Abstract
Two IgG subfractions of horse antilymphocyte serum (ALS) were obtained by DEAE Sephadex chromatography. Although the fractions did not differ antigenically, they differed on amino acid and carbohydrate analysis, and in electrophoretic mobility. As demonstrated by binding studies, only the most positively charged population of IgG molecules (fraction 1) obtained from anti-lymphocyte serum had specificity for the small lymphocyte; 50 per cent of the molecules in this population bound specifically to lymphocytes in vitro. As determined by an in vitro correlate of immunosuppressive potency (rosette inhibition), fraction 1 (F1) IgG from ALS contained approximately 4 times the specific activity of fraction 2 (F2). F1 was significantly more effective in prolonging skin graft survival than F2, whereas F2 contained the major component of the non-specific anti-inflammatory activity of serum. The anti-inflammatory effect was mediated by anticomplement activity.
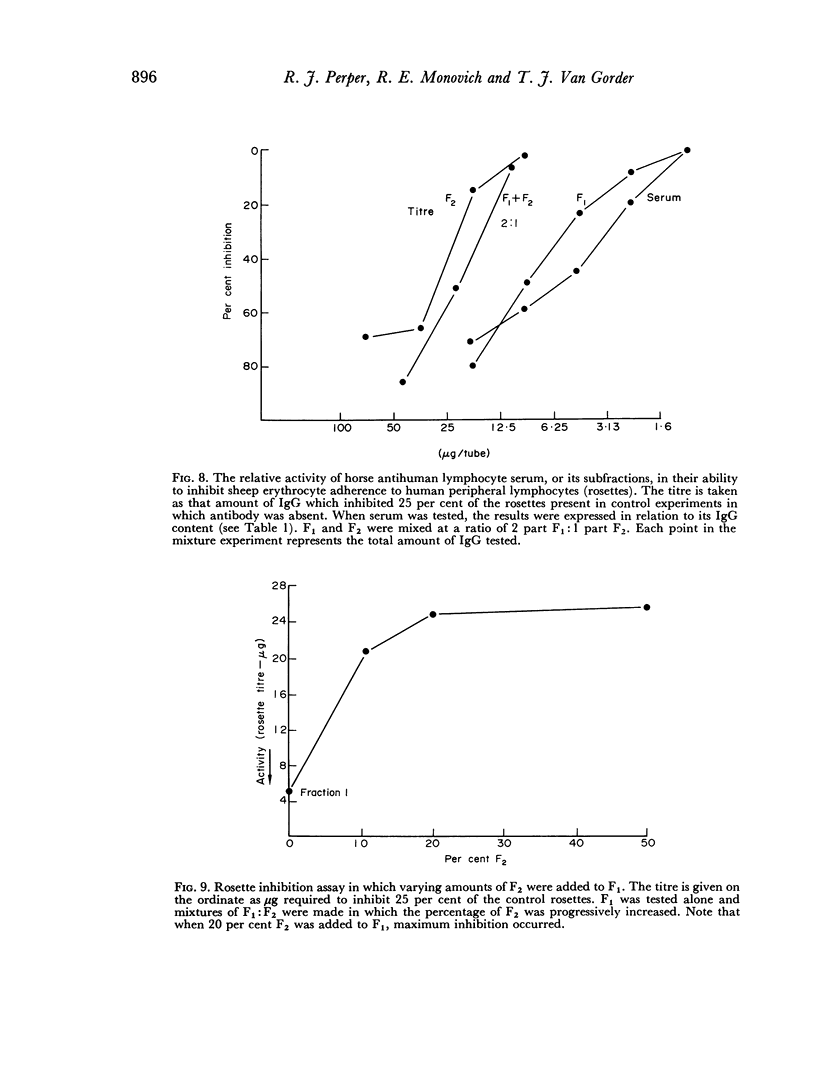
F2 was found to be an effective inhibitor of the immunosuppressive activity of F1 both in vivo and in vitro. Quantitative studies indicated that 1 part of F2 could maximally inhibit 4 parts of F1. The percentage of F2 present in serum IgG was inversely related to the skin graft survival elicited by the serum, which indicated that F2 was active as an inhibitor when tested as purified fraction as well as in unfractionated serum. Following immunization when F1 gained immunosuppressive potency, it lost non-specific anti-inflammatory activity. These observations indicated that not only was there a quantitative, as well as a qualitative concentration of immunosuppressive antibodies in F1, but also that this activity was controlled by the concentration of F2. This report, therefore, describes an IgG control mechanism which can limit the expression of antibody induced biological activity.
It is suggested that in ALS the immunosuppressive antibody molecules possess a greater net positive charge than the remaining population, and that this is due to the degree of the negative charge on the immunizing antigen. Using DEAE Sephadex chromatography, these populations could be separated into two differently charged populations of molecules, only one of which had significant immunosuppressive capability. This increase in activity resulted from the increase of specific molecules, the loss of non-specific molecules, and was manifest upon the removal of an IgG inhibitor.
Full text
PDF















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bach J. F., Dormont J., Dardenne M., Balner H. In vitro rosette inhibition by antihuman antilymphocyte serum. Correlation with skin graft prolongation in subhuman primates. Transplantation. 1969 Sep;8(3):265–280. doi: 10.1097/00007890-196909000-00008. [DOI] [PubMed] [Google Scholar]
- Balner H., Dersjant H., Van Bekkum D. W. Testing of antihuman lymphocyte sera in chimpanzees and lower primates. Transplantation. 1969 Sep;8(3):281–290. doi: 10.1097/00007890-196909000-00009. [DOI] [PubMed] [Google Scholar]
- Binaghi R. A., Oriol R., Boussac-Aron Y. Immunogenicity of heterologous Fc and Fab immunoglobulin fragments in rabbits, guinea-pigs and rats. Immunology. 1967 Jul;13(1):63–69. [PMC free article] [PubMed] [Google Scholar]
- Bokisch V. A., Müller-Eberhard H. J., Cochrane C. G. Isolation of a fragment (C3a) of the third component of human complement containing anaphylatoxin and chemotactic activity and description of an anaphylatoxin inactivator of human serum. J Exp Med. 1969 May 1;129(5):1109–1130. doi: 10.1084/jem.129.5.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currey H. L., Ziff M. Suppression of adjuvant disease in the rat by heterologous antilymphocyte globulin. J Exp Med. 1968 Jan 1;127(1):185–203. doi: 10.1084/jem.127.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Einstein E. R., Cerutti P. Quantitative determination of gamma-globulin in human sera by column chromatography. Anal Biochem. 1968 Oct 10;26(1):183–184. doi: 10.1016/0003-2697(68)90043-2. [DOI] [PubMed] [Google Scholar]
- FLEISCHMAN J. B., PAIN R. H., PORTER R. R. Reduction of gamma-globulins. Arch Biochem Biophys. 1962 Sep;Suppl 1:174–180. [PubMed] [Google Scholar]
- Feinstein A., Hobart M. J. Structural relationship and complement fixing activity of sheep and other ruminant immunoglobulin G subclasses. Nature. 1969 Aug 30;223(5209):950–952. doi: 10.1038/223950a0. [DOI] [PubMed] [Google Scholar]
- Fong S. W., Masouredis S. P. Physicochemical and immunologic properties of eluates containing I-125 anti-Rho(D). J Immunol. 1967 Feb;98(2):374–385. [PubMed] [Google Scholar]
- Grob P. J., Frommel D., Isliker H. C., Masouredis S. P. Interaction of IgG and its fragments with red cells. Immunology. 1967 Nov;13(5):489–499. [PMC free article] [PubMed] [Google Scholar]
- HOOK W. A., MUSCHEL L. H. ANTICOMPLEMENTARY EFFECTS AND COMPLEMENT ACTIVITY OF HUMAN SERA. Proc Soc Exp Biol Med. 1964 Oct;117:292–297. doi: 10.3181/00379727-117-29561. [DOI] [PubMed] [Google Scholar]
- JOHANSEN P. G., MARSHALL R. D., NEUBERGER A. Carbohydrates in protein. 2. The hexose, hexosamine, acetyl and amide-nitrogen content of hen's-egg albumin. Biochem J. 1960 Nov;77:239–247. doi: 10.1042/bj0770239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James K., James V. S., Pullar D. M., Wood A., Logan E. In vitro studies on the heterogeneity of equine antibodies to human lymphoid tissue. Immunochemistry. 1969 Sep;6(5):659–680. doi: 10.1016/0019-2791(67)90131-0. [DOI] [PubMed] [Google Scholar]
- James K. The preparation and properties of anti-lymphocytic sera. Prog Surg. 1969;7:140–217. doi: 10.1159/000386301. [DOI] [PubMed] [Google Scholar]
- Klinman N. R., Rockey J. H., Frauenberger G., Karush F. Equine anti-hapten antibody. 3. The comparative properties of gamma G- and gammaA-antibodies. J Immunol. 1966 Apr;96(4):587–595. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lance E. M. The mechanism of action of anti-lymphocyte serum. Studies of antibody eluate. J Exp Med. 1969 Jul 1;130(1):49–76. doi: 10.1084/jem.130.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConahey P. J., Dixon F. J. A method of trace iodination of proteins for immunologic studies. Int Arch Allergy Appl Immunol. 1966;29(2):185–189. doi: 10.1159/000229699. [DOI] [PubMed] [Google Scholar]
- Mozes E., Sela M. Resolution of mouse and human immunoglobulin G into two fractions by chromatography on diethylaminoethyl-sephadex. Relation of mouse antibody type to the antigen charge. Isr J Med Sci. 1969 Mar-Apr;5(2):267–270. [PubMed] [Google Scholar]
- POLLACK W., HAGER H. J., RECKEL R., TOREN D. A., SINGHER H. A STUDY OF THE FORCES INVOLVED IN THE SECOND STAGE OF HEMAGGLUTINATION. Transfusion. 1965 Mar-Apr;5:158–183. doi: 10.1111/j.1537-2995.1965.tb01152.x. [DOI] [PubMed] [Google Scholar]
- Perper R. J., Lyster S. C., Monovich R. E., Bowersox B. E. Analysis of the biological activity of antilymphocyte serum. II. Influence of cell type and method of immunization. Transplantation. 1970 May;9(5):447–456. doi: 10.1097/00007890-197005000-00003. [DOI] [PubMed] [Google Scholar]
- Perper R. J., Okimoto J. T., Cochrum K. C., Ramsey N., Najarian J. S. A rapid method for purification of large quantities of anti-lymphocytic serum. Proc Soc Exp Biol Med. 1967 Jun;125(2):575–580. doi: 10.3181/00379727-125-32150. [DOI] [PubMed] [Google Scholar]
- Perper R. J., Zee T. W., Mickelson M. M. Purification of lymphocytes and platelets by gradient centrifugation. J Lab Clin Med. 1968 Nov;72(5):842–848. [PubMed] [Google Scholar]
- ROCKEY J. H., KLINMAN N. R., KARUSH F. EQUINE ANTIHAPTEN ANTIBODY. I. 7S BETA-2A- AND 1OS GAMMA-1- GLOBULIN COMPONENTS OF PURIFIED ANTI-BETA-LACTOSIDE ANTIBODY. J Exp Med. 1964 Oct 1;120:589–609. doi: 10.1084/jem.120.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhenstroth-Bauer G., Lücke-Huhle C. Two populations of small lymphocytes. J Cell Biol. 1968 Apr;37(1):196–199. doi: 10.1083/jcb.37.1.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sela M., Mozes E. Dependence of the chemical nature of antibodies on the net electrical charge of antigens. Proc Natl Acad Sci U S A. 1966 Feb;55(2):445–452. doi: 10.1073/pnas.55.2.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skutelsky E., Danon D. Reduction in surface charge as an explanation of the recognition by macrophages of nuclei expelled from normoblasts. J Cell Biol. 1969 Oct;43(1):8–15. doi: 10.1083/jcb.43.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starzl T. E., Brettschneider L., Penn I., Schmidt R. W., Bell P., Kashiwagi N., Townsend C. M., Putnam C. W. A trial with heterologous antilymphocyte globulin in man. Transplant Proc. 1969 Mar;1(1):448–454. [PMC free article] [PubMed] [Google Scholar]
- WASSERMAN E., LEVINE L. Quantitative micro-complement fixation and its use in the study of antigenic structure by specific antigen-antibody inhibition. J Immunol. 1961 Sep;87:290–295. [PubMed] [Google Scholar]
- Willoughby D. A., Coote E., Turk J. L. Complement in acute inflammation. J Pathol. 1969 Feb;97(2):295–305. doi: 10.1002/path.1710970215. [DOI] [PubMed] [Google Scholar]
- Willoughby D. A., Giroud J. P. The role of polymorphonuclear leucocytes in acute inflammation in agranulocytic rats. J Pathol. 1969 May;98(1):53–60. doi: 10.1002/path.1710980107. [DOI] [PubMed] [Google Scholar]
- Woodruff M. F., Reid B. L., James K. Quantitative studies with antilymphocytic antibody. Nature. 1967 Nov 25;216(5117):758–762. doi: 10.1038/216758a0. [DOI] [PubMed] [Google Scholar]