Abstract
Recombinant protein subunit AIDS vaccines have been based predominantly on the virus envelope protein. Such vaccines elicit neutralizing antibody responses that can provide type-specific sterilizing immunity, but in most cases do not confer protection against divergent viruses. In this report we demonstrate that a multiantigen subunit protein vaccine was able to prevent the development of disease induced in rhesus monkeys by a partially heterologous AIDS virus. The vaccine was composed of recombinant human immunodeficiency virus type 1 (HIV-1) gp120, NefTat fusion protein, and simian immunodeficiency virus (SIV) Nef formulated in the clinically tested adjuvant AS02A. Upon challenge of genetically unselected rhesus monkeys with the highly pathogenic and partially heterologous SIV/HIV strain SHIV89.6p the vaccine was able to reduce virus load and protect the animals from a decline in CD4-positive cells. Furthermore, vaccination prevented the development of AIDS for more than 2.5 years. The combination of the regulatory proteins Nef and Tat together with the structural protein gp120 was required for vaccine efficacy.
The development of recombinant subunit protein vaccines for AIDS was previously focused on the envelope glycoprotein (Env) of the virus. Sterile protection against homologous virus challenge has been achieved in primate models (11, 13, 26, 37, 48, 54, 56, 64, 74). However, this neutralizing antibody-mediated protection was highly strain specific, directed mostly against laboratory-adapted human immunodeficiency virus (HIV) strains and did not prevent infection with heterologous viruses (75). More recent data suggest that novel envelope-based immunogens were able to elicit antibodies that appeared to be more broadly reactive and were able to neutralize HIV primary isolates (6) or prevent the development of disease in macaques (17, 18).
In order to overcome the strain-specific immunogenicity of recombinant subunit protein vaccines, antigens or individual epitopes that are conserved among different virus strains and clades could be combined in multiantigen formulations (55). Furthermore, immune responses directed against antigens that are expressed very early in the virus life cycle may trigger immediate elimination of infected cells. One protein that meets these criteria is the viral transactivator protein Tat. Tat has been proposed as a vaccine antigen (reviewed in reference 27) because it is believed to contribute to viral pathogenesis and immunosuppression through inhibition of antigen-specific lymphoproliferation and induction of apoptosis (46, 79, 81). Furthermore, the presence of Tat-specific antibodies (82) and cytotoxic T lymphocytes (CTL) (77) has been inversely correlated with disease progression.
Evaluation of Tat-containing vaccines in nonhuman primate simian immunodeficiency virus (SIV) and chimeric simian-human immunodeficiency virus (SHIV) models has yielded conflicting results. Various recombinant Tat protein (15, 63) or peptide (28) vaccines as well as Tat-encoding DNA (16) and recombinant live vectors (59) have demonstrated some impact on virus infection and disease progression in these models (55). However, combined vaccination with DNA and modified vaccinia virus Ankara (1) or different forms of recombinant Tat protein (73) did not demonstrate any efficacy against virus challenge in spite of good CTL or antibody responses. A Tat toxoid vaccine has been administered to humans and shown to be safe and immunogenic (32).
The Nef protein of HIV and SIV plays an important role in AIDS pathogenesis. Nef-defective SIV is highly attenuated in macaques (42). Furthermore, Nef-defective HIV-1 has been detected in long-term-nonprogressing patients (43, 45), although disease progression is eventually observed (31). Nef is also known to be a frequent CTL target (reviewed in reference 68), and Nef-directed CTL have been correlated with favorable clinical outcome in macaque studies (25). Interestingly, Nef downregulates major histocompatibility complex (MHC) class I and CD4 molecules in vitro (reviewed in reference 65). Based on this observation, it has been suggested that Nef contributes to viral immune escape, but the relevance of the in vitro findings has not been fully understood in light of the apparent immunogenicity of Nef for CTL. Because of its role in pathogenesis and as a target for CTL, Nef represents a promising component of a multiantigen AIDS vaccine.
We have previously developed a recombinant subunit protein vaccine containing the Env protein gp120W61D derived from the dualtropic CCR5/CXCR4 HIV-1 isolate ACH320 (33) formulated in the proprietary adjuvant AS02A. This vaccine has been shown to induce neutralizing antibodies and helper T-cell responses in a phase I clinical trial (9, 39, 53). It also provided sterile immunity against challenge with a homologous in vivo-passaged SHIV virus in rhesus macaques (56) and prevented loss of CD4-positive cells after pathogenic SHIV89.6p rechallenge (54). Furthermore, the adjuvant AS02A, in combination with the circumsporozoite protein-derived antigen RTS,S, has been shown to protect human volunteers from experimental challenge with the malaria parasite Plasmodium falciparum (76) and from human malarial infection in a field trial in The Gambia (12).
Based on this previous experimental evidence, we have now developed an AS02A-adjuvanted multiantigen subunit protein AIDS vaccine that incorporates the HIV Nef and Tat proteins in addition to the gp120 immunogen. The Nef and Tat proteins were expressed recombinantly in yeast cells and evaluated separately or in combination with gp120 in a vaccine efficacy study using a highly pathogenic and partially heterologous (for gp120 and Tat) rhesus macaque SHIV challenge model. The data revealed that only the combination of gp120, a NefTat fusion protein, and SIV Nef conferred solid protection from SHIV-induced disease in nonhuman primates.
MATERIALS AND METHODS
Animals and veterinary procedures.
The SHIV challenge study was performed at Primedica Laboratories, Worcester, Mass. Rhesus monkeys (Macaca mulatta) were received from Covance Research Center, Alice, Tex. Prior to the study, all animals were tested and determined to be seronegative for antibodies to SIV, type D retrovirus, and simian T-cell-lymphotropic virus type 1. All animal care and use procedures conformed to the revised Public Health Service Policy on Humane Care and Use of Laboratory Animals. The animals were anesthetized with ketamine prior to all procedures. All animals from the study were characterized for their MHC class I haplotypes by PCR-single-strand polymorphism analysis (44) based on primers specific for particular MHC class I alleles. Animals were typed for the MHC class I alleles Mamu-A*01, -A*02, -A*08, -A*11, -B*01, -B*03, -B*04, and -B*17. Routine hematology and clinical chemistry was performed after each vaccination. Peripheral blood mononuclear cells (PBMC) were analyzed by standard flow cytometry methods for the detection of CD4- and CD8-positive lymphocytes and additional cell subsets (80).
Vaccines.
Groups of four rhesus monkeys were immunized thrice intramuscularly at 0, 1, and 3 months. The composition of the vaccines is depicted in Table 1. The recombinant gp120W61D antigen was derived from the Dutch clinical HIV isolate ACH320 (33), expressed in CHO cells, and used at 100 μg per dose. All Nef- and Tat-related antigens were expressed in the yeast Pichia pastoris as His-tagged proteins and used at 20 μg per dose. The HIV-1 nef gene was derived from the clone Bru/Lai (78), SIV nef was derived from the clone SIVmac239 (66) without a premature stop codon (kindly provided by R. Desrosiers), and the HIV-1 tat gene was derived from the clone BH10 (4). Nef-Tat is a full-length fusion protein of the two viral proteins. In order to adapt the experimental vaccines to the SHIV challenge virus carrying the SIV nef gene, the HIV-1 NefTat fusion protein was always used for immunization in conjunction with the SIV Nef protein.
TABLE 1.
Neutralizing antibody responses after immunization and challengea
| Group | Antigen(s) | Adjuvant | Animal no. | SHIV89.6p neutralizing antibody titer
|
HIV-1w6.1D neutralization | ||
|---|---|---|---|---|---|---|---|
| Wk 0 | Wk 4 | Wk 13 | |||||
| 1 | gp120 | AS02A | 008 | <20 | <20 | <20 | + |
| 046 | <20 | ND | ND | + | |||
| 087 | <20 | <20 | <20 | + | |||
| 443 | <20 | 30 | 132 | + | |||
| 2 | gp120/NefTat/SIV Nef | AS02A | 009 | <20 | <20 | 268 | + |
| 032 | <20 | <20 | <20 | + | |||
| 102 | <20 | 26 | 159 | + | |||
| 358 | <20 | 128 | 305 | + | |||
| 3 | gp120/NefTat/SIV Nef | AS06 | 118 | <20 | <20 | 1,088 | − |
| 249 | 66 | 90 | 1,627 | − | |||
| 382 | <20 | <20 | 1,835 | − | |||
| 383 | <20 | <20 | 1,287 | + | |||
| 4 | NefTat/SIV Nef | AS02A | 010 | <20 | <20 | <20 | − |
| 047 | <20 | <20 | 190 | − | |||
| 155 | <20 | <20 | <20 | − | |||
| 305 | <20 | <20 | <20 | − | |||
| 5 | None | AS02A | 024 | <20 | <20 | <20 | − |
| 041 | <20 | ND | 134 | − | |||
| 173 | <20 | <20 | 541 | − | |||
| 228 | <20 | <20 | <20 | − | |||
Antibodies capable of neutralizing the challenge virus SHIV89.6p were measured in an MT-2 cell-killing assay at the day of challenge (week 0) and at 4 and 13 weeks thereafter. Neutralization of HIV-1w6.1D was tested in a PBMC-killing assay on the day of challenge at a 1:4 dilution. A reduction in p24 production of >80% was considered a positive sample (+), ≤80% reduction was negative (−). ND, not determined.
The adjuvant AS02A contains 250 μl of an oil-in-water emulsion, 50 μg of 3D-monophosphoryl lipid A, and 50 μg of the saponin QS21. The novel adjuvant AS06 comprises 500 μg of the CpG sequence-containing oligonucleotide 1826 (52) and 500 μg of aluminum hydroxide. The adjuvant components and the antigens were formulated to yield a 500-μl volume per vaccine dose.
Virus challenge.
Four weeks after the last immunization, the macaques were challenged intravenously with chimeric SHIV89.6p (67). The virus stock was diluted 1:1,000 in RPMI 1640 supplemented with 10% fetal bovine serum, 1% l-glutamine, and 1% penicillin-streptomycin. This virus stock was previously titrated in vivo, and a 1:1,000 dilution represents 5 to 50 50% infectious doses for macaques (kindly provided by N. Letvin, Beth Israel Deaconess Medical Center, Boston, Mass.). The sequence similarities between the vaccine antigens and those produced by the challenge virus (clone 89.6KB9 [40]) were 79.8% for Env, 89.7% for Tat, 38.2% for HIV Nef, and 100% for SIV Nef.
Determination of plasma virus load.
The amount of SHIV RNA in plasma was analyzed with the Bayer SIV branched DNA (bDNA) assay. Plasma was separated from whole blood by centrifugation and stored at −70°C until shipment. Samples were analyzed in the 50-μl and 1.0-ml formats of the assay. The lower limit of detection of the assay was 1,500 RNA copies per ml until study week 24 and 500 RNA copies per ml for subsequent samples.
Virus neutralization assays.
Serum neutralization of SHIV89.6p and the T-cell line-adapted (TCLA) HIV-1MN was measured in an MT-2 cell-killing assay as described previously (19). Briefly, 50 μl of cell-free virus containing 500 50% tissue culture infectious doses was added to multiple dilutions of test serum in 100 μl of growth medium in triplicate in 96-well culture plates. The mixtures were incubated at 37°C for 1 h, followed by the addition of MT-2 cells (5 × 104 cells in 100 μl) to each well. Infection led to extensive syncytium formation and virus-induced cell killing in approximately 4 to 6 days in the absence of antibodies. Neutralization was measured by staining viable cells with Finter's neutral red in poly-l-lysine-coated plates. Percent protection was determined by calculating the difference in absorption (A540) between test wells (cells plus serum plus virus) and virus control wells (cells plus virus), dividing this result by the difference in absorption between cell control wells (cells only) and virus control wells and multiplying by 100. Serum neutralization of HIVW6.1D TCLA isolate was performed in human PBMC as described previously (14).
Neutralization was measured at a time when virus-induced cell killing in virus control wells was greater than 70% but less than 100%. Neutralization titers are given as the reciprocal dilution required to protect 50% of cells from virus-induced killing; this cutoff corresponds to an approximately 90% reduction in Gag antigen synthesis (14). Virus stocks were produced in either human peripheral blood mononuclear cells (SHIV89.6p or HIVW6.1D) or H9 cells (HIV-1MN).
Antigen-specific serology.
The presence of antigen-specific antibodies in the serum of immunized monkeys was determined with a standard enzyme-linked immunosorbent assay (ELISA) method. Briefly, ELISA plates were coated with the recombinant HIV-1 proteins Nef, Tat, gp120, and SIV Nef and incubated overnight. Then the plates were washed and saturated with blocking buffer. This was followed by an incubation with prediluted monkey serum, additional washes, and subsequent incubation with horseradish peroxidase-coupled mouse anti-human immunoglobulin G antibody. After a final washing step and a color reaction with 3,3′,5,5′-tetramethylbenzidine, the plates were read in an ELISA reader. The titers were calculated from a standard curve generated with an immune serum pool with the software Softmaxpro and expressed as midpoint titers.
Statistical analysis.
Plasma virus load and CD4-positive cell data were used for statistical analysis to examine differences between experimental groups. To compare arithmetic means (from log10) of groups, one-way analysis of variance was used. When there was a statistically significant difference between group means (P value of <0.05), an adapted multiple comparison of means was employed (Tukey's studentized range test). Assumptions of normality were always checked by Shapiro-Wilk's test, and homogeneity of variances was always checked by the Cochran test. ELISA antibody data were subjected to the statistical approach described above. Analysis of the individual antibody titers revealed that the study was not powered sufficiently to detect less than 3.5-fold differences between groups.
Linear regression analysis was performed on ELISA titers at weeks −2, 0, and 3 and on SHIV89.6p neutralizing antibody titers at weeks 4 and 13, each versus virus load or CD4 levels at week 13.
RESULTS
Immunizations and vaccine safety.
Groups of four rhesus monkeys received three immunizations with the various study vaccines (Table 1) over a 3-month period. No significant changes in hematology or clinical chemistry values that were attributed to the administration of the vaccines were observed.
Vaccine efficacy in rhesus monkeys against challenge with SHIV89.6p.
Four weeks after the last immunization, all animals were subjected to an intravenous challenge with the pathogenic strain SHIV89.6p. Virus infection was determined through analysis of plasma virus load by the bDNA assay, and cell-associated virus load was determined by peripheral blood mononuclear cell coculture. The two parameters indicated that none of the animals were protected from virus infection (Fig. 1 and data not shown). The evaluation of plasma virus loads revealed an initial peak of virus replication of between 107 and 4 × 108 viral copies per ml at 2 weeks postinfection in all animals (Fig. 1).
FIG. 1.
Plasma virus load from rhesus monkeys. Animals were immunized three times and challenged with SHIV89.6p 4 weeks after the last immunization. Postchallenge plasma virus loads were determined at the indicated time points with a bDNA assay. The data from individual animals are grouped per vaccine. When lines end within the 68-week observation period, the animals were euthanized before the next time point. A bDNA assay with a sensitivity of 1,500 copies per ml was used until week 24; subsequent testing was performed with an improved assay with a detection limit of 500 copies per ml.
After the initial peak of viremia, three control animals showed a sustained virus titer of about 106 copies per ml, while the fourth control animal had its virus load reduced to undetectable levels. All animals in group 4, which received only the combination of Nef-Tat and SIV Nef, as well as three out of four animals in group 1, immunized with gp120 alone, maintained high virus loads. In contrast, all group 2 animals, which had received the complete antigen combination composed of gp120, NefTat, and SIV Nef formulated in AS02A, exhibited a rapid reduction of plasma virus load. In three animals it declined to undetectable levels by week 13 postchallenge, whereas the remaining animal maintained a detectable but largely reduced virus load until week 24, which declined thereafter to undetectable levels.
Statistical analysis at 13 and 16 weeks postchallenge revealed that this group of animals was significantly different from the control group (P = 0.0183 and 0.0292, respectively). The group 3 animals, which also received the full antigen combination but formulated in a different experimental adjuvant (AS06), did not show any significant difference from the control group. When a more sensitive bDNA assay was used for subsequent study samples, most of the animals from the different groups with virus levels below 1,500 copies/ml revealed sporadic virus replication of between 500 and 1,000 copies per ml.
The enumeration of virus-infected cells by limiting-dilution coculture assays indicated that all animals had high titers of infected cells at 2 weeks after the virus challenge (data not shown). Most of the animals from the control group and the groups which received separately gp120 or NefTat and SIV Nef remained positive in the cell coculture test until euthanasia. Interestingly, the group 2 animals eventually became negative in this assay except for the one animal that remained sporadically positive in the plasma viremia assay (Fig. 1). The group 3 animals continued to be cell coculture positive (data not shown).
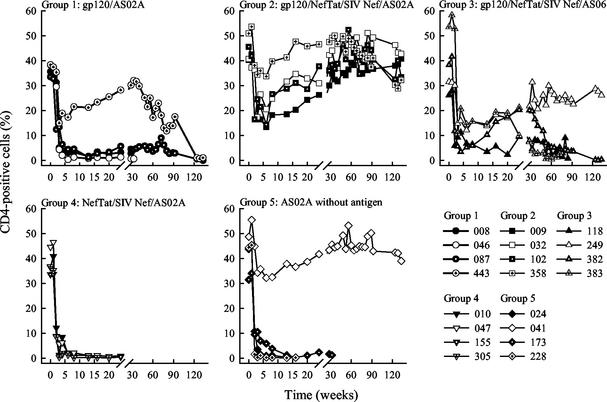
Analysis of CD4-positive cells in the peripheral blood revealed that all animals in the control group except the one controlling its virus load displayed a dramatic decrease in this cell population within a month after infection (Fig. 2). Likewise, all animals from group 4 immunized with NefTat and SIV Nef proteins alone and three out of four animals from group 1 immunized with gp120 alone experienced a similar drop in CD4-positive cells. Interestingly, all four animals from group 2, which received the full antigen combination with gp120, NefTat, and SIV Nef formulated in the adjuvant AS02A, showed an initial decrease in CD4-positive cells but then stabilized their CD4 counts, which eventually recovered to prechallenge levels. Statistical analysis at 13 and 16 weeks postchallenge indicated that the group 2 animals were significantly different from the control animals (P = 0.024 and 0.0078, respectively). A similar but not significant trend was observed with the animals from group 3, which had received the same antigen combination as those in group 2 but formulated in the experimental adjuvant AS06.
FIG. 2.
Evolution of CD4-positive cells in rhesus monkeys. Animals were immunized three times and challenged with SHIV89.6p 4 weeks after the last immunization. The percentage of CD4-positive cells among whole T cells was determined after challenge at the indicated time points from whole blood samples by standard flow cytometry. The data from individual animals are grouped per vaccine. When lines end within the 76-week observation period, the animals were euthanized before the next time point.
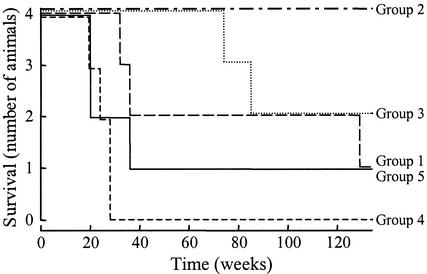
Most of the animals in groups 1, 4, and 5 had to be euthanized within 48 weeks postchallenge due to the development of AIDS-like disease symptoms except for one control group animal (group 5) and two animals in group 1, which received gp120 alone (Fig. 3). In contrast, all animals from groups 2 and 3, which had been immunized with the complete antigen combination, were alive and healthy at that time. Subsequently, two group 3 monkeys (383 and 118) and one group 1 animal (087) were euthanized at weeks 74, 85, and 129, respectively. All monkeys from group 2 and two from group 3 as well as one animal from group 1 and one control animal are still alive more than 2.5 years after the SHIV challenge (week 134 postchallenge, Fig. 3).
FIG. 3.
Survival of rhesus monkeys after challenge with SHIV89.6p. Animals were immunized three times and challenged with SHIV89.6p 4 weeks after the last immunization. SHIV infection-related mortality was monitored through 134 weeks postchallenge.
Humoral immune responses induced by vaccination.
The induction of antibodies specific for the different vaccine antigens was investigated during the immunization period and after virus challenge. Substantial antibody titers to the vaccine antigens were observed as early as after the second immunization in most animals (Fig. 4). The third immunization boosted the antibody responses, and all animals had seroconverted to all respective vaccine antigens. Thereafter, the antibody titers decreased until the day of challenge. The antibody titers varied considerably within the animal groups. Only the reduced immunogenicity of the AS06 adjuvant in group 3 reached statistical significance (P < 0.05) in comparison to the other groups that had received the AS02A adjuvant. All other differences in mean titers were not significant.
FIG. 4.
Vaccine antigen-specific antibody responses. Serum samples were taken from the study animals throughout the immunization period (weeks −16 to week 0) and after challenge with SHIV89.6p at the indicated time points and analyzed for the presence of antibodies directed against the different vaccine antigens. The samples were tested in an ELISA format in serial dilutions, and the midpoint titers were calculated for each serum sample. Results are presented as group geometric means per vaccine antigen. Arrows indicate the times of immunization and challenge.
Most animals with preexisting gp120 antibody titers exhibited considerable anamnestic immune responses after challenge (Fig. 4). Similarly, the animals with vaccine-induced SIV Nef antibody titers displayed a substantial anamnestic immune response. In contrast, an anamnestic immune response was not observed for the HIV Nef and Tat antigens, and the antibody titers continued to decline after virus challenge.
The presence of antibodies capable of neutralizing the standard HIV-1MN isolate, the envelope-homologous HIV-1W6.1D T-cell line-adapted strains, or the challenge virus SHIV89.6p was investigated after two vaccine doses, at the end of the immunization period, and after virus challenge. Neutralizing antibodies specific for HIV-1MN were readily detectable after two immunizations and were not tested further (data not shown). Serum collected after the third vaccine dose was able to neutralize the homologous HIV-1W6.1D T-cell line-adapted virus (Table 1). Selected serum samples with reactivity against the T-cell line-adapted virus were tested against the corresponding primary isolate and failed to demonstrate any activity (data not shown). Only one sample from the prechallenge period was able to neutralize the challenge virus (Table 1). However, after virus infection, SHIV89.6p-neutralizing antibodies were present in several animals, and most of the animals that remained healthy for a prolonged period developed this antibody response.
Regression analysis of binding antibody titers and neutralization activity versus virus load or CD4 level at week 13 did not indicate any significant correlation.
DISCUSSION
This report describes an adjuvanted recombinant protein vaccine containing multiple HIV antigens that has prevented the development of AIDS for more than 2.5 years in genetically unselected rhesus monkeys after highly pathogenic and partially heterologous SHIV challenge. While sterile immunity was not achieved, vaccination prevented the loss of CD4-positive cells and led to a significant reduction in virus load and prevention of AIDS-like disease. The lack of sterile immunity after immunization with this envelope-containing vaccine is probably related to the heterologous nature of the challenge virus (20.2% sequence difference for gp120), since the same gp120 had previously protected macaques from infection with a homologous SHIV (56). Novel envelope-based antigens aimed at inducing broadly neutralizing activity against HIV primary isolates are being developed (6) but have also failed to provide sterile immunity in macaque efficacy studies (18). A vaccine based on an envelope mimotope that is shared by several HIV clades contained development of disease in macaques but again did not induce sterile protection (17).
A variety of other vaccine modalities have been shown to reduce virus loads and/or maintain the number of CD4-positive cells in macaque SIV and SHIV challenge models. These modalities include recombinant subunit vaccines (38, 57), peptides (10), DNA immunization (8, 18, 22, 24, 29, 30), recombinant live vector vaccination (20, 21, 25, 36, 58, 60, 61, 69, 71, 72), or a combination thereof (3, 35, 41, 51, 72). However, almost all of these approaches were evaluated by using homologous challenge viruses. In contrast, the data presented in this report demonstrated protection from SHIV-induced disease with partially heterologous Env (79.8% homology) and Tat (89.7% homology) antigens. Since virus strain variability is a major obstacle for the development of a widely cross-protective AIDS vaccine, our observation suggests that this problem may be partially overcome with the described antigen combination vaccine.
Furthermore, most of the above-mentioned vaccine strategies that utilize DNA and/or recombinant live vectors aim predominantly at inducing powerful cell-mediated immune responses, particularly CD8-positive cells. Even though such effector cell responses have been demonstrated to control AIDS virus-induced disease in rhesus monkeys (47, 70), recent data suggest that such immune surveillance can be circumvented over time by mutations in CD8-positive cell epitopes and lead to disease induction (7). More encouragingly, the adjuvanted multicomponent protein vaccine was still able to control the SHIV infection after 2.5 years, even in the face of sporadic low-level virus replication.
One particularly important factor for the susceptibility to HIV and SIV infection may be the MHC genetic background. In order to investigate such a potential confounding effect of particular MHC alleles, the study monkeys were typed for their MHC alleles. A correlation between any MHC class allele and resistance to SHIV-induced disease could not be detected (data not shown). Similarly, Pal et al. could correlate the MHC class I allele Mamu-A*01 with resistance only to SIV- but not SHIV-induced disease in rhesus monkeys (62).
The rhesus monkey vaccine efficacy study described in this report demonstrated that only the full antigen combination of gp120, NefTat, and SIV Nef conferred protection from SHIV-induced disease. Notably, gp120 formulated in the adjuvant AS02A had previously induced sterile immunity against homologous and nonpathogenic SHIVW6.1D challenge (56) and heterologous SHIVHAN2 and SHIVSF13 rechallenge, and some protection from CD4-positive cell decline was observed after pathogenic SHIV89.6p rechallenge (54). Tat has been described as a protective antigen in some nonhuman primate challenge experiments (15, 63), while other studies did not demonstrate any efficacy of Tat-based vaccines (1, 73), in agreement with our current study, in which no protection was observed after immunization with gp120 or Nef-Tat and SIV Nef antigens alone.
Therefore, the full combination of gp120 and the regulatory proteins Nef and/or Tat appears to be necessary, and the components seem to act additively or synergistically in preventing SHIV-induced disease. This hypothesis is further supported by the observation that the animals from group 3 in particular, which had also received the full antigen combination, exhibited some preservation of CD4-positive cells and prolonged survival. By the same token, the comparison of the group 2 and group 3 animals demonstrated the importance of the adjuvant components, since the group 2 animals received the same antigen combination but the apparently more powerful adjuvant AS02A.
The combination of the regulatory proteins Nef-Tat, SIV Nef, and the structural protein gp120 formulated in AS02A prevented the development of AIDS in a stringent rhesus monkey challenge model, as evidenced by the analysis of CD4-positive cells, virus load, and survival. In an attempt to identify potential correlates of the observed vaccine efficacy, several serological parameters were investigated. The analysis of antigen-specific antibodies prior to and after challenge did not reveal any obvious correlation with vaccine efficacy. Interestingly, anamnestic antibody responses were observed with the gp120 and SIV Nef antigens, while they did not develop for HIV Nef and Tat. The lack of an anamnestic response directed against HIV Nef may be explained by the sequence differences between the HIV Nef immunogen and the SIV Nef expressed by the challenge virus (61.8% divergence).
More surprisingly, an anamnestic antibody response to Tat was absent, while Tat must be present in vivo because it is essential for virus replication. Even though the sequence differences between the vaccine antigen and the challenge virus were small (10.3%), these differences may have affected potential antibody epitopes on the Tat protein. Alternatively, Tat might be present in vivo only in quantities insufficient to induce an anamnestic immune response, or the protein produced in infected cells may not be accessible to antibody-generating B-cell responses. Moreover, since Tat appears to evade host CTL responses through mutations in CTL epitopes that occur rapidly after SIV infection (2), this may also happen with antibody epitopes.
The evaluation of antibody responses at the day of challenge showed that vaccination with the recombinant gp120 did not induce antibodies capable of neutralizing SHIV89.6p. Interestingly, neutralizing antibody responses developed after virus challenge in most of the animals that remained healthy for a prolonged period. These antibodies may be a reflection of the ability of the healthy animals to mount strong immune responses evoked by the replicating challenge virus. However, it has been suggested that the challenge virus SHIV89.6p is highly sensitive to neutralization and an established virus infection easily controlled by this immune effector mechanism (23). Furthermore, passively transferred polyclonal AIDS virus-specific antisera and monoclonal antibodies can either prevent SIV and SHIV infection or reduce disease manifestations (5, 34, 49, 50). Thus, the antibody responses observed after challenge in our study may well be causally linked to the long-term control of viremia.
Additional immune mechanisms may be required for the observed efficacy of the vaccine formulations, since little or no neutralizing antibody was detected at a time when peak viremia started to decrease, and the best-protected group 2 animals clearly did not exhibit the highest neutralization titers. The SHIV89.6p challenge virus model has been criticized for being artificial, because early vaccine-induced events that partially protect against the rapid loss of CD4-positive helper cells may have a profound impact on the overall outcome of the virus challenge (23). In that respect, it appears interesting that the group 3 animals maintained relatively high virus loads despite partial CD4-positive cell preservation, the highest neutralizing antibody titers, and prolonged survival. Furthermore, the fact that some of the surviving animals from the different groups eventually progressed to disease suggests that early events in SHIV89.6p infection do not necessarily predict the long-term clinical outcome.
This report demonstrates that an adjuvanted multivalent protein AIDS vaccine prevented disease caused by a partially heterologous pathogenic SHIV for more than 2.5 years in rhesus monkeys. However, the immune correlates of protection of this vaccine remain to be defined to better understand the mechanism of action underlying the observed protective efficacy. In particular, CD8-positive cell responders appear to contribute to the control of AIDS viruses in rhesus monkeys (70). To that end, further studies in the SHIV rhesus monkey model will be conducted to elucidate the role of cell-mediated immunity and to evaluate the relative importance of the regulatory protein vaccine components.
In an effort to confirm the vaccine efficacy of the antigen combination gp120, Nef-Tat and SIV Nef formulated in AS02A adjuvant, a second challenge study with groups of six animals was initiated in an unrelated monkey population of divergent genetic origins (unpublished data). As in the first study, all animals were challenged with the pathogenic SHIV89.6p 4 weeks after the last immunization, and vaccination with gp120, Nef-Tat, and SIV Nef prevented the loss of CD4-positive cells.
In conclusion, the vaccine appeared to be safe and efficacious in rhesus monkeys, and the combination of gp120 and Nef-Tat without SIV Nef has not shown any adverse effects in preclinical toxicology studies (data not shown). Furthermore, the adjuvant AS02A has been tested clinically with other antigens in more than 1,000 volunteers. On this basis, the clinical evaluation of a gp120 and Nef-Tat protein vaccine formulated in the adjuvant AS02A has been initiated.
Acknowledgments
This study was partially funded by the Région Wallonne (contracts 3498 and 3909). David Montefiori was supported by NIH (contract AI85343).
We thank Nancy Miller (NIH, NIAID, DAIDS, Bethesda, Md.) for continuous support of the study, N. Letvin (Beth Israel Deaconess Medical Center, Boston, Mass.) for providing the challenge virus, and R. Desrosiers (New England Regional Primate Research Center, Southborough, Mass.) for providing the SIVmac239 nef clone. We also thank the dedicated members of the GlaxoSmithKline Biologicals HIV vaccine program who contributed to this work.
REFERENCES
- 1.Allen, T. M., L. Mortara, B. R. Mothé, M. Liebl, P. Jing, B. Calore, M. Piekarczyk, R. Ruddersdorf, D. H. O'Connor, X. Wang, C. Wang, D. B. Allison, J. D. Altman, A. Sette, R. C. Desrosiers, G. Sutter, and D. I. Watkins. 2002. Tat-vaccinated macaques do not control simian immunodeficiency virus SIVmac239 replication. J. Virol. 76:4108-4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen, T. M., D. H. O'Connor, P. Jing, J. L. Dzuris, B. R. Mothé, T. U. Vogel, E. Dunphy, M. E. Liebl, C. Emerson, N. Wilson, K. J. Kunstman, X. Wang, D. B. Allison, A. L. Hughes, R. C. Desrosiers, J. D. Altman, S. M. Wolinsky, A. Sette, and D. I. Watkins. 2000. Tat-specific cytotoxic T lymphocytes select for SIV escape variants during resolution of primary viraemia. Nature 407:386-390. [DOI] [PubMed] [Google Scholar]
- 3.Amara, R. R., F. Villinger, J. D. Altman, S. L. Lydy, S. P. O'Neil, S. I. Staprans, D. C. Montefiori, Y. Xu, J. G. Herndon, L. S. Wyatt, M. A. Candido, N. L. Kozyr, P. L. Earl, J. M. Smith, H.-L. Ma, B. D. Grimm, M. L. Hulsey, J. Miller, H. M. McClure, J. M. McNicholl, B. Moss, and H. L. Robinson. 2001. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/modified vaccinia virus Ankara vaccine. Science 292:69-74. [DOI] [PubMed] [Google Scholar]
- 4.Arya, S. K., C. Guo, S. F. Josephs, and F. Wong-Staal. 1985. Trans-activator gene of human T-lymphotropic virus type III (HTLV-III). Science 229:69-73. [DOI] [PubMed] [Google Scholar]
- 5.Baba, T. W., V. Liska, R. Hofmann-Lehmann, J. Vlasak, W. Xu, S. Ayehunie, L. A. Cavacini, M. R. Posner, H. Katinger, G. Stiegler, B. J. Bernacky, T. A. Rizvi, R. Schmidt, L. R. Hill, M. E. Keeling, Y. Lu, J. E. Wright, T.-C. Chou, and R. M. Ruprecht. 2000. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nat. Med. 6:200-206. [DOI] [PubMed] [Google Scholar]
- 6.Barnett, S. W., S. Lu, I. Srivastava, S. Cherpelis, A. Gettie, J. Blanchard, S. Wang, I. Mboudjeka, L. Leung, Y. Lian, A. Fong, C. Buckner, A. Ly, S. Hilt, J. Ulmer, C. T. Wild, J. R. Mascola, and L. Stamatatos. 2001. The ability of an oligomeric human immunodeficiency virus type 1 (HIV-1) envelope antigen to elicit neutralizing antibodies against primary HIV-1 isolates is improved following partial deletion of the second hypervariable region. J. Virol. 75:5526-5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barouch, D. H., J. Kunstman, M. J. Kuroda, J. E. Schmitz, S. Santra, F. W. Peyerl, G. R. Krivulka, K. Beaudry, M. A. Lifton, D. A. Gorgone, D. C. Montefiori, M. G. Lewis, S. M. Wolinsky, and N. L. Letvin. 2002. Eventual AIDS vaccine failure in a rhesus monkey by viral escape from cytotoxic T lymphocytes. Nature 415:335-339. [DOI] [PubMed] [Google Scholar]
- 8.Barouch, D. H., S. Santra, J. E. Schmitz, M. J. Kuroda, T.-M. Fu, W. Wagner, M. Bilska, A. Craiu, X. X. Zheng, G. R. Krivulka, K. Beaudry, M. A. Lifton, C. E. Nickerson, W. L. Trigona, K. Punt, D. C. Freed, L. Guan, S. Dubey, D. Casimiro, A. Simon, M.-E. Davies, M. Chastain, T. B. Strom, R. S. Gelman, D. C. Montefiori, M. G. Lewis, E. A. Emini, J. W. Shiver, and N. L. Letvin. 2000. Control of viremia and prevention of clinical AIDS in rhesus monkeys by cytokine-augmented DNA vaccination. Science 290:486-492. [DOI] [PubMed] [Google Scholar]
- 9.Beddows, S., S. Lister, R. Cheingsong, C. Bruck, and J. Weber. 1999. Comparison of the antibody repertoire generated in healthy volunteers following immunization with a monomeric recombinant gp120 construct derived from a CCR5/CXCR4-with human immunodeficiency virus type 1 isolate with sera from naturally infected individuals. J. Virol. 73:1740-1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Belyakov, I. M., Z. Hel, B. Kelsall, V. A. Kuznetsov, J. D. Ahlers, J. Nacsa, D. I. Watkins, T. M. Allen, A. Sette, J. Altman, R. Woodward, P. D. Markham, J. D. Clements, G. Franchini, W. Strober, and J. A. Berzofsky. 2001. Mucosal AIDS vaccine reduces disease and viral load in gut reservoir and blood after mucosal infection of macaques. Nat. Med. 7:1320-1326. [DOI] [PubMed] [Google Scholar]
- 11.Berman, P. W., T. J. Gregory, L. Riddle, G. R. Nakamura, M. A. Champe, J. P. Porter, F. M. Wurm, R. D. Hershberg, E. K. Cobb, and J. W. Eichberg. 1990. Protection of chimpanzees from infection by HIV-1 after vaccination with recombinant glycoprotein gp120 but not gp160. Nature 345:622-625. [DOI] [PubMed] [Google Scholar]
- 12.Bojang, K. A., P. J. M. Milligan, M. Pinder, L. Vigneron, A. Alloueche, K. E. Kester, W. R. Ballou, D. J. Conway, W. H. H. Reece, P. Gothard, L. Yamuah, M. Delchambre, G. Voss, B. M. Greenwood, A. Hill, K. P. W. J. McAdam, N. Tornieporth, J. D. Cohen, and T. Doherty. 2001. Efficacy of RTS,S/AS02 malaria vaccine against Plasmodium falciparum infection in semi-immune adult men in the Gambia: a randomised trial. Lancet 358:1927-1934. [DOI] [PubMed] [Google Scholar]
- 13.Bruck, C., C. Thiriart, L. Fabry, M. Francotte, P. Pala, O. Van Opstal, J. Culp, M. Rosenberg, M. De Wilde, P. Heidt, and J. L. Heeney. 1994. HIV-1 envelope-elicited neutralizing antibody titres correlate with protection and virus load in chimpanzees. Vaccine 12:1141-1148. [DOI] [PubMed] [Google Scholar]
- 14.Bures, R., A. Gaitan, T. Zhu, C. Graziosi, K. M. McGrath, J. Tartaglia, P. Caudrelier, R. El Habib, M. Klein, A. Lazzarin, D. M. Stablein, M. Deers, L. Corey, M. L. Greenberg, D. H. Schwartz, and D. C. Montefiori. 2000. Immunization with recombinant canarypox vectors expressing membrane-anchored glycoprotein 120 followed by glycoprotein 160 boosting fails to generate antibodies that neutralize R5 primary isolates of human immunodeficiency virus type 1. AIDS Res. Hum. Retroviruses 16:2019-2035. [DOI] [PubMed] [Google Scholar]
- 15.Cafaro, A., A. Caputo, C. Fracasso, M. T. Maggiorella, D. Goletti, S. Baroncelli, M. Pace, L. Sernicola, M. L. Koanga-Mogtomo, M. Betti, A. Borsetti, R. Belli, L. Åkerblom, F. Corrias, S. Buttò, J. Heeney, P. Verani, F. Titti, and B. Ensoli. 1999. Control of SHIV-89.6P-infection of cynomolgus monkeys by HIV-1 Tat protein vaccine. Nat. Med. 5:643-650. [DOI] [PubMed] [Google Scholar]
- 16.Cafaro, A., F. Titti, C. Fracasso, M. T. Maggiorella, S. Baroncelli, A. Caputo, D. Goletti, A. Borsetti, M. Pace, E. Fanales-Belasio, B. Ridolfi, D. R. M. Negri, L. Sernicola, R. Belli, F. Corrias, I. Macchia, P. Leone, Z. Michelini, P. ten Haaft, S. Buttò, P. Verani, and B. Ensoli. 2001. Vaccination with DNA containing tat coding sequences and unmethylated CpG motifs protects cynomolgus monkeys upon infection with simian/human immunodeficiency virus (SHIV89.6P). Vaccine 19:2862-2877. [DOI] [PubMed] [Google Scholar]
- 17.Chen, X., G. Scala, I. Quinto, W. Liu, T.-W. Chun, J. S. Justement, O. J. Cohen, T. C. VanCott, M. Iwanicki, M. G. Lewis, J. Greenhouse, T. Barry, D. Venzon, and A. S. Fauci. 2001. Protection of rhesus macaques against disease progression from pathogenic SHIV-89.6PD by vaccination with phage-displayed HIV-1 epitopes. Nat. Med. 7:1225-1231. [DOI] [PubMed] [Google Scholar]
- 18.Cherpelis, S., I. Shrivastava, A. Gettie, X. Jin, D. D. Ho, S. W. Barnett, and L. Stamatatos. 2001. DNA vaccination with the human immunodeficiency virus type 1 SF162ΔV2 envelope elicits immune responses that offer partial protection from simian/human immunodeficiency virus infection to CD8+ T-cell-depleted rhesus macaques. J. Virol. 75:1547-1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crawford, J. M., P. L. Earl, B. Moss, K. A. Reimann, M. S. Wyand, K. H. Manson, M. Bilska, J. T. Zhou, C. D. Pauza, P. W. H. I. Parren, D. R. Burton, J. G. Sodroski, N. L. Letvin, and D. C. Montefiori. 1999. Characterization of primary isolate-like variants of simian-human immunodeficiency virus. J. Virol. 73:10199-10207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crotty, S., C. J. Miller, B. L. Lohman, M. R. Neagu, L. Compton, D. Lu, F. X. S. Lü, L. Fritts, J. D. Lifson, and R. Andino. 2001. Protection against simian immunodeficiency virus vaginal challenge by with Sabin poliovirus vectors. J. Virol. 75:7435-7452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davis, N. L., I. J. Caley, K. W. Brown, M. R. Betts, D. M. Irlbeck, K. M. McGrath, M. J. Connell, D. C. Montefiori, J. A. Frelinger, R. Swanstrom, P. R. Johnson, and R. E. Johnston. 2000. Vaccination of macaques against pathogenic simian immunodeficiency virus with Venezuelan equine encephalitis virus replicon particles. J. Virol. 74:371-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Egan, M. A., W. A. Charini, M. J. Kuroda, J. E. Schmitz, P. Racz, K. Tenner-Racz, K. Manson, M. Wyand, M. A. Lifton, C. E. Nickerson, T. Fu, J. W. Shiver, and N. L. Letvin. 2000. Simian immunodeficiency virus (SIV) gag DNA-vaccinated rhesus monkeys develop secondary cytotoxic T-lymphocyte responses and control viral replication after pathogenic SIV infection. J. Virol. 74:7485-7495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feinberg, M. B., and J. P. Moore. 2002. AIDS vaccine models: challenging challenge viruses. Nat. Med. 8:207-210. [DOI] [PubMed] [Google Scholar]
- 24.Fuller, D. H., P. A. Rajakumar, L. A. Wilson, A. M. Trichel, J. T. Fuller, T. Shipley, M. S. Wu, K. Weis, C. R. Rinaldo, J. R. Haynes, and M. Murphey-Corb. 2002. Induction of mucosal protection against primary, heterologous simian immunodeficiency virus by a DNA vaccine. J. Virol. 76:3309-3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gallimore, A., M. Cranage, N. Cook, N. Almond, J. Bootman, E. Rud, P. Silvera, M. Dennis, T. Corcoran, J. Stott, A. McMichael, and F. Gotch. 1995. Early suppression of SIV replication by CD8+ nef-specific cytotoxic T-cells in vaccinated macaques. Nat. Med. 1:1167-1173. [DOI] [PubMed] [Google Scholar]
- 26.Girard, M., M.-P. Kieny, A. Pinter, F. Barre-Sinoussi, P. Nara, H. Kolbe, K. Kusumi, A. Chaput, T. Reinhart, E. Muchmore, J. Ronco, M. Kaczorek, E. Gomard, J.-C. Gluckman, and P. N. Fultz. 1991. Immunization of chimpanzees confers protection against challenge with human immunodeficiency virus. Proc. Natl. Acad. Sci. USA 88:542-546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goldstein, G. 1996. HIV-1 Tat protein as a potential AIDS vaccine. Nat. Med. 2:960-964. [DOI] [PubMed] [Google Scholar]
- 28.Goldstein, G., K. Manson, G. Tribbick, and R. Smith. 2000. Minimization of chronic plasma viremia in rhesus macaques immunized with synthetic HIV-1 Tat peptides and infected with a chimeric simian/human immunodeficiency virus (SHIV33). Vaccine 18:2789-2795. [DOI] [PubMed] [Google Scholar]
- 29.Gorelick, R. J., R. E. Benveniste, J. D. Lifson, J. L. Yovandich, W. R. Morton, L. Kuller, B. M. Flynn, B. A. Fisher, J. L. Rossio, M. Piatak, Jr., J. W. Bess, Jr., L. E. Henderson, and L. O. Arthur. 1949. 2000. Protection of Macaca nemestrina from disease following pathogenic simian immunodeficiency virus (SIV) challenge: utilization of SIV nucleocapsid mutant DNA vaccines with and without an SIV protein boost. J. Virol. 74:11935-11941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gorelick, R. J., J. D. Lifson, J. L. Yovandich, J. L. Rossio, M. Piatak, Jr., A. J. Scarzello, W. B. Knott, J. W. Bess, Jr., B. A. Fisher, B. M. Flynn, L. E. Henderson, L. O. Arthur, and R. E. Benveniste. 2000. Mucosal challenge of Macaca nemestrina with simian immunodeficiency virus (SIV) following SIV nucleocapsid mutant DNA vaccination. J. Med. Primatol. 29:209-219. [DOI] [PubMed] [Google Scholar]
- 31.Greenough, T. C., J. L. Sullivan, and R. C. Desrosiers. 1999. Declining CD4 T-cell counts in a person infected with nef-deleted HIV-1. N. Engl. J. Med. 340:236-237. [DOI] [PubMed] [Google Scholar]
- 32.Gringeri, A., E. Santagostino, M. Muça-Perja, P. M. Mannucci, J. F. Zagury, B. Bizzini, A. Lachgar, M. Carcagno, J. Rappaport, M. Criscuolo, W. Blattner, A. Burny, R. C. Gallo, and D. Zagury. 1998. Safety and immunogenicity of HIV-1 Tat toxoid in immunocompromised HIV-1-infected patients. J. Hum. Virol. 1:293-298. [PubMed] [Google Scholar]
- 33.Groenink, M., R. A. M. Fouchier, R. E. Y. de Goede, F. de Wolf, R. A. Gruters, H. T. M. Cuypers, H. G. Huisman, and M. Tersmette. 1991. Phenotypic heterogeneity in a panel of infectious molecular human immunodeficiency virus type 1 clones derived from a single individual. J. Virol. 65:1968-1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haigwood, N. L., A. Watson, W. F. Sutton, J. McClure, A. Lewis, J. Ranchalis, B. Travis, G. Voss, N. L. Letvin, S.-L. Hu, V. M. Hirsch, and P. R. Johnson. 1996. Passive immune globulin therapy in the SIV/macaque model: early intervention can alter disease profile. Immunol. Lett. 51:107-114. [DOI] [PubMed] [Google Scholar]
- 35.Hanke, T., R. V. Samuel, T. J. Blanchard, V. C. Neumann, T. M. Allen, J. E. Boyson, S. A. Sharpe, N. Cook, G. L. Smith, D. I. Watkins, M. P. Cranage, and A. J. McMichael. 1999. Effective induction of simian immunodeficiency virus-specific cytotoxic T lymphocytes in macaques by with a multiepitope gene and DNA prime-modified vaccinia virus Ankara boost vaccination regimen. J. Virol. 73:7524-7532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hirsch, V. M., T. R. Fuerst, G. Sutter, M. W. Carroll, L. C. Yang, S. Goldstein, M. Piatak, Jr., W. R. Elkins, W. G. Alvord, D. C. Montefiori, B. Moss, and J. D. Lifson. 1996. Patterns of viral replication correlate with outcome in simian immunodeficiency virus (SIV)-infected macaques: effect of prior immunization with a trivalent SIV vaccine in modified vaccinia virus Ankara. J. Virol. 70:3741-3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hu, S.-L., K. Abrams, G. N. Barber, P. Moran, J. M. Zarling, A. J. Langlois, L. Kuller, W. R. Morton, and R. E. Benveniste. 1992. Protection of macaques against SIV infection by subunit vaccines of SIV envelope glycoprotein gp160. Science 255:456-459. [DOI] [PubMed] [Google Scholar]
- 38.Israel, Z. R., P. F. Edmonson, D. H. Maul, S. P. O'Neil, S. P. Mossman, C. Thiriart, L. Fabry, O. Van Opstal, C. Bruck, and F. Bex. 1994. Incomplete protection, but suppression of virus burden, elicited by subunit simian immunodeficiency virus vaccines. J. Virol. 68:1843-1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jones, G. J., P. Von Hoegen, J. Weber, and A. D. Rees. 1999. Immunization with human immunodeficiency virus type 1 rgp120W61D in QS21/MPL adjuvant primes T-cell proliferation and C-C chemokine production to multiple epitopes within variable and conserved domains of gp120W61D. J. Infect. Dis. 179:558-566. [DOI] [PubMed] [Google Scholar]
- 40.Karlsson, G. B., M. Halloran, J. Li, I. W. Park, R. Gomila, K. A. Reimann, M. K. Axthelm, S. A. Iliff, N. L. Letvin, and J. Sodroski. 1997. Characterization of molecularly cloned simian-human immunodeficiency viruses causing rapid CD4+ lymphocyte depletion in rhesus monkeys. J. Virol. 71:4218-4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kent, S. J., A. Zhao, S. J. Best, J. D. Chandler, D. B. Boyle, and I. A. Ramshaw. 1998. Enhanced T-cell immunogenicity and protective efficacy of a human immunodeficiency virus type 1 vaccine regimen consisting of consecutive priming with DNA and boosting with recombinant fowlpox virus. J. Virol. 72:10180-10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kestler, H. W. 3., D. J. Ringler, K. Mori, D. L. Panicali, P. K. Sehgal, M. D. Daniel, and R. C. Desrosiers. 1991. Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell 65:651-662. [DOI] [PubMed] [Google Scholar]
- 43.Kirchhoff, F., T. C. Greenough, D. B. Brettler, J. L. Sullivan, and R. C. Desrosiers. 1995. Absence of intact nef sequences in a long-term survivor with nonprogressive HIV-1 infection. N. Engl. J. Med. 332:228-232. [DOI] [PubMed] [Google Scholar]
- 44.Knapp, L. A., E. Lehmann, M. S. Piekarczyk, J. A. Urvater, and D. I. Watkins. 1997. A high frequency of Mamu-A*01 in the rhesus macaque detected by polymerase chain reaction with sequence-specific primers and direct sequencing. Tissue Antigens 50:657-661. [DOI] [PubMed] [Google Scholar]
- 45.Learmont, J. C., A. F. Geczy, J. Mills, L. J. Ashton, C. H. Raynes-Greenow, R. J. Garsia, W. B. Dyer, L. McIntyre, R. B. Oelrichs, D. I. Rhodes, N. J. Deacon, J. S. Sullivan, D. A. McPhee, S. Crowe, A. E. Solomon, C. Chatfield, S. Blasdall, and H. Kuipers. 1999. Immunologic and virologic status after 14 to 18 years of infection with an attenuated strain of HIV-1. A report from the Sydney Blood Bank Cohort. N. Engl. J. Med. 340:1715-1722. [DOI] [PubMed] [Google Scholar]
- 46.Li, C. J., D. J. Friedman, C. Wang, V. Metelev, and A. B. Pardee. 1995. Induction of apoptosis in uninfected lymphocytes by HIV-1 Tat protein. Science 268:429-431. [DOI] [PubMed] [Google Scholar]
- 47.Lifson, J. D., J. L. Rossio, M. Piatak, Jr., T. Parks, L. Li, R. Kiser, V. Coalter, B. Fisher, B. M. Flynn, S. Czajak, V. M. Hirsch, K. A. Reimann, J. E. Schmitz, J. Ghrayeb, N. Bischofberger, M. A. Nowak, R. C. Desrosiers, and D. Wodarz. 2001. Role of CD8+ lymphocytes in control of simian immunodeficiency virus infection and resistance to rechallenge after transient early antiretroviral treatment. J. Virol. 75:10187-10199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lüke, W., C. Coulibaly, U. Dittmer, G. Voss, R. Oesterle, B. Makoschey, U. Sauermann, E. Jurkiewicz, C. Stahl-Henning, H. Petry, and G. Hunsmann. 1996. Simian immunodeficiency virus (SIV) gp130 oligomers protect rhesus macaques (Macaca mulatta) against the infection with SIVmac32H grown on T-cells or derived ex vivo. Virology 216:444-450. [DOI] [PubMed] [Google Scholar]
- 49.Mascola, J. R., M. G. Lewis, G. Stiegler, D. Harris, T. C. VanCott, D. Hayes, M. K. Louder, C. R. Brown, C. V. Sapan, S. S. Frankel, Y. Lu, M. L. Robb, H. Katinger, and D. L. Birx. 1999. Protection of macaques against pathogenic simian/human immunodeficiency virus 89.6PD by passive transfer of neutralizing antibodies. J. Virol. 73:4009-4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mascola, J. R., G. Stiegler, T. C. VanCott, H. Katinger, C. B. Carpenter, C. E. Hanson, H. Beary, D. Hayes, S. S. Frankel, D. L. Birx, and M. G. Lewis. 2000. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat. Med. 6:207-210. [DOI] [PubMed] [Google Scholar]
- 51.Matano, T., M. Kano, H. Nakamura, A. Takeda, and Y. Nagai. 2001. Rapid appearance of secondary immune responses and protection from acute CD4 depletion after a highly pathogenic immunodeficiency virus challenge in macaques vaccinated with a DNA prime/Sendai virus vector boost regimen. J. Virol. 75:11891-11896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McCluskie, M. J., and H. L. Davis. 2000. Oral, intrarectal and intranasal immunizations with CpG and non-CpG oligodeoxynucleotides as adjuvants. Vaccine 19:413-422. [DOI] [PubMed] [Google Scholar]
- 53.McCormack, S., A. Tilzey, A. Carmichael, F. Gotch, J. Kepple, A. Newberry, G. Jones, S. Lister, S. Beddows, R. Cheingsong, A. Rees, A. Babiker, J. Banatvala, C. Bruck, J. Darbyshire, D. Tyrrell, C. Van Hoecke, and J. Weber. 2000. A phase I trial in HIV negative healthy volunteers evaluating the effect of potent adjuvants on immunogenicity of a recombinant gp120W61D derived from dual tropic R5X4 HIV-1ACH320. Vaccine 18:1166-1177. [DOI] [PubMed] [Google Scholar]
- 54.Mooij, P., W. M. J. M. Bogers, H. Oostermeijer, W. Koornstra, P. J. F. Ten Haaft, B. E. Verstrepen, G. Van Der Auwera, and J. L. Heeney. 2000. Evidence for viral virulence as a predominant factor limiting human immunodeficiency virus vaccine efficacy. J. Virol. 74:4017-4027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mooij, P., and J. L. Heeney. 2001. Rational development of prophylactic HIV vaccines based on structural and regulatory proteins. Vaccine 20:304-321. [DOI] [PubMed] [Google Scholar]
- 56.Mooij, P., M. van der Kolk, W. M. J. M. Bogers, P. J. F. Ten Haaft, P. Van Der Meide, N. Almond, J. Stott, M. Deschamps, D. Labbe, P. Momin, G. Voss, P. Von Hoegen, C. Bruck, and J. L. Heeney. 1998. A clinically relevant HIV-1 subunit vaccine protects rhesus macaques from in vivo passaged simian-human immunodeficiency virus infection. AIDS 12:F15-F22. [DOI] [PubMed] [Google Scholar]
- 57.Mossman, S. P., F. Bex, P. Berglund, J. Arthos, S. P. O'Neil, D. Riley, D. H. Maul, C. Bruck, P. Momin, A. Burny, P. N. Fultz, J. I. Mullins, P. Liljestrom, and E. A. Hoover. 1996. Protection against lethal simian immunodeficiency virus SIVsmmPBj14 disease by a recombinant Semliki Forest virus gp160 vaccine and by a gp120 subunit vaccine. J. Virol. 70:1953-1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Murphy, C. G., W. T. Lucas, R. E. Means, S. Czajak, C. L. Hale, J. D. Lifson, A. Kaur, R. P. Johnson, D. M. Knipe, and R. C. Desrosiers. 2000. Vaccine protection against simian immunodeficiency virus by recombinant strains of herpes simplex virus. J. Virol. 74:7745-7754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Osterhaus, A. D. M. E., C. A. van Baalen, R. A. Gruters, M. Schutten, C. H. J. Siebelink, E. G. J. Hulskotte, E. J. Tijhaar, R. E. R. Randall, G. van Amerongen, A. Fleuchaus, V. Erfle, and G. Sutter. 1999. Vaccination with Rev and Tat against AIDS. Vaccine 17:2713-2714. [DOI] [PubMed] [Google Scholar]
- 60.Ourmanov, I., M. Bilska, V. M. Hirsch, and D. C. Montefiori. 2000. Recombinant modified vaccinia virus Ankara expressing the surface gp120 of simian immunodeficiency virus (SIV) primes for a rapid neutralizing antibody response to SIV infection in macaques. J. Virol. 74:2960-2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ourmanov, I., C. R. Brown, B. Moss, M. Carroll, L. Wyatt, L. Pletneva, S. Goldstein, D. Venzon, and V. M. Hirsch. 2000. Comparative efficacy of recombinant modified vaccinia virus Ankara expressing simian immunodeficiency virus (SIV) Gag-Pol and/or Env in macaques challenged with pathogenic SIV. J. Virol. 74:2740-2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pal, R., D. Venzon, N. L. Letvin, S. Santra, D. C. Montefiori, N. R. Miller, E. Tryniszewska, M. G. Lewis, T. C. VanCott, V. Hirsch, R. Woodward, A. Gibson, M. Grace, E. Dobratz, P. D. Markham, Z. Hel, J. Nacsa, M. Klein, J. Tartaglia, and G. Franchini. 2002. ALVAC-SIV-gag-pol-env-based vaccination and macaque major histocompatibility complex class I (A*01) delay simian immunodeficiency virus SIVmac-induced immunodeficiency. J. Virol. 76:292-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pauza, C. D., P. Trivedi, M. Wallace, T. J. Ruckwardt, H. Le Buanec, W. Lu, B. Bizzini, A. Burny, D. Zagury, and R. C. Gallo. 2000. Vaccination with Tat toxoid attenuates disease in simian/HIV-challenged macaques. Proc. Natl. Acad. Sci. USA 97:3515-3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Petry, H., U. Dittmer, D. Jones, G. Farrar, H. Wachter, D. Fuchs, T. Nisslein, E. Jurkiewicz, G. Hunsmann, C. Stahl-Hennig, and W. Lüke. 1998. Prechallenge high neutralizing antibodies and long-lasting immune reactivity to gp41 correlate with protection of rhesus monkeys against productive simian immunodeficiency virus infection or disease development. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 19:441-450. [DOI] [PubMed] [Google Scholar]
- 65.Piguet, V., O. Schwartz, S. Le Gall, and D. Trono. 1999. The downregulation of CD4 and MHC-I by primate lentiviruses: a paradigm for the modulation of cell surface receptors. Immunol. Rev. 168:51-63. [DOI] [PubMed] [Google Scholar]
- 66.Regier, D. A., and R. C. Desrosiers. 1990. The complete nucleotide sequence of a pathogenic molecular clone of simian immunodeficiency virus. AIDS Res. Hum. Retroviruses 6:1221-1231. [DOI] [PubMed] [Google Scholar]
- 67.Reimann, K. A., J. T. Li, R. Veazey, M. Halloran, I. W. Park, G. B. Karlsson, J. Sodroski, and N. L. Letvin. 1996. A chimeric simian/human immunodeficiency virus expressing a primary patient human immunodeficiency virus type 1 isolate env causes an AIDS-like disease after in vivo passage in rhesus monkeys. J. Virol. 70:6922-6928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Riviere, Y., M. N. Robertson, and F. Buseyne. 1994. Cytotoxic T lymphocytes in human immunodeficiency virus infection: regulator genes. Curr. Top. Microbiol. Immunol. 189:65-74. [DOI] [PubMed] [Google Scholar]
- 69.Rose, N. F., P. A. Marx, A. Luckay, D. F. Nixon, W. J. Moretto, S. M. Donahoe, D. Montefiori, A. Roberts, L. Buonocore, and J. K. Rose. 2001. An effective AIDS vaccine based on live attenuated vesicular stomatitis virus recombinants. Cell 106:539-549. [DOI] [PubMed] [Google Scholar]
- 70.Schmitz, J. E., M. J. Kuroda, S. Santra, V. G. Sasseville, M. A. Simon, M. A. Lifton, P. Racz, K. Tenner-Racz, M. Dalesandro, B. J. Scallon, J. Ghrayeb, M. A. Forman, D. C. Montefiori, E. P. Rieber, N. L. Letvin, and K. A. Reimann. 1999. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 283:857-860. [DOI] [PubMed] [Google Scholar]
- 71.Seth, A., I. Ourmanov, J. E. Schmitz, M. J. Kuroda, M. A. Lifton, C. E. Nickerson, L. Wyatt, M. Carroll, B. Moss, D. Venzon, N. L. Letvin, and V. M. Hirsch. 2000. Immunization with a modified vaccinia virus expressing simian immunodeficiency virus (SIV) Gag-Pol primes for an anamnestic Gag-specific cytotoxic T-lymphocyte response and is associated with reduction of viremia after SIV challenge. J. Virol. 74:2502-2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shiver, J. W., T.-M. Fu, L. Chen, D. R. Casimiro, M.-E. Davies, R. K. Evans, Z.-Q. Zhang, A. J. Simon, W. L. Trigona, S. A. Dubey, L. Huang, V. A. Harris, R. S. Long, X. Liang, L. Handt, W. A. Schleif, L. Zhu, D. C. Freed, N. V. Persaud, L. Guan, K. S. Punt, A. Tang, M. Chen, K. A. Wilson, K. B. Collins, G. J. Heidecker, V. R. Fernandez, H. C. Perry, J. G. Joyce, K. M. Grimm, J. C. Cook, P. M. Keller, D. S. Kresock, H. Mach, R. D. Troutman, L. A. Isopi, D. M. Williams, Z. Xu, K. E. Bohannon, D. B. Volkin, D. C. Montefiori, A. Miura, G. R. Krivulka, M. A. Lifton, M. J. Kuroda, J. E. Schmitz, N. L. Letvin, M. J. Caulfield, A. J. Bett, R. Youil, D. C. Kaslow, and E. A. Emini. 2002. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature 415:331-335. [DOI] [PubMed] [Google Scholar]
- 73.Silvera, P., M. W. Richardson, J. Greenhouse, J. Yalley-Ogunro, N. Shaw, J. Mirchandani, K. Khalili, J.-F. Zagury, M. G. Lewis, and J. Rappaport. 2002. Outcome of simian-human immunodeficiency virus strain 89.6p challenge following vaccination of rhesus macaques with human immunodeficiency virus Tat protein. J. Virol. 76:3800-3809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stahl-Hennig, C., C. Coulibaly, H. Petry, G. Voss, U. Dittmer, W. Bodemer, B. Makoschey, E. Jurkiewicz, W. Luke, and G. Hunsmann. 1994. Immunization with virion-derived glycoprotein 130 from HIV-2 or SIV protects macaques against challenge virus grown in human or simian cells or prepared ex vivo. AIDS Res. Hum. Retroviruses 10:S27-S32. [PubMed] [Google Scholar]
- 75.Stott, E. J., N. Almond, K. Kent, B. Walker, R. Hull, J. Rose, P. Silvera, R. Sangster, T. Corcoran, J. Lines, K. Silvera, P. Luciw, M. Murphy-Corb, P. Momin, and C. Bruck. 1998. Evaluation of a candidate human immunodeficiency virus type 1 (HIV-1) vaccine in macaques: effect of vaccination with HIV-1 gp120 on subsequent challenge with heterologous simian immunodeficiency virus-HIV-1 chimeric virus. J. Gen. Virol. 79:423-432. [DOI] [PubMed] [Google Scholar]
- 76.Stoute, J. A., M. Slaoui, D. G. Heppner, P. Momin, K. E. Kester, P. Desmons, B. T. Wellde, N. Garçon, U. Krzych, M. Marchand, W. R. Ballou, and J. D. Cohen. 1997. A preliminary evaluation of a recombinant circumsporozoite protein vaccine against Plasmodium falciparum malaria. N. Engl. J. Med. 336:86-91. [DOI] [PubMed] [Google Scholar]
- 77.van Baalen, C. A., O. Pontesilli, R. C. Huisman, A. M. Geretti, M. R. Klein, F. de Wolf, F. Miedema, R. A. Gruters, and A. D. M. E. Osterhaus. 1997. Human immunodeficiency virus type 1 Rev- and Tat-specific cytotoxic T lymphocyte frequencies inversely correlate with rapid progression to AIDS. J. Gen. Virol. 78:1913-1918. [DOI] [PubMed] [Google Scholar]
- 78.Wain-Hobson, S., P. Sonigo, O. Danos, S. Cole, and M. Alizon. 1985. Nucleotide sequence of the AIDS virus, LAV. Cell 40:9-17. [DOI] [PubMed] [Google Scholar]
- 79.Westendorp, M. O., R. Frank, C. Ochsenbauer, K. Stricker, J. Dhein, H. Walczak, K.-M. Debatin, and P. H. Krammer. 1995. Sensitization of T-cells to CD95-mediated apoptosis by HIV-1 Tat and gp120. Nature 375:497-500. [DOI] [PubMed] [Google Scholar]
- 80.Wyand, M. S., K. H. Manson, M. Garcia-Moll, D. Montefiori, and R. C. Desrosiers. 1996. Vaccine protection by a triple deletion mutant of simian immunodeficiency virus. J. Virol. 70:3724-3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zagury, D., A. Lachgar, V. Chams, L. S. Fall, J. Bernard, J.-F. Zagury, B. Bizzini, A. Gringeri, E. Santagostino, J. Rappaport, M. Feldman, A. Burny, and R. C. Gallo. 1998. Interferon α and Tat involvement in the immunosuppression of uninfected T-cells and C-C chemokine decline in AIDS. Proc. Natl. Acad. Sci. USA 95:3851-3856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zagury, J. F., A. Sill, W. Blattner, A. Lachgar, H. Le Buanec, M. Richardson, J. Rappaport, H. Hendel, B. Bizzini, A. Gringeri, M. Carcagno, M. Criscuolo, A. Burny, R. C. Gallo, and D. Zagury. 1998. Antibodies to the HIV-1 Tat protein correlated with nonprogression to AIDS: a rationale for the use of Tat toxoid as an HIV-1 vaccine. J. Hum. Virol. 1:282-292. [PubMed] [Google Scholar]