Abstract
Previous studies have shown that thymectomized lethally irradiated bone marrow grafted mice, reconstituted with thymocytes and pretreated with a large dose of sheep red blood cells (SRBC), are unable to respond to a subsequent immunizing injection of SRBC even after an inoculation of normal thymocytes. If, however, the mice are not thymocyte reconstituted prior to the pretreatment with SRBC, they can respond almost normally to an immunizing injection of SRBC if inoculated with normal thymocytes after the termination of antigen pretreatment.
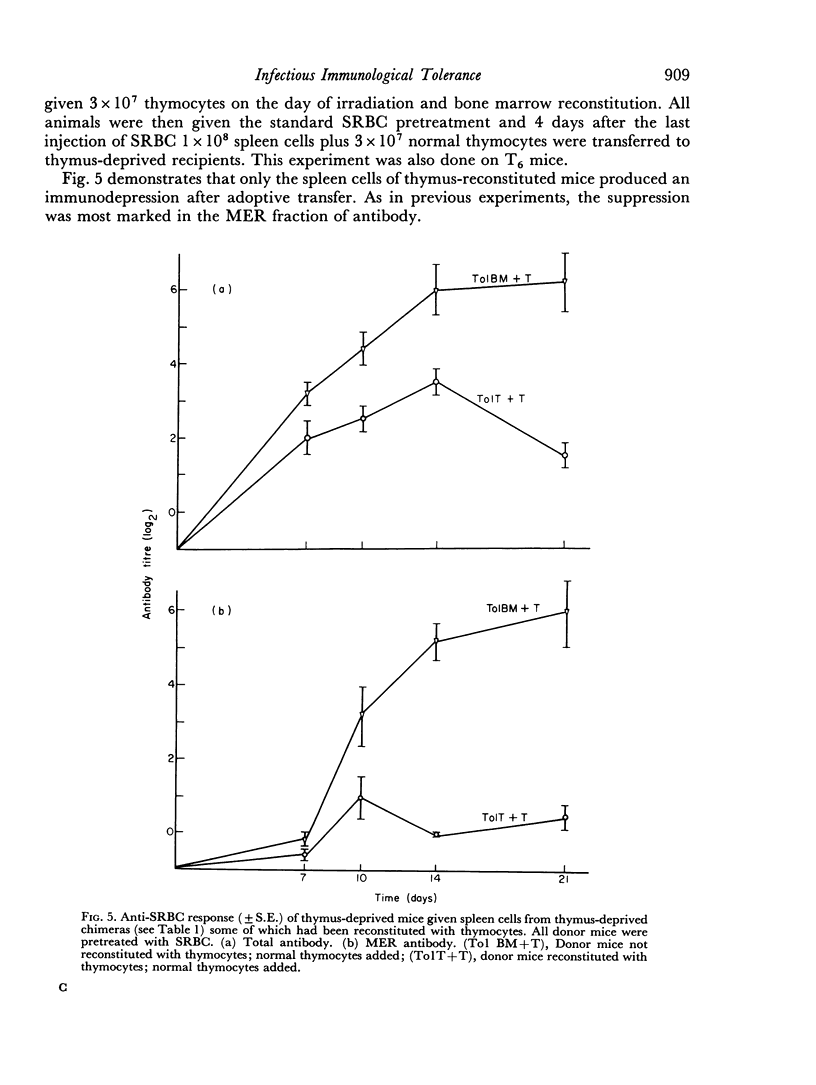
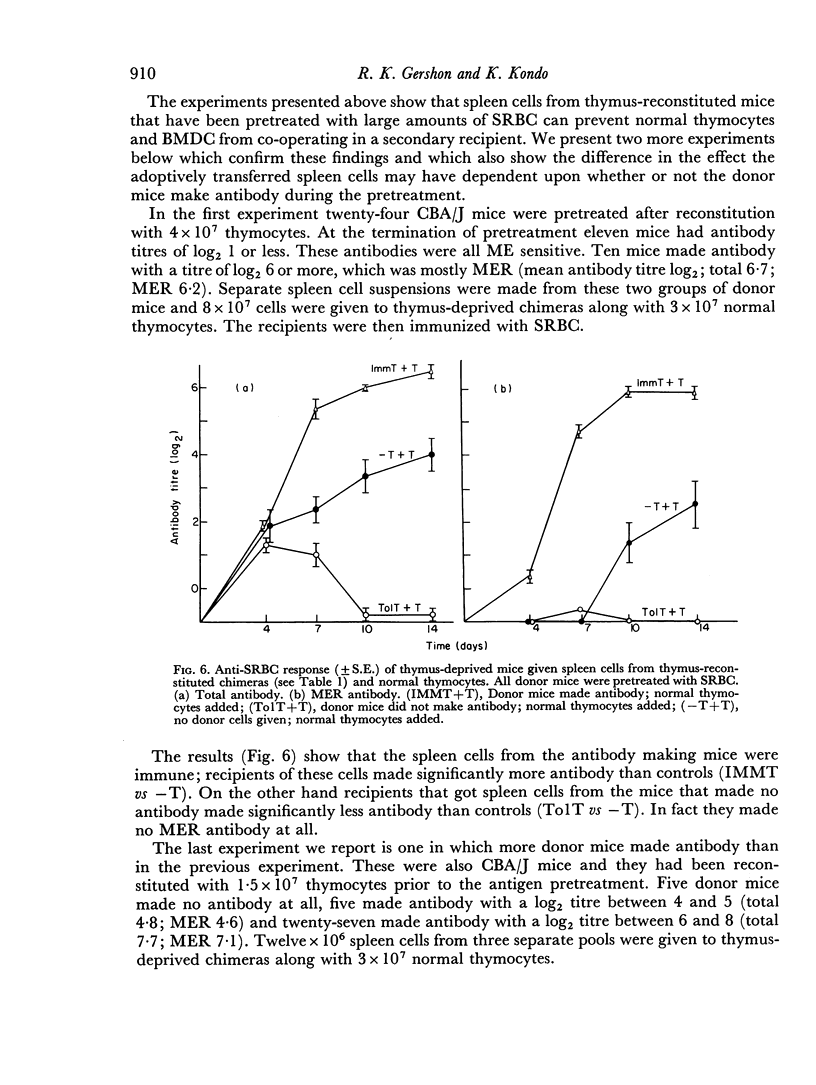
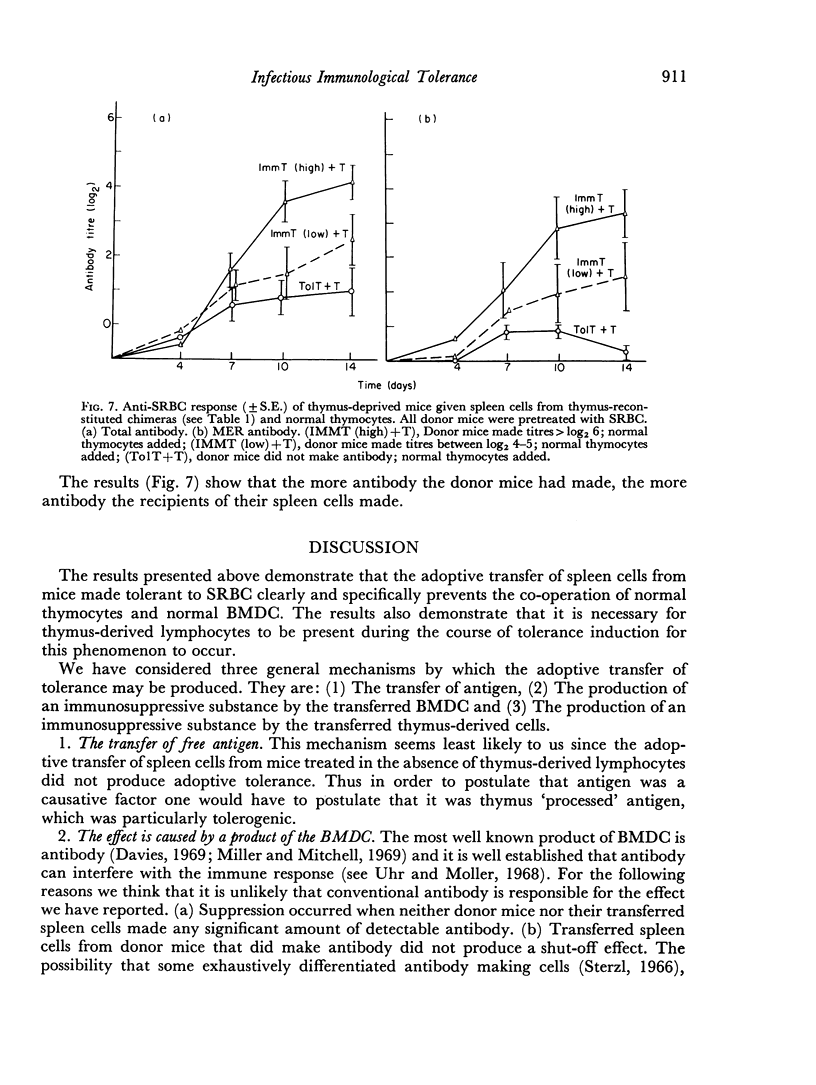
In the present study the immunosuppressive effect of the presence of thymocytes during the antigen pretreatment was studied by adoptively transferring the spleen cells of the antigen pretreated mice to thymus-deprived chimeras. These spleen cells not only did not co-operate with normal thymocytes in the secondary hosts, but they also prevented the co-operation of normal thymocytes with normal bone marrow derived cells. Untreated spleen cells or treated spleen cells from mice not reconstituted with thymocytes did not affect cell co-operation in the secondary hosts. The abrogation of the co-operation in the secondary host was specific in that the addition of spleen cells did not affect the anti-horse red blood cell response. If the primary host made antibody as a result of the pretreatment, the transfer of their spleen cells did not prevent antibody production in the secondary host.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker P. J., Stashak P. W., Amsbaugh D. F., Prescott B., Barth R. F. Evidence for the existence of two functionally distinct types of cells which regulate the antibody response to type 3 pneumococcal polysaccharide. J Immunol. 1970 Dec;105(6):1581–1583. [PubMed] [Google Scholar]
- Chiller J. M., Habicht G. S., Weigle W. O. Cellular sites of immunologic unresponsiveness. Proc Natl Acad Sci U S A. 1970 Mar;65(3):551–556. doi: 10.1073/pnas.65.3.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claman H. N., Bronsky E. A. Inhibition of antibody production and acquired immunologic tolerance by actinomycin. J Immunol. 1965 Oct;95(4):718–721. [PubMed] [Google Scholar]
- Crowle A. J., Hu C. C. Adoptive transfer of immunologic tolerance into normal mice. J Immunol. 1969 Dec;103(6):1242–1247. [PubMed] [Google Scholar]
- Davies A. J. The thymus and the cellular basis of immunity. Transplant Rev. 1969;1:43–91. doi: 10.1111/j.1600-065x.1969.tb00136.x. [DOI] [PubMed] [Google Scholar]
- Gershon R. K., Kondo K. Antigenic competition between heterologous erythrocytes. I. Thymic dependency. J Immunol. 1971 Jun;106(6):1524–1531. [PubMed] [Google Scholar]
- Gershon R. K., Kondo K. Cell interactions in the induction of tolerance: the role of thymic lymphocytes. Immunology. 1970 May;18(5):723–737. [PMC free article] [PubMed] [Google Scholar]
- Horiuchi A., Waksman B. H. Role of the thymus in tolerance. 8. Relative effectiveness of nonaggregated and heat-aggregated bovine gamma globulin, injected directly into lymphoid organs of normal rats, in suppressing immune responsiveness. J Immunol. 1968 Dec;101(6):1322–1332. [PubMed] [Google Scholar]
- MILLER J. F. Studies on mouse leukaemia. The role of the thymus in leukaemogenesis by cell-free leukaemic filtrates. Br J Cancer. 1960 Mar;14:93–98. doi: 10.1038/bjc.1960.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullagh P. J. The immunological capacity of lymphocytes from normal donors after their transfer to rats tolerant of sheep erythrocytes. Aust J Exp Biol Med Sci. 1970 Aug;48(4):369–379. doi: 10.1038/icb.1970.39. [DOI] [PubMed] [Google Scholar]
- McGregor D. D., McCullagh P. J., Gowans J. L. The role of lymphocytes in antibody formation. I. Restoration of the haemolysin response in x-irradiated rats with lymphocytes from normal and immunologically tolerant donors. Proc R Soc Lond B Biol Sci. 1967 Sep 12;168(1012):229–243. doi: 10.1098/rspb.1967.0063. [DOI] [PubMed] [Google Scholar]
- Miller J. F., Mitchell G. F. Thymus and antigen-reactive cells. Transplant Rev. 1969;1:3–42. doi: 10.1111/j.1600-065x.1969.tb00135.x. [DOI] [PubMed] [Google Scholar]
- Morris A., Möller G. Regulation of cellular antibody synthesis effect of adoptively transferred antibody-producing spleen cells on cellular antibody synthesis. J Immunol. 1968 Sep;101(3):439–445. [PubMed] [Google Scholar]
- Playfair J. H. Specific tolerance to sheep erythrocytes in mouse bone marrow cells. Nature. 1969 May 31;222(5196):882–883. doi: 10.1038/222882a0. [DOI] [PubMed] [Google Scholar]
- Raff M. Theta isoantigen as a marker of thymus-derived lymphocytes in mice. Nature. 1969 Oct 25;224(5217):378–379. doi: 10.1038/224378a0. [DOI] [PubMed] [Google Scholar]
- SAHIAR K., SCHWARTZ R. S. INHIBITION OF 19S ANTIBODY SYNTHESIS BY 7S ANTIBODY. Science. 1964 Jul 24;145(3630):395–397. doi: 10.1126/science.145.3630.395. [DOI] [PubMed] [Google Scholar]
- SEVER J. L. Application of a microtechnique to viral serological investigations. J Immunol. 1962 Mar;88:320–329. [PubMed] [Google Scholar]
- Scott D. W., Gershon R. K. Determination of total and merecaptothanol-resistant antibody in the same serum sample. Clin Exp Immunol. 1970 Feb;6(2):313–316. [PMC free article] [PubMed] [Google Scholar]
- Shellam G. R., Nossal G. J. Mechanism of induction of immunological tolerance. IV. The effects of ultra-low doses of flagellin. Immunology. 1968 Feb;14(2):273–284. [PMC free article] [PubMed] [Google Scholar]
- Sterzl J. Immunological tolerance as the result of terminal differentiation of immunologically competent cells. Nature. 1966 Jan 22;209(5021):416–417. doi: 10.1038/209416a0. [DOI] [PubMed] [Google Scholar]
- Theis G. A., Siskind G. W. Selection of cell populations in induction of tolerance: affinity of antibody formed in partially tolerant rabbits. J Immunol. 1968 Jan;100(1):138–141. [PubMed] [Google Scholar]
- Tong J. L., Boose D. Immunosuppressive effect of serum from CBA mice made tolerant by the subernatant from ultracentrifuged bovine gamma globulin. J Immunol. 1970 Aug;105(2):426–430. [PubMed] [Google Scholar]
- Uhr J. W., Möller G. Regulatory effect of antibody on the immune response. Adv Immunol. 1968;8:81–127. doi: 10.1016/s0065-2776(08)60465-4. [DOI] [PubMed] [Google Scholar]
- Walker J. G., Siskind G. W. Studies on the control of antibody synthesis. Effect of antibody affinity upon its ability to suppress antibody formation. Immunology. 1968 Jan;14(1):21–28. [PMC free article] [PubMed] [Google Scholar]
- Wigzell H. Antibody synthesis at the cellular level. Antibody-induced suppression of 7S antibody synthesis. J Exp Med. 1966 Nov 1;124(5):953–969. doi: 10.1084/jem.124.5.953. [DOI] [PMC free article] [PubMed] [Google Scholar]


