 100 μl. Three days later, animals were sacrificed and organs were harvested, weighed, and resuspended in 2 volumes of PBS by weight. Tissue lysates were prepared by homogenizing organs and centrifuging for 30 min at 3,000 × g followed by a 10-min incubation at 65°C. Total tissue CAT activity was assayed as described above.
RESULTS
Construction of fiber 41 Ad5 chimeras.
Amino acid sequence comparison of the Ad5 fiber protein tail region to analogous regions of the two Ad41 proteins showed a remarkable conservation of 65 and 69% identities. Since assembly of the fiber homotrimer into the capsid is primarily dependent on the tail domain, the high sequence identity suggests the Ad41 fibers would form a penton with Ad5 penton base. Additionally, the long fiber, but not the short fiber, can bind to the CAR (25). Chimeric Ad5 viruses were constructed which expressed individual or tandem Ad41 fiber genes as they occur in the wt Ad41 virus. The Ad41 fiber regions (consisting of the 5′ RNA processing elements, ORF, and 3′ RNA processing elements) were PCR amplified singly or together and subcloned into the pAd70-100dlE3 plasmid, replacing the Ad5 L5 region (12). These plasmids, corresponding to Ad41 short, Ad41 long, both Ad41 genes (as authentic Ad41 sequence), or both Ad41 genes with the long ORF knocked out [F41T (L KO)] were transfected into HEK-293 cells with a left-end subgenomic fragment from dlAd5NCAT.F7 as described by Gall et al. (12). Lysates were screened by restriction digestion; positive lysates were plaque purified (two times), and purity was verified by Southern blot analysis. Only recombinant viruses expressing the long fiber protein were isolated, i.e., dlAd5NCAT.F41Long (F41L) and dlAd5NCAT.F41Tandem (F41T) (Fig. 1A). These chimeric viruses were grown as large-scale preparations multiple times and cesium-band purified to establish particle yields. The parental Ad5 virus, dlAd5NCAT (12), and F41T yielded ∼2.5 × 1013 particles per 4 × 108 HEK-293 cells, while that with F41L was consistently about twofold lower (data not shown).
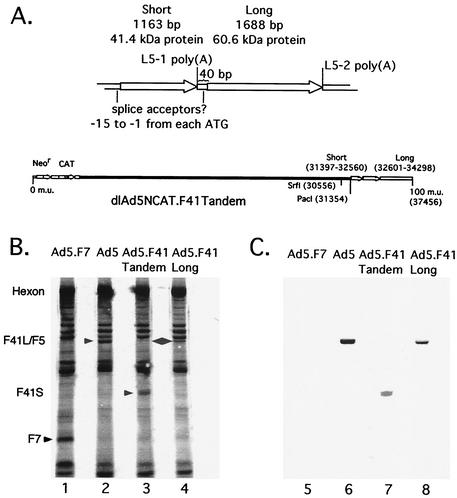
FIG. 1.
Chimeric F41 tandem adenovirus dlAd5NCAT.F41T. (A) Genetic structure of the short and long fiber protein genes. Poly(A) site nomenclature is from Davison et al. (7), and putative splice acceptor sites are from Pieniazek et al. (22). In fiber proteins, the translation start and stop codons (ATG and AAU) are often integrated into the splice acceptor site GA and the polyadenylation hexanucleotide AAUAAA recognized by the RNA processing machinery. Insertion or replacement at the Ad5 L5 site maintains the order of the F41 L5-1 and L5-2 terminal exons. (B and C) Determination of the polypeptide composition of chimeric virus particles. Aliquots of 5 × 105 cpm of [35S]methionine-labeled, cesium chloride-banded virus particles mixed with 5 × 1011 unlabeled particles were electrophoresed on an SDS-10% PAGE gel, transferred to nitrocellulose, and viewed by autoradiography (B) or Western blotting with anti-Ad2 fiber serum (C). The dlAd5NCAT samples were not mixed with 5 × 1011 unlabeled particles, due to the much higher sensitivity of the anti-Ad2 fiber serum for Ad5 fiber. Lanes 1 and 5, Ad5.F7; lanes 2 and 6, Ad5; lanes 3 and 7, Ad5.F41T; lanes 4 and 8, Ad5.F41L.
Repeated attempts to construct a short-fiber-only virus did not meet with success except in one instance, where the expected restriction pattern for the dlAd5NCAT.F41Short (F41S) genome was observed among an excess of contaminating dlAd5NCAT.F7 (parental) DNA. The other short fiber virus, dlAd5NCAT.F41T (L KO) was never detected, even though the parental construct (F41T) was functional. The F41S virus could not be isolated from dlAd5NCAT.F7 by plaque purification, limiting dilution, anti-Ad7 immunoselection, or a combination of approaches, and the percentage of F41S could be enriched to only ∼20% of that of a mixed lysate (data not shown). Attempts to further purify the maximally enriched lysate always resulted in a complete loss of the F41S recombinant genome. The inability to isolate a virus expressing only the Ad41 short fiber led us to conclude that under the experimental conditions used in construction of these viruses, the short fiber protein either was unable to interact with Ad5 penton base, was unable to bind to existing receptors available on the 293 cell line used to construct the recombinants, or it cannot function as a primary attachment protein and mediate virus entry.
The polypeptide components of the Ad5/F41 chimeric virions were analyzed by [35S]methionine labeling of Ad-infected 293 cells and purification of mature virus particles over cesium chloride gradients. Purified particles were separated by SDS-PAGE and blotted onto a nylon membrane. The membrane was exposed to film for autoradiography and then used for Western blot analysis (Fig. 1B and C, respectively). Ad5 (lane 2) and Ad7 (lane 1) fiber polypeptides represent two size extremes due to differences in the number of shaft region repeat elements. The F41L virus particles (lane 4) contain a band corresponding in size to the Ad41 60.5-kDa (long) protein, which is predicted to be very similar to the size of Ad5 fiber. Proteins present in F41T virion particles (lane 3) include a band corresponding to the 41.4-kDa (short) fiber protein of Ad41 and to a lesser degree a band corresponding to the 60.5-kDa (long) fiber polypeptide. Based on the intensity of the F41S and F41L bands in this gel, the F41S is not only expressed and incorporated into the Ad5 virion capsid, but is also incorporated at higher levels than F41L. The inability to generate a chimera based solely on F41S was not due to an apparent incompatibility with the Ad5 penton base used in this system. To confirm the presence of the fiber proteins, Western blotting was performed using R72 polyclonal antibody (generously provided by M. Horowitz), which was generated using purified Ad2 fiber. R72 cross-reacted with F5, F41L, and F41S as expected (15), but not the F7 subgroup B fiber. At the level of antigenic identity, the subgroup F viruses are clearly more closely related to the subgroup C viruses than to the subgroup B viruses.
The Western blotting results support the observation, made with [35S]methionine labeling of the capsid proteins, that F41L is underrepresented in isolated chimeric dlAd5NCAT-F41T virions. The short protein may be expressed at higher levels during infection due to the location of its gene in front of the long fiber protein gene, or it may be preferentially packaged into virions. To determine if the packaging dominance of F41S over F41L occurs at the level of gene expression, we examined cytoplasmic levels of mRNA and protein corresponding to F41S and F41L. Northern blot analysis of RNA from late-stage infection of 293 cells indicated that both L5-1 (short) and L5-2 (long) mRNAs are expressed at roughly equivalent molar ratios (data not shown) and reflect the steady-state level of each fiber expressed during late infection by labeling with [35S]methionine (data not shown). Since the two terminal exons are expressed at similar levels, we are led to believe that there exists a packaging bias favoring the incorporation of Ad41 short fiber over Ad41 long fiber into the Ad5 capsid.
Transduction and binding characteristics of subgroup F fiber chimeras.
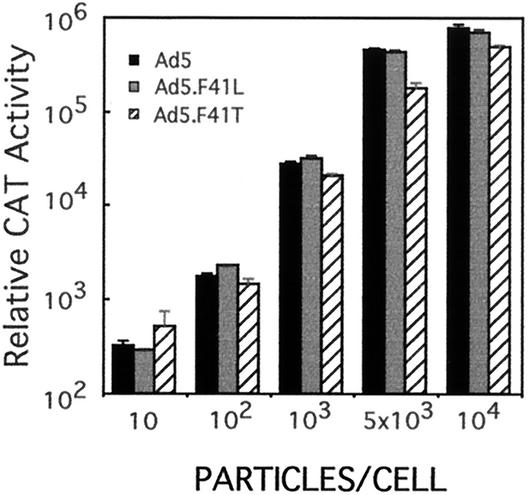
The transduction efficiencies of dlAd5NCAT, F41L, and F41T were determined on A549 lung carcinoma epithelial cells to establish the general influence of F41 gene replacement on transduction. All three viruses were able to effectively transduce A549 cells (Fig. 2) and HeLa cells (data not shown). Although similar, the transduction of A549 cells by dlAd5NCAT-F41T was decreased by approximately twofold compared with Ad5 control vector.
FIG. 2.
Transduction efficiency of Ad5-Ad41 fiber chimeras. A549 cells were infected with the indicated amounts of dlAd5NCAT, dlAd5NCAT.F41L, or dlAd5NCAT.F41T. After 24 h, cell extracts were prepared and CAT gene expression was determined for each infection. CAT activity is given as the total activity in the extract. Data are means of triplicates ± sample standard deviation.
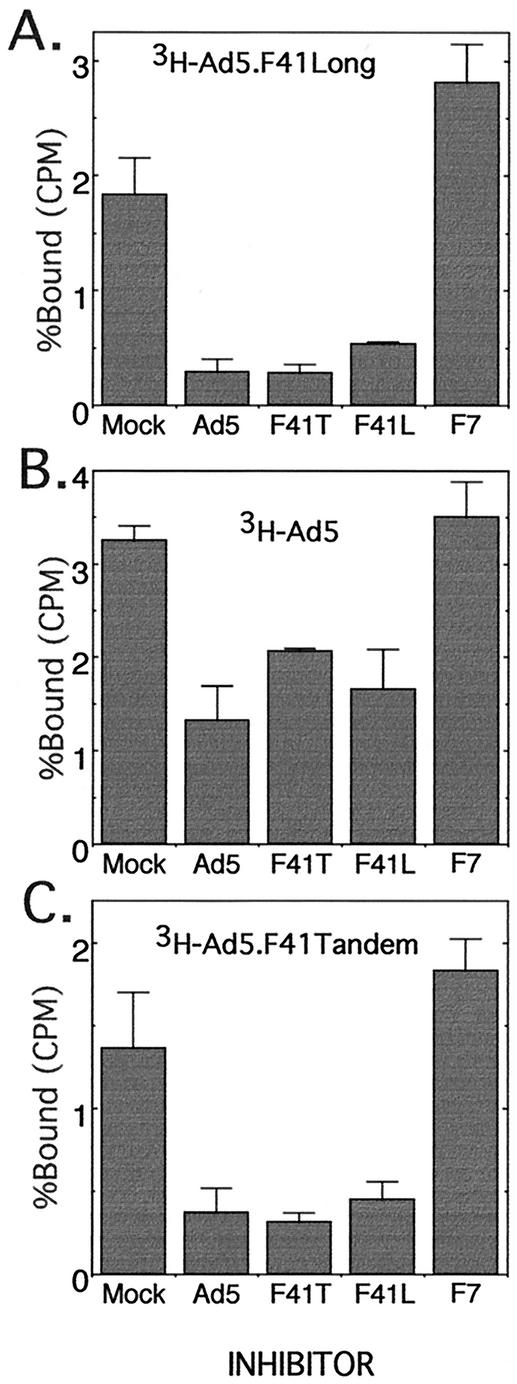
To characterize the role of the Ad41 long and short fiber proteins in attachment of the virus to the cell, binding inhibition assays were performed at 4°C. A 500-fold excess of unlabeled virus (either dlAd5NCAT, F41T, F41L, or F7) was prebound to A549 cells for 1 h, and 3H-labeled virus (either dlAd5NCAT, F41T, or F41L) was then added for 1 h and the percentage of bound 3H-labeled virus was determined (Fig. 3). In all cases, dlAd5NCAT, F41T, and F41L inhibited the binding of each other, while F7, previously shown to not compete for dlAd5NCAT (12), did not inhibit the three viruses. It is clear that the Ad41 long fiber protein is competing with the Ad5 fiber protein for binding to the adenovirus receptor, CAR, in agreement with the binding characteristics of the purified knob region of Ad41 long fiber (25). Since Ad5 and F41L inhibited the binding of 3H-labeled F41T to A549 cells as well as F41T (Fig. 4C), it is unlikely that the short protein present in F41T is binding to the cells via a receptor different than CAR. Studies by Roelvink et al. with purified fiber 41S (25) indicated that F41S does not bind to CAR, which would argue that the F41T virus is binding primarily through F41L interactions with CAR.
FIG. 3.
Fiber binding inhibition assay. Competitor virus (dlAd5NCAT, dlAd5NCAT.F41T, dlAd5NCAT.F41L, or dlAd5NCAT.F7) was bound to A549 cells at 4°C prior to addition of labeled dlAd5NCAT (A), dlAd5NCAT.F41L (B), or dlAd5NCAT.F41T (C). The competitor virus was at a 500-fold excess to labeled virus. After 1 hour at 4°C, virus binding was measured as described in Materials and Methods. Data are means of triplicates ± sample standard deviation.
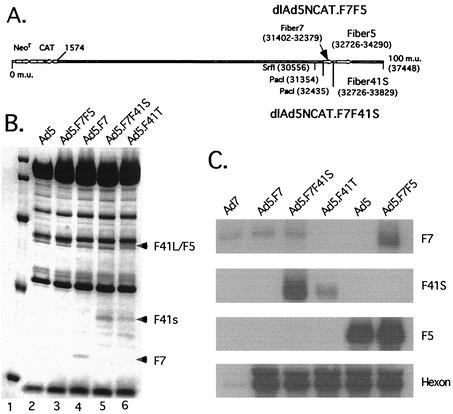
FIG. 4.
F7F41S and F7F5 tandem fiber chimeras. (A) Diagram of chimeric viruses constructed using F7 inserted 5′ to either F41S or F5 terminal exons into the 3′ end of the MLTU. (B) Coomassie blue staining of SDS-polyacrylamide gel loaded with 5 × 1011 virus particles of dlAd5NCAT (lane 2), F7F5 (lane 3), F7 (lane 4), (F7F41S) lane 5, or F4T (lane 6). Arrows indicate designated fiber polypeptides. (C) Northern blot analysis of 293 cell RNA isolated 24 h postinfection with 103 particles/cell of Ad7, Ad5.F7, Ad5.F741S, Ad5.F41T, Ad5, or Ad5.F7F5. Membranes were probed with radiolabeled DNA fragments corresponding to the indicated fiber terminal exons. An Ad5 hexon-labeled DNA fragment was used as a control for MLTU viral products and has limited homology with Ad7 hexon.
Altering terminal exon insertions to influence capsid fiber content and function.
The construction of a virus encoding two fiber proteins in the MLTU, F41T, showed that the Ad5 MLTU has considerable flexibility with respect to expression of novel terminal exons. This raises the possibility of constructing Ad5 vectors expressing our choice of two adenovirus fiber proteins for gene transfer applications. Based on previous characterizations, several elements contribute to the bioactivity of fiber incorporated into the Ad capsid as a high-affinity ligand. The dominant function is ligand-receptor interaction: either CAR binding F5 or F41L, or the uncharacterized subgroup B receptor binding F7 and finally F41S, which currently does not bind to a known receptor. Another consideration is fiber length. The CAR binding fibers with longer shaft domains have an advantage in receptor binding over short-shafted fibers (27), and it is unknown how shaft length influences function in tandem constructs. Additionally, interactions between fiber and secondary receptors have been characterized with subgroup C, and the different structure of the subgroup B and F fibers may provide a distinct advantage in having less nonspecific binding. Finally, we have found the number of bioactive fibers localized in a single capsid can vary. Based on the F41 tandem virus where the nonbinding F41S clearly dominates, virus propagation is only modestly compromised even though a small fraction of the fibers (F41L) are contributing to high-affinity attachment. Using the tandem fiber strategy, we can examine how some of these factors can be used to control and target tandem fiber viruses.
In considering our design of new Ad5 tandem fiber vectors, we were interested in controlling the expression and function of the inserted terminal exons and chose to establish the F7 fiber as our fixed fiber terminal exon. Since the Ad7a fiber protein represents a short-shafted fiber (F7 contains 6 shaft repeat elements, F41S contains 12, and F41L and F5 both contain 22 shaft repeat elements), we would consider it to be at a disadvantage with respect to binding over a longer-shafted fiber. Additionally, we have found that the wt F7 terminal exon is comparatively inefficient for recognition by the RNA processing machinery (E. Falck-Pedersen, unpublished data). In one proposed construct, F7F41S, the virus would have 41S as the dominant fiber and bind preferentially through the F7 receptor. However, because this virus would contain the nonbinding F41S as the dominant fiber, we would predict that such a tandem virus might be severely compromised in terms of infectivity. In contrast to the F7F41S construct, an F7F5 construct targets through both the F5 and the F7 receptor pathways, and we would predict that F5 would dominate as a targeting fiber because of the vast difference in shaft length as well as the bias in RNA processing predicted to favor F5 mRNA production.
Dual-fiber-expressing viruses were constructed by the cotransfection of pAd70-100dlE3.F7F5 or pAD70-100dlE3.F7F41S with the left-end fragment of dlAd5NCAT.F7 to generate the chimeric viruses dlAd5NCAT.F7F5 (F7F5) and dlAd5NCAT.F7F41S (F7F41S) (Fig. 4A). Tandem viruses were isolated by plaque purification and determined to be free of contaminating parental virus by diagnostic PCR (data not shown). The yield of F7F5 was similar to that of F41L, i.e., approximately twofold lower than with dlAd5NCAT and F41T. However, under similar growth conditions (low MOI), overall F7F41S virus yield was routinely an order of magnitude below that with the F7F5 or F41T vectors (relative F7F41S virus particle yields increased with higher input MOI [data not shown]). To determine capsid composition, cesium chloride-banded tandem viruses were prepared in parallel and 5 × 1011 particles of each virus was applied to an SDS-polyacrylamide gel. Consistent with our prediction, the fiber composition of cesium chloride-purified F7F5 and F7F41S particles was strongly biased against F7 (Fig. 4B). Northern blot analysis confirmed low levels of F7 terminal exon expression from the MLTU of wt Ad7 as well as in the chimeric virus constructs (Fig. 4C). Using viruses prepared in parallel (Fig. 4B), we calculated the average particle/PFU ratio for each tandem construct in three to five different plaque assays on 293 monolayer cells. Only the F7F41S virus was significantly different from dlAd5NCAT, with a 45-fold increase in nonproductive particles (Ad5, 135 particles/PFU; Ad5.F7F5, 165 particles/PFU; Ad5.F7, 232 particles/PFU; Ad5.F41T, 216 particles/PFU; Ad5.F7F41S, 6,175 particles/PFU). The plaques generated by F7F41S were also extremely small by comparison, which may have influenced the overall accuracy of the plaquing assay.
Diminished gene transduction by dlAd5NCAT.F7F41S.
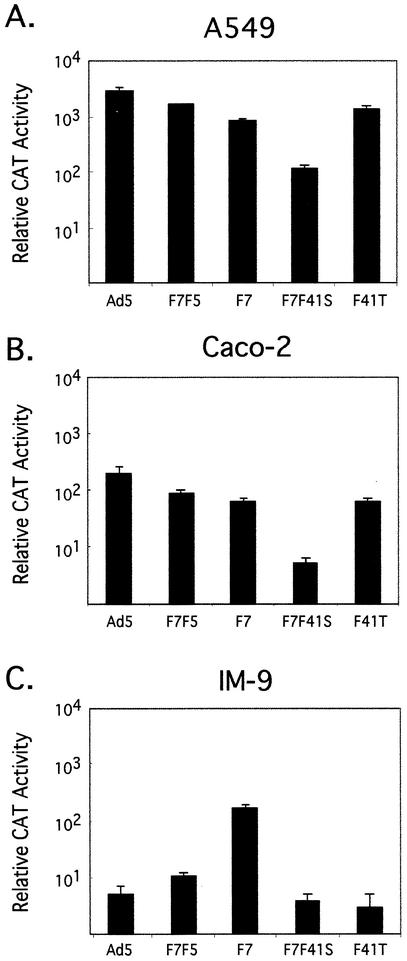
To determine the influence of the various tandem fiber gene arrangements on the efficiency of gene transduction, we used each construct to infect a variety of cell lines. When our standard transduction cell line A549 lung fibroblasts were assayed for CAT gene expression mediated by the tandem fiber virus constructs, F41T, F7F5, and F7F41S demonstrated predictable transduction efficiencies based on fiber function. The F41T and F7F5 viruses were slightly less efficient (CAT reporter gene expression) than wt Ad5-based vectors when equal particle numbers were used to infect A549 lung fibroblasts (Fig. 5A) and HeLa cells (data not shown) under identical conditions. Both of these cell types are known to express CAR as well as the Ad7 (subgroup B) fiber receptor at approximately equal levels (12). However, transduction of these cells by the F7F41S virus was reduced 10- to 100-fold. The decrease in transduction is consistent with decreased high-affinity binding due to dominance of the nonbinding F41S fiber and potentially limited access of the F7 fiber to its target receptor.
FIG. 5.
Transduction efficiency of tandem fiber viruses in A549, Caco-2, and IM-9 lymphoblast cell lines. Aliquots of 2 × 105 A549 fibroblast cells (A), Caco-2 intestinal epithelial cells (B), or IM-9 lymphoblast cells (C) were incubated with 103 virus particles/cell for each of the indicated virus constructs. After 24 h cells were harvested and assayed for total relative CAT enzymatic activity as previously described. Data are means of triplicates ± sample standard deviation.
The natural site for productive infection of Ad41 is the gastrointestinal tract, although the virus may be able to infect other cell types. Replacing the Ad5 fiber protein with the Ad41 fiber proteins could influence the tropism of the Ad5-based vectors to more efficiently infect differentiated intestinal epithelium, which is relatively resistant to infection by Ad5-based vectors. Intestinal epithelial cell monolayers were infected with dlAd5NCAT, F7F5, F7F41S, and F41T to determine if vectors with both Ad41 fiber proteins (F41T) or with F41S could transduce this cell type at a higher efficiency than Ad5 or Ad7 fiber-containing viruses. The results on two different cell lines, Caco-2 (Fig. 5B) and T84 (data not shown) were the same: the viruses had an overall lower transduction efficiency (as measured by CAT activity) than A549 cells and demonstrated a similar pattern of transduction, with F741S being most severely compromised. Based on these studies, we do not find the Ad41 fibers to mediate preferential transduction of intestinal epithelial cells.
Recent studies indicate that hematopoietic cells can be transduced by subgroup B serotype viruses more efficiently than by CAR binding serotypes (4, 5, 33). To determine if the array of tandem fiber viruses had altered transduction capacity when targeting a hematopoietic cell line, we infected IM-9 cells, a B-cell lymphoblast cell line, with each of the tandem viruses as well as the Ad5 and F7 control viruses. Overall, transduction of this cell type by CAR binding vectors was lower than either the A549 cell line or the Caco-2 cell line (compare Fig. 5A and C). Of the viruses used to infect the IM-9 cells, the F7 fiber virus was more effective in mediating CAT gene expression than any of the other chimeric constructs. CAT gene transduction mediated by the F7F41S virus was decreased compared to F7, reflecting low levels of F7 fiber in this virus. The F41T fiber virus also demonstrated low levels of transduction efficiency in these cells. Based on these observations, we find that tandem constructs whose fiber expression is biased towards F41S have severely compromised in vitro gene transduction capabilities compared to single fiber viruses that are targeting through either subgroup B fiber receptor or CAR.
Reduced levels of liver and spleen transduction with systemically administered chimeric vectors.
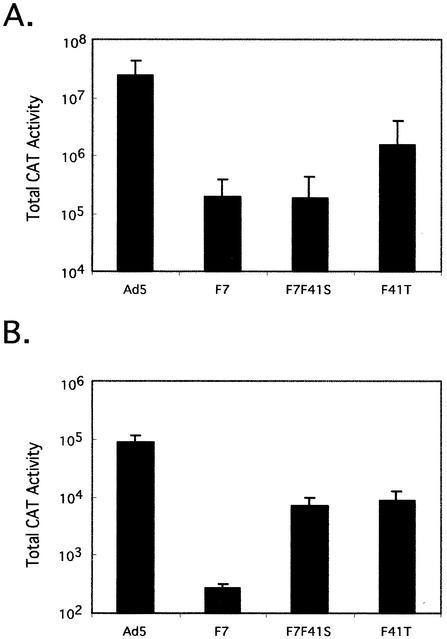
Studies using recombinant Ad5-based vectors have demonstrated that systemically administered Ad5-based viruses localize predominantly to the liver. The localization to the liver occurs in constructs that are mutated in CAR binding activity as well as those which are mutated in integrin binding by mutation of penton (1, 10, 17, 21, 28). To determine if the decreased transduction observed in vitro with our chimeric viruses translates into a reduced level of hepatocyte transduction in vivo, B129 mice were infected with 1010 virus particles of Ad5, F7, F7F41S, or F41T. Three days postinfection, animals were sacrificed and CAT activity was determined for liver and spleen. The results from this assay were both interesting and consistent with our in vitro characterization of the chimeric viruses. As previously demonstrated, Ad5-based vector yields extremely high levels of liver CAT transduction (Fig. 6A). Interestingly, each of the chimeric constructs demonstrated dramatically lower levels of liver CAT gene expression. The F41T construct, which in vitro was approximately half as effective as Ad5, had less than 10% the level of Ad5 CAT gene expression in vivo. The basis of this additional decrease when comparing viruses in vitro to in vivo is considered in the Discussion. Even greater reduction was seen with F7F41S. CAT gene expression resulting from infection with this construct was less than 1% of that with Ad5. Transduction assays using the F7 virus as a control revealed a modestly unexpected result. The F7 virus had previously been shown to efficiently infect rat cardiac myocytes following direct myocardial injection (12). When used in a systemic infection, we found the level of hepatocyte transduction to be at a level of less then 1% of Ad5 CAT.
FIG. 6.
Transduction efficiency of tandem viruses in mice. Female B129 mice (n = 5 per group) were infected retroorbitally with 1010 particles of the indicated virus. Three days postinfection, mice were killed and total CAT activity in liver (A) and spleen (B) was determined as described in Materials and Methods. Data are means of five animals (n = 4 for Ad5 spleen data) ± sample standard deviation.
Total Ad5 CAT transduction of spleen was less than 1% of that found in liver (compare Fig. 6A and B), in agreement with previous studies. Interestingly, transduction of the splenic cell population by the chimeric tandem fiber viruses was 10-fold less than that with Ad5 CAT, and transduction of splenic cells by the F7 virus was 300-fold decreased compared to that of Ad5 CAT. CAT activity assays from kidney, lung, and intestine tissue samples of animals infected with CAR binding viruses were marginally above background levels, whereas the F7 and F7F41S vectors were at background levels in these tissues (data not shown). Therefore, each of the chimeric fiber constructs demonstrates greatly reduced transduction of the primary and secondary target tissues that are normally transduced by traditional Ad5-based vectors following systemic administration.
DISCUSSION
This study continues our interest in exploring the use of Ad serotypes other than Ad2 or Ad5 for gene transfer applications and, specifically, to determine their usefulness in Ad-retargeting strategies. One of the necessary objectives associated with retargeting an Ad vector is to eliminate or greatly reduce normal virus binding to cells other than the desired target cell type. In the series of experiments presented in this study, we have made a significant step toward achieving this goal. The Ad5.F7F41S virus demonstrates greatly reduced binding activity in a variety of cell types in vitro and, more importantly, compared to an Ad5-based vector it demonstrates a dramatic reduction in liver and spleen gene expression following systemic administration in a murine model.
The subgroup F viruses are inherently interesting serotypes of adenovirus. They have a natural tropism for the intestinal tract, are comparatively difficult to grow in culture, have a novel tandem fiber arrangement, and express a distinct short fiber trimer that has been shown to be non-CAR binding. Insertion of Ad41 fiber terminal exons into the MLTU has allowed us to analyze the contributions of F41L and F41S to vector transduction in a well-characterized Ad5 background. Assuming expression and F41 incorporation into the Ad5 capsid is a reflection of the fiber function in wt serotype 41 virus, we did not find that the subgroup F fibers enhanced transduction into polarized (or nonpolarized) intestinal epithelial cell lines (Caco-2 or T-84). We have demonstrated that in a virus, F41L binds through CAR while F41S lacks distinct binding activity, in agreement with the characterization of baculovirus His tag-expressed F41S and F41L (25). In a study by Croyle et al. (6), it was shown that fluorescently labeled wt Ad41 could bind and undergo internalization into differentiated Caco-2 cells more efficiently than an Ad5 vector. This indicates there may be unique capsid elements associated with wt Ad41 binding to differentiated intestinal epithelial cells that are lacking in our F41T virus.
The F41T virus yielded an unexpected result. Both fiber terminal exons were transcribed into appropriate mRNAs, and the level of each mRNA was translated into representative pools of steady-state fiber protein; however, a disproportionate amount of F41S was incorporated into purified virions. Recently, structural studies by Favier et al. (11) concluded that Ad41 virions contain approximately a 1:1 ratio of long and short fibers. While these results were estimated from electron micrographs, the authors encountered difficulties in quantitating the fiber content of purified virions by SDS-PAGE due to incomplete denaturation of fiber molecules from Ad41 penton. In our study, the bias favoring F41S is based on visualizing fiber from purified virus by Coomassie staining, Western blotting, and [35S]methionine labeling. The combination of physical data, as well as the distinctive binding and CAT transduction profile that we have found with the chimeric F41 viruses, support our conclusion that in the chimeric constructs (F7F41S and F41T) F41S is the dominant fiber present in the purified virions.
Because there has yet to be a high-affinity binding function associated with F41S, its incorporation as the dominant fiber seems counterintuitive. One explanation may simply be that F41S interacts with Ad5 penton more readily than F41L and the bias is specific to our chimeras. Alternatively, the bias favoring F41S may represent a natural bias that serves a biological function. A high level of F41S in the Ad virion may afford a structural advantage in promoting F41L-CAR binding. In our studies we found that the F41T virus grows extremely well, infects through a CAR pathway with great efficiency, and that its greatest distinction from an Ad5 vector is at the level of liver transduction in vivo. These are the characteristics of a virus that is dominated by having 5- to 10-fold more F41S than F41L. Since others have shown that several non-CAR binding pathways may contribute to Ad5 vector transduction (1, 17, 21, 28), the limited presentation of F41L may afford a greater degree of specific CAR binding than with a virus that is exclusively expressing an Ad5-type fiber, such as F41L. The dominant presence of the F41S fiber (12 shaft repeats) may provide a fiber population with lower avidity for non-CAR interactions. Productive virus-cell interactions are therefore primarily mediated through high-affinity CAR interactions. Since the F41L fiber (22 shaft repeats) is longer than F41S, the F41L knob extends beyond the shell provided by F41S and exclusively transduces through a CAR pathway.
In comparison to the F41T virus, the F7F41S construct presents a virus where, once again, F41S is the dominant fiber incorporated into the virion capsid. However, the alternative fiber, F7 (six shaft repeats), is expressed at lower levels and is significantly shorter in length than F41S. In the case of the F7F41S virus, the F41S provides an inert shell to the environment but also shields F7 from binding to its target receptor. This model would predict that the presence of a low concentration of F7 in the virion would yield a highly defective virus with respect to cell transduction. All of the experiments presented in this study support this hypothesis. The F7F41S virus is 10- to 100-fold less effective in transduction in vitro, and greater than 100-fold less infectious in liver cell transduction in vivo.
Our original fiber-replacement strategy (12) demonstrated the principle of fiber manipulation as a method to redirect an Ad5-based vector. The in vivo data presented in this study extend our insight into the F7-based Ad5 vector. Based on total CAT transduction to liver and spleen, 99.9% of the F7 vector is not localizing to the normal targets of Ad5 vectors. We are currently extending our assays of these vectors to determine the fate of the F7 vectors in vivo and to extend our understanding of how the F7 vector may influence other aspects of tissue-specific cell interactions. However, we have clearly demonstrated that the F7 vector has a dramatic effect on Ad5 vector detargeting, which substantiates its use as a platform for retargeting (in place of a mutated CAR binding fiber). Subgroup B viruses have been shown to have distinct cell targets, as previously described (20, 23-25, 33), and it will be important to identify the receptor used by the subgroup B viruses as well as the functional ligand domain present in the F7 homotrimer. The extension of the non-CAR binding vectors to the F7F41S tandem platform represents a novel class of modified Ad5-based vectors that provides distinct advantages over traditional single-fiber swap chimeras. The low level of F7 fiber and the lack of receptor binding by F41S not only reduces binding through the CAR uptake pathway but also diminishes uptake through the subgroup B pathway. Importantly, these vectors will provide a useful platform for the construction of retargeted Ad5-based vectors. Both the F41T and F7F41S systems have characteristics that can be used to enhance cell-specific targeting. We are currently testing several options for construction of retargeted Ad5-based vectors based on these constructs.
In addition to the expression of multiple adenovirus L5 terminal exons, our observations indicate that the MLTU has the flexibility to potentially express any inserted terminal exon. Our studies are focused on fiber, but insertion of any cDNA as a terminal exon in the region of the L5 domain (or using the L5 processing elements) would result in expression of the target gene during the late stage of virus infection. Expression of capsid proteins such as hexon, penton, or protein IX could be carried out, allowing a complete redesign of the virion capsid. Alternatively, use of Ad vectors that are cell restricted with respect to the replicative cycle could express a designated gene product in a stage-specific manner. Adenovirus vectors present a host of challenges if they are to be productively employed for gene transfer applications. Based on the observations presented in this study, we feel the terminal exon insertion paradigm presented by the subgroup F viruses provides a novel and useful genetic approach to reengineer adenovirus vectors.
Acknowledgments
We thank Janette Gomos-Kline for her assistance with the animal studies.
This work was supported by National Institutes of Health Public Health Service grant HL51746 to E.F.-P.
REFERENCES
- 1.Alemany, R., and D. T. Curiel. 2001. CAR-binding ablation does not change biodistribution and toxicity of adenoviral vectors. Gene Ther. 8:1347-1353. [DOI] [PubMed] [Google Scholar]
- 2.Bergelson, J. M., J. A. Cunningham, G. Droguett, E. A. Kurt-Jones, A. Krithivas, J. S. Hong, M. S. Horwitz, R. L. Crowell, and R. W. Finberg. 1997. Isolation of a common receptor for coxsackie B viruses and adenoviruses 2 and 5. Science 275:1320-1323. [DOI] [PubMed] [Google Scholar]
- 3.Bewley, M. C., K. Springer, Y. B. Zhang, P. Freimuth, and J. M. Flanagan. 1999. Structural analysis of the mechanism of adenovirus binding to its human cellular receptor, CAR. Science 286:1579-1583. [DOI] [PubMed] [Google Scholar]
- 4.Chu, Y., K. Sperber, L. Mayer, and M. T. Hsu. 1992. Persistent infection of human adenovirus type 5 in human monocyte cell lines. Virology 188:793-800. [DOI] [PubMed] [Google Scholar]
- 5.Cripe, T. P., E. J. Dunphy, A. D. Holub, A. Saini, N. H. Vasi, Y. Y. Mahller, M. H. Collins, J. D. Snyder, V. Krasnykh, D. T. Curiel, T. J. Wickham, J. DeGregori, J. M. Bergelson, and M. A. Currier. 2001. Fiber knob modifications overcome low, heterogeneous expression of the coxsackievirus-adenovirus receptor that limits adenovirus gene transfer and oncolysis for human rhabdomyosarcoma cells. Cancer Res. 61:2953-2960. [PubMed] [Google Scholar]
- 6.Croyle, M. A., M. Stone, G. L. Amidon, and B. J. Roessler. 1998. In vitro and in vivo assessment of adenovirus 41 as a vector for gene delivery to the intestine. Gene Ther. 5:645-654. [DOI] [PubMed] [Google Scholar]
- 7.Davison, A. J., E. A. Telford, M. S. Watson, K. McBride, and V. Mautner. 1993. The DNA sequence of adenovirus type 40. J. Mol. Biol. 234:1308-1316. [DOI] [PubMed] [Google Scholar]
- 8.Dechecchi, M. C., P. Melotti, A. Bonizzato, M. Santacatterina, M. Chilosi, and G. Cabrini. 2001. Heparan sulfate glycosaminoglycans are receptors sufficient to mediate the initial binding of adenovirus types 2 and 5. J. Virol. 75:8772-8780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Defer, C., M. T. Belin, M. L. Caillet-Boudin, and P. Boulanger. 1990. Human adenovirus-host cell interactions: comparative study with members of subgroups B and C. J. Virol. 64:3661-3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Einfeld, D. A., R. Schroeder, P. W. Roelvink, A. Lizonova, C. R. King, I. Kovesdi, and T. J. Wickham. 2001. Reducing the native tropism of adenovirus vectors requires removal of both CAR and integrin interactions. J. Virol. 75:11284-11291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Favier, A. L., G. Schoehn, M. Jaquinod, C. Harsi, and J. Chroboczek. 2002. Structural studies of human enteric adenovirus type 41. Virology 293:75-85. [DOI] [PubMed] [Google Scholar]
- 12.Gall, J., A. Kass-Eisler, L. Leinwand, and E. Falck-Pedersen. 1996. Adenovirus type 5 and 7 capsid chimera: fiber replacement alters receptor tropism without affecting primary immune neutralization epitopes. J. Virol. 70:2116-2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greber, U. F., M. Willetts, P. Webster, and A. Helenius. 1993. Stepwise dismantling of adenovirus 2 during entry into cells. Cell 75:477-486. [DOI] [PubMed] [Google Scholar]
- 14.Hong, S. S., L. Karayan, J. Tournier, D. T. Curiel, and P. A. Boulanger. 1997. Adenovirus type 5 fiber knob binds to MHC class Iα2 domain at the surface of human epithelial and B lymphoblastoid cells. EMBO J. 16:2294-2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kidd, A. H., J. Chroboczek, S. Cusack, and R. W. Ruigrok. 1993. Adenovirus type 40 virions contain two distinct fibers. Virology 192:73-84. [DOI] [PubMed] [Google Scholar]
- 16.Kirby, I., E. Davison, A. J. Beavil, C. P. Soh, T. J. Wickham, P. W. Roelvink, I. Kovesdi, B. J. Sutton, and G. Santis. 1999. Mutations in the DG loop of adenovirus type 5 fiber knob protein abolish high-affinity binding to its cellular receptor CAR. J. Virol. 73:9508-9514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leissner, P., V. Legrand, Y. Schlesinger, D. A. Hadji, M. van Raaij, S. Cusack, A. Pavirani, and M. Mehtali. 2001. Influence of adenoviral fiber mutations on viral encapsidation, infectivity and in vivo tropism. Gene Ther. 8:49-57. [DOI] [PubMed] [Google Scholar]
- 18.Li, E., S. L. Brown, D. G. Stupack, X. S. Puente, D. A. Cheresh, and G. R. Nemerow. 2001. Integrin αvβ1 is an adenovirus coreceptor. J. Virol. 75:5405-5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mathias, P., T. Wickham, M. Moore, and G. Nemerow. 1994. Multiple adenovirus serotypes use alpha v integrins for infection. J. Virol. 68:6811-6814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mei, Y. F., K. Lindman, and G. Wadell. 2002. Human adenoviruses of subgenera B, C, and E with various tropisms differ in both binding to and replication in the epithelial A549 and 293 cells. Virology 295:30-43. [DOI] [PubMed] [Google Scholar]
- 21.Mizuguchi, H., N. Koizumi, T. Hosono, A. Ishii-Watabe, E. Uchida, N. Utoguchi, Y. Watanabe, and T. Hayakawa. 2002. CAR- or αv integrin-binding ablated adenovirus vectors, but not fiber-modified vectors containing RGD peptide, do not change the systemic gene transfer properties in mice. Gene Ther. 9:769-776. [DOI] [PubMed] [Google Scholar]
- 22.Pieniazek, N. J., S. B. Slemenda, D. Pieniazek, J. J. Velarde, and R. B. Luftig. 1990. Human enteric adenovirus type 41 (Tak) contains a second fiber protein gene. Nucleic Acids Res. 18:1901.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rea, D., M. J. Havenga, M. van Den Assem, R. P. Sutmuller, A. Lemckert, R. C. Hoeben, A. Bout, C. J. Melief, and R. Offringa. 2001. Highly efficient transduction of human monocyte-derived dendritic cells with subgroup B fiber-modified adenovirus vectors enhances transgene-encoded antigen presentation to cytotoxic T cells. J. Immunol. 166:5236-5244. [DOI] [PubMed] [Google Scholar]
- 24.Roelvink, P. W., I. Kovesdi, and T. J. Wickham. 1996. Comparative analysis of adenovirus fiber-cell interaction: adenovirus type 2 (Ad2) and Ad9 utilize the same cellular fiber receptor but use different binding strategies for attachment. J. Virol. 70:7614-7621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roelvink, P. W., A. Lizonova, J. G. Lee, Y. Li, J. M. Bergelson, R. W. Finberg, D. E. Brough, I. Kovesdi, and T. J. Wickham. 1998. The coxsackievirus-adenovirus receptor protein can function as a cellular attachment protein for adenovirus serotypes from subgroups A, C, D, E, and F. J. Virol. 72:7909-7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roelvink, P. W., G. Mi Lee, D. A. Einfeld, I. Kovesdi, and T. J. Wickham. 1999. Identification of a conserved receptor-binding site on the fiber proteins of CAR-recognizing adenoviridae. Science 286:1568-1571. [DOI] [PubMed] [Google Scholar]
- 27.Shayakhmetov, D. M., and A. Lieber. 2000. Dependence of adenovirus infectivity on length of the fiber shaft domain. J. Virol. 74:10274-10286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith, T., N. Idamakanti, H. Kylefjord, M. Rollence, L. King, M. Kaloss, M. Kaleko, and S. C. Stevenson. 2002. In vivo hepatic adenoviral gene delivery occurs independently of the coxsackievirus-adenovirus receptor. Mol. Ther. 5:770-779. [DOI] [PubMed] [Google Scholar]
- 29.Stevenson, S. C., M. Rollence, B. White, L. Weaver, and A. McClelland. 1995. Human adenovirus serotypes 3 and 5 bind to two different cellular receptors via the fiber head domain. J. Virol. 69:2850-2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tomko, R. P., C. B. Johansson, M. Totrov, R. Abagyan, J. Frisen, and L. Philipson. 2000. Expression of the adenovirus receptor and its interaction with the fiber knob. Exp. Cell. Res. 255:47-55. [DOI] [PubMed] [Google Scholar]
- 31.Tomko, R. P., R. Xu, and L. Philipson. 1997. HCAR and MCAR: the human and mouse cellular receptors for subgroup C adenoviruses and group B coxsackieviruses. Proc. Natl. Acad. Sci. USA 94:3352-3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Raaij, M. J., E. Chouin, H. van der Zandt, J. M. Bergelson, and S. Cusack. 2000. Dimeric structure of the coxsackievirus and adenovirus receptor D1 domain at 1.7 Å resolution. Structure Fold Des. 8:1147-1155. [DOI] [PubMed] [Google Scholar]
- 33.Von Seggern, D. J., S. Huang, S. K. Fleck, S. C. Stevenson, and G. R. Nemerow. 2000. Adenovirus vector pseudotyping in fiber-expressing cell lines: improved transduction of Epstein-Barr virus-transformed B cells. J. Virol. 74:354-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wickham, T. J., P. Mathias, D. A. Cheresh, and G. R. Nemerow. 1993. Integrins alpha v beta 3 and alpha v beta 5 promote adenovirus internalization but not virus attachment. Cell 73:309-319. [DOI] [PubMed] [Google Scholar]
- 35.Yeh, H. Y., N. Pieniazek, D. Pieniazek, H. Gelderblom, and R. B. Luftig. 1994. Human adenovirus type 41 contains two fibers. Virus Res. 33:179-198. [DOI] [PubMed] [Google Scholar]