Abstract
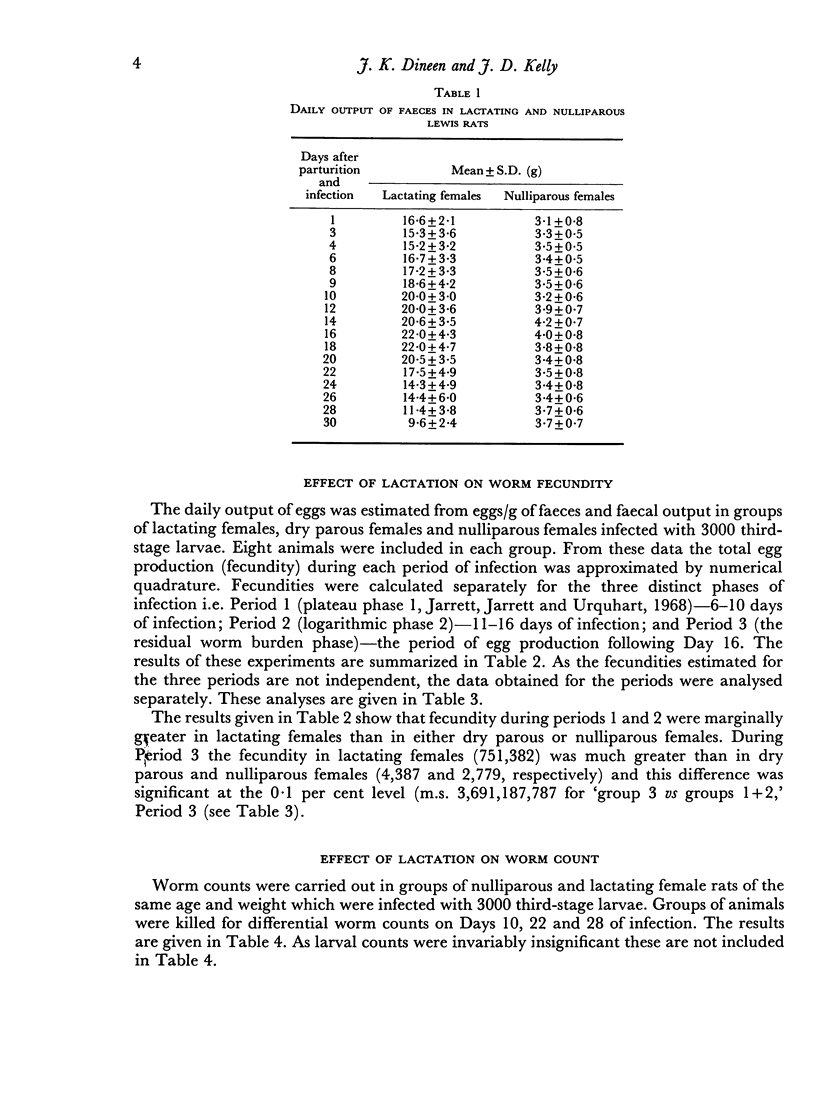
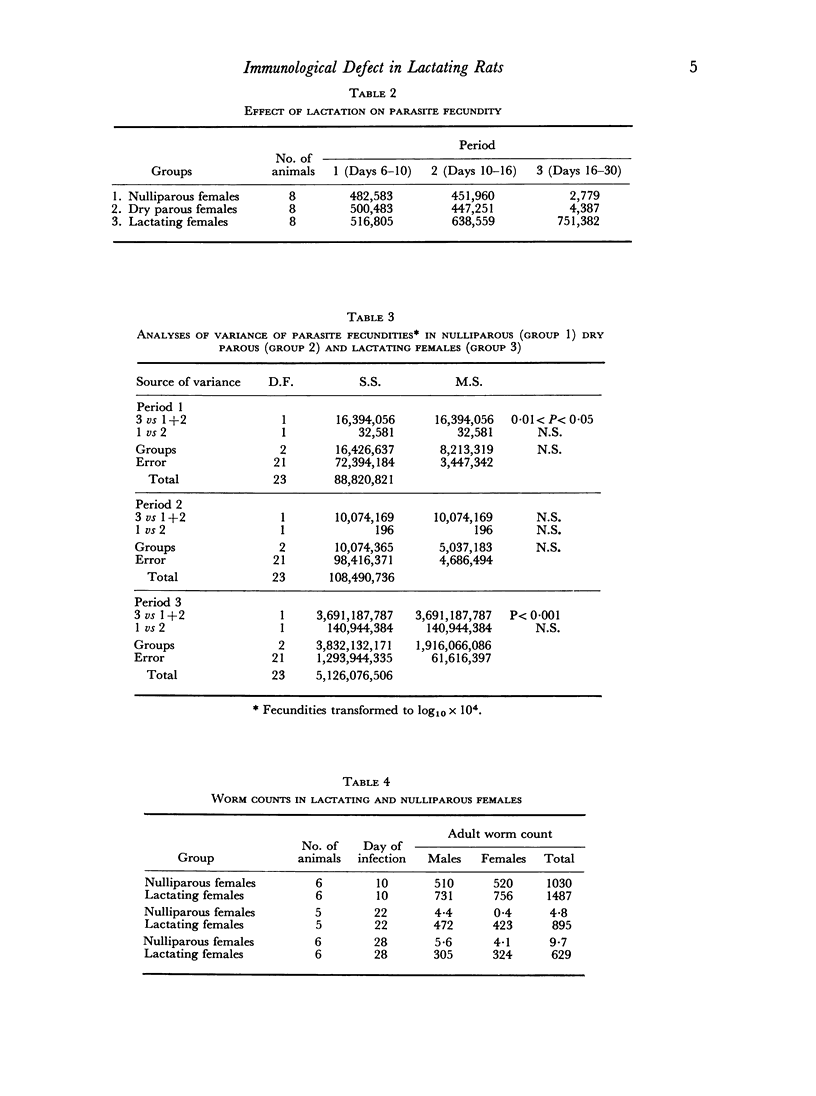
Lactating female rats infected with 3000 third-stage larvae of Nippostrongylus brasiliensis showed significant increases in worm fecundity and total worm burdens when compared with infected nulliparous controls. Statistically significant differences were recorded for each of the three periods of infection, although these differences were of greatest magnitude during Period 3 (16–30 days of infection).
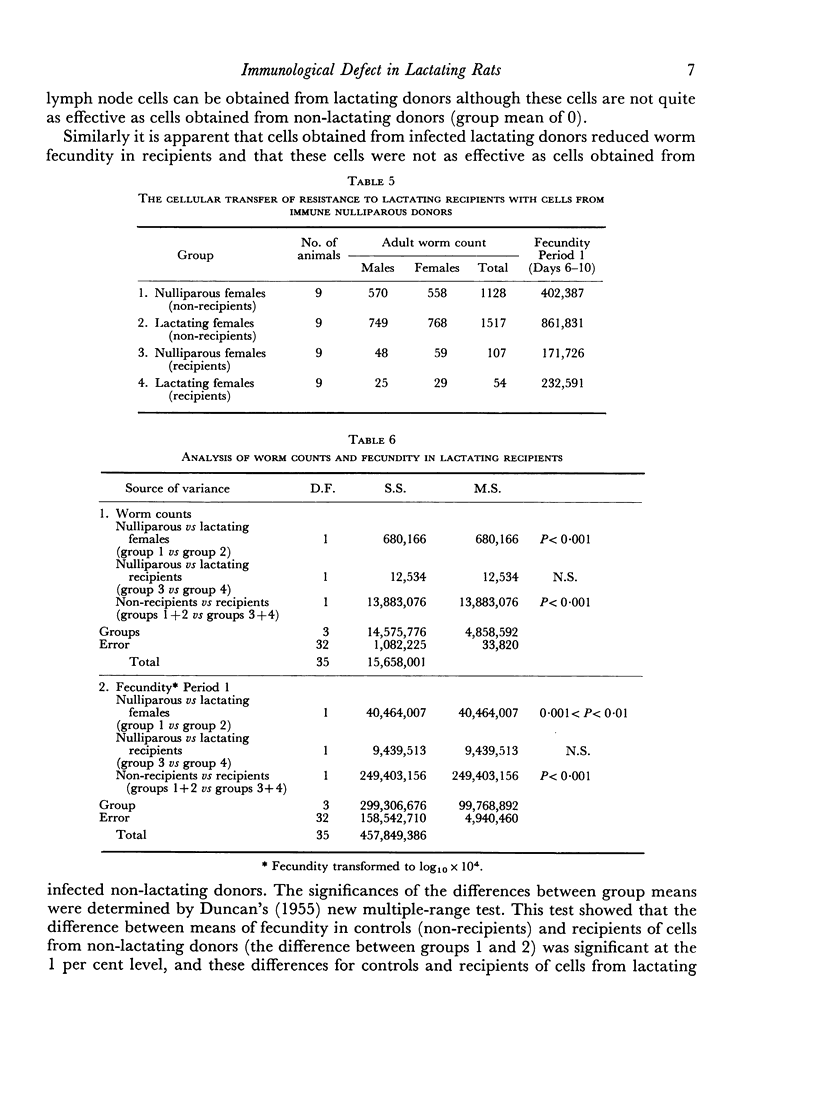
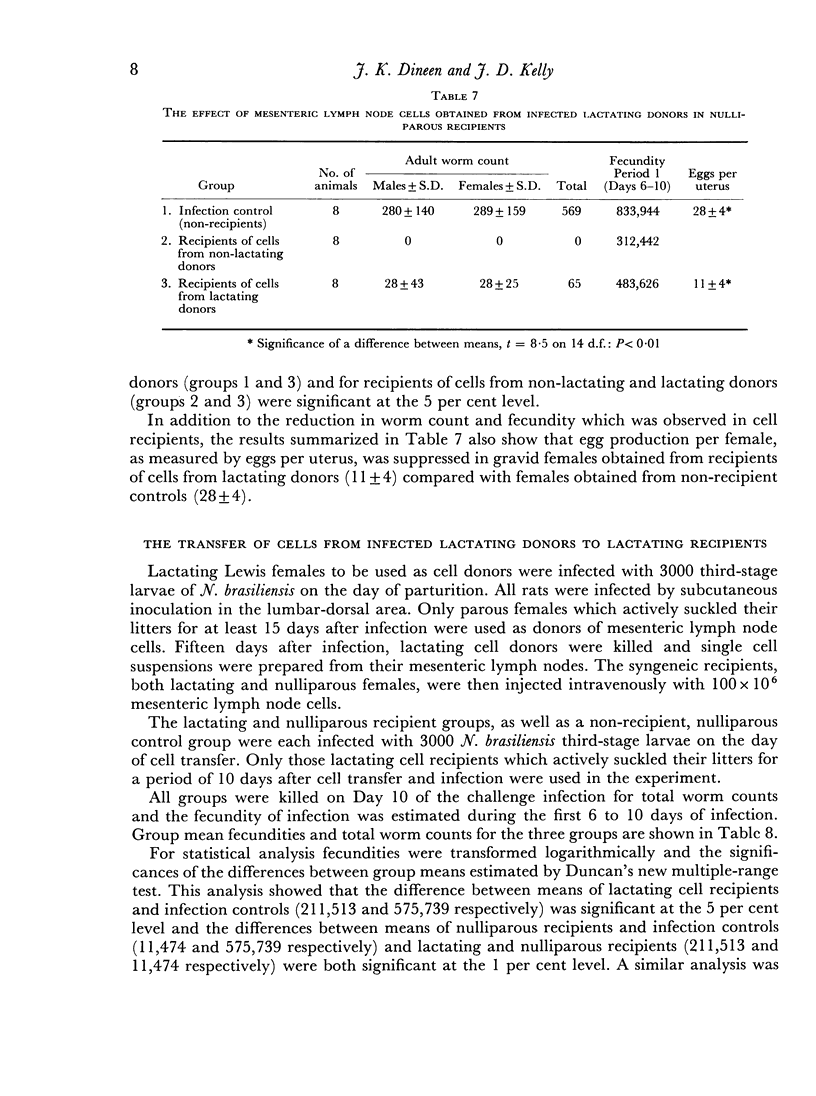
Immune mesenteric lymph node cells (100 × 106), obtained from nulliparous female donors on Day 15 of a primary infection, were transferred syngeneically to lactating female recipients. The transferred cells invariably caused suppression of worm fecundity, reduction in the number of eggs per uterus in gravid female worms and rejection of a substantial proportion of worms by Day 10 of a challenge infection in the lactating recipients. The results of this study showed that immune cells were functional in lactating female recipients and that transfer of immune cells repaired the deficit in the rejection mechanism.
Mesenteric lymph node cells (100 × 106), obtained from lactating female donors on Day 15 of a primary infection, were transferred syngeneically to nulliparous female recipients. The transferred cells caused suppression of worm fecundity, reduction in the number of eggs per uterus in gravid female worms and rejection of the majority of parasites by Day 10 of a challenge infection in the nulliparous recipients. Clearly, potentially immune lymphoid cells were present in the mesenteric nodes of lactating females at the time that the rejection mechanism was severely impaired.
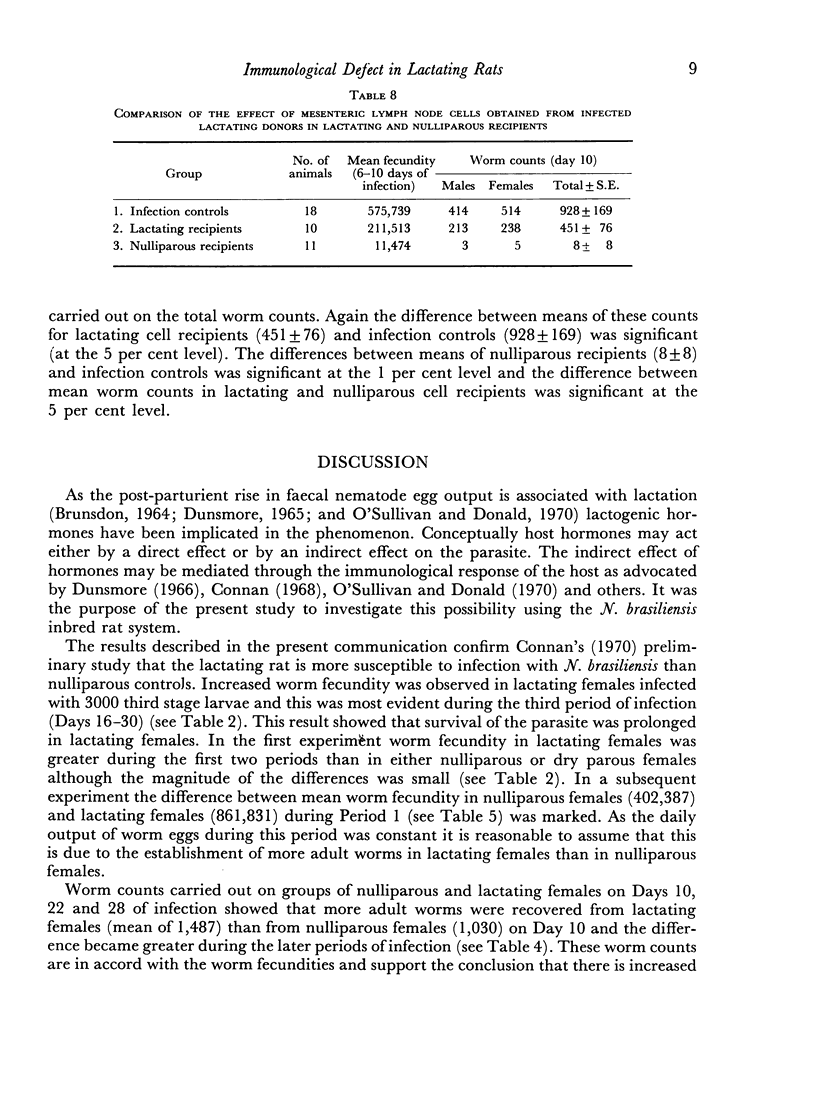
Mesenteric lymph node cells obtained from infected lactating donors were substantially less effective in lactating recipients than in nulliparous recipients. These cells caused the expulsion of 51 per cent of worms by Day 10 in lactating recipients, whereas they caused expulsion of 99 per cent of worms in nulliparous recipients.
These observations suggest that the inductive processes of the immune response occur normally, but that differentiation of induced cells to effector cells is impaired in lactating animals.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brunsdon R. V. The seasonal variations in the nematode EGG counts of sheep: a comparison of the spring rise phenomenon in breeding and unmated ewes. N Z Vet J. 1964 Aug;12(4):75–80. doi: 10.1080/00480169.1964.33556. [DOI] [PubMed] [Google Scholar]
- Brunsdon R. V. The spring-rise phenomenon: seasonal changes in the worm burdens of breeding ewes and in the availability of pasture infection. N Z Vet J. 1970 Apr;18(4):47–54. doi: 10.1080/00480169.1970.33861. [DOI] [PubMed] [Google Scholar]
- CROFTON H. D. Nematode parasite populations in sheep on lowland farms. 1. Worm egg counts in ewes. Parasitology. 1954 Nov;44(3-4):465–477. doi: 10.1017/s0031182000019144. [DOI] [PubMed] [Google Scholar]
- CROFTON H. D. Nematode parasite populations in sheep on lowland farms. V. Further observations on the post-parturient rise and a discussion of its significance. Parasitology. 1958 Nov;48(3-4):243–250. doi: 10.1017/s0031182000021211. [DOI] [PubMed] [Google Scholar]
- Connan R. M. Observations on the epidemiology of parasitic gastro-enteritis due to Oesophagostomum spp. and Hyostrongylus rubidus in the pig. Vet Rec. 1967 Apr 8;80(14):424–429. doi: 10.1136/vr.80.14.424. [DOI] [PubMed] [Google Scholar]
- Connan R. M. Observations on the post-parturient rise in the faecal nematode egg count of ewes. Vet Rec. 1967 Apr 1;80(13):401–405. doi: 10.1136/vr.80.13.401. [DOI] [PubMed] [Google Scholar]
- Dunsmore J. D. Influence of host reproduction on numbers of trichostrongylid nematodes in the European rabbit, Oryctolagus cuniculus (L.). J Parasitol. 1966 Dec;52(6):1129–1133. [PubMed] [Google Scholar]
- Dunsmore J. D. Ostertagia spp. in lambs and pregnant ewes. J Helminthol. 1965;39(2):159–184. doi: 10.1017/s0022149x00020575. [DOI] [PubMed] [Google Scholar]
- Gibbs H. C. Observations on an Outbreak of Clinical Parasitism in Ewes During the Winter Months. Can Vet J. 1964 Jan;5(1):8–11. [PMC free article] [PubMed] [Google Scholar]
- Jarrett E. E., Jarrett W. F., Urquhart G. M. Quantitative studies on the kinetics of establishment and expulsion of intestinal nematode populations in susceptible and immune hosts. Nippostrongylus brasiliensis in the rat. Parasitology. 1968 Aug;58(3):625–639. doi: 10.1017/s0031182000028924. [DOI] [PubMed] [Google Scholar]
- Keller R. Immune reactions to Nippostrongylus brasiliensis in the rat. I. Characteristics of primary and secondary immune response in vivo. Int Arch Allergy Appl Immunol. 1970;37(2):197–215. doi: 10.1159/000230233. [DOI] [PubMed] [Google Scholar]
- Keller R. On the mechanism of expulsion of helminths. Clin Exp Immunol. 1970 Feb;6(2):207–210. [PMC free article] [PubMed] [Google Scholar]
- Kelly J. D., Dineen J. K. The cellular transfer to immunity to Nippostrongylus brasiliensis in inbred rats (Lewis strain). Immunology. 1972 Feb;22(2):199–210. [PMC free article] [PubMed] [Google Scholar]
- O'Sullivan B. M., Donald A. D. A field study of nematode parasite populations in the lactating ewe. Parasitology. 1970 Oct;61(2):301–315. doi: 10.1017/s0031182000041135. [DOI] [PubMed] [Google Scholar]
- Ogilvie B. M., Hockley D. J. Effects of immunity of Nippostrongylus brasiliensis adult worms: reversible and irreversible changes in infectivity, reproduction, and morphology. J Parasitol. 1968 Dec;54(6):1073–1084. [PubMed] [Google Scholar]
- PIKE R. L., SUDER H. B., ROSS M. L. The influence of protein and energy intakes upon nitrogen retention in the pregnant rat. J Nutr. 1954 Feb 10;52(2):297–309. doi: 10.1093/jn/52.2.297. [DOI] [PubMed] [Google Scholar]
- Salisbury J. R., Arundel J. H. The relationship between lactation and post-parturient rise in faecal nematode egg counts of ewes. Aust Vet J. 1970 Jun;46(6):267–271. doi: 10.1111/j.1751-0813.1970.tb15775.x. [DOI] [PubMed] [Google Scholar]
- Urquhart G. M., Mulligan W., Eadie R. M., Jennings F. W. Immunological studies on Nippostrongylus brasiliensis infection in the rat: the role of local anaphylaxis. Exp Parasitol. 1965 Oct;17(2):210–217. doi: 10.1016/0014-4894(65)90023-8. [DOI] [PubMed] [Google Scholar]


