Abstract
Unmethylated CpG dinucleotides in bacterial DNA or synthetic oligodeoxynucleotides (ODNs) are known as potent activators of the immune system and inducers of several Th1-associated immunomodulatory cytokines. We therefore investigated whether such a CpG-containing ODN (CpG ODN) given mucosally in the female genital tract could enhance innate immunity and protect against genital herpes infection. Groups of C57BL/6 mice were treated intravaginally with either CpG ODN or a non-CpG ODN control in the absence of any antigen either 2 days before or 4 h after an intravaginal challenge with a normally lethal dose of herpes simplex virus type 2 (HSV-2). Mice treated with CpG ODN exhibited significantly decreased titers of HSV-2 in their vaginal fluids compared with non-CpG ODN-treated mice. Furthermore, CpG ODN pretreatment significantly protected against development of disease and death compared to non-CpG ODN pretreatment. Most strikingly, CpG ODN conferred protection against disease and death even when given after the viral challenge. The CpG ODN-induced protection was associated with a rapid production of gamma interferon (IFN-γ), interleukin-12 (IL-12), IL-18, and RANTES in the genital tract mucosa following CpG ODN treatment. The observed protection appeared to be dependent on IFN-γ, IL-12, IL-18, and T cells, as CpG ODN pretreatment did not confer any significant protection in mice deficient in IFN-γ, IL-12, IL-18, or T cells. Further, a complete protective immunity to reinfection was elicited in CpG ODN-treated, HSV-2-challenged mice, suggesting a role for mucosally administered CpG ODN in inducing the development of an acquired immune response in addition to its potent stimulation of innate immunity.
Herpes simplex virus type 2 (HSV-2) is a sexually transmitted pathogen that invades the human genital tract mucosa and is the most common causative agent of genital ulcer disease in humans. The prevalence of HSV-2 infection is high in many countries (22), and the incidence is increasing worldwide (33). Genital ulcers are common, but disseminating disease and life-threatening complications such as meningitis may also occur in immunocompromised individuals. In early pregnancy, HSV-2 can cross the placental barrier and affect the fetus, which may lead to spontaneous abortion or serious damage to the fetus, including mental retardation (51). Genital herpes is also implicated in the transmission and acquisition of human immunodeficiency virus (6, 15).
The standard treatment and prevention of genital herpes infection have depended upon antiviral drugs targeted against HSV, such as acyclovir and other guanosine analogues (9). Despite the efficacy of such drugs in reducing both virus shedding and duration of the symptoms, some of the HSV strains have now developed resistance to the drugs, and there are few new antiherpes drug candidates on the horizon (32). Thus, there remains a serious need to develop new strategies to prevent and treat genital herpes infection.
Over the last decades, considerable efforts have been made to develop vaccines to induce HSV-specific protective immune responses. Despite significant progress in this enterprise, no effective vaccine against genital herpes infection has yet been licensed. The vast majority of efforts in the development of an effective antiherpes vaccine have focused on the effector phase of the antigen-specific adaptive immune response (3, 7, 44). Here, we have instead investigated the potential of stimulating the innate mucosal immune response for induction of protective immunity against genital herpes infection.
Bacterial DNA contains unmethylated CpG motifs, which distinguish bacterial DNA from vertebrate DNA. Vertebrate genomes uniformly contain a low frequency of CpG motifs and such CpG dinucleotides are usually methylated (27). Vertebrate immune systems appear to have evolved a specific Toll-like receptor (TLR), TLR9, that distinguishes bacterial DNA from self DNA. Interactions between CpG motifs in bacterial DNA and TLR9 rapidly activate the antigen-presenting cells through the Toll/interleukin-1 (IL-1) receptor signaling pathway to up-regulate costimulatory molecules and to produce Th1-polarizing cytokines (19, 49). In vitro, bacterial DNA or synthetic oligonucleotides that contain CpG-containing oligodeoxynucleotide (CpG ODN) can directly stimulate dendritic cells, splenocytes, monocytes, and macrophages to secrete a variety of cytokines, such as gamma interferon (IFN-γ) (53), IL-1β (29), tumor necrosis factor alpha (42), IL-12 (29), and IL-18 (35), and can also activate B cells for proliferation and IL-6 secretion (55). Further, CpG activates NK cells, macrophages (43), B cells (27), and dendritic cells (41) to up-regulate major histocompatibility complex class I and II, as well as costimulatory molecules such as CD80 and CD86. In vivo, systemic administration of CpG ODN was demonstrated to promote NK cell activity (53), to increase the total number of B lymphocytes in the spleen (27), and to increase plasma levels or tissue mRNA expression of IFN-γ (54), IL-12 (58), and tumor necrosis factor alpha (43). Overall, CpG DNA induces a predominantly Th1 pattern of immune stimulation.
Several recent reports have established the potent adjuvant activity of CpG ODN in induction of antigen-specific Th1-like immune responses to coadministered antigens (2, 25). In addition to induction of such antigen-specific immune responses, systemically administered CpG ODN has been shown to induce nonspecific Th1-like innate immune responses of a protective nature. Thus, systemic pretreatment of mice with CpG ODN was shown to confer protection against infection with Plasmodium yoelii, Listeria monocytogenes, and Leishmania major (13, 14, 26).
Despite the documented effects of CpG ODN on systemic innate and adaptive immunity, little is known about the role of CpG ODN in induction of immunity at mucosal surfaces, particularly in the female genital tract mucosa. The present study investigated the effects of vaginal mucosal administration of CpG ODN, in the absence of any antigen, on induction of immune responses against genital herpes infection as a model system for sexually transmitted viral diseases. We show here for the first time that vaginal treatment of mice with CpG ODN, in the absence of any viral antigen, confers protective immunity against genital herpes infection and disease and that such treatment rapidly elicits strong mucosal-genital Th1 cytokine and chemokine responses of innate immunity. Further, our results indicate that such CpG ODN-induced protective immunity is dependent on IFN-γ, IL-12, IL-18, and T cells. Moreover, a complete protective immunity to reinfection was elicited in CpG ODN-treated, HSV-2 challenged mice, suggesting a contribution of CpG ODN to the development of specific acquired immunity.
MATERIALS AND METHODS
ODNs.
The ODNs were purchased from Cybergene AB (Novum Research Park, Sweden). The CpG ODN used in this study was 1826 (TCC ATG ACG TTC CTG ACG TT), a 20-mer which has a nuclease-resistant phosphorothioate backbone and which contains two copies of a CpG motif known to have potent immunostimulatory effects on the murine immune system (56). The control ODN was TCC AGG ACT TCT CTC AGG TT, a 20-mer that has a nuclease-resistant phosphorothioate backbone but contains no CpG motif. The ODNs were tested for endotoxin by using the Limulus amebocyte lysate assay (Associates of Cape Cod, Inc.). All dilutions were conducted with pyrogen-free reagents.
Mice.
Six- to 8-week-old female mice were used for all experiments. C57BL/6 wild-type (WT) mice (M&B); IFN-γ−/− (8), B-cell-deficient (μMT) (23) (a gift from Nils Lycke, Göteborg University, Göteborg, Sweden), IL-12 p40−/− (30) (a gift from Elisabeth Svanberg, Göteborg University), IL-18−/− (45) (a gift from Shizuo Akira, Osaka University, Osaka, Japan), and nude (M&B) mice on a C57BL/6 background; and SCID mice on a CB-17 background (M&B) were kept in ventilated cages under specific-pathogen-free conditions at the EBM Animal Facility, Sahlgrenska Academy, Göteborg University. All experiments were performed with the approval from the Ethical Committee for Animal Experimentation in Göteborg, Sweden.
CpG ODN treatment.
Mice were pretreated by subcutaneous (s.c.) injection with 3.0 mg of Depo-Provera (Upjohn s.a., Puurs, Belgium) in 150 μl of phosphate-buffered saline (PBS). Six days later and 2 days prior to vaginal HSV-2 challenge, the mice were anesthetized with isofluran (Baxter Medical AB) and received a single administration of 60 μg of CpG ODN or non-CpG control ODN in distilled water by either the s.c., intraperitoneal (i.p.), combined s.c. and i.p., intravaginal (i.vag.) or combined i.vag. and intramuscular (i.m.) route. In some experiments, CpG ODN was instead administered at 4 h after virus challenge.
Virus and virus challenge.
HSV-2 strain 333 (39) was grown and titrated in monolayers of African green monkey kidney cells (GMK-AH1) and prepared by one cycle of freezing and thawing and subsequent removal of cellular debris by centrifugation. Before HSV-2 challenge, each mouse was pretreated by s.c. injection with 3.0 mg of Depo-Provera in PBS. Six days later, the mice were anesthetized with isofluran (Baxter Medical AB) and challenged by i.vag. inoculation of 9 × 104 PFU of HSV-2 strain 333 in 10 μl of Hanks' balanced salt solution.
Monitoring of infection. (i) Viral replication.
Following i.vag. HSV-2 challenge, vaginal fluids were collected by pipetting 40 μl of sterile Hanks' balanced salt solution in and out of the vagina until a discrete clump of mucus was retrieved, and then a second wash was performed. The two washes were pooled and stored at −70°C. HSV-2 titers were determined by plaque assay on GMK-AH1 cell monolayers by using a standard method.
(ii) Inflammation and disease.
Mice were examined daily for vaginal inflammation, neurological illness and death after HSV-2 challenge. The severity of disease was graded as follows: 0, healthy; 1, genital erythema; 2, moderate genital inflammation; 3, severe and purulent genital lesion; 4, hind-limb paralysis; and 5, death or sacrifice due to paralysis
Extraction of cytokines, chemokines, and antibodies from tissues.
Extraction of IFN-γ, IL-12, IL-18, IL-4, RANTES, and antibodies from the vagina and genital lymph nodes (gLN) was performed by using a modified version of a PERFEXT method (21). Briefly, mice were sacrificed at various intervals after CpG ODN treatment, and the vaginas and gLN were excised and weighed before storage at −70°C in a PBS solution containing 2 mM phenylmethylsulfonyl fluoride, 0.1 mg of soybean trypsin inhibitor (Sigma) per ml, and 0.05 M EDTA. The tissue samples were thawed and then permeabilized with saponin (Sigma) at a final concentration of 2% (wt/vol) in PBS at 4°C overnight. The tissue samples were then centrifuged at 16,000 × g for 5 min, and the supernatants were analyzed for cytokine, chemokine, and antibody contents by enzyme-linked immunosorbent assay (ELISA).
Proliferation assays.
Mononuclear cell suspensions of spleen cells isolated from CpG ODN-treated mice 3 to 4 weeks after a vaginal challenge with a lethal dose of HSV-2 were seeded in triplicate wells in Iscove's medium supplemented with l-glutamine, 50 μM 2-mercaptoethanol, gentamicin, and 10% fetal calf serum and incubated at 37°C in the presence of UV-inactivated HSV-2 or mock antigen (17). After 48 h of incubation, culture supernatants were collected and assayed for cytokine contents. On day 3, cells were pulsed with 1 μCi of [3H]thymidine (Amersham Pharmacia) for the last 6 h of culture, and the cellular DNA was harvested on glass fiber filters and then assayed by liquid scintillation counting. Data are expressed as stimulation indices, corresponding to the mean counts per minute for UV-inactivated HSV-2-treated cultures divided by the mean counts per minute for mock-treated cultures.
Cytokine and chemokine quantification.
Concentrations of IFN-γ, IL-12, IL-18, IL-4, and RANTES in the tissue extracts, the serum samples, and the culture supernatants were determined by using Duoset cytokine ELISA kits from R&D Systems (Abingdon, United Kingdom) according to the manufacturer's recommendations.
Flow cytometry.
gLN and spleen cells were analyzed for the expression of various cell surface molecules by dual-color immunofluorescent staining with fluorescence-labeled antibodies for B220, CD3, CD4, CD8, and NK1.1 (PharMingen) followed by use of a FACSCalibur (Becton Dickinson).
Antibody measurements.
Microtiter plate wells (Maxisorp; Nunc) were coated with 100 μl of a deoxycholate-solubilized membrane fraction of HSV-1-infected cells (20) (a gift from Bo Svennerholm, Göteborg University) for 4 h at room temperature in 0.05 M carbonate buffer at pH 9.6. The plates were blocked with 2% bovine serum albumin in PBS for 30 min at 37°C. Serial dilutions of sera obtained 3 weeks postchallenge with a lethal dose of HSV-2 were incubated for 1 h at 37°C. After being washed with 0.05% Tween 20, the plates were incubated for another 1 h at 37°C with goat anti-mouse immunoglobulin G (IgG) coupled to horseradish peroxidase (1/1,000; Southern Biotechnology Associates, Inc., Birmingham, Ala.) in 1% bovine serum albumin in PBS. The plates were washed with 0.05% Tween 20 and developed with 100 μl of 1-mg/ml o-phenylene diamine dihydrochloride (Sigma) in 0.1 M citrate buffer (pH 4.5) containing 0.04% H2O2. After 20 min of incubation at room temperature, the absorbance was read at 450 nm. The sample IgG titer was defined as the reciprocal of the sample dilution giving an optical density of 0.4 above the background value.
Statistical analysis.
Statistical analyses were done by Student's t test or the log rank test where applicable.
RESULTS
Protection against genital herpes infection by CpG ODN.
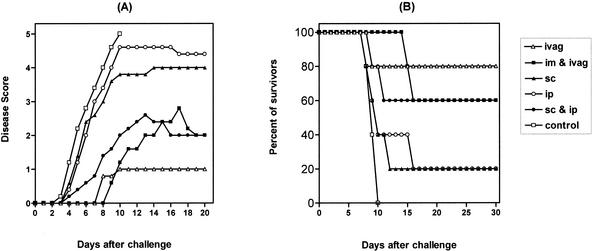
To investigate the impact of CpG ODN on induction of protective immunity against genital HSV-2 infection, WT mice were treated with CpG ODN by different routes, including s.c., i.p., s.c. plus i.p., i.vag., and i.vag. plus i.m., 2 days prior to a genital challenge with a normally lethal dose of HSV-2. As shown in Fig. 1, control mice started to show macroscopic signs of the disease as early as 3 days following the HSV-2 challenge, and all animals had died as a result of neurological illness within 10 days of infection. In contrast, i.vag. administration of CpG ODN protected 80% of the mice against a normally lethal HSV-2 challenge (Fig. 1). Combined i.vag. plus i.m. or s.c. plus i.p. CpG ODN administration conferred protection in 60% of the mice, while s.c. or i.p. administration of CpG ODN led to survival in only 20% of the animals (Fig. 1).
FIG. 1.
Impact of CpG ODN pretreatment on disease progression after a genital HSV-2 challenge. Groups of female C57BL/6 mice (6 to 12 mice/group) were injected s.c. with 3.0 mg of Depo-Provera. Six days later, the mice received a single dose of 60 μg of CpG ODN 1826 by either the s.c., i.p., combined s.c. and i.p., i.vag., or combined i.vag. and i.m. route, and they were challenged 2 days later by i.vag. inoculation of 9 ×104 PFU of a virulent HSV-2 strain. Animals were scored daily for macroscopic signs of the disease (A) and mortality (B). Disease progression was scored as healthy (0), genital erythema (1), moderate genital inflammation (2), severe and purulent genital lesion (3), hind-limb paralysis (4), and death or sacrifice due to paralysis (5).
We then examined the dependence of such protection on the CpG motif. Mice were treated i.vag. with either CpG ODN or a non-CpG control ODN 2 days before challenge. As depicted in Fig. 2A, mice treated with the control ODN had significantly higher titers of HSV-2 in their vaginal fluids than CpG ODN-treated mice (P < 0.05) (Fig. 2A), and the majority of the non-CpG ODN-treated animals rapidly developed signs of disease (Fig. 2B) and died (Fig. 2C). These data clearly demonstrate that such protection is dependent on the CpG motif and not simply due to the phosphorothioate oligonucleotide backbone.
FIG. 2.
CpG ODN-induced protection against genital herpes infection is dependent on the CpG motif. Groups of C57BL/6 mice were injected s.c. with 3.0 mg of Depo-Provera. Six days later, the mice were inoculated i.vag. with a single dose of either 60 μg of CpG ODN 1826 or a non-CpG control ODN. The animals were challenged 2 days later with 9 × 104 PFU of a virulent HSV-2 strain. (A) Vaginal HSV-2 titers on day 3 after viral challenge (n = 5 to 7). Data are expressed as the mean virus load (PFU per sample) and standard error of the mean. The difference was statistically significant at P value of <0.05 (*) by Student's t test. (B and C) The mice were monitored daily for macroscopic signs of the disease (B) and mortality (C) (n ≥ 12) as described in Fig. 1. ○, CpG ODN treatment; •, non-CpG ODN treatment.
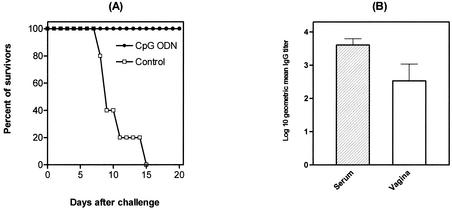
In another set of experiments, we examined the impact of CpG ODN on development of genital herpes infection and disease in mice already exposed to the virus. Mice were treated i.vag. with either CpG ODN or vehicle at 4 h after a normally lethal vaginal challenge with HSV-2. The results show that the control-treated mice exhibited high vaginal titers of the virus and all animals died within a week of infection, while the majority of CpG ODN-treated mice had significantly lower titers of the virus in their genital tract (not shown) and survived throughout the experiment (Fig. 3).
FIG. 3.
Effect of CpG ODN treatment on survival in mice already exposed to HSV-2. C57BL/6 mice were injected s.c. with 3.0 mg of Depo-Provera and challenged 6 days later with 9 × 104 PFU of a virulent HSV-2 strain. Four hours later, the mice were treated i.vag. with either vehicle or 60 μg of CpG ODN 1826 and monitored daily for disease progression and death (12 mice/group). ○, CpG ODN treatment; •, control.
In total, these findings indicate that CpG ODN is a potent inducer of protective immunity against genital herpes infection when applied i.vag. and that the antiherpes protective effect of CpG ODN is specifically due to the CpG motif.
Enlargement of local lymph nodes induced by i.vag. CpG ODN treatment.
It has been shown that systemic CpG ODN administration induces extramedullary splenic hematopoiesis and splenomegaly (36). Thus, we sought to examine whether mucosal-vaginal CpG ODN administration would affect the size and cell contents of both the gLN and spleen at 48 h after treatment (i.e., at the time of i.vag. viral challenge). i.vag. administration of CpG ODN to mice led to a marked enlargement of draining lymph nodes that was associated with a dramatic increase in gLN cell counts; the gLN cell number in CpG ODN-treated mice was sixfold greater that that in control animals. Further, as defined by flow cytometry, the total number of each of the major cell types, i.e., NK cells, NKT cells, B cells, and T cells, was increased by seven-, six-, seven-, and fourfold, respectively. In contrast, neither the size nor the cell contents of the spleen were altered appreciably 2 days after i.vag. CpG ODN treatment, reflecting the local nature of the response.
Role of IFN-γ in protective effect of CpG ODN.
Systemic CpG ODN administration is known to induce IFN-γ production, suggesting that IFN-γ may be involved in the CpG ODN-induced innate immunity (54). Therefore, we wanted to determine the role of IFN-γ in mucosal CpG ODN-induced immunity to genital herpes infection. We first determined the production of IFN-γ at various intervals after vaginal CpG ODN treatment. Following such treatment, levels of IFN-γ in the genital tract showed a twofold increase on day 1 that declined on day 2 and was followed by a second peak on day 4 (Fig. 4); levels of IL-4, on the other hand, remained unchanged (not shown). Unlike those in the genital tract, the levels of IFN-γ in sera of CpG ODN-treated animals remained virtually unaltered throughout the test period (not shown). We next examined the need for IFN-γ in CpG ODN-induced protective immunity against genital herpes infection. IFN-γ−/− and WT mice were given CpG ODN i.vag. 2 days before a normally lethal genital HSV-2 challenge. In CpG ODN-treated IFN-γ−/− mice, the level of shed virus was 15 times higher than that in CpG ODN-treated WT animals (P < 0.01) (Fig. 5A), and all CpG ODN-treated IFN-γ−/− mice, in sharp contrast to the WT animals, showed macroscopic signs of the disease and died within 9 days of challenge (Fig. 5B). These data establish that the CpG ODN-induced protection against genital herpes infection is dependent on IFN-γ.
FIG. 4.
Induction of vaginal IFN-γ response after i.vag. CpG ODN administration. C57BL/6 mice were injected s.c. with 3.0 mg of Depo-Provera. Six days later (day 0), CpG ODN 1826 was inoculated i.vag. (60 μg/mouse), and the vaginas were removed and saponin extracted at the indicated time points (two mice per time point). The vaginal extracts were analyzed for IFN-γ content by ELISA. The data are expressed as fold increase over the values for day 0 control mice. The data are representative of those from two independent experiments with comparable results.
FIG. 5.
The protective effect of CpG ODN is abolished in the absence of IFN-γ, IL-12, or IL-18. Groups of C57BL/6 WT, IFN-γ−/− (GKO), IL-12−/−, and IL-18−/− mice were injected s.c. with 3.0 mg of Depo-Provera. Six days later, the mice were inoculated i.vag. with a single dose of 60 μg of CpG ODN 1826. The animals were challenged 2 days later with 9 × 104 PFU of a virulent HSV-2 strain. (A) The vaginal HSV-2 titers were examined on day 3 after viral challenge (n = 5 to 7). Data are expressed as the mean virus load (PFU per sample) and standard error of the mean. Differences were statistically significant at P values of <0.05 (*) and <0.01 (**) by Student's t test compared with WT mice. (B) The mice were monitored daily for mortality (12 to 17 mice/group).
Roles of IL-12 and IL-18 in the protective effect of CpG ODN.
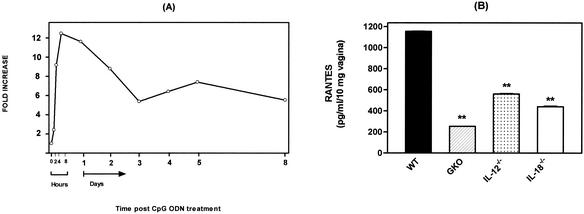
We have recently reported that both of the IFN-γ inducing cytokines IL-12 and IL-18 are important components of the innate immunity against genital herpes infection (18). Thus, we examined whether CpG ODN-induced protection is also dependent on IL-12 and IL-18. To this end, we first determined the levels of these cytokines in the genital tracts, gLN, and sera of CpG ODN-treated mice at various intervals after treatment. A low level of IL-12 was found in the genital tract mucosa of naive mice (12.2 ± 6.6 pg/ml/10 mg of tissue). However, as early as 2 h after i.vag. administration of CpG ODN, the concentration of IL-12 in the genital tract rose by 75-fold; it peaked at 8 h (112-fold) and remained markedly elevated for at least 8 days after CpG ODN administration (Fig. 6A). The levels of IL-12 in the gLN increased 4-fold at 2 h, peaked at 4 h (15-fold), and remained high for 24 h (12-fold), followed by a second wave of IL-12 production on day 5 after CpG ODN administration (10-fold) that stayed elevated for at least 8 days (Fig. 6B). The levels of IL-12 in serum dramatically increased within 2 h of i.vag. administration of CpG ODN (44-fold), peaked at 4 h (55-fold), stayed elevated for 24 h (>20-fold), and then gradually waned, reaching the baseline level on day 5 after CpG ODN treatment (Fig. 6C).
FIG. 6.
Kinetics of IL-12 response to i.vag. CpG ODN administration. C57BL/6 mice were injected s.c. with 3.0 mg of Depo-Provera. Six days later (day 0), CpG ODN 1826 was inoculated i.vag. (60 μg/mouse). (A and B) The vaginas (A) and gLN (B) were excised and saponin extracted at the indicated time points (two to four mice per time point). (C) The tissue extracts and serum samples (C) (taken at indicated time points) were analyzed for IL-12 content by ELISA. The data are expressed as fold increase over values for day 0 control mice.
A low level of IL-18 was detected in the genital tract mucosa of naive mice (15.8 ± 1.2 pg/ml/10 mg of tissue). The level of IL-18 in the vagina rose by 3-fold within 24 h and peaked on day 8 (61-fold) (Fig. 7), and then declined by day 14 after CpG ODN treatment (4-fold) (not shown). The level of IL-18 in the gLN increased by twofold on day 5 and then waned by day 8 of CpG ODN treatment; however, the levels of IL-18 in sera of CpG ODN-treated animals remained virtually unaltered throughout the test period (not shown).
FIG. 7.
Time course of vaginal IL-18 response to i.vag. CpG ODN administration. C57BL/6 mice were injected s.c. with 3.0 mg of Depo-Provera. Six days later (day 0), CpG ODN 1826 was inoculated i.vag. (60 μg/mouse), and the vaginas were removed and saponin extracted at the indicated time points (two to four mice per time point). The vaginal extracts were analyzed for IL-18 content by ELISA, and the data are presented as the fold increase over values for day 0 control mice.
We next tested the requirement for IL-12 and IL-18 in CpG ODN-induced protection. IL-12−/− and IL-18−/− mice as well as WT mice were treated with CpG ODN 2 days prior to a lethal HSV-2 challenge. The vaginal HSV-2 titers in CpG ODN-treated IL-12−/− and IL-18−/− mice were 8 and 14 times higher than those in CpG ODN-treated WT mice, respectively (P < 0.01) (Fig. 5A). Further, both the CpG ODN-treated IL-12- and IL-18-deficient mice developed macroscopic signs of the disease in the genital tract, and the majority of them had died by day 14 (Fig. 5B). Thus, similar to the case for IFN-γ, IL-12 and IL-18 are both required for the CpG ODN-induced protective immunity against genital herpes infection.
Role of RANTES in protective effect of CpG ODN.
Recent studies have shown that RANTES is an important factor involved in the development of mucosal immune responses (28). To investigate the possible involvement of RANTES in CpG ODN-induced protective immunity in the genital tract mucosa, we measured the levels of RANTES at various intervals after i.vag. administration of CpG ODN. Vaginal levels of RANTES had increased 9-fold within 2 to 4 h after intravaginal administration of CpG ODN, peaked at 8 h (12-fold), and remained markedly elevated for 8 days (5- to 11-fold) (latest time point examined) (Fig. 8A); however, the levels of RANTES in gLN extracts and sera of CpG ODN-treated animals remained virtually unaltered throughout the test period (not shown). Thus, CpG ODNs stimulate rapid and sustained local RANTES production in the female genital tract mucosa.
FIG. 8.
RANTES production in the vaginas of C57BL/6 WT, IFN-γ−/−, IL-12−/−, and IL-18−/− mice after i.vag. CpG ODN administration. C57BL/6 WT, IFN-γ−/− (GKO), IL-12−/−, and IL-18−/− mice were injected s.c. with 3.0 mg of Depo-Provera. Six days later, CpG ODN 1826 was inoculated i.vag. (60 μg/mouse), and the vaginas were removed and saponin extracted. The vaginal extracts were analyzed for RANTES content by ELISA. (A) Kinetics of vaginal RANTES production in WT mice after i.vag. CpG ODN administration. Data are presented as the fold increase over values for day 0 control mice (two to four mice per time point). (B) Vaginal production of RANTES on day 2 after i.vag. CpG ODN administration. Data are expressed as the mean concentration of RANTES per 10 mg of vagina, with the standard error of the mean. Differences were statistically significant at P values of <0.05 (*) and <0.01 (**) by Student's t test compared with CpG ODN-treated WT mice (five to seven mice per group).
To examine if a correlation exists between CpG ODN-induced protective immunity and local production of RANTES, we compared the levels of RANTES in the genital tracts of WT, IFN-γ−/−, IL-12−/−, and IL-18−/− mice 48 h after CpG ODN administration (i.e., at the time of HSV-2 challenge). We found that in CpG ODN-treated IFN-γ−/−, IL-12−/−, and IL-18−/− mice, which succumbed to subsequent lethal HSV-2 challenge, the genital levels of RANTES were significantly lower than those in CpG ODN-treated WT animals (P < 0.01) (Fig. 8B).
These data indicate that vaginal mucosal administration of CpG ODN elicits rapid and sustained local production of RANTES in the female genital tract mucosa, which is associated with protective immunity against genital herpes infection.
Role of T and B cells in protective effect of CpG ODN.
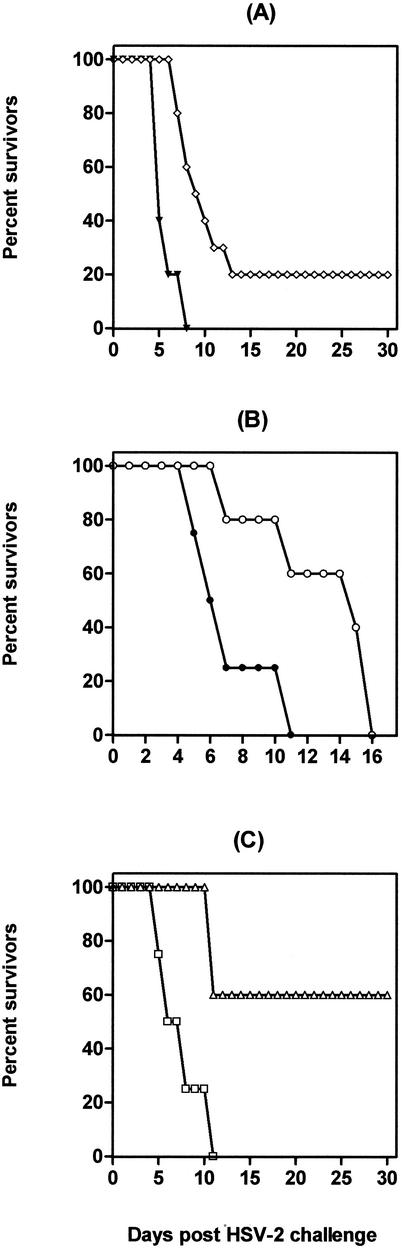
To investigate the requirement for T and B cells in the CpG ODN-induced protection, SCID mice were treated with CpG ODN 2 days prior to a lethal vaginal challenge with HSV-2 or left untreated before challenge. Untreated SCID mice exhibited high vaginal titers of HSV-2, and all animals developed disease and died within 8 days of challenge (Fig. 9A). Unexpectedly, the absence of T and B cells appeared to significantly reduce the CpG ODN-induced protection, as only 20% of the CpG ODN-treated SCID mice resisted the subsequent lethal HSV-2 challenge (Fig. 9A). However, CpG ODN treated SCID mice had lower HSV-2 titers in their genital tracts and developed signs of the disease 2 to 3 days later than untreated SCID mice (not shown).
FIG. 9.
Effects of i.vag. CpG ODN administration on disease progression in mice deficient in T and/or B cells after a genital HSV-2 challenge. Groups of SCID (A), nude (B), and μMT (C) mice were injected s.c. with 3.0 mg of Depo-Provera. Six days later, the mice were inoculated i.vag. with a single dose of 60 μg of CpG ODN 1826. The animals were challenged 2 days later with 9 × 104 PFU of a virulent HSV-2 strain and monitored daily for disease progression and mortality (5 to 10 mice per group). ⋄, ○, and ▵, CpG ODN treatment; ▾, •, and □, control.
To examine further the absolute requirement for T and B cells in CpG ODN-induced protection, nude mice and μMT mice were treated with either CpG ODN or vehicle and subsequently challenged with HSV-2 by using the same protocol. All CpG ODN-treated nude and control nude mice developed macroscopic signs of the disease and died (Fig. 9B); however, the CpG ODN-treated nude mice developed signs of the disease 3 days later than control nude mice and died 2 to 7 days later. All control μMT mice developed rapidly progressive disease requiring euthanization by day 7, whereas 60% of the CpG ODN-treated μMT mice resolved the vaginal lesions and survived for the whole 30-day follow-up period (Fig. 9C).
In total, these data indicate that T cells, but not B cells, are required for development of the CpG ODN-induced protective immunity against genital herpes infection.
Induction of acquired or memory HSV-2-specific immunity by CpG ODN.
We next examined whether genital mucosal administration of CpG ODN fostered the subsequent development of virus-specific acquired immunity. To achieve this goal, CpG ODN-treated WT mice that survived a primary genital HSV-2 challenge were further examined. Thus, CpG ODN-treated, HSV-2-challenged mice were rechallenged 3 to 4 weeks later with a lethal dose of the virus, without any further CpG-ODN application. These mice showed no local viral replication (<10 PFU/sample) and no macroscopic signs of the disease throughout the experiment (not shown), and all animals survived (Fig. 10A).
FIG. 10.
Induction of acquired specific immunity by CpG ODN. Groups of C57BL/6 mice were injected s.c. with 3.0 mg of Depo-Provera. Six days later, the mice were inoculated i.vag. with 60 μg of CpG ODN and challenged 2 days later with 9 × 104 PFU of a virulent HSV-2 strain. Four weeks after primary challenge, the mice were either rechallenged (n = 10 to 15), followed by a daily basis monitoring for disease progression and death (A), or the mice were sacrificed and the titers of HSV-specific IgG in the sera and genital tract extracts were determined (B). Error bars indicate standard errors of the means.
We next examined the immune status of CpG ODN-treated mice 4 weeks after a primary challenge with a lethal dose of the virus by analyzing HSV-specific T- and B-cell responses. We found a profound HSV-2-specific proliferative response of spleen cells (stimulation indices, 18.2 ± 2) and also markedly enhanced IFN-γ production by spleen cells (5.0 ± 0.4 ng/ml per million cells) upon restimulation with UV-inactivated HSV-2 in vitro. Further, both sera and genital tract extracts of these mice contained high titers of HSV-specific IgG (Fig. 10B); however, only low levels of HSV-specific IgA were detected in the sera and genital tract extracts of CpG ODN-treated, HSV-2-challenged mice (not shown).
In total, these results demonstrate that i.vag. administration of mice with CpG ODN followed by a primary HSV-2 challenge facilitates the development of HSV-2-specific acquired or memory immunity, which conferred complete protective immunity to a later genital HSV-2 reinfection.
DISCUSSION
In the present study, we show that in mice, a single vaginal administration of CpG ODN, in the absence of any viral antigen, stimulates a rapid mucosal genital immune response that confers protective immunity against genital herpes infection. The protective effects were found to be associated with a rapid local production of both the Th1-associated cytokines IFN-γ, IL-12, and IL-18 and the chemokine RANTES in the genital tract mucosa. Importantly, in addition to the role of CpG ODN in induction of nonspecific innate immunity against genital herpes infection, i.vag. administration of CpG ODN prior to a HSV-2 challenge also facilitated the subsequent development of HSV-2-specific acquired or memory immunity, which conferred complete protective immunity to a genital herpes reinfection. Our results suggest that the local vaginal administration of CpG ODN may serve as a potential strategy to prevent genital herpes infection in individuals who are either at risk of being exposed to or already exposed to the virus; however, the utility of this approach may be limited by the fact that it has to be applied locally, i.e., in the genital tract.
We examined the possible involvement of Th1-associated cytokines IFN-γ, IL-12, and IL-18, which we have recently shown to contribute to innate immunity to genital herpes infection (18), in the CpG ODN-induced protection. This was done by both measuring the production of these cytokines in response to the CpG ODN administration and by determining the protective effect of CpG ODN treatment in mice deficient in IFN-γ, IL-12, or IL-18.
Our kinetic cytokine expression studies showed that a single mucosal vaginal administration of CpG ODN induces rapid production of the Th1-associated cytokines IFN-γ, IL-12, and IL-18 in the genital tract mucosa.
We show that IFN-γ is an essential component of innate anti-HSV-2 protective immunity elicited by CpG ODN. Thus, upon a normally lethal vaginal challenge with HSV-2, CpG ODN-treated IFN-γ−/− mice developed markedly increased vaginal HSV-2 titers compared with CpG ODN-treated WT mice, and all animals succumbed to the infection. The role of IFN-γ in the innate control of viral infections is pleiotropic, and several additional mechanisms might be involved. IFN-γ can directly inhibit HSV replication by blocking transactivation-induced transcription of so-called immediate-early genes (10). IFN-γ further augments the proliferation of NK cells (4) and activates their functions, which can limit HSV-2 spread (1). IFN-γ can also up-regulate adhesion molecules such as ICAM-1 on endothelial cells (12), thereby directing the influx of immune cells into the target tissue. Further, IFN-γ up-regulates expression of major histocompatibility complex class I and II molecules on the target cells, facilitating the recognition and subsequent destruction of infected cells by T cells.
We found that the protective effect of CpG ODN is abolished in the absence of IL-12 or IL-18, as CpG ODN pretreatment did not confer any significant level of protection in mice lacking either IL-12 or IL-18. The most prominent functions of IL-12 and IL-18 in innate defense are the enhancements of NK cell activity and IFN-γ production (48, 52). Moreover, IL-12 and IL-18 rapidly and synergistically induce both naive T cells and B cells to develop into IFN-γ-producing cells without engaging their antigen receptors (57). Similar coordinated effects of IL-12 and IL-18 on IFN-γ production by macrophages have recently been demonstrated (31, 37). IL-18 can also induce ICAM-1 expression by an IFN-γ-independent pathway, promoting immune cell recruitment to the target tissue (24).
The present observations support our previous data that IFN-γ, IL-12, and IL-18 are important in innate immunity against genital HSV-2 infection (18) and thus confirm and extend previous findings by others concerning the importance of Th1 cytokines in CpG ODN-induced immunity (5, 14, 26, 38, 50).
We could show that i.vag. CpG ODN treatment also stimulates rapid and sustained RANTES production in the female genital tract mucosa and that such a local RANTES response is associated with protection. This observation is in line with our previous findings in which protective mucosal vaccination of mice with an attenuated strain of HSV-2 (HSV-2 TK−) was shown to be associated with a rapid induction of local RANTES production after a vaginal challenge with a lethal strain of the virus (16). Moreover, i.vag. CpG ODN treatment also elicits robust MIP-1α secretion in the genital tract mucosa as early as 2 h after CpG ODN administration (unpublished data). Recently, it was demonstrated that i.m. administration of CpG ODN rapidly increases the mRNA levels of RANTES and MIP-1α both at the site of injection and in the draining lymph nodes (46). RANTES and MIP-1α are particularly important in the induction of an immune response due to their preferential capacity to recruit and activate NK cells, dendritic cells, and monocytes (11, 34, 40, 47). Thus, a single i.vag. administration of CpG ODN elicits rapid and sustained secretion of certain Th1-associated cytokines and chemokines in the genital tract mucosa.
The CpG ODN-induced enhancement of innate immunity appeared to be of a local nature. Thus, i.vag. CpG ODN administration rapidly increased the levels of Th1 cytokines and chemokines in the genital tract and/or the draining lymph nodes, while such treatment did not affect the systemic levels of IFN-γ, IL-18, or RANTES at any time points tested; however, a transiently elevated level of IL-12 in serum was detected. Further, such treatment led to a local lymph node enlargement as well as a dramatic increase in the total cell numbers of B cells, NK cells, NKT cells, and T cells in the gLN in the absence of any appreciable changes in either size or cell contents of the spleen. In total, our observations provide strong evidence that the protective effects of CpG ODN are not restricted to the systemic immune response against pathogens, as reported earlier (13, 14, 26), but, in addition, after mucosal vaginal administration also involve the amplification of the innate immune response in the female genital tract mucosa. Further, the Th1-biased cytokine milieu in the gLN, comprising IL-12 and IL-18, may contribute to the subsequent development of a specific acquired immunity following HSV-2 challenge.
Surprisingly, the protection was dependent on the presence of lymphocytes, particularly T cells; SCID mice or nude mice treated with CpG ODN were unable to survive challenge with HSV-2, while the majority of CpG ODN-treated B-cell-deficient mice were protected and survived the subsequent viral challenge. Similar CpG ODN-induced protection that is also strongly lymphocyte dependent operates in L. monocytogenes infection (13). The results presented here imply that T lymphocytes, in addition to IFN-γ, IL-12, and IL-18, contribute significantly to CpG ODN-induced innate immunity against genital herpes infection.
Our results indicate that genital mucosal administration of CpG ODN fostered the subsequent development of HSV-2-specific acquired or memory immunity. Thus, the spleen cells from CpG ODN-treated, HSV-2 challenged mice showed a profound HSV-2-specific proliferative response as well as markedly enhanced IFN-γ production. Further, both sera and genital tract extracts of these mice contained high titers of HSV-specific IgG comparable to those observed in mice vaccinated with an attenuated strain of the virus (HSV-2 TK−) (17). Consistently, these mice were completely protected against genital HSV-2 reinfection. Thus, it seems likely that the primary genital HSV-2 challenge provides an internal source of antigens, while CpG ODN acts as strong immunostimulator or adjuvant to facilitate the subsequent development of an HSV-2-specific acquired or memory immune response. In other words, CpG ODN as a strong innate immunostimulator provides a rapid, short-term antiviral innate defense until the slower, antigen-specific acquired or memory immune response develops.
In summary, the present study demonstrates that i.vag. administration of DNA sequences containing CpG motifs increases resistance against genital herpes infection and that immune protection appears to be mediated by both the effector Th1-associated cytokines IFN-γ, IL-12, and IL-18 and the chemokine RANTES of innate immunity. Importantly, CpG ODN conferred protection against disease and death even when given after the viral challenge. Further, i.vag. administration of CpG ODN prior to an HSV-2 challenge fostered the subsequent development of HSV-2-specific acquired or memory immunity, which conferred complete protection against genital herpes reinfection. Thus, our study suggests that vaginal mucosal administration of CpG ODN may represent a valuable and potent strategy for prevention of genital herpes infection and other sexually transmitted viral diseases.
Acknowledgments
We are grateful to Margareta Frediksson for skilled technical assistance. We gratefully acknowledge Shizuo Akira, Osaka University, Osaka, Japan, for providing the IL-18−/− mice.
This study was supported by the Swedish Medical Research Council, SIDA/SAREC's Special Program for AIDS and Related Diseases, The Swedish Strategic Foundation Program in Infection and Vaccinology, Socialstyrelsens Fonder, the Swedish Society for Medical Research, the Wilhelm and Martina Lundgrens Science Foundation, Magn. Bergvalls' Foundation, and support to the Göteborg University Vaccine Research Institute from the Knut and Alice Wallenberg Foundation.
REFERENCES
- 1.Adler, H., J. L. Beland, N. C. Del-Pan, L. Kobzik, R. A. Sobel, and I. J. Rimm. 1999. In the absence of T-cells, natural killer cells protect from mortality due to HSV-1 encephalitis. J. Neuroimmunol. 93:208-213. [DOI] [PubMed] [Google Scholar]
- 2.Ban, E., L. Dupre, E. Hermann, W. Rohn, C. Vendeville, B. Quatannens, P. Ricciardi-Castagnoli, A. Capron, and G. Riveau. 2000. CpG motifs induce Langerhans cell migration in vivo. Int. Immunol. 12:737-745. [DOI] [PubMed] [Google Scholar]
- 3.Bernstein, D. I., and L. R. Stanberry. 1999. Herpes simplex virus vaccines. Vaccine 17:1681-1689. [DOI] [PubMed] [Google Scholar]
- 4.Biron, C. A., G. Sonnenfeld, and R. M. Welsh. 1984. Interferon induces natural killer cell blastogenesis in vivo. J. Leukoc. Biol. 35:31-37. [DOI] [PubMed] [Google Scholar]
- 5.Bohle, B., B. Jahn-Schmid, D. Maurer, D. Kraft, and C. Ebner. 1999. Oligodeoxynucleotides containing CpG motifs induce IL-12, IL-18 and IFN-gamma production in cells from allergic individuals and inhibit IgE synthesis in vitro. Eur. J. Immunol. 29:2344-2353. [DOI] [PubMed] [Google Scholar]
- 6.Chen, C. Y., R. C. Ballard, C. M. Beck-Sague, Y. Dangor, F. Radebe, S. Schmid, J. B. Weiss, V. Tshabalala, G. Fehler, Y. Htun, and S. A. Morse. 2000. Human immunodeficiency virus infection and genital ulcer disease in South Africa: the herpetic connection. Sex. Transm. Dis. 27:21-29. [DOI] [PubMed] [Google Scholar]
- 7.Corey, L., A. G. Langenberg, R. Ashley, R. E. Sekulovich, A. E. Izu, J. M. J. Douglas, H. H. Handsfield, T. Warren, L. Marr, S. Tyring, R. DiCarlo, A. A. Adimora, P. Leone, C. L. Dekker, R. L. Burke, W. P. Leong, and S. E. Straus. 1999. Recombinant glycoprotein vaccine for the prevention of genital HSV-2 infection. Two randomized controlled trials. JAMA 282:331-340. [DOI] [PubMed] [Google Scholar]
- 8.Dalton, D. K., S. Pitt-Meek, S. Keshav, I. S. Figari, A. Bradley, and T. A. Stewart. 1993. Multiple defects of immune cell function in mice with disrupted interferon-gamma genes. Science 259:1739.. [DOI] [PubMed] [Google Scholar]
- 9.De Clercq, E. 2000. Guanosine analogues as anti-herpesvirus agents. Nucleosides Nucleotides Nucleic Acids 19:1531-1541. [DOI] [PubMed] [Google Scholar]
- 10.De Stasio, P. R., and M. W. Taylor. 1990. Specific effect of interferon on the herpes simplex virus type 1 transactivation event. J. Virol. 64:2588-2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dieu, M., B. Vanbervliet, A. Vicari, J. Bridon, E. Oldham, S. Ait-Yahia, F. Briere, A. Zlotnik, S. Lebecque, and C. Caux. 1998. Selective recruitment of immature and mature dendritic cells by distinct chemokines expressed in different anatomic sites. J. Exp. Med. 188:373-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dustin, M. L., R. Rothlein, A. K. Bhan, C. A. Dinarello, and T. A. Springer. 1986. Induction by IL 1 and interferon-γ: tissue distribution, biochemistry, and function of a natural adherence molecule (ICAM-1). J. Immunol. 137:245-254. [PubMed] [Google Scholar]
- 13.Elkins, K., T. Rhinehart-Jones, S. Stibitz, J. Conover, and D. Klinman. 1999. Bacterial DNA containing CpG motifs stimulates lymphocyte-dependent protection of mice against lethal infection with intracellular bacteria. J. Immunol. 162:2291-2298. [PubMed] [Google Scholar]
- 14.Gramzinski, R., D. Doolan, M. Sedegah, H. Davis, A. Krieg, and S. Hoffman. 2001. Interleukin-12- and gamma interferon-dependent protection against malaria conferred by CpG oligodeoxynucleotide in mice. Infect. Immun. 69:1643-1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gwanzura, L., W. McFarland, D. Alexander, R. L. Burke, and D. Katzenstein. 1998. Association between human immunodeficiency virus and herpes simplex virus type 2 seropositivity among male factory workers in Zimbabwe. J. Infect. Dis. 177:481-484. [DOI] [PubMed] [Google Scholar]
- 16.Harandi, A., B. Svennerholm, J. Holmgren, and K. Eriksson. 2001. Protective vaccination against genital herpes simplex virus type 2 (HSV-2) infection in mice is associated with a rapid induction of local IFN-gamma-dependent RANTES production following a vaginal viral challenge. Am. J. Reprod. Immunol. 46:420-424. [DOI] [PubMed] [Google Scholar]
- 17.Harandi, A. M., B. Svennerholm, J. Holmgren, and K. Eriksson. 2001. Differential roles of B-cells and IFN-γ-secreting CD4+ T-cells in innate and adaptive immune control of genital HSV-2 infection in mice. J. Gen. Virol. 82:845-853. [DOI] [PubMed] [Google Scholar]
- 18.Harandi, A. M., B. Svennerholm, J. Holmgren, and K. Eriksson. 2001. Interleukin-12 (IL-12) and IL-18 are important in innate defense against genital herpes simplex virus type 2 infection in mice but are not required for the development of acquired gamma interferon-mediated protective immunity. J. Virol. 75:6705-6709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hemmi, H., O. Takeuchi, T. Kawai, T. Kaisho, S. Sato, H. Sanjo, M. Matsumoto, K. Hoshino, H. Wagner, K. Takeda, and S. Akira. 2000. A Toll-like receptor recognizes bacterial DNA. Nature 408:740-745. [DOI] [PubMed] [Google Scholar]
- 20.Jeansson, S., M. Forsgren, and B. Svennerholm. 1983. Evaluation of solubilized herpes simplex virus membrane antigen by enzyme-linked immunosorbent assay. J. Clin. Microbiol. 18:1160-1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johansson, E. L., C. Rask, M. Fredriksson, K. Eriksson, C. Czerkinsky, and J. Holmgren. 1998. Antibodies and antibody-secreting cells in the female genital tract after vaginal or intranasal immunization with cholera toxin B subunit or conjugates. Infect. Immun. 66:514-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kinghorn, G. R. 1994. Epidemiology of genital herpes. J. Int. Med. Res. 22:14A-23A. [PubMed] [Google Scholar]
- 23.Kitamura, D., J. Roes, R. Kuhn, and K. Rajewsky. 1991. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature 350:423-426. [DOI] [PubMed] [Google Scholar]
- 24.Kohka, H., T. Yoshini, H. Iwagaki, I. Sakuma, T. Tanimoto, Y. Matsuo, M. Kurimoto, K. Orita, T. Akagi, and N. Tanaka. 1998. Interleukin-18/interferon-gamma-inducing factor, a novel cytokine, up-regulates ICAM-1 (CD54) expression in KG-1 cells. J. Leukoc. Biol. 64:519-527. [DOI] [PubMed] [Google Scholar]
- 25.Krieg, A., and H. Davis. 2001. Enhancing vaccine with immune stimulatory CpG DNA. Curr. Opin. Mol. Ther. 3:15-24. [PubMed] [Google Scholar]
- 26.Krieg, A., L. Love-Homan, A. Yi, and J. Harty. 1998. CpG DNA induces sustained IL-12 expression in vivo and resistance to Listeria monocytogenes challenge. J. Immunol. 161:2428-2434. [PubMed] [Google Scholar]
- 27.Krieg, A., A. Yi, S. Matson, T. Waldschmidt, G. Bishop, R. Teasdale, G. Koretzky, and D. Klinman. 1995. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature 374:546-549. [DOI] [PubMed] [Google Scholar]
- 28.Lillard, J. W., P. N. Boyaka, D. D. Taub, and J. R. McGhee. 2001. RANTES potentiates antigen-specific mucosal immune responses. J. Immunol. 166:162-169. [DOI] [PubMed] [Google Scholar]
- 29.Lipford, G., T. Sparwasser, M. Bauer, S. Zimmermann, E. Koch, K. Heeg, and H. Wagner. 1997. Immunostimulatory DNA: sequence-dependent production of potentially harmful or useful cytokines. Eur. J. Immunol. 27:3420-3426. [DOI] [PubMed] [Google Scholar]
- 30.Magram, J., S. E. Connaughton, R. R. Warrier, D. M. Carvajal, C. Y. Wu, J. Ferrante, C. Stewart, U. Sarmiento, D. A. Faherty, and M. K. Gately. 1996. IL-12-deficient mice are defective in IFN gamma production and type 1 cytokine responses. Immunity 4:471-481. [DOI] [PubMed] [Google Scholar]
- 31.Munder, M., M. Mallo, K. Eichmann, and M. Modolell. 1998. Murine macrophages secrete interferon-gamma upon combined stimulation with interleukin (IL)-12 and IL-18: a novel pathway of autocrine macrophage activation. J. Exp. Med. 187:2103-2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Naesens, L., and E. De Clercq. 2001. Recent developments in herpesvirus therapy. Herpes 8:12-16. [PubMed] [Google Scholar]
- 33.Nahmias, A. J., F. K. Lee, and S. Beckman-Nahmias. 1990. Sero-epidemiological and -sociological patterns of herpes simplex virus infection in the world. Scand. J. Infect. Dis. Suppl. 69:19-36. [PubMed] [Google Scholar]
- 34.Rollins, B. J. 1997. Chemokines. Blood 90:909-928. [PubMed] [Google Scholar]
- 35.Roman, M., E. Martin-Orozco, J. Goodman, M. Nguyen, Y. Sato, A. Ronaghy, R. Kornbluth, D. Richman, D. Carson, and E. Raz. 1997. Immunostimulatory DNA sequences function as T helper-1-promoting adjuvants. Nat. Med. 3:849-854. [DOI] [PubMed] [Google Scholar]
- 36.Saparwasser, T., L. Hultner, E. Koch, A. Luz, G. Lipford, and H. Wagner. 1999. Immunostimulatory CpG-oligodeoxynucleotides cause extramedullary murine hemopoiesis. J. Immunol. 162:2368-2374. [PubMed] [Google Scholar]
- 37.Schindler, H., M. B. Lutz, M. Röllinghoff, and C. Bogdan. 2001. The production of IFN-gamma by IL-12/IL-18-activated macrophages requires STAT4 signaling and is inhibited by IL-4. J. Immunol. 166:3075-3082. [DOI] [PubMed] [Google Scholar]
- 38.Schwartz, D., C. Wohlford-Lenane, T. Quinn, and A. Krieg. 1999. Bacterial DNA or oligonucleotides containing unmethylated CpG motifs can minimize lipopolysaccharide-induced inflammation in the lower respiratory tract through an IL-12-dependent pathway. J. Immunol. 163:224-231. [PubMed] [Google Scholar]
- 39.Seth, P., W. E. Rawls, R. Duff, F. Rap, E. Adam, and J. L. Melnick. 1974. Antigenic differences between isolates of herpesvirus type 2. Intervirology 3:1-14. [DOI] [PubMed] [Google Scholar]
- 40.Sozzani, S., W. Luini, A. Borsatti, N. Polentarutti, D. Zhou, L. Piemonti, G. D. Amico, C. A. Power, T. N. Wells, M. Gobbi, et al. 1993. Receptor expression and responsiveness of human dendritic cells to a defined set of CC and CXC chemokines. J. Immunol. 159:1993-2000. [PubMed] [Google Scholar]
- 41.Sparwasser, T., E. S. Koch, R. M. Vabulas, K. Heeg, G. B. Lipford, G. B. Ellwart, and H. Wagner. 1998. Bacterial DNA and immunostimulatory CpG oligonucleotides trigger maturation and activation of murine dendritic cells. Eur. J. Immunol. 28:2045-2054. [DOI] [PubMed] [Google Scholar]
- 42.Sparwasser, T., T. Miethke, G. Lipford, K. Borschert, H. Hacker, K. Heeg, and H. Wagner. 1997. Bacterial DNA causes septic shock. Nature 386:336-337. [DOI] [PubMed] [Google Scholar]
- 43.Sparwasser, T., T. Miethke, G. Lipford, A. Erdmann, H. Hacker, K. Heeg, and H. Wagner. 1997. Macrophages sense pathogens via DNA motifs: induction of tumor necrosis factor-alpha-mediated shock. Eur. J. Immunol. 27:1671-1679. [DOI] [PubMed] [Google Scholar]
- 44.Stephenson, J. 2000. Genital herpes vaccine shows limited promise. JAMA. 284:1913-1914. [PubMed] [Google Scholar]
- 45.Takeda, K., H. Tsutsui, T. Yoshimoto, O. Adachi, N. Yoshida, T. Kishimoto, H. Okamura, K. Nakanishi, and S. Akira. 1998. Defective NK cell activity and Th1 response in IL-18-deficient mice. Immunity 8:383-390. [DOI] [PubMed] [Google Scholar]
- 46.Takeshita, S., F. Takeshita, D. Haddad, K. Ishii, and D. Klinman. 2000. CpG oligodeoxynucleotides induce murine macrophages to up-regulate chemokine mRNA expression. Cell. Immunol. 206:101-106. [DOI] [PubMed] [Google Scholar]
- 47.Taub, D. D., T. J. Sayers, C. R. Carter, and J. R. Ortaldo. 1995. Alpha and beta chemokines induce NK cell migration and enhance NK-mediated cytolysis. J. Immunol. 155:3877-3888. [PubMed] [Google Scholar]
- 48.Tomura, M., X. Zhou, S. Maruo, H. Ahn, T. Hamaoka, H. Okamura, K. Nakanishi, T. Tanimoto, M. Kurimoto, and H. Fujiwara. 1998. A critical role for IL-18 in the proliferation and activation of NK1.1+ CD3− cells. J. Immunol. 160:4738-4746. [PubMed] [Google Scholar]
- 49.Wagner, H. 2002. Interactions between bacterial CpG-DNA and TLR9 bridge innate and adaptive immunity. Curr. Opin. Immunol. 5:62-69. [DOI] [PubMed] [Google Scholar]
- 50.Walker, P., T. Scharton-Kersten, A. Krieg, L. Love-Homan, E. Rowton, M. Udey, and J. Vogel. 1999. Immunostimulatory oligodeoxynucleotides promote protective immunity and provide systemic therapy for leishmaniasis via IL-12- and IFN-gamma-dependent mechanisms. Proc. Natl. Acad. Sci. USA 96:6970-6975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Whitley, R. J. 1991. Perinatal herpes simplex virus infections. Rev. Med. Virol. 1:101-110. [Google Scholar]
- 52.Wolf, S., P. Temple, M. Kobayashi, D. Young, M. Dicig, L. Lowe, R. Dzialo, L. Fitz, C. Ferenz, and R. Hewick. 1991. Cloning of cDNA for natural killer cell stimulatory factor, a heterodimeric cytokine with multiple biologic effects on T and natural killer cells. J. Immunol. 146:3074-3081. [PubMed] [Google Scholar]
- 53.Yamamoto, S., T. Yamamoto, T. Kataoka, E. Kuramoto, O. Yano, and T. Tokunaga. 1992. Unique palindromic sequences in synthetic oligonucleotides are required to induce IFN-gamma and augment IFN-gamma-mediated natural killer activity. J. Immunol. 148:4072-4076. [PubMed] [Google Scholar]
- 54.Yamamoto, S., T. Yamamoto, S. Shimada, E. Kuramoto, O. Yano, T. Kataoka, and T. Tokunaga. 1992. DNA from bacteria, but not from vertebrates, induces interferons, activates natural killer cells and inhibits tumor growth. Microbiol. Immunol. 36:983-997. [DOI] [PubMed] [Google Scholar]
- 55.Yi, A., D. Klinman, T. Martin, S. Matson, and A. Krieg. 1996. Rapid immune activation by CpG motifs in bacterial DNA. Systemic induction of IL-6 transcription through an antioxidant-sensitive pathway. J. Immunol. 157:5394-5402. [PubMed] [Google Scholar]
- 56.Yi, A.-K., M. Chang, D. Peckham, A. Krieg, and R. Ashman. 1998. CpG oligodeoxyribonucleotides rescue mature spleen B cells from spontaneous apoptosis and promote cell cycle entry. J. Immunol. 160:5898-5906. [PubMed] [Google Scholar]
- 57.Yoshimoto, T., K. Takeda, T. Tanaka, K. Ohkusu, S. Kashiwamura, H. Okamura, S. Akira, and K. Nakanishi. 1998. IL-12 up-regulates IL-18 receptor expression on T cells, Th1 cells, and B cells: synergism with IL-18 for IFN-gamma production. J. Immunol. 161:3400-3407. [PubMed] [Google Scholar]
- 58.Zimmermann, S., O. Egeter, S. Hausmann, G. Lipford, M. Röcken, H. Wagner, and K. Heeg. 1998. CpG oligodeoxynucleotides trigger protective and curative Th1 responses in lethal murine leishmaniasis. J. Immunol. 160:3627-3630. [PubMed] [Google Scholar]