Abstract
New approaches to increase the efficiency of non-viral gene delivery are still required. Here we report a simple approach that enhances gene delivery using permanent and pulsating magnetic fields. DNA plasmids and novel DNA fragments (PCR products) containing sequence encoding for green fluorescent protein were coupled to polyethylenimine coated superparamagnetic nanoparticles (SPIONs). The complexes were added to cells that were subsequently exposed to permanent and pulsating magnetic fields. Presence of these magnetic fields significantly increased the transfection efficiency 40 times more than in cells not exposed to the magnetic field. The transfection efficiency was highest when the nanoparticles were sedimented on the permanent magnet before the application of the pulsating field, both for small (50 nm) and large (200–250 nm) nanoparticles. The highly efficient gene transfer already within 5 min shows that this technique is a powerful tool for future in vivo studies, where rapid gene delivery is required before systemic clearance or filtration of the gene vectors occurs.
INTRODUCTION
Non-viral gene vectors such as cationic polymers, are less immunogenic, can be mass produced, easily shipped and targeted to organs effectively (1,2). Among the non-viral vectors, polyethylenimines (PEI) are known to exhibit efficient transfection properties both in vitro and in vivo owing to their ability to condense DNA and RNA into stable complexes, polyplex, via electrostatic interactions. Polyplex formation protects RNA and DNA from degradation by enzymes (1,3–6). The nucleotide/polymer polyplexes are taken up into the cells via receptor-mediated endocytosis into the endosomes. PEI apparently induces a massive proton accumulation in the endosomes followed by passive chloride influx leading to osmotic swelling of the endosomes (proton sponge effect). Finally endosomes burst releasing detectable fraction of polyplexes into the cytoplasm and the DNA translocates into the nucleus (6–8). PEI also easily associates with superparamagnetic iron oxide nanoparticles (SPIONs), which is an added advantage in delivery, since their combination with magnets can draw particles to a desired location and keep them at a specific site. The uptake of DNA/SPIONs complexes into the cells is by unspecific endocytosis similar to that of DNA/PEI polyplexes (8).
The coupling of magnetic nanoparticles to gene vectors in presence of static (permanent) magnets (magnetofection™) has been shown to result in dramatic increase in transfection efficiency of reporter genes when compared with conventional transfection system (8–11); however, the transfection rates of PEI-based vectors have been shown to be lower than those with viral gene vectors (12). In order to maximize the transfection efficiencies, the DNA entry into the cells and the nuclear uptake of the DNA for its expression has to be enhanced. The cellular uptake, endocytosis and cytoplasmic movement of smaller polyplexes (<150–200 nm) have been shown to be higher than larger ones (13,14). We thus synthesized smaller (50 nm) SPIONs since the particles used previously were larger with an average size of 200 nm. We then explored the effect of a pulsating magnetic field, in which alternating horizontal, perpendicular and oscillating movements of the magnetic particles are induced, on the transfection efficiency with the 50 nm SPIONs and larger (200–250 nm) commercially available polyMAG nanoparticles.
The nuclear uptake of DNA is dependent on the size of DNA, and shorter DNA fragments are easily transported across the nuclear pores by diffusion, whereas plasmids tend to remain in the cytoplasm (15–17). We generated novel shorter, 1.6 kb, DNA fragments (PCR products) whose sequence included only the GFP gene, 5′human cytomegalovirus (CMV) immediate early promoter and 3′ SV40 early mRNA polyadenylation signal, eliminating the requirement of biotin–streptavidin binding (18). The PCR products were used for transfection in presence of pulsating and static magnetic fields. Our study demonstrated for the first time that the use of magnets also enhances the transfection of PCR products containing only the gene of interest. The application of a pulsating magnetic field proved to be a powerful tool for the enhancement of gene delivery, already within 5 min after exposure to magnetic field.
MATERIALS AND METHODS
Coating of superparamagnetic iron oxide nanoparticles
SPIONs were prepared by alkaline co-precipitation of ferric and ferrous chlorides in aqueous solution as described previously (19). Briefly, solutions of FeCl3·6H2O (0.086 M) and FeCl2·4H2O (0.043 M) were mixed and precipitated with concentrated ammonia while stirring vigorously. The black precipitate, which immediately formed was washed several times with ultra-pure water until the pH decreased from 10 to 7. The solid was collected and refluxed in a mixture of 0.8 M nitric acid and 0.21 M aqueous Fe(NO3)3·9H2O for 1 h. During this step the initial black slurry turned brown and the formation of nitric oxide could be observed. The system was allowed to cool to room temperature, the remaining liquid was discarded, and 100 ml of ultra-pure water was added to the slurry mixture, which immediately dispersed. The brown suspension was dialyzed for 2 days against 0.01 M nitric acid, and stored at 4°C. Aqueous colloidal dispersions of SPIONs coated with 25 kDa PEI (Aldrich) with an average size of 50 nm were prepared according to the following procedure. A PEI solution (20% w/w) was prepared and 0.1 ml of this solution was added to 0.9 ml of standard iron oxide dispersion (iron content 10.6 mg iron/ml dispersion) in 1.5 ml Eppendorf tubes. After 12 h of incubation the particles were used for transfection experiments. The particles were diluted (1:800) in phosphate-buffered saline (PBS) (pH 7.4) just before the transfections. Characterization of the coated SPIONs was done by transmission electron microscope and photon correlation spectroscopy. In addition, the transfection efficiency using commercially available nanoparticles, polyMAG® (Chemicell), with a mean size of 200–250 nm, was also determined.
Plasmid and PCR products
The pEGFP-C2 plasmid (Clontech) was propagated in Escherichia coli and endotoxin-free plasmid DNA was purified using an Endotoxin-free Maxiprep plasmid Kit (Qiagen).
PCR products from pEGFP-C2 plasmid (Clontech) were amplified using primers designed to include the 5′human CMV immediate early promoter and a 3′SV40 early mRNA polyadenylation signal, (forward primer, 3′-CCG TAT TAC CGC CAT GCA T-5′; reverse primer, 3′-GCC GAT TTC GGC CTA TTG GT-5′). The PCR products (1.6 kb) were purified using PCR purification Kit (Qiagen).
Cell cultures
HeLa (human cervix carcinoma cells), 293T and Cos7 (fibroblasts) cells were purchased from America Type Culture Collection (ATCC) and were grown in DMEM (Gibco-BRL), supplemented with 10% fetal calf serum (FCS; Gibco-BRL) and 1% penicillin/streptomycin. Primary ovine synovial membrane cells (synoviocytes) were harvested and prepared for cell culture as described previously (20,21). Briefly, synovial membranes of healthy sheep were collected under sterile conditions from the stifle joints immediately after slaughtering. Subsequently, the synoviocytes were isolated in a digestion chamber at 37°C, using trypsin and collagenase under constant stirring, washed by centrifugation and re-suspended in Nutrient Mixture F-12 (HAM) medium with l-Glutamine, supplemented with 10% FCS and 1% penicillin/streptomycin (22). Cells were grown at 37°C in a water saturated atmosphere and 5% CO2.
Transfection with plasmid DNA and exposure to permanent magnetic field
The transfection procedure (10) was optimized and 0.3–0.5 × 106 cells were seeded in 6-well plates, one day before transfection to obtain 80–90% confluence. All incubations were done at 37°C in a water saturated atmosphere and 5% CO2. Before transfection, the medium was removed and the cells washed once with PBS (pH 7.4) before addition of 2 ml serum-free medium.
The DNA was diluted in autoclaved distilled water to get a final concentration of 25 and 50 µg/ml, for polyMAG and SPIONs transfections, respectively. Hundred microliters of 25 µg/ml DNA solution was aliquoted into eppendorf tubes and an equal volume (100 µl) of polyMAG diluted with PBS (1:50), was added. SPIONs (100 µl) diluted 1:800 in PBS were added to 100 µl aliquots of 50 µg/ml DNA solution. The samples were prepared in triplicates. The DNA/particle mixture was gently pipetted up and down about five times and incubated at room temperature for 30 min, before the 200 µl mixture was added to each well. After mixing, unless otherwise stated, the cell culture plates were placed on a neodymium–iron–boron (NdFeB) permanent magnet (Br = 1.1 T, Maurer Magnets, Switzerland) for 20 min. The cells were at a distance of 2 mm from the magnet surface, which leads to a magnetic flux density of 250 mT and a magnetic field gradient perpendicular to the well plate of 10 T/m in the area of the cells for 20 min. After a further incubation of 4 h, medium was removed and new medium containing 10% FCS was added.
Concurrently, transfection was done using 20% (w/w) PEI, which was diluted 1:1000 in PBS and aliquots of 100 µl were added to an equal volume of DNA (100 µg/ml). After mixing and incubation for 30 min at room temperature, the mixture (200 µl) was added to each well. After 20 min incubation, the medium was replaced with new medium containing 10% FCS. 2.5 µg DNA/well was complexed to the lipofectamine and transfection done according to the manufacturer's protocol (Invitrogen). An aliquot of 10 µg DNA/well was used for a routine calcium phosphate transfection (23). As controls, PBS was added as a sham solution instead of the polymer or the particles. To determine if short exposure to magnetic field would influence transfection efficiency, the above transfection methods were used; however, medium was replaced 5 min after each transfection protocol.
Application of pulsating magnetic field
Transfection was done as stated above with the exception that a pulsating magnetic field was applied for 5 min before or after placing the cells on a static magnetic plates for 5 min. The pulsating field was generated using the dynamic magnetic field generator ‘Dynamic Marker’ developed by Stetter-Elektronik, Seeheim-Jugenheim, Germany. The applicator was modified in order to accommodate the entire 6-well plate within the magnetic field. More details regarding the equipment can be found on the company's website http://www.feldkraft.de.
For these investigations, a stationary wave of sinus type was applied with a maximum amplitude of 27 mT and a field gradient of 10 T/m perpendicular to the well plate. As a control, cells were either exposed to the pulsating magnetic field only for 5 min, or placed only on static magnetic plates for 5 min, or exposed to neither of these for 5 min. The medium was subsequently removed and replaced with new medium containing 10% FCS.
Determination of green fluorescent protein (GFP) expression and viability by microscopy and flow cytometry
After 24 h of incubation, the medium was discarded and replaced with PBS, 20 µg/ml propidium iodide (PI) was added and cells were subsequently incubated at 37°C for 30 min. Transfected cells expressing GFP and red non-viablecells stained with PI were detected using a Leica fluorescence microscope. Cells were harvested by trypsinization, washed twice with PBS, and at least 10 000 cells were acquired using Beckman Coulter FC500 cytometer and analyzed using a CXP analysis software.
RESULTS
Increased transfection of EGFP plasmid in presence of a static magnetic field
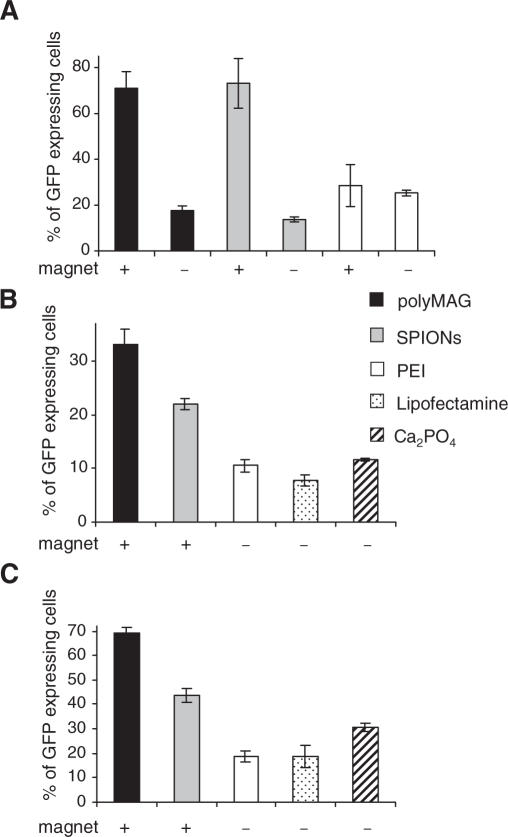
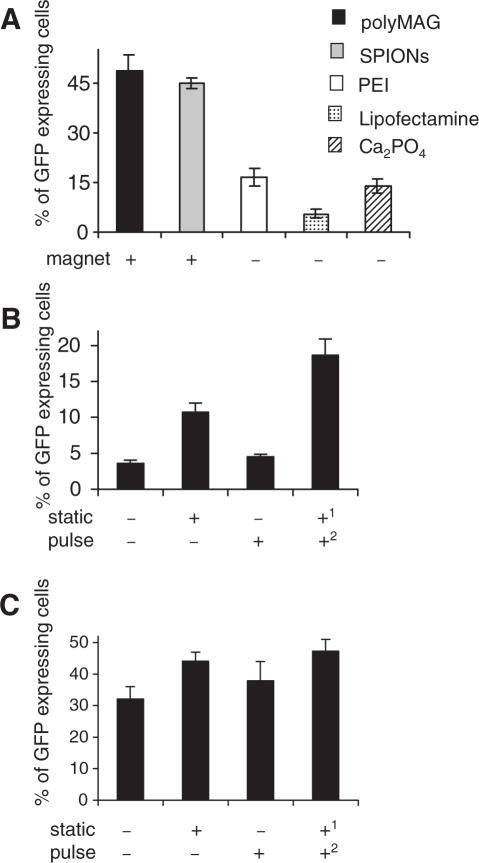
Cells were transfected as stated above with either DNA/polyMAG or DNA/SPIONs complexes, or DNA/PEI polyplexes. Exposure to a permanent (static) magnetic field for 20 min followed by a 4 h incubation, resulted in transfections rates of 71.0 ± 7.3% and 73.2 ± 10.9% in 293T cells treated with DNA/polyMAG and DNA/SPIONs complexes, respectively (Figure 1A). In contrast, in cells not exposed to magnetic field, the rates were significantly lower, corresponding to 17.7 ± 2.0% and 13.7 ± 1.1%, respectively. In cells treated with DNA/PEI polyplexes, medium was replaced after 20 min of incubation, since longer incubation period resulted in high toxicity. The transfection rate of 28.5 ± 9.2% was obtained. Presence of a magnetic field did not influence the transfection efficiency (Figure 1A).
Figure 1.
Effect of magnetic field on transfection efficiency of GFP plasmid. (A) The 293T cells were transfected with either DNA/polyMAG or DNA/SPIONs in presence or absence of permanent magnetic field for 20 min and medium was replaced after 4 h, or cells were transfected with DNA/PEI polyplexes with or without magnetic field for 20 min. (B) The 293T cells and (C) synoviocytes were transfected with either DNA/polyMAG or DNA/SPIONs in presence or absence of permanent magnetic field for 5 min before medium was replaced, or transfected with either PEI, lipofectamine or calcium phosphates for the same duration. Data represent the mean values of at least three independent experiments. Results are shown as means and SD values from at least three independent experiments.
When 293T cells were transfected with DNA/polyMAG or plasmid/SPIONs complexes and exposed to a static magnetic field for 5 min before the medium was changed, the transfection rates were significantly enhanced over those of the conventional transfection methods (Figure 1B). The transfection rates were 1.5 fold higher, using the larger polyMAG nanoparticles than with the smaller SPIONs. Similarly, significantly higher transfection rates with nanoparticles were also obtained with synoviocytes within 5 min of exposure to a magnetic field (Figure 1C), and with Cos7 and HeLa cells (data not shown).
Pulsating magnetic field enhances transfection efficiency of plasmid DNA
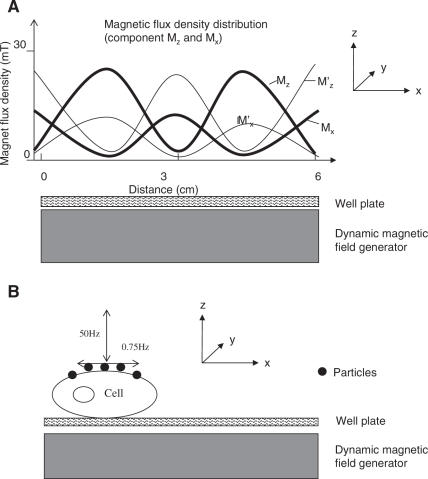
When a stationary wave of sinus type was applied with maximum amplitude of 27 mT and a field gradient of 10 T/m perpendicular to the 6-well plate. The magnetic field strength in z-direction Mz (Figure 2A) was modulated so that two maxima with a distance of 3 cm in x-directions occurred. The component of the magnetic field strength in x-direction Mx showed the same modulation. Since the magnetic field was generated by electromagnets, the applied frequency of the field corresponded to the frequency of the applied current of the coils, in our case 50 Hz. In addition to this oscillation, an oscillation of the entire magnetic field in x-direction was generated. This oscillation in x-direction had a frequency of 0.75 Hz with amplitude 1.5 cm. The two extreme positions of this movement of the magnetic field in x-direction are shown in Figure 2A (Mz and ; Mx and ). The field gradient perpendicular to the wave direction was negligible.
Figure 2.
Magnetic flux densities after the application of pulsating field on cells. (A) The dynamic field generator, [Dynamic Marker, Stetter Electronik, Seeheim-Jugenheim, Germany (http://www.feldkraft.de)] was placed under the 6-well plate; the distance between the surface of the generator and the cells is 2 mm. The 6-well plate is placed in the x, y plane. The z-direction is perpendicular to the plate. The applied magnetic field strength is described in its three main directions x, y and z, as indicated in the figure, whereas the field strength in x-direction is called Mx, the field strength in y-direction (perpendicular to the plane of the figure) My, and the field strength z perpendicular to the 6-well plate Mz. Mz and Mx are sinusoidal in x-direction, whereas My is homogeneous and very low (data not shown). Additionally, the magnetic field is alternating with a frequency of 0.75 Hz between two extreme positions in the x-direction. The magnetic field strength distribution for these two extreme positions is shown in the figure (Mz and ; Mx and ). (B) Schematic illustration of the cell/particle interaction within the pulsating magnetic field.
This dynamic magnetic field forced the particles to oscillate perpendicular and parallel to the surface of the well with a frequency of 50 and 0.75 Hz, respectively. The movement is schematically shown in Figure 2B. Additional rotational movements of the particles could not be excluded. A gradual temperature increase from 37 to 42.5°C was recorded on the surface of the generator during the 5 min pulse application.
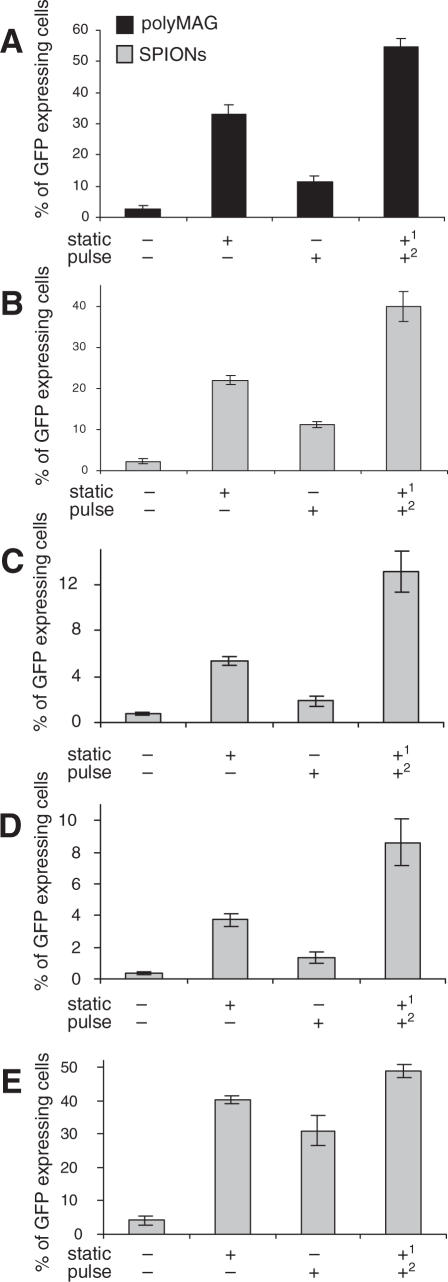
Exposure of 293T cells, transfected with DNA/polyMAG or DNA/SPIONs complexes, to static magnetic field alone for 5 min increased the transfection rates by 12- and 10-fold, respectively (Figure 3A and B). In HeLa, Cos7 cells and synoviocytes transfected with DNA/SPIONs, the increase was 10-, 7- and 10-fold, respectively, when compared with cells not exposed to any magnetic field (Figure 3C–E). Application of a pulsating magnetic field alone for 5 min resulted in 4- and 5-fold increase in transfection in 293T cells treated with DNA/polyMAG and DNA/SPIONs, respectively (Figure 3A and B), while in HeLa, Cos7 cells and synoviocytes, the increase was 3-, 4- and 8-fold, respectively (Figure 3C–E). The effect of the pulsating field on other transfection methods was also determined. In 293T cells transfected with PEI/DNA polyplexes, this increase was insignificant, while in cells transfected with lipofectamine and calcium phosphate, the increase was 1.8- and 1.6-fold, respectively, when compared with cells not exposed to a magnetic field (data not shown).
Figure 3.
Effect of pulsating magnetic field on expression of GFP. The 293T cells were transfected with either DNA/polyMAG (A) or DNA/SPIONs (B), and placed either on a static magnetic plate for 5 min, or a pulsating magnetic field was applied for 5 min, or the pulsating field was applied after the static field. (C) HeLa cells, (D) Cos7 cells and (E) synoviocytes were transfected with DNA/SPIONs as stated above. Control cells were not exposed to any magnetic field. Numbers 1 and 2 represent the application sequence of the magnetic fields. Results are shown as means and SD values from at least three independent experiments.
Application of a pulsating magnetic field to cells already exposed to a static field, resulted in 2-fold increase on average of the transfection rates, over cells exposed only to the static field (Figure 3A–E). This corresponded to 20-fold increase in transfection efficiency over cells not exposed to any magnetic field. Exposure to a static magnetic field after application of the pulsating field increased the transfections 1.4-fold, when compared to that obtained in presence of a static magnetic field alone (data not shown).
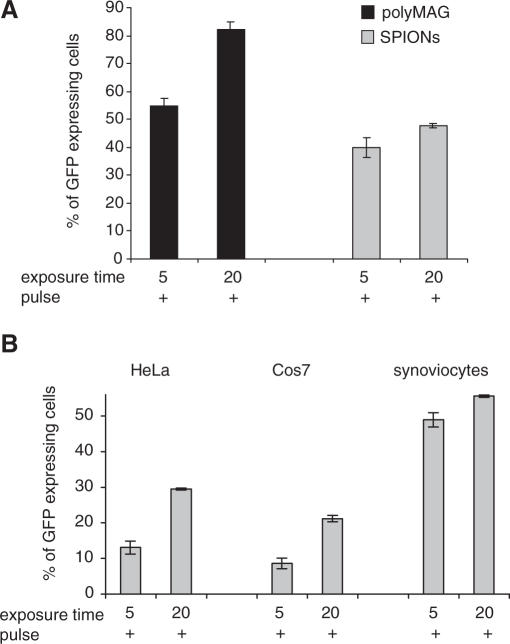
The duration of the exposure to static magnetic field was increased from 5 to 20 min before the application of the pulsating field. In 293T cells treated with larger polyMAG, the transfection efficiency increased 1.5-fold, from 54.7 ± 2.8 to 82.2 ± 2.8%, corresponding to a 40-fold increase, when compared to cells not exposed to a magnetic field. This was the highest transfection rate achieved in these cells and it surpassed the efficiency achieved after routine magnetofection of 20 min exposure to permanent magnetic field, followed by 4 h incubation, before medium was replaced (Figure 1A). With smaller SPIONs, the increase was 1.2-fold, from 39.9 ± 3.6 to 47.8 ± 0.9% (Figure 4A), while in HeLa and Cos7 cells the increase was 2.4- and 1.1-fold in synoviocytes, when compared to cells exposed to a static field for 5 min before the application of the pulsating field (Figure 4B).
Figure 4.
Dependence of transfection on duration of exposure to magnetic field. (A) The 293T cells transfected with either DNA/polyMAG or DNA/SPIONs, and subsequently placed on a static magnetic plate either for 5 or 20 min before application of a pulsating magnetic field. (B) HeLa, Cos7 cells and synoviocytes were transfected with DNA/SPIONs similarly. Results are shown as means and SD values from at least three independent experiments.
Magnetic field enhances transfection with EGFP PCR products
To determine if enhanced transfection efficiency by magnetic field was not limited only to plasmid vectors, shorter DNA fragments (1.6 kb), PCR products of amplified EGFP gene were used for transfections. The 293T cells were treated with either PCR-DNA/polyMAG or PCR-DNA/SPIONs and placed on static magnet for 20 min. Medium was replaced after 4 h incubation. Concurrently cells were routinely transfected using conventional methods. In cells transfected with PCR-DNA/polyMAG and PCR-DNA/SPIONs complexes, the transfection rates were 48.4 ± 4.8 and 44.8 ± 1.5%, respectively. Significantly lower rates of 16.6 ± 2.7, 5.5 ± 1.5 and 14.0 ± 2.1% resulted from transfection with PEI, lipofectamine and calcium phosphate, respectively (Figure 5A). The GFP expression and protein distribution within cells transfected with PCR products was similar to cells transfected with the plasmid (data not shown).
Figure 5.
Effect of magnetic field on transfection of PCR products. (A) The 293T cells were transfected with PCR-DNA/polyMAG or PCR-DNA/SPIONs in presence of a static magnetic field for 20 min, and medium replaced after 4 h, or cells were routinely transfected with either PEI, lipofectamine or calcium phosphate. (B) The 293T cells and (C) synoviocytes were transfected with PCR-DNA/polyMAG and placed either on a static magnetic plate for 5 min, or a pulsating magnetic field was applied for 5 min, or the pulsating field was applied after the static field. Control cells were not exposed to any magnetic field. Numbers 1 and 2 represent the sequence of application of the magnetic fields. Results are shown as means and SD values from at least three independent experiments.
The effect of pulsating magnetic field on transfection efficiency of PCR products was also determined. The 293T cells and synoviocytes treated with PCR-DNA/polyMAG were exposed to both static and pulsating magnetic field for 5 min as stated above. Exposure to the static magnetic field alone resulted in a 3-fold increase of the transfection efficiency in 293T, when compared to control cells not exposed to magnet. This efficiency was further enhanced by 1.7-fold after the application of the pulsating magnetic field (Figure 5B). This was six times higher than in cells not exposed to a magnetic field. Enhanced transfection efficiency was also obtained in synoviocytes (Figure 5C). As observed with the plasmid, the application of pulsating magnetic field on its own or its use before the exposure to a static field, increased the transfection; however, the rates were lower than when static field exposure was followed by application of a pulsating magnetic field (Figure 5B and C and data not shown).
DISCUSSION
We have enhanced non-viral gene delivery by using a novel technique of combining the application a pulsating magnetic field to a static field. The presence of the static magnetic field for 5 min was adequate enough to significantly increase the transfection efficiency over that obtained with standard transfection methods. This is consistent with results from previous studies, which showed that use of static magnet (magnetofection) enhanced the transfection efficiency (8–10,24–26).
The application of a pulsating magnetic field proved to be a powerful tool for the enhancement of gene delivery. This was most obvious in cells treated with larger DNA/polyMAG and exposed for 20 min to a static magnetic field prior to the application of the pulsating field. The highest transfection efficiency was achieved, which surpassed that of routine magnetofection, which required at least 4 h incubation, and that with conventional non-viral transfection methods. The combined effect of the pulsating and static magnetic fields eliminated the necessity of longer incubation for effective gene delivery, and the enhancement was evident within 5 min after the sedimentation of the particles on the cells by a static magnet. This efficiency was attributed to the horizontal, perpendicular and additional oscillating movements of the particles within the pulsating magnetic field on the surface of the cells. These movements seemed to enhance the translocation of the particles across the cell membrane.
Although the temperature increase on the surface of the magnetic pulse generator could also have contributed to enhanced transfection as reported previously (27), it should be noted however, that this increase was most significant in cells transfected with superparamagnetic particles and thus can be attributed to nanoparticle properties in the presence of the pulsating magnetic field. The alternating magnetic field, used in our study, was at lower frequencies and thus any possibility that the superparamagnetic particles are producing heat when exposed to higher frequencies (28) can be ruled out.
The higher transfection efficiencies with polyMAG particles after 5 min in a magnetic field was attributed to their size (200–250 nm), since they required less time to sediment on the cells. This was consistent with earlier observations that larger particles faster sedimentated on the cells (8,9,29,30) and this resulted in higher cellular uptake. In contrast, the 50 nm SPIONs had a 10 times lower velocity in z-direction, when compared to the polyMAG particles, since transport velocity in the magnetic field gradient is dependent on the amount of magnetic material and the hydrodynamic radius, including attached DNA (Equation 1).
| 1 |
where umag represents the velocity of the particle in a magnetic field gradient; Vmag, the volume of magnetically active material; M, magnetic field strengths; dB/dz, gradient of the magnetic flux density in z-direction; η, viscosity of the cell medium and rhydr, hydrodynamic radius of the particle.
The content of magnetic material in the SPIONs was nevertheless high enough, allowing them to have high and similar transfection efficiency as the larger particles, after longer incubation of 4 h. Time dependency of transfection efficiency on magnetic field was also shown previously in measles virus gene delivery (26). The pulsating magnetic field also enhanced the transfection efficiency of the 50 nm SPIONs, after shorter incubation period. This observation is beneficial for future studies, since preliminary work done by our group has shown that the smaller nanoparticles are better tolerated in vivo than the larger ones, after intraarticular application.
The transfection rates in synoviocytes was higher than in other cells and we attributed this to their macrophage-like characteristics (31–33). This was also shown in a previous study by our group, where >80% of the synoviocytes took up SPIONs within 24 h (20).
Our study exploited the superior DNA binding properties of SPIONs, for transfection of PCR products whose sequence included only the gene of interest, 5′human CMV immediate early promoter and 3′ SV40 early mRNA polyadenylation signal, thus eliminating the requirement of biotin–streptavidin binding (18). The transfection efficiencies of PCR products were slightly lower than plasmid, consistent with earlier observation (18,34). This could be due to PEI's preference to complex supercoiled DNA rather than linearized DNA (35) or presence of free DNA ends, which are more prone to exonuclease-mediated DNA degradation. Nevertheless, our results clearly showed that the PCR products are effective gene vectors on their own and the application of pulsating magnetic field enhanced their efficacy. This is a step forward towards safer clinical gene therapy. Transfection rates achieved using polyMAG and SPIONs particles were significantly higher than those with conventional methods, indicating that the complexation of DNA to SPIONs protects against cleavage by nucleases a feature, which PEI was reported previously to possess (4–6).
Our study shows for the first time that use of magnets also enhances the transfection of PCR products, which would be more suited for clinical gene therapy. The application of a pulsating magnetic field proved to be a powerful tool for the enhancement of gene delivery, already within 5 min after exposure to magnetic field. This technique proved to be an efficient tool for future in vivo gene delivery studies, where rapid gene delivery is required before systemic clearance or filtration of the gene vectors occurs.
Acknowledgments
This project was funded by the Vetsuisse-Faculty of the Universities of Berne and Zurich, Switzerland. Funding to pay the Open Access publication charges for this article was provided by the Vetsuisse-Faculty Switzerland and the Kanton of Zurich, Switzerland.
Conflict of interest statement. None declared.
REFERENCES
- 1.Hildebrandt I.J., Iyer M., Wagner E., Gambhir S.S. Optical imaging of transferrin targeted PEI/DNA complexes in living subjects. Gene Ther. 2003;10:758–764. doi: 10.1038/sj.gt.3301939. [DOI] [PubMed] [Google Scholar]
- 2.Huang L.V.E., Viroonchaatapan E. Introduction. In: Huang L., Hung M-C., Wagner E., editors. Nonviral Vectors for Gene Therapy. London: Academic Press; 1999. pp. 4–22. [Google Scholar]
- 3.Aigner A., Fischer D., Merdan T., Brus C., Kissel T., Czubayko F. Delivery of unmodified bioactive ribozymes by an RNA-stabilizing polyethylenimine (LMW-PEI) efficiently down-regulates gene expression. Gene Ther. 2002;9:1700–1707. doi: 10.1038/sj.gt.3301839. [DOI] [PubMed] [Google Scholar]
- 4.Neu M., Fischer D., Kissel T. Recent advances in rational gene transfer vector design based on poly(ethylene imine) and its derivatives. J. Gene Med. 2005;7:992–1009. doi: 10.1002/jgm.773. [DOI] [PubMed] [Google Scholar]
- 5.Brus C., Petersen H., Aigner A., Czubayko F., Kissel T. Efficiency of polyethylenimines and polyethylenimine-graft-poly (ethylene glycol) block copolymers to protect oligonucleotides against enzymatic degradation. Eur. J. Pharm. Biopharm. 2004;57:427–430. doi: 10.1016/j.ejpb.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 6.Lungwitz U., Breunig M., Blunk T., Gopferich A. Polyethylenimine-based non-viral gene delivery systems. Eur. J. Pharm. Biopharm. 2005;60:247–266. doi: 10.1016/j.ejpb.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 7.Godbey W.T., Wu K.K., Mikos A.G. Tracking the intracellular path of poly(ethylenimine)/DNA complexes for gene delivery. Proc. Natl Acad. Sci. USA. 1999;96:5177–5181. doi: 10.1073/pnas.96.9.5177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huth S., Lausier J., Gersting S.W., Rudolph C., Plank C., Welsch U., Rosenecker J. Insights into the mechanism of magnetofection using PEI-based magnetofectins for gene transfer. J. Gene Med. 2004;6:923–936. doi: 10.1002/jgm.577. [DOI] [PubMed] [Google Scholar]
- 9.Gersting S.W., Schillinger U., Lausier J., Nicklaus P., Rudolph C., Plank C., Reinhardt D., Rosenecker J. Gene delivery to respiratory epithelial cells by magnetofection. J. Gene Med. 2004;6:913–922. doi: 10.1002/jgm.569. [DOI] [PubMed] [Google Scholar]
- 10.Scherer F., Anton M., Schillinger U., Henke J., Bergemann C., Kruger A., Gansbacher B., Plank C. Magnetofection: enhancing and targeting gene delivery by magnetic force in vitro and in vivo. Gene Ther. 2002;9:102–109. doi: 10.1038/sj.gt.3301624. [DOI] [PubMed] [Google Scholar]
- 11.Plank C., Anton M., Rudolph C., Rosenecker J., Krotz F. Enhancing and targeting nucleic acid delivery by magnetic force. Expert Opin. Biol. Ther. 2003;3:745–758. doi: 10.1517/14712598.3.5.745. [DOI] [PubMed] [Google Scholar]
- 12.Varga C.M., Tedford N.C., Thomas M., Klibanov A.M., Griffith L.G., Lauffenburger D.A. Quantitative comparison of polyethylenimine formulations and adenoviral vectors in terms of intracellular gene delivery processes. Gene Ther. 2005;12:1023–1032. doi: 10.1038/sj.gt.3302495. [DOI] [PubMed] [Google Scholar]
- 13.Ogris M., Steinlein P., Carotta S., Brunner S., Wagner E. DNA/polyethylenimine transfection particles: influence of ligands, polymer size, and PEGylation on internalization and gene expression. AAPS PharmSci. 2001;3:E21. doi: 10.1208/ps030321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mahato R.I., Furgeson D.Y., Maheshwari A., Han S.O., Kim S.W. In: Polymeric Gene Delivery for Cancer Treatment. Park K.K.I., Yui N., Jeong S., Park K., editors. Seoul, Korea: Han Rim Wonn Publishing; 2000. [Google Scholar]
- 15.Van De Parre T.J., Martinet W., Schrijvers D.M., Herman A.G., de Meyer G.R. mRNA but not plasmid DNA is efficiently transfected in murine J774A.1 macrophages. Biochem. Biophys. Res. Commun. 2005;327:356–360. doi: 10.1016/j.bbrc.2004.12.027. [DOI] [PubMed] [Google Scholar]
- 16.Hebert E. Improvement of exogenous DNA nuclear importation by nuclear localization signal-bearing vectors: a promising way for non-viral gene therapy? Biol. Cell. 2003;95:59–68. doi: 10.1016/s0248-4900(03)00007-8. [DOI] [PubMed] [Google Scholar]
- 17.Lukacs G.L., Haggie P., Seksek O., Lechardeur D., Freedman N., Verkman A.S. Size-dependent DNA mobility in cytoplasm and nucleus. J. Biol. Chem. 2000;275:1625–1629. doi: 10.1074/jbc.275.3.1625. [DOI] [PubMed] [Google Scholar]
- 18.Isalan M., Santori M.I., Gonzalez C., Serrano L. Localized transfection on arrays of magnetic beads coated with PCR products. Nature Methods. 2005;2:113–118. doi: 10.1038/nmeth732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chastellain M., Petri A., Hofmann H. Particle size investigations of a multistep synthesis of PVA coated superparamagnetic nanoparticles. J. Colloid. Interface Sci. 2004;278:353–360. doi: 10.1016/j.jcis.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 20.Schulze K., Koch A., Schöpf B., Petri A., Steitz B., Chastellain M., Hofmann M., Hofmann H., von Rechenberg B. Intraarticular application of superparamagnetic nanoparticles and their uptake by synovial membrane—an experimental study in sheep. J. Magn. Mag. Mat. 2005;293:419–432. [Google Scholar]
- 21.Schöpf B., Neuberger T., Schulze K., Petri A., Chastellain M., Hofmann M., Hofmann H., von Rechenberg B. Methodology description for detection of cellular uptake of PVA coated superparamagnetic iron oxide nanoparticles (SPION) in synovial cells of sheep. J. Magn. Mag. Mat. 2005;293:411–418. [Google Scholar]
- 22.Green D., Ryan C., Malandruccolo N., Nadler H.L. Characterization of the coagulant activity of cultured human fibroblasts. Blood. 1971;37:47–51. [PubMed] [Google Scholar]
- 23.James R.F.L., Grosveld F.G. DNA-mediated gene transfer into mammalian cells. In: Walker J.M., Gaastra W., editors. Techniques in Molecular Biology. Croom Helm, London and Sydney: 1987. pp. 187–202. [Google Scholar]
- 24.Krotz F., Sohn H.Y., Gloe T., Plank C., Pohl U. Magnetofection potentiates gene delivery to cultured endothelial cells. J. Vasc. Res. 2003;40:425–434. doi: 10.1159/000073901. [DOI] [PubMed] [Google Scholar]
- 25.Krotz F., de Wit C., Sohn H.Y., Zahler S., Gloe T., Pohl U., Plank C. Magnetofection—a highly efficient tool for antisense oligonucleotide delivery in vitro and in vivo. Mol. Ther. 2003;7:700–710. doi: 10.1016/s1525-0016(03)00065-0. [DOI] [PubMed] [Google Scholar]
- 26.Kadota S., Kanayama T., Miyajima N., Takeuchi K., Nagata K. Enhancing of measles virus infection by magnetofection. J. Virol. Methods. 2005;128:61–66. doi: 10.1016/j.jviromet.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 27.Pipes B.L., Vasanwala F.H., Tsang T.C., Zhang T., Luo P., Harris D.T. Brief heat shock increases stable integration of lipid-mediated DNA transfections. Biotechniques. 2005;38:48–52. doi: 10.2144/05381BM05. [DOI] [PubMed] [Google Scholar]
- 28.Berry C.C., Curtis A.S.G. Functionalisation of magnetic nanoparticles for applications in biomedicine. J. Phys. D. Appl. Phys. 2003;36:R198–R206. [Google Scholar]
- 29.Sung S.J., Min S.H., Cho K.Y., Lee S., Min Y.J., Yeom Y.I., Park J.K. Effect of polyethylene glycol on gene delivery of polyethylenimine. Biol. Pharm. Bull. 2003;26:492–500. doi: 10.1248/bpb.26.492. [DOI] [PubMed] [Google Scholar]
- 30.Ogris M., Steinlein P., Kursa M., Mechtler K., Kircheis R., Wagner E. The size of DNA/transferrin-PEI complexes is an important factor for gene expression in cultured cells. Gene Ther. 1998;5:1425–1433. doi: 10.1038/sj.gt.3300745. [DOI] [PubMed] [Google Scholar]
- 31.Berger I., Weckauf H., Helmchen B., Ehemann V., Penzel R., Fink B., Bernd L., Autschbach F. Rheumatoid arthritis and pigmented villonodular synovitis: comparative analysis of cell polyploidy, cell cycle phases and expression of macrophage and fibroblast markers in proliferating synovial cells. Histopathology. 2005;46:490–497. doi: 10.1111/j.1365-2559.2005.01959.x. [DOI] [PubMed] [Google Scholar]
- 32.Sarkissian M., Lafyatis R. Integrin engagement regulates proliferation and collagenase expression of rheumatoid synovial fibroblasts. J. Immunol. 1999;162:1772–1779. [PubMed] [Google Scholar]
- 33.Aigner J., Tegeler J., Hutzler P., Campoccia D., Pavesio A., Hammer C., Kastenbauer E., Naumann A. Cartilage tissue engineering with novel nonwoven structured biomaterial based on hyaluronic acid benzyl ester. J. Biomed. Mater. Res. 1998;42:172–181. doi: 10.1002/(sici)1097-4636(199811)42:2<172::aid-jbm2>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 34.Zanta M.A., Belguise-Valladier P., Behr J.P. Gene delivery: a single nuclear localization signal peptide is sufficient to carry DNA to the cell nucleus. Proc. Natl Acad. Sci. USA. 1999;96:91–96. doi: 10.1073/pnas.96.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bronich T., Kabanov A., Marky L. A thermodynamic characterization of the interaction of a cationic copolymer with DNA. J. Phys. Chem. 2001;105:6041–6050. [Google Scholar]