Abstract
A hydrophilic region consisting of strikingly clustered charged amino acids is present at the center of human immunodeficiency virus type 1 (HIV-1) Vif. In this study, the role for this central hydrophilic region (E88WRKKR93) in the virus replication in nonpermissive H9 cells was investigated by extensive deletion and substitution analysis. A total of 31 mutants were constructed. Deletion of the E88 or W89 residue alone abolished viral infectivity in H9 cells and impaired virus replication in primary macrophage cultures. Substitution analysis indicated that the hydrophilicity and charge of the central region are insignificant for the function of Vif. Of the 16 substitution mutants, 3 mutants with substitution of E88 and W89 with an A residue did not grow in H9 cells. Upon transfection, four mutants (i.e., two mutants with deletion of E88 or W89; a mutant with substitution of E88 and W89 with A; and a mutant with substitution of E88, W89, and R90 with A) were found to express Vif at a very reduced level relative to that by the wild-type clone. These results have thus demonstrated that amino acid residues 88 and 89 of Vif are critical for the replication of HIV-1 in target cells by enhancing the steady-state expression of Vif. In addition, E88 and W89 residues were found to be extremely conserved among the Vif proteins of naturally occurring HIV-1 field isolates as well as those of laboratory HIV-1 strains.
Vif is one of the human immunodeficiency virus type 1 (HIV-1) accessory proteins, but is conserved in the primate lentivirus groups (29) and is essential for virus replication in a certain type of cells (9, 10, 22). It acts during the stage of assembly, budding, or maturation to augment the infectivity of progeny virions in a producer cell-dependent manner (6, 8, 12, 17, 31, 34, 38). Producer cells are therefore divided into permissive and nonpermissive, and HIV-1 grown in nonpermissive cells, such as H9 (27) and peripheral blood mononuclear cells (PBMCs), in the absence of Vif cannot replicate in any type of target cells. Recent evidence has demonstrated that vif-deficient HIV-1 is impaired in endogenous reverse transcription and in its ability to form proviral DNA in newly infected cells (20, 34, 36, 38). HIV-1 Vif is a highly basic, 23-kDa protein composed of approximately 190 amino acids (HIV Sequence Compendium 2000 [http://hiv-web.lanl.gov]) that is synthesized at a late phase of virus replication. The basic residues are predominantly present in the N- and C-terminal regions. At the center of HIV-1 Vif, however, there is a striking cluster of basic and acidic residues. This major hydrophilic region consists of six amino acids and contains four basic residues and one acidic residue in the case of laboratory HIV-1 strains (HIV Sequence Compendium 2000). Another feature of HIV-1 Vif is that it contains two cysteine residues. These are conserved among HIV-1, HIV-2, and some simian immunodeficiency virus isolates (HIV Sequence Compendium 2000).
Quite surprisingly, extensive systemic studies on the structure-function relationship of HIV-1 Vif to identify functional domains, motifs, and residues have not been amply carried out. Some studies described below have demonstrated the regions or amino acid residues important for the Vif function. It has been shown by scanning mutagenesis of HIV-1 Vif that there could be an effector domain in the internal region containing the two cysteine residues (35), although the characterizations of many deletion, insertion, and substitution mutants have indicated that amino acids dispersed throughout Vif are important for function (14, 32, 35). The two cysteine residues have been reported to be critical for the replication of HIV-1 in nonpermissive cells (14, 15, 26, 32). Deletion analysis has shown that HIV-1 Vif is packaged into a nucleoprotein complex through an interaction of its central region with viral genomic RNA and suggested that the incorporation of Vif into virions is important for its function (24). It has been demonstrated by mutational analysis that the C-terminal region of Vif is important for its function (19, 21), which appears to be inconsistent with the results of the others (13, 30, 32). Another report has shown that deletion of C-terminal region of Vif abolishes its interaction with Gag precursor (7). To the best of our knowledge, an extensive structure-function analysis of the central hydrophilic region of HIV-1 Vif (E88WRKKR93) has not yet been described. In this study, we systematically analyzed the functional significance of the region. Various deletions and substitutions were introduced into the pNL432 (1) vif gene to generate mutants of the region. The proviral mutants constructed were then examined for virus replication in various target cells and for the expression of Vif. We demonstrate here that amino acid residues 88 and 89 of HIV-1 Vif are important for virus replication in H9 cells and monocyte-derived macrophages (MDMs). We also show that these two residues are critical for steady-state expression of Vif.
Generation and characterization of various mutants of the HIV-1 Vif hydrophilic region.
As shown in Table 1, to delineate the appreciate amino acid residues in the central hydrophilic region crucial for the Vif function in T-lymphocytic cells and MDMs, 15 deletion and 16 substitution mutants were designed and constructed from wild-type pNL432 (1) with a QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, Calif.). Construction of the deletion mutants, which lack one of the six amino acids in the hydrophilic region, aimed at direct evaluation of the importance of the amino acid in question. To obtain more drastic mutational effects, mutants with deletion of three amino acids were also constructed. Substitution mutants in the present study were designed to change authentic amino acids into those with distinct or similar biophysical characteristics, such as hydrophilicity and charge. Drastic changes could be introduced into the hydrophilic region by alanine, arginine, and glutamic acid substitutions. By alanine substitution, both hydrophilicity and charge were lost. Only charge was changed by substitution of arginine or lysine with glutamic acid and by substitution of glutamic acid with arginine.
TABLE 1.
Deletion and substitution mutants of HIV-1 vif gene used in this study
| Clone | Sequence (amino acids 88-93 of Vif)a | Growth inb:
|
Expressionc | ||
|---|---|---|---|---|---|
| M8166 | H9 | MDM | |||
| NL432 (WT) | EWRKKR | + | + | − | WT |
| NL-Nd (Δ Vif) | ∗∗∗∗∗∗ | + | − | − | |
| NL-fE88del | ∗WRKKR | + | − | Low | |
| NL-fW89del | E∗RKKR | + | − | Low | |
| NL-fR90del | EW∗KKR | + | + | WT | |
| NL-fK91del | EWR∗KR | + | + | WT | |
| NL-fK92del | EWRK∗R | + | + | WT | |
| NL-fR93del | EWRKK∗ | + | + | WT | |
| NL-f90/3del | EW∗∗∗R | + | − | WT | |
| NL-f91/3del | EWR∗∗∗ | + | − | WT | |
| NF462 (WT) | EWRKKR | − | − | + | |
| NF-Nd (Δ Vif) | ∗∗∗∗∗∗ | − | |||
| NF-fE88del | ∗WRKKR | − | |||
| NF-fW89del | E∗RKKR | − | |||
| NF-fR90del | EW∗KKR | + | |||
| NF-fK91del | EWR∗KR | + | |||
| NF-fK92del | EWRK∗R | + | |||
| NF-fR93del | EWRKK∗ | + | |||
| NF-f90/3del | EW∗∗∗R | − | |||
| NL-fE88A | AWRKKR | + | + | WT | |
| NL-fE88R | RWRKKR | + | + | WT | |
| NL-fE88D | DWRKKR | + | + | WT | |
| NL-fW89A | EARKKR | + | + | WT | |
| NL-fW89Y | EYRKKR | + | + | WT | |
| NL-f88/2A | AARKKR | + | − | Low | |
| NL-f88/3A | AAAKKR | + | − | Low | |
| NL-fE88R/R90E | RWEKKR | + | + | WT | |
| NL-f90/3A | EWAAAR | + | + | WT | |
| NL-f90/3E | EWEEER | + | + | WT | |
| NL-f91/3A | EWRAAA | + | + | WT | |
| NL-f91/3E | EWREEE | + | + | WT | |
| NL-f90/4A | EWAAAA | + | + | WT | |
| NL-f90/4E | EWEEEE | + | + | WT | |
| NL-f88/6A | AAAAAA | + | − | WT | |
| NL-fE88R/90/4E | RWEEEE | + | + | WT | |
The amino acid sequence of NL432 Vif is from the GenBank database (accession no. M19921). Clone pNF462 was constructed from pNL432 (23). Generation and characterization of Δ Vif of NL432 and NF462 have been previously described (2, 23). Asterisks indicate deletion of amino acids.
+, virus replication comparable to that of wild-type virus; −, no or poor virus replication similar to that of ΔVif. Blank spaces indicate that virus replication was not monitored.
WT, wild-type level of Vif expression in transfected 293T cells; Low, low level of expression relative to that by the wild-type clone; −, no expression. Blank spaces indicate that the expression level of Vif was not determined.
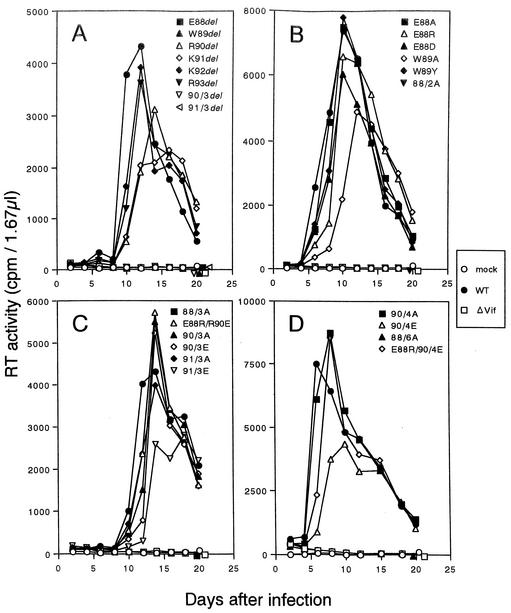
The mutants thus generated were examined for their ability to grow, first in M8166 (33) cells and then in H9 cells nonpermissive for vif-negative mutants (ΔVif). Input cell-free mutant viruses for infection were prepared from 293T (25) cells transfected with various proviral clones (Table 1) by the calcium-phosphate coprecipitation method (1). As shown in Table 1, wild-type and mutant viruses grew similarly well in M8166 cells. In sharp contrast, in H9 cells, some mutant viruses did not grow at all like ΔVif virus. As clearly observed in Fig. 1 and summarized in Table 1, the mutants with a growth phenotype similar to that of ΔVif virus included NL-fE88del, -fW89del, -f90/3del, -f91/3del, -f88/2A, -f88/3A, and -f88/6A. Thus, deletion of E88 or W89, but not of the other residues in the hydrophilic region, completely abolished viral infectivity in H9 cells. Deletion of three amino acids in the region also destroyed the ability of the virus to grow. As for substitution mutants, it was necessary to change both E88 and W89 into A residues to inactivate the virus. Alteration of E88 into A, R, or D and alteration of W89 into A or Y were not enough to impair viral infectivity.
FIG. 1.
Growth kinetics in nonpermissive H9 cells of various vif mutants. H9 cells (1 × 106) were infected with equivalent reverse transcriptase (RT) units of cell-free viruses (5 × 107) as previously described (11), and virus replication was monitored at intervals by RT production in the culture supernatants (39). Input viruses were prepared from 293T cells transfected with 20 μg of various pNL clones (Table 1). The results for deletion mutants (A), substitution mutants involving one or two amino acids (B), substitution mutants involving two or three amino acids (C), and substitution mutants of involving four to six amino acids (D) are shown. WT, NL432 (wild type); ΔVif, NL-Nd.
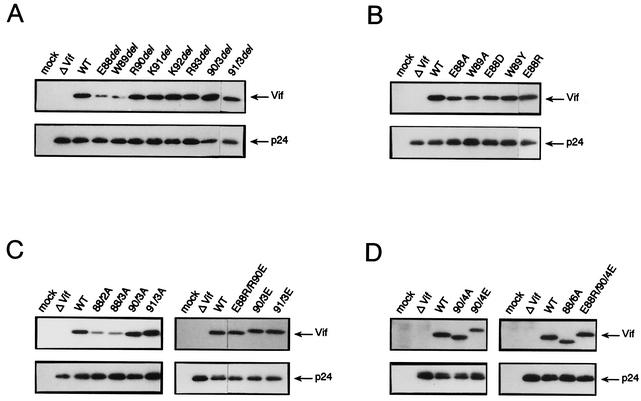
The expression of mutant Vif proteins in cells was monitored by Western blot analysis (3-5, 16, 39). 293T cells were transfected with various proviral clones (Table 1), and 2 days later, cell lysates were prepared for analysis. As shown in Fig. 2, all of the proviral clones tested produced a comparable level of p24gag upon transfection. In contrast, as clearly observed in Fig. 2 and summarized in Table 1, mutant Vif was expressed at a very reduced level in cells transfected with pNL-fE88del, -fW89del, -f88/2A, or -f88/3A. The low level of expression of mutant Vif (NL-fE88del) became normal when transfected 293T cells were cultured in the presence of proteasome inhibitors (M. Fujita and A. Adachi, unpublished results). The reduced level of Vif expression was also observed in H9 cells transfected with pNL-fE88del, -fW89del, or -f88/2A by electroporation (M. Fujita, A. Sakurai, and A. Adachi, unpublished results). Of note here is that NL-fE88del, -fW89del, -f88/2A, and -f88/3A were unable to replicate in H9 cells (Fig. 1). No major reduction in the level of expression of mutant Vif was observed for the other mutants, including pNL-f90/3del, -f91/3del, and -f88/6A, which produce viruses noninfectious for H9 cells (Fig. 1) upon transfection into 293T cells.
FIG. 2.
Monitoring of expression of various mutant Vif proteins by Western blotting. 293T cells were transfected with 20 μg of various pNL clones (Table 1), and 2 days later, cell lysates were prepared as described previously (39). Proteins were resolved on sodium dodecyl sulfate-12.5% polyacrylamide gels, followed by electrophoretic transfer to polyvinylidene fluoride membranes (Immobilon-P, Millipore Co., Bedford, Mass.). The membranes were treated with anti-HIV-1 antibodies as previously described (4, 5) and visualized with an ECL Plus enhanced chemiluminescence Western blotting detection system (Amersham Pharmacia Biotech, Inc., Buckinghamshire, United Kingdom) (3-5, 16). The results for deletion mutants (A), substitution mutants involving one amino acid (B), substitution mutants involving two or three amino acids (C), and substitution mutants involving four to six amino acids (D) are shown in panels A to D, respectively. WT, pNL432 (wild type); ΔVif, pNL-Nd.
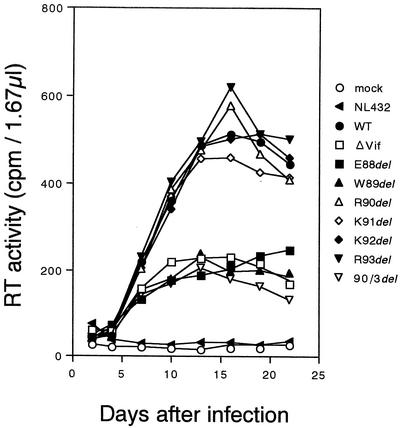
The growth of deletion mutants in human MDMs was monitored to determine whether amino acid residues in the hydrophilic region are important for virus replication in nonlymphocytic cells. To obtain differentiated MDMs for infection, monocytes were separated from PBMCs by adhesion to the plastic essentially as previously described (37) and cultured in RPMI 1640 medium supplemented with 10% heat-inactivated human serum AB (Nabi, Boca Raton, Fla.) for 3 days and then in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum for 7 days in the presence of 5 ng of granulocyte-macrophage colony-stimulating factor (GM-CSF [PeproTech EC, Ltd., London, England]) per ml. For infection of MDMs, macrophage-tropic NF viruses (23) were used. It has been reported that MDMs are nonpermissive for ΔVif (18, 23, 28, 38). In our infection system here (Fig. 3), however, MDMs were semipermissive. While ΔVif did not grow at all in four preparations of peripheral blood lymphocytes, it did at a reduced level relative to that of wild-type virus in four preparations of MDMs (M. Fujita and A. Adachi, unpublished results). Figure 3 shows the growth kinetics in MDMs of various NF deletion mutants (Table 1) with ΔVif as a control. As clearly illustrated and summarized in Table 1, NF-fE88del, -fW89del, and -f90/3del displayed a growth defect similar to that of ΔVif. On the contrary, the other mutant viruses grew normally in MDMs.
FIG.3.
Growth kinetics in MDMs of vif deletion mutants. Human MDMs (2 × 105) in a well of 24-well tissue culture plates were infected with equivalent reverse transcriptase (RT) units of cell-free viruses (1 × 106) in the presence of 5 μg of DEAE-dextran per ml, and virus replication was monitored at intervals by RT production in the culture supernatants (39). MDMs were cultured in the presence of GM-CSF (5 ng/ml) throughout the infection experiment. Input viruses were prepared from 293T cells transfected with 20 μg of various pNF clones (Table 1). HIV-1 NL432 virus (1), which is known to be highly infectious for T-lymphocytic cells, but not for macrophages, did not replicated at all in the MDMs, as shown. WT, NF462 (wild type); ΔVif, NF-Nd.
Variation in amino acid sequence of the HIV-1 Vif hydrophilic region.
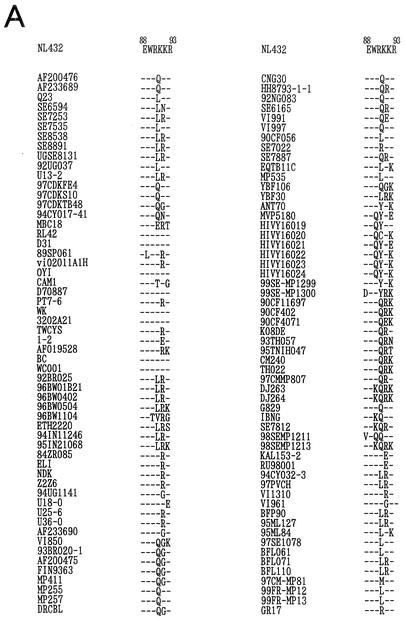
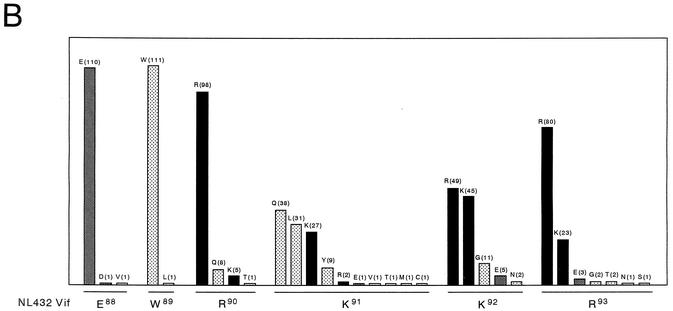
We then asked whether our results described above are applicable to naturally occurring viruses. The importance of the E88 and W89 residues for the Vif function in vivo was evaluated by examining amino acid sequences of the hydrophilic region of 112 nonlaboratory HIV-1 strains in the HIV Sequence Compendium 2000. The sequence alignment in the Compendium was carefully constructed by balancing the number of representatives of all HIV-1 subtypes and by maintaining geographical diversity. Figure 4A shows the alignment of amino acid sequences of the Vif hydrophilic region with the NL432 sequence as a standard. When similar amino acids (K and R or D and E) occurring at identical positions in the region were considered to be same, 21, 65, 22, and 4 clones had zero to three amino acid changes relative to the NL432 sequence, respectively. It was therefore concluded that the E88WRKKR93 motif is quite conserved in the Vif sequence. Figure 4B shows the frequency of amino acids at positions 88 to 93 based on the sequence data in Fig. 4A. As clearly shown, there was almost no variation in amino acids at positions 88 and 89. Positively charged R and K residues were present quite frequently at positions 90, 92, and 93, but not at position 91. In conclusion, amino acids at positions 88, 89, 90, 92, and 93 were much more conserved than the amino acid at position 91.
FIG. 4.
Variation in amino acid sequence of the HIV-1 Vif hydrophilic region. (A) Amino acids in the Vif hydrophilic regions of various nonlaboratory HIV-1 strains. The sequences in the hydrophilic region of 112 nonlaboratory HIV-1 strains are compared with that of a laboratory HIV-1 strain, NL432. A dash represents an amino acid identical to that of NL432. The sequences of nonlaboratory strains are from the HIV Sequence Compendium 2000. (B) Frequency of amino acids at specific positions in the HIV-1 Vif hydrophilic region. The frequency of each amino acid at positions 88 to 93 in the Vif sequence was determined for 112 nonlaboratory HIV-1 strains in panel A and depicted as a bar graph. Amino acids and their frequencies (in parentheses) are shown at the top of the bars. Black, gray, and dotted bars indicate the frequency of amino acids with a positive charge, negative charge, or without a charge, respectively.
Role of a highly charged region of HIV-1 Vif spanning residues 88 to 93.
To determine whether the E88WRKKR93 motif in the HIV-1 Vif, the central hydrophilic region, is crucial for the Vif function, we have generated a number of in-frame deletion and missense substitution mutant proteins. These were all evaluated for function in infectivity and expression assays. In this study, amino acid residues 88 and 89 were demonstrated to be important for the steady-state expression of HIV-1 Vif and therefore were critical for virus replication in nonpermissive lymphocytic H9 cells and semipermissive MDMs (Table 1). The highly conserved nature of E88 and W89 in vivo (Fig. 4) is consistent with the observations described above. However, alterations in hydrophilicity and charge profiles of the hydrophilic region by various mutations did not affect the virus replication potential in H9 cells (Table 1).
In this study, two types of vif mutants were found to be replication incompetent in H9 cells. While mutants pNL-f90/3del, -f91/3del, and -f88/6A expressed Vif at a level comparable to that of the wild-type clone upon transfection, mutants pNL-fE88del, -fW89del, -f88/2A, and -f88/3A did so at a very reduced level (Table 1). Of particular note are the results obtained for pNL-f88/2A, -f88/3A, and -f88/6A. The low level of expression of Vif observed for pNL-f88/2A and -f88/3A was apparently restored to a high level by substitution of all six amino acids, including E88 and W89, with A (pNL-f88/6A). It has been reported that a deletion in HIV-1 Vif encompassing the whole hydrophilic region does not affect its level of expression in cells (24). Although the molecular basis for these observations is currently unknown, it is conceivable that E88W89 and 90RKKR93 regions are involved in the suppression of instability of Vif and the instability of Vif, respectively. The structure or structures of HIV-1 Vif responsible for its steady-state expression remain to be determined. Another important point here is that NL-f90/3del and -f91/3del displayed impaired Vif function in the absence of Vif instability. These mutant Vif proteins need to be analyzed for their subcellular localization and virion incorporation.
In our in vitro assays using substitution mutants, a combination of E88 and W89 in the hydrophilic region was critical for the Vif function, and one of the two residues was changeable without loss of viral infectivity in H9 cells (Table 1). In addition, the 90RKKR93 motif was found not to be important for the Vif function (Table 1). However, analysis of Vif sequences of HIV-1 field isolates (Fig. 4) revealed that almost no variation is found at positions 88 and 89 and that charged amino acids such as R and K strikingly cluster at positions 90 to 93. It is therefore conceivable that each of the two residues and the RK motif are critically required for the Vif function in vivo. Further study is necessary to understand fully the structure-function relationship of HIV-1 Vif.
Acknowledgments
We thank Tokushima Red Cross Blood Center, Tokushima, Japan, for buffy coats from HIV-seronegative blood donors. We are indebted to Kazuko Yoshida for editorial assistance.
This work was supported by a Grant-in-Aid for Scientific Research on Priority Areas from the Ministry of Education, Science, Sports and Culture of Japan (14021078), a Grant-in-Aid for Scientific Research (B) from the Japan Society for the Promotion of Science (14370103), and a Health Sciences Research Grant from the Ministry of Health, Labour and Welfare of Japan (Research on HIV/AIDS 13110201).
REFERENCES
- 1.Adachi, A., H. E. Gendelman, S. Koenig, T. Folks, R. Willey, A. Rabson, and M. A. Martin. 1986. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J. Virol. 59:284-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adachi, A., N. Ono, H. Sakai, K. Ogawa, R. Shibata, T. Kiyomasu, H. Masuike, and S. Ueda. 1991. Generation and characterization of the human immunodeficiency virus type 1 mutants. Arch. Virol. 117:45-58. [DOI] [PubMed] [Google Scholar]
- 3.Akari, H., S. Arold, T. Fukumori, T. Okazaki, K. Strebel, and A. Adachi. 2000. Nef-induced major histocompatibility complex class I down-regulation is functionally dissociated from its virion incorporation, enhancement of viral infectivity, and CD4 down-regulation. J. Virol. 74:2907-2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akari, H., T. Fukumori, and A. Adachi. 2000. Cell-dependent requirement of human immunodeficiency virus type 1 gp41 cytoplasmic tail for Env incorporation into virions. J. Virol. 74:4891-4893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akari, H., T. Uchiyama, T. Fukumori, S. Iida, A. H. Koyama, and A. Adachi. 1999. Pseudotyping human immunodeficiency virus type 1 by vesicular stomatitis virus G protein does not reduce the cell-dependent requirement of Vif for optimal infectivity: functional difference between Vif and Nef. J. Gen. Virol. 80:2945-2949. [DOI] [PubMed] [Google Scholar]
- 6.Borman, A. M., C. Quillent, P. Charneau, C. Dauguet, and F. Clavel. 1995. Human immunodeficiency virus type 1 Vif− mutant particles from restrictive cells: role of Vif in correct particle assembly and infectivity. J. Virol. 69:2058-2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bouyac, M., M. Courcoul, G. Bertoia, Y. Baudat, D. Gabuzda, D. Blanc, N. Chazal, P. Boulanger, J. Sire, R. Vigne, and B. Spire. 1997. Human immunodeficiency virus type 1 Vif protein binds to the Pr55Gag precursor. J. Virol. 71:9358-9365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bouyac, M., F. Rey, M. Nascimbeni, M. Courcoul, J. Sire, D. Blanc, F. Clavel, R. Vigne, and B. Spire. 1997. Phenotypically Vif− human immunodeficiency virus type 1 is produced by chronically infected restrictive cells. J. Virol. 71:2473-2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cullen, B. R. 1998. HIV-1 auxiliary proteins: making connections in a dying cell. Cell 93:685-692. [DOI] [PubMed] [Google Scholar]
- 10.Emerman, M., and M. H. Malim. 1998. HIV-1 regulatory/accessory genes: keys to unraveling viral and host cell biology. Science 280:1880-1884. [DOI] [PubMed] [Google Scholar]
- 11.Folks, T., S. Benn, A. Rabson, T. Theodore, M. D. Hoggan, M. Martin, M. Lightfoote, and K. Sell. 1985. Characterization of a continuous T-cell line susceptible to the cytopathic effects of the acquired immune deficiency syndrome (AIDS)-associated retrovirus. Proc. Natl. Acad. Sci. USA 82:4539-4543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fouchier, R. A. M., J. H. M. Simon, A. B. Jaffe, and M. H. Malim. 1996. Human immunodeficiency virus type 1 Vif does not influence expression or virion incorporation of gag-, pol-, and env-encoded proteins. J. Virol. 70:8263-8269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fujita, M., S. Matsumoto, A. Sakurai, N. Doi, M. Miyaura, A. Yoshida, and A. Adachi. Apparent lack of trans-dominant negative effects of various vif mutants on the replication of HIV-1. Microbes Infect., in press. [DOI] [PubMed]
- 14.Fujita, M., A. Sakurai, N. Doi, M. Miyaura, A. Yoshida, K. Sakai, and A. Adachi. 2001. Analysis of the cell-dependent replication potentials of human immunodeficiency virus type 1 vif mutants. Microbes Infect. 3:1093-1099. [DOI] [PubMed] [Google Scholar]
- 15.Fujita, M., A. Sakurai, A. Yoshida, S. Matsumoto, M. Miyaura, and A. Adachi. 2002. Subtle mutations in the cysteine region of HIV-1 Vif drastically alter the viral replication phenotype. Microbes Infect. 4:621-624. [DOI] [PubMed] [Google Scholar]
- 16.Fujita, M., A. Yoshida, M. Miyaura, A. Sakurai, H. Akari, A. H. Koyama, and A. Adachi. 2001. Cyclophilin A-independent replication of a human immunodeficiency virus type 1 isolate carrying a small portion of the simian immunodeficiency virus SIVMAC gag capsid region. J. Virol. 75:10527-10531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gabuzda, D. H., K. Lawrence, E. Langhoff, E. Terwilliger, T. Dorfman, W. A. Haseltine, and J. Sodroski. 1992. Role of vif in replication of human immunodeficiency virus type 1 in CD4+ T lymphocytes. J. Virol. 66:6489-6495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gabuzda, D. H., H. Li, K. Lawrence, B. S. Vasir, K. Crawford, and E. Langhoff. 1994. Essential role of vif in establishing productive HIV-1 infection in peripheral blood T lymphocytes and monocyte/macrophages. J. Acquir. Immune Defic. Syndr. 7:908-915. [PubMed] [Google Scholar]
- 19.Goncalves, J., P. Jallepalli, and D. H. Gabuzda. 1994. Subcellular localization of the Vif protein of human immunodeficiency virus type 1. J. Virol. 68:704-712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goncalves, J., Y. Korin, J. Zack, and D. Gabuzda. 1996. Role of Vif in human immunodeficiency virus type 1 reverse transcription. J. Virol. 70:8701-8709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goncalves, J., B. Shi, X. Yang, and D. Gabuzda. 1995. Biological activity of human immunodeficiency virus type 1 Vif requires membrane targeting by C-terminal basic domains. J. Virol. 69:7196-7204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inubushi, R., M. Tamaki, R. Shimano, A. H. Koyama, H. Akari, and A. Adachi. 1998. Functional roles of HIV accessory proteins for viral replication. Int. J. Mol. Med. 2:429-433. [DOI] [PubMed] [Google Scholar]
- 23.Kawamura, M., T. Ishizaki, A. Ishimoto, T. Shioda, T. Kitamura, and A. Adachi. 1994. Growth ability of human immunodeficiency virus type 1 auxiliary gene mutants in primary blood macrophage cultures. J. Gen. Virol. 75:2427-2431. [DOI] [PubMed] [Google Scholar]
- 24.Khan, M. A., C. Aberham, S. Kao, H. Akari, R. Gorelick, S. Bour, and K. Strebel. 2001. Human immunodeficiency virus type 1 Vif protein is packaged into the nucleoprotein complex through an interaction with viral genomic RNA. J. Virol. 75:7252-7265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lebkowski, J. S., S. Clancy, and M. P. Calos. 1985. Simian virus 40 replication in adenovirus-transformed human cells antagonizes gene expression. Nature 317:169-171. [DOI] [PubMed] [Google Scholar]
- 26.Ma, X.-Y., P. Sova, W. Chao, and D. J. Volsky. 1994. Cysteine residues in the Vif protein of human immunodeficiency virus type 1 are essential for viral infectivity. J. Virol. 68:1714-1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mann, D. L., S. J. O'Brien, D. A. Gilbert, Y. Reid, M. Popovic, E. Read-Connole, R. C. Gallo, and A. F. Gazdar. 1989. Origin of the HIV-susceptible human CD4+ cell line H9. AIDS Res. Hum. Retrovir. 5:253-255. [DOI] [PubMed] [Google Scholar]
- 28.Michaels, F., N. Hattori, R. Gallo, and G. Franchini. 1993. The HIV-1 vif protein is located in the cytoplasm of infected cells and its effect on viral replication is equivalent in HIV-2. AIDS Res. Hum. Retrovir. 9:1025-1029. [DOI] [PubMed] [Google Scholar]
- 29.Miller, R. J., J. S. Cairns, S. Bridges, and N. Sarver. 2000. Human immunodeficiency virus and AIDS: insights from animal lentiviruses. J. Virol. 74:7187-7195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ochsenbauer, C., V. Bosch, I. Oelze, and U. Wieland. 1996. Unimpaired function of a naturally occurring C terminally truncated vif gene product of human immunodeficiency virus type 1. J. Gen. Virol. 77:1389-1395. [DOI] [PubMed] [Google Scholar]
- 31.Sakai, H., R. Shibata, J.-I. Sakuragi, S. Sakuragi, M. Kawamura, and A. Adachi. 1993. Cell-dependent requirement of human immunodeficiency virus type 1 Vif protein for maturation of virus particles. J. Virol. 67:1663-1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sakai, K., M. Horiuchi, S. Iida, T. Fukumori, H. Akari, and A. Adachi. 1999. Mutational analysis of the HIV-1 vif gene. Virus Genes 18:179-181. [DOI] [PubMed] [Google Scholar]
- 33.Shibata, R., M. Kawamura, H. Sakai, M. Hayami, A. Ishimoto, and A. Adachi. 1991. Generation of a chimeric human and simian immunodeficiency virus infectious to monkey peripheral blood mononuclear cells. J. Virol. 65:3514-3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simon, J. H. M., and M. H. Malim. 1996. The human immunodeficiency virus type 1 Vif protein modulates the postpenetration stability of viral nucleoprotein complexes. J. Virol. 70:5297-5305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simon, J. H. M., A. M. Sheehy, E. A. Carpenter, R. A. M. Fouchier, and M. H. Malim. 1999. Mutational analysis of the human immunodeficiency virus type 1 Vif protein. J. Virol. 73:2675-2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sova, P., and D. J. Volsky. 1993. Efficiency of viral DNA synthesis during infection of permissive and nonpermissive cells with vif-negative human immunodeficiency virus type 1. J. Virol. 67:6322-6326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsurutani, N., M. Kubo, Y. Maeda, T. Ohashi, N. Yamamoto, M. Kannagi, and T. Masuda. 2000. Identification of critical amino acid residues in human immunodeficiency virus type 1 IN required for efficient proviral DNA formation at steps prior to integration in dividing and nondividing cells. J. Virol. 74:4795-4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.von Schwedler, U., J. Song, C. Aiken, and D. Trono. 1993. vif is crucial for human immunodeficiency virus type 1 proviral DNA synthesis in infected cells. J. Virol. 67:4945-4955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Willey, R. L., D. H. Smith, L. A. Lasky, T. S. Theodore, P. L. Earl, B. Moss, D. J. Capon, and M. A. Martin. 1988. In vitro mutagenesis identifies a region within the envelope gene of the human immunodeficiency virus that is critical for infectivity. J. Virol. 62:139-147. [DOI] [PMC free article] [PubMed] [Google Scholar]