Abstract
We report here the responses of mice with symptomatic pneumovirus infection to combined antiviral and specific immunomodulatory agents. Mice infected with pneumonia virus of mice, a natural mouse pathogen that replicates the signs and symptoms of severe infection with respiratory syncytial virus (RSV), responded to the antiviral agent ribavirin when it was administered in the setting of endogenous (gene deletion) or exogenous (antibody-mediated) blockade of the MIP-1α proinflammatory signaling cascade. Although neither treatment is effective alone, together they offer a dramatic reduction in symptoms and pathology, the most impressive of which is a significant reduction in morbidity and mortality. The findings presented are consistent with the notion of unique and independent contributions of virus replication and ongoing inflammation to the pathogenesis of severe respiratory virus infection, and they provide the impetus for the study of this treatment regimen in RSV-infected humans.
The human pneumovirus pathogen respiratory syncytial virus (RSV) is among the most important respiratory pathogens worldwide and is currently responsible for 90,000 hospitalizations and 3,000 deaths per year in the United States alone (5, 22, 25). While there have been significant improvements in preventive measures utilized for specific high-risk groups (1, 23), there is no safe and effective vaccine for RSV, nor are there any specific interventions, even for the most severe manifestations of this disease. Among the most interesting of the therapeutic failures is ribavirin, a nucleoside analog that inhibits virus replication in vivo (19, 23, 32) but does not alter the overall pathogenesis and outcome of severe RSV disease (7, 29). This finding has contributed to the current understanding of severe RSV infection as a disease with detrimental inflammatory, as well as infectious, components (34).
Progress in understanding the pathogenesis of severe RSV infection in vivo has been limited by the lack of an appropriate rodent model. While the BALB/c presensitization model has been invaluable for studies aimed at elucidating the pathogenesis of allergic responses to inactivated RSV virions and individual RSV components (2, 24, 26), RSV itself is not a natural mouse pathogen and induces only a limited, minimally symptomatic, and rapidly aborted primary infection in response to a massive, nonphysiologic inoculum of the virus (6). In an attempt to address this issue, we have recently established a model of infection by using the natural mouse pathogen pneumonia virus of mice (PVM), intranasal inoculation as few as 30 PFU of which results in an infection that replicates many of the signs and symptoms of the most severe forms of RSV in human infants (12, 14, 15). RSV and PVM are both viruses of the family Paramyxoviridae, subfamily Pneumovirinae, and are each other's closest known phylogenetic relatives. In wild-type mice, PVM infection is characterized by rapid virus replication accompanied by a massive inflammatory response that leads to mucus plugging, respiratory failure, and death. PVM infection induces local production of MIP-1α (14, 15), the proinflammatory chemokine that promotes recruitment of granulocytes to the lungs of infected mice (14). Local production of MIP-1α is also a feature of human RSV disease, correlating with disease severity (16) and accumulation of neutrophil (18, 21) and eosinophil degranulation products (3, 21, 31).
The inflammatory response has been compared metaphorically to a “double-edged sword,” referring to the fact that it is a complex yet primitive physiologic response with both beneficial and detrimental sequelae (30). With respect to pneumovirus infection, we have shown previously that virus replication in lung tissue proceeds at a more rapid pace in MIP-1α−/− mice, suggesting a role for the MIP-1α-mediated inflammatory response in providing at least a rudimentary level of innate immunity in this setting (14). Similarly, the enhanced severity of RSV disease in immunodeficient (20) and inflammation-deficient (33) hosts has been well documented. In this work, we report that acute respiratory disease associated with pneumovirus infection results both from ongoing virus replication and from the prolonged inflammatory response. Interestingly, we have found that these two pathological elements are not linked inextricably to one another during the course of infection. The experiments described here demonstrate that appropriate therapy for pneumovirus infection in vivo combines elements that combat both the virus replication and the ongoing inflammatory responses, as both promote the morbidity and mortality characteristic of the most severe forms of this respiratory virus disease.
MATERIALS AND METHODS
Mouse and virus stocks.
Wild-type C57Black/6 mice were obtained from Taconic Laboratories, Germantown, N.Y. Gene deletion-containing MIP-1α−/− mice, initially described by Cook and colleagues (9), were bred on site (Syracuse, N.Y.) from breeder pairs obtained from Jackson Laboratories (Bar Harbor, Maine). Mouse-passaged stocks of PVM (strain J3666, ∼106 PFU/ml) were obtained from clarified mouse lung homogenates as described previously (15) and stored in liquid nitrogen. Virus stocks were defrosted and diluted in phosphate-buffered saline (PBS) immediately prior to intranasal inoculation.
Antiviral activity of ribavirin in vitro.
BS-C-1 epithelial cells (American Type Culture Collection, Manassas, Va.) were grown in Iscove's modified Dulbecco's medium supplemented with 10% fetal bovine serum, 2 mM l-glutamine, and antibiotics in 5% CO2 at 32°C. At ∼50% confluence, the cell monolayers were inoculated with PVM at a multiplicity of infection of ∼1 and incubated at 32°C for 2 days, at which time ribavirin (0 to 1,000 μg/ml) was added. To accommodate the slow replication of PVM in vitro, 1-ml aliquots of culture supernatant were sampled on days 10 and 14, flash frozen in dry ice-ethanol, and stored in liquid nitrogen. Viral titers were determined by standard plaque assay (lower limit of detection, ∼10 PFU/ml) as previously described (15).
Establishment of PVM infections in mice and treatment interventions.
Six- to eight-week-old mice were used in all experiments. Mice subjected to brief isoflurane anesthesia were inoculated intranasally with 60 PFU of mouse-passaged PVM strain J3666 in a 50-μl volume of PBS at day 0. All treatment interventions were initiated on day 3 and continued until day 14. Animals were weighed and observed daily. Clinical scoring of infected mice was done as initially devised by Easton and colleagues (10), with modifications (13), as follows: 1, healthy; 2, ruffled fur at neck; 3, piloerection and difficulty breathing, less alert; 4, lethargic with labored breathing; 5, premorbid with emaciation and cyanosis; 6, death. Clinical scores as determined by two independent observers (C.A.B. and J.B.D.) were found to be highly reproducible. In the event that clinical scores were discrepant, the less severe score was used. Treatment interventions included (i) daily intraperitoneal ribavirin (two daily doses of 37.5 mg/kg separated by 6 to 8 h; Sigma Aldrich, St. Louis, Mo.) or an equal volume of diluent control (PBS) and (ii) intraperitoneal administration of purified goat anti-mMIP-1α neutralizing antibody (11) or a goat immunoglobulin G isotype control (5 μg daily in a single dose; R&D Systems, Minneapolis, Minn.). For all experiments, treatments were initiated on day 3 postinfection and continued until day 14. Mice were sacrificed on days 0 through 7 for determination of viral lung titers and on days 0, 3, 5, and 7 for determination of total lung chemokine concentrations and analysis of bronchoalveolar lavage (BAL) fluid cell counts. All procedures were reviewed and approved by the Committee on the Humane Use of Animals, SUNY Upstate Medical University (CHUA no. 634).
BAL and differential cell counts.
At the time points indicated, mice were sacrificed by cervical dislocation (three mice per condition per time point) and BAL fluid was harvested by transtracheal instillation and removal of prechilled phosphate-buffered saline with 0.25% bovine serum albumin (2 × 0.80-ml instillation with recovery of 1.2 to 1.5 ml per mouse). Total and differential leukocyte counts were obtained by light microscopic quantitative analysis of methanol-fixed cytospin preparations stained with Diff-Quik (Fisher Scientific, Pittsburgh, Pa.).
Lung homogenates and chemokine and plaque assays.
Mice were sacrificed as described above (three mice per condition per time point), and lungs were removed and transferred into 1 ml of prechilled Iscove's modified Dulbecco's medium. Lung tissue suspensions were subjected to blade homogenization (Tissumizer; Tekmar, Cincinnati, Ohio), and cellular debris was removed by low-speed centrifugation (500 × g at 4°C). Clarified supernatants were flash frozen in a dry ice and ethanol slurry and stored at −80°C or liquid nitrogen prior to analysis. Assays for mouse MIP-1α and mouse JE/MCP-1 were performed in accordance with the manufacturer's (R&D Systems) instructions, and results were corrected for total protein determined by the Bradford colorimetric assay with bovine serum albumin standards. Viral recovery was determined by standard plaque assay on the BS-C-1 epithelial cell line (American Type Culture Collection).
Statistical analysis.
Datum points represent the average ± the standard error of the mean of samples from three or more trials. Fisher's exact test was employed for categorical (clinical) data. Unpaired t tests were used to compare continuous data in accordance with the algorithms of the Microsoft Excel data analysis program. Kaplan Meier Analyses were performed by using Statistica Software (StatSoft, Tulsa, Okla.).
RESULTS
Replication of PVM in vitro and in vivo in the presence of ribavirin.
Ribavirin treatment results in dose-dependent inhibition of PVM replication both in vitro (Table 1) and in vivo (Table 2). At a concentration of 50 μg/ml, ribavirin administration resulted in a 25- to 50-fold reduction in active virus, with complete inhibition at 500 μg/ml and higher concentrations. No cytotoxicity was observed at any of the ribavirin concentrations evaluated. For in vivo studies, mice received intranasal inoculations of 60 PFU of PVM on day 0, with twice-daily intraperitoneal ribavirin (37.5 mg/kg/dose) or diluent control (PBS) beginning on day 3. In the absence of ribavirin, PVM replication proceeded as anticipated, reaching 1.5 × 108 ± 0.6 × 108 PFU/g of lung tissue on day 6. Virus titers in the lungs of mice receiving twice-daily doses of ribavirin were ∼1,000-fold lower on day 6, measured at 1.3 × 105 ± 0.6 × 105 PFU/g (P < 0.001). From these data, we conclude that replication of PVM both in vitro in cell culture and in vivo in its natural host responds to ribavirin administration in a manner similar to that reported for RSV both in culture (8) and in clinical settings (19).
TABLE 1.
Ribavirin-mediated inhibition of PVM replication in vitroa
| Time (days) postinfection | Avg no. of PVM PFU/ml (104) ± SEM at ribavirin concn (μg/ml) of:
|
|||||
|---|---|---|---|---|---|---|
| 0 | 10 | 50 | 100 | 500 | 1,000 | |
| 10 | 37 ± 2 | 2 ± 1 | 0.8 ± 0.1b | 0c | 0 | 0 |
| 14 | 51 ± 6 | 10 ± 7 | 1.5 ± 0.5b | <0.5 ± 0.2b | 0 | 0 |
Ribavirin was added at the concentrations indicated on day 2 after inoculation of a BS-C-1 epithelial cell monolayer with PVM strain J3666 (multiplicity of infection = 1) on day 0. Virus titers in culture supernatants were determined in triplicate by standard plaque assay.
P < 0.01 compared to diluent control (0 μg of ribavirin per ml:).
0, none detected.
TABLE 2.
Ribavirin-mediated inhibition of PVM replication in vivoa
| Mouse strain and time (days) postinfection | Avg no. of PVM PFU/g (106) ± SEM at ribavirin dose (mg/kg/day) of:
|
Fold reduction | |
|---|---|---|---|
| 0 | 75 | ||
| C57BLACK/6 | |||
| 0 | 0 | 0 | |
| 3 | 0.8 ± 0.01 | 0 | |
| 4 | 1.4 ± 0.7 | 0 | |
| 5 | 24 ± 12 | 0.09 ± 0.01b | 260 |
| 6 | 150 ± 60 | 0.13 ± 0.06b | 1,150 |
| 7 | 140 ± 21 | 0.10 ± 0.03b | 1,400 |
| MIP-1α−/− | |||
| 0 | 0 | 0 | |
| 3 | 34 ± 16 | 0 | |
| 6 | 805 ± 320 | 0.41 ± 0.18b | 1,960 |
Wild-type C57Black/6 or MIP-1α−/− mice were inoculated intranasally with 60 PFU of PVM on day 0, followed by intraperitoneal administration of ribavirin (37.5 mg/kg/dose × 2 doses/day = 75 mg/kg/day) or diluent control beginning on day 3 and continuing to day 14 (six mice per data μm point). Virus titers were determined by standard plaque assay.
P < 0.01 compared to control.
Production of proinflammatory chemokines and leukocyte recruitment in PVM-infected mice with or without ribavirin.
We have shown previously that the proinflammatory chemokines MIP-1α and MCP-1 are produced locally in mouse lung tissue in response to PVM infection (13, 14). While the role of MCP-1 in the pathogenesis of this infection has not been clarified, we have shown that MIP-1α plays a crucial role in recruiting granulocytes (neutrophils and eosinophils) to lungs of PVM-infected mice (14). Diminished quantities of both MIP-1α and MCP-1 were detected in lung homogenates from ribavirin-treated mice (Table 3), but the reductions were surprisingly small (about two- to eightfold) given the dramatic inhibition of virus replication observed. These results suggest that the inflammatory response, once initiated, is not tightly linked to the extent of ongoing virus replication.
TABLE 3.
Detection of proinflammatory chemokines MIP-1α and MCP-1 in lung tissue homogenates of PVM-infected wild-type and MIP-1α−/− micea
| Mice and time (days) postinfection | Avg MIP-1α concn (pg/ml/mg of protein) ± SEM
|
Fold reduction | Avg MCP-1 concn (pg/ml/mg of protein) ± SEM
|
Fold reduction | ||
|---|---|---|---|---|---|---|
| Control | Ribavirin at 75 mg/kg/day | Control | Ribavirin at 75 mg/kg/day | |||
| Wild-type C57Black/6 | ||||||
| 0 | 0c | 0 | 0 | 0 | ||
| 3 | 0 | 0 | 0 | 0 | ||
| 5 | 167 ± 32 | 20 ± 9b | 8.4 | 1,750 ± 260 | 458 ± 90b | 3.8 |
| 7 | 407 ± 87 | 287 ± 64b | 1.4 | 2,780 ± 430 | 875 ± 100b | 3.2 |
| MIP-1a−/− | ||||||
| 0 | 0 | 0 | 0 | 0 | ||
| 3 | 0 | 0 | 0 | 0 | ||
| 5 | 0 | 0 | 1,240 ± 310 | 602 ± 190b | 2.1 | |
| 7 | 0 | 0 | 2,090 ± 510 | 1,020 ± 150b | 2.0 | |
Chemokine concentrations were determined in three mice per datum point.
P < 0.01 compared to diluent-treated controls.
0, none detected.
As was expected given the ongoing production of MIP-1α, we observed a remarkably small decline in the number of proinflammatory leukocytes recruited to the lungs of PVM-infected mice treated with ribavirin (Table 4). Ribavirin had no effect whatsoever on the differential recruitment of leukocyte subsets, shown here and previously to be virtually all granulocytes. Consistent with our interpretation of the data presented in Table 3, these results likewise suggest that the degree and duration of the inflammatory response are not precisely linked to ongoing virus replication, a point that becomes crucial when considering therapeutic intervention strategies.
TABLE 4.
Analysis of leukocytes in BAL fluid of PVM-infected micea
| Day postinfection | Avg no. of leukocytes (102) ml of BAL fluid ± SEM
|
|||||
|---|---|---|---|---|---|---|
| Control
|
Ribavirin at 75 mg/kg/day
|
|||||
| Total | Granulocytesb | Lymphocytes | Total | Granulocytesb | Lymphocytes | |
| 3 | 2.10 ± 0.21 | 1.90 ± 0.55 | 0.19 ± 0.04 | 1.80 ± 0.11 | 1.60 ± 0.09 | 0.23 ± 0.06 |
| 5 | 5,630 ± 270 | 5,630 ± 270 | 0c | 3,520 ± 150 | 3,520 ± 150 | 0 |
| 7 | 6,420 ± 430 | 6,420 ± 430 | 0 | 5,120 ± 510 | 5,120 ± 510 | 0 |
Total and differential leukocyte counts were determined in stained cytospin preparations of BAL fluid (three mice per datum point). Statistical significance does not reach P < 0.01 compared to diluent-treated controls.
Neutrophils and eosinophils.
0, none detected.
Clinical scoring of ribavirin- and control-treated mice.
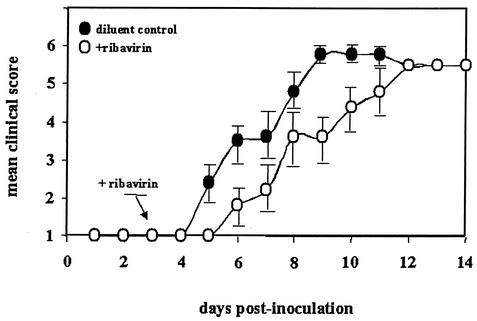
Two groups of 10 mice were each inoculated with PVM on day 0 and then treated twice daily with ribavirin or diluent control beginning on day 3. Symptoms were scored by a modification (13) of the scale devised by Easton and colleagues (10) that is described Materials and Methods (Fig. 1). As shown, mice that received ribavirin exhibited a brief lag in the development of morbidity but both groups eventually experienced the progression of symptoms characteristic of this infection, including respiratory distress leading to death, reaching 90% in each group by day 14. It is interesting that although ribavirin clearly inhibits PVM replication in vivo (Table 2), this activity alone does not alter the clinical outcome. This is similar to what has been observed in severe RSV infections in human infants—although ribavirin is effective at reducing virus replication in vivo, its overall efficacy in the treatment of the disease process is limited (7, 29). Indeed, our data thus far suggest that the progression of disease in this setting is more closely linked to the ongoing inflammatory response than to the absolute viral burden at any time.
FIG. 1.
Mean clinical scores of wild-type mice infected with PVM strain J3666 on day 0 and treated twice daily by intraperitoneal administration of ribavirin (75 mg/kg/day in two divided doses; open circles) or diluent control (PBS; filled circles) beginning on day 3. Clinical scoring system (10, 13): 1, healthy; 2, ruffled fur at neck; 3, piloerection and difficulty breathing, less alert; 4, lethargic with labored breathing; 5, premorbid with emaciation and cyanosis; 6, death. Error bars indicate the standard error of the mean. No statistically significant differences were observed.
Virus replication in MIP-1α−/− gene deletion-containing mice treated with ribavirin.
In previous work, we have demonstrated that MIP-1α−/− mice respond to infection with accelerated rates of virus replication in lung tissue (14). Our present data are consistent with these findings, as the lung virus titer determined on day 6 in the control-treated MIP-1α−/− mice (805 × 106 ± 320 × 106 PFU/g) was significantly higher than that measured in the control-treated MIP-1α+/+ counterparts (153 × 106 ± 60 × 106 PFU/g, P < 0.01; Table 2). We have also determined that ribavirin functions just as well at inhibiting virus replication in PVM-infected MIP-1α−/− mice as it does in MIP-1α+/+ mice, with an approximately 2,000-fold reduction in lung virus titer observed among the ribavirin-treated MIP-1α−/− mice on day 6, compared to the control-treated MIP-1α−/− counterparts (Table 2). Interestingly, ribavirin treatment of PVM-infected MIP-1α−/− mice does not reduce the viral load to the level observed in ribavirin-treated MIP-1α+/+ mice (0.41 × 106 ± 0.18 × 106 PFU/g for MIP-1α−/− mice versus 0.13 × 106 ± 0.06 × 106 PFU/g for MIP-1α+/+ mice, P < 0.01), again consistent with a role for the inflammatory response in reducing the rate of virus replication in vivo.
Production of proinflammatory chemokines and leukocyte recruitment in PVM-infected MIP-1α−/− mice with or without ribavirin.
As observed among MIP-1α+/+ mice, ribavirin administration resulted in a statistically significant but comparatively small reduction (about twofold) in MCP-1 produced in the lungs of PVM-infected MIP-1α−/− mice (Table 3); the levels of MCP-1 detected overall were indistinguishable from those detected among MIP-1α+/+ mice, as were the fold reductions (Table 3). As anticipated, no MIP-1α was detected in any of the MIP-1α−/− mice. Also consistent with our previous findings, we observed minimal leukocyte recruitment in response to PVM among MIP-1α−/− mice (105-fold reduction compared to MIP-1α+/+ mice; Table 4). We observed no difference in either the quantity of leukocytes recruited or the subset differential between the ribavirin- and control-treated MIP-1α−/− groups (Table 5).
TABLE 5.
Analysis of leukocytes in BAL fluid of PVM-infected MIP-1α−/− micea
| Day postinfection | Avg no. of leukocytes (102) ml of BAL fluid ± SEM
|
|||||
|---|---|---|---|---|---|---|
| Control
|
Ribavirin at 75 mg/kg/day
|
|||||
| Totalb | Granulocytesb,c | Lymphocytes | Totalb | Granulocytesb,c | Lymphocytes | |
| 3 | 0.33 ± 0.01 | 0.15 ± 0.01 | 0.18 ± 0.04 | 0.28 ± 0.04 | 0.20 ± 0.04 | 0.08 ± 0.01 |
| 5 | 0.47 ± 0.02 | 0.47 ± 0.06 | 0d | 0.34 ± 0.02 | 0.28 ± 0.03 | 0.07 ± 0.03 |
| 7 | 0.38 ± 0.01 | 0.38 ± 0.01 | 0 | 0.22 ± 0.05 | 0.20 ± 0.08 | 0.02 ± 0.01 |
Total and differential leukocyte counts were determined in stained cytospin preparations of BAL fluid from three mice per datum point.
There are no significant differences between the control and ribavirin-treated groups of MIP-1α−/− mice; totals and granulocyte counts all differ significantly (P < 0.001, ∼102- to 104-fold reduction) from the respective MIP-1α+/+ groups (control versus control, ribavirin versus ribavirin) shown in Table 4.
Neutrophils and eosinophils.
0, none detected.
Clinical scoring of ribavirin- and control-treated MIP-1α−/− mice.
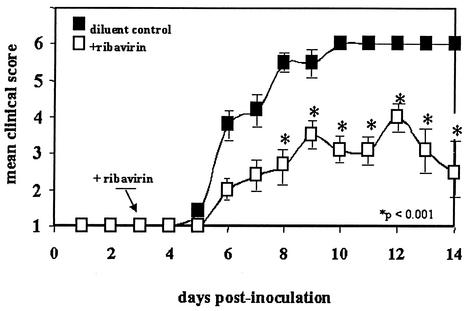
Two groups of 10 MIP-1α−/− mice were inoculated intranasally with 60 PFU of PVM per mouse on day 0 and then treated with ribavirin or diluent control beginning on day 3 as was done for all previous experiments. The clinical scoring system used was that described in the legend to Fig. 1 and in Materials and Methods. In contrast to the clinical scores displayed by the MIP-1α+/+ wild-type mice (Fig. 1), the mice in the MIP-1α−/− ribavirin-treated group fared significantly better at day 8 and all subsequent time points than did those in the MIP-1α−/− control-treated group (Fig. 2; P < 0.001). It is worth noting that all of the mice in all four arms of this study (Fig. 1 and 2) demonstrated some clinical symptoms, each scoring at least 2 during the course of the experiment. The MIP-1α−/− ribavirin-treated group is the only group of the four tested in which the majority of the mice not only survived but clearly recovered from an established, symptomatic infection.
FIG. 2.
Mean clinical scores of MIP-1α−/− mice infected with PVM on day 0 and treated intraperitoneally with ribavirin (75 mg/kg/day administered in two divided doses; open squares) or diluent control (filled squares) beginning on day 3. The clinical scoring system used and the error bars are described in the legend to Fig. 1. P < 0.001 at the time points indicated.
Survival of ribavirin- or control-treated MIP-1α+/+ and MIP 1α−/− mice.
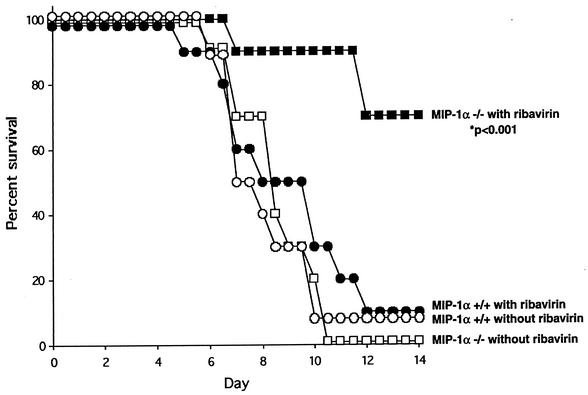
The aforementioned point is established more clearly by formal survival analysis (Fig. 3). Of the four groups studied (10 mice per group), 100% mortality was observed only in the MIP-1α−/− control-treated group, in which neither exogenous (ribavirin) nor endogenous (inflammatory response) limits on virus replication were set. Ninety percent mortality was observed in both MIP-1α+/+ (ribavirin- and control-treated) groups. The mean survival time of the MIP-1α+/+ ribavirin-treated group (8.5 ± 0.8 days) was only slightly longer than that of the MIP-1α+/+ control-treated group (7.9 ± 0.4 days), a difference that does not reach statistical significance. In contrast, the only mice to fare significantly better (P < 0.001) were those in the MIP-1α−/− ribavirin-treated group, with only 30% mortality observed at day 14. The seven (70%) mice remained alive and apparently healthy (score of 1) at 28 days postinoculation.
FIG. 3.
Survival analysis of wild-type (MIP-1α+/+; all circles) and gene deletion-containing (MIP-1α−/−; all squares) mice inoculated with 60 PFU of PVM on day 0 and treated with ribavirin (75 mg/kg/day administered in two divided doses; filled symbols) or diluent control (open symbols) beginning on day 3 (10 mice per group). Significantly improved survival of ribavirin-treated MIP-1α−/− mice, compared independently with that of each of the other three groups (P < 0.001), was observed.
Survival of ribavirin- or control-treated mice in response to administration of anti-MIP-1α neutralizing antibody.
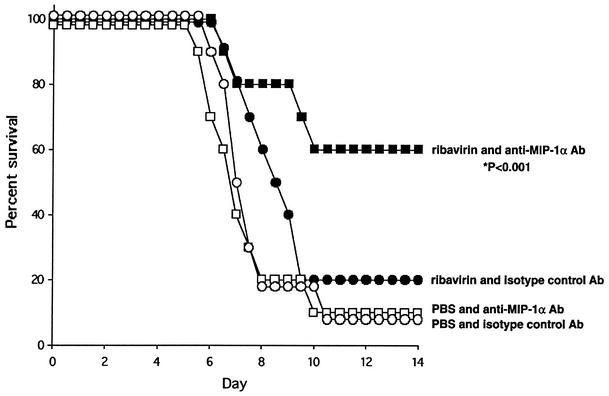
A means of translating these findings into a clinically achievable therapeutic regimen is shown in Fig. 4. This experiment was performed with four groups of 10 wild-type mice with intraperitoneal administration of anti-mouse-MIP-1α neutralizing antibody as an immunomodulatory agent in an attempt to replicate a MIP-1α−/− state in a therapeutically relevant fashion. Both the twice-daily ribavirin or PBS and once-daily antibody or isotype control (5 μg/dose) regimens were initiated on day 3 after infection had been established via inoculation with PVM as described above. Similar to our findings with the gene deletion-containing mice, 60% of the mice that received both ribavirin and neutralizing anti-mMIP-1α antibody became long-term survivors with no clinical signs of illness after day 11 (P < 0.01 compared to each of the other groups). The mean survival time of the group that received ribavirin alone (8.3 ± 0.4 days) was slightly longer than that of the double-control group (7.4 ± 0.4 days) or the group that received the neutralizing anti-mMIP-1α antibody alone (7.1 ± 0.5 days), but neither of these differences reached statistical significance.
FIG. 4.
Survival analysis of wild-type mice inoculated with 60 PFU of PVM on day 0 and treated with ribavirin (75 mg/kg/day administered in two divided doses; filled symbols) or diluent control (open symbols) and goat anti-mMIP-1α (5 μg/day administered intraperitoneally; squares) or goat isotype control (same dose; circles) beginning on day 3 (10 mice per group). Significantly improved survival of combined ribavirin- and anti-mMIP-1α-treated mice, compared independently to that of each of the other three groups (P < 0.001), was observed.
DISCUSSION
In this work, we have demonstrated significant reductions in morbidity and mortality from severe pneumovirus infection in response to a combined antiviral-immunomodulatory approach. For these and related studies, we have developed a new mouse model of pneumovirus infection using the natural mouse pathogen PVM. PVM is a virus of the same family (Paramyxoviridae) and subfamily (Pneumovirinae) as RSV and is the closest known phylogenetic relative of RSV. Unlike RSV, PVM is a natural pathogen of mice and, when it is inoculated in quantities as small as 30 PFU, replicates the signs and symptoms of the most severe forms of RSV in humans (14, 15). The PVM model represents a unique tool for the study of severe pneumovirus infection. While most human RSV infections are mild, moderate and severe cases lead to the greatest morbidity. Infants with severe lower respiratory tract RSV disease would benefit the most from effective antiviral and specific immunomodulatory intervention.
In this work, we demonstrate that the nucleoside analog ribavirin has potent activity against PVM both in vitro and in vivo but does not appear to offer a substantial clinical benefit when used alone to treat PVM-infected mice. This is reflective of current pediatric practice. Although ribavirin was once used routinely for therapy of RSV bronchiolitis, enthusiasm for ribavirin has decreased as concerns over clinical efficacy have grown. While ribavirin has potent antiviral activity against viruses of several divergent families, including pneumoviruses, the current consensus is that the overall clinical benefit of reducing viral replication in symptomatic patients through ribavirin administration alone has been unimpressive (7, 29).
Although ribavirin is not effective when used as a sole treatment modality, we have demonstrated that a dual approach, combining specific immunomodulatory intervention with potent antiviral therapy in vivo, does offer a dramatic clinical benefit, the most impressive of which is a significant reduction in mortality. Our approach to this combined strategy stems from earlier observations on the pathogenesis of pneumovirus disease based on the PVM-mouse model (12, 13). In previous work, we identified the proinflammatory chemokine MIP-1α as an essential link between virus infection and granulocyte recruitment to infected lung tissue (14). However, we demonstrate here that once the virus infection is established, the continued production of MIP-1α and the cellular inflammatory response that ensues are not tightly linked to virus replication and that while ribavirin has significant efficacy in combating the virus infection, it does nothing to ameliorate the parts of this disease state that result from prolonged inflammation. As symptomatic individuals appear to have reached the point at which the disease process involves both ongoing and independent infectious and inflammatory components, simultaneous administration of both antiviral and specific immunomodulatory agents is clearly warranted. Our findings are analogous to those obtained by Prince and colleagues (27, 28) in their evaluation of the combination of a specific antivirus antibody and a systemic anti-inflammatory agent for the treatment of parainfluenza virus type 3 and RSV infections in rodent models.
Given the role of detrimental inflammatory responses in the pathogenesis of pneumovirus disease, it is interesting that systemic glucocorticoids have a marginal, if any, benefit in the treatment of human RSV infection (reviewed in reference 4). A recent meta-analysis of five of these studies (17) demonstrated that systemic glucocorticoid therapy reduced the total length of the hospital stay by only 0.43 day per patient, an effect reduced to only 0.29 day per patient when patients with previous episodes of wheezing were excluded from the analysis. In an attempt to understand this clinical observation, we have recently demonstrated that glucocorticoids have no effect whatsoever on the production or release of MIP-1α or MCP-1 from RSV-infected epithelial cells in vitro (4), nor do they inhibit the production of MIP-1α and MCP-1 in, or the recruitment of neutrophils to, the lungs of PVM-infected mice (13).
In light of these findings, we proposed that specific agents that target the MIP-1α signaling pathway might be more effective than systemic anti-inflammatory agents in combating these detrimental inflammatory sequelae. Our initial studies with gene deletion-containing MIP-1α−/− mice demonstrated proof of principle—that administration of a potent antiviral agent in the setting of a blockade of this crucial proinflammatory pathway resulted in clinical improvement of pneumovirus-infected mice, with statistically significant reductions in morbidity and mortality. Our ability to replicate these clinical improvements by systemic administration of an anti-MIP-1α antibody suggests that we should be able to translate these laboratory findings into a clinically achievable therapeutic strategy for the management of severe RSV infection in human infants, a proposal that merits consideration for an initial phase I therapeutic trial.
Acknowledgments
This work was supported in part by an American Heart Association Scientist Development Grant to J.B.D. and the Central New York Children's Miracle Network.
REFERENCES
- 1.AAP Committee on Infectious Diseases and Committee on Fetus and Newborn. 1998. Prevention of respiratory syncytial virus infections: indications for the use of palivizumab and update on the use of RSV-IVIG. Pediatrics 5:1211-1216. [DOI] [PubMed] [Google Scholar]
- 2.Aung, S., J. A. Rutigliano, and B. S. Graham. 2001. Alternative mechanisms of respiratory syncytial virus clearance in perforin knockout mice lead to enhanced disease. J. Virol. 75:9918-9924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonville, C. A., H. F. Rosenberg, and J. B. Domachowske. 1999. Macrophage inflammatory protein-1α and RANTES are present in nasal secretions during ongoing upper respiratory tract infection. Pediatr. Allergy Immunol. 10:39-44. [DOI] [PubMed] [Google Scholar]
- 4.Bonville, C. A., P. A. Mehta, L. Krilov, H. F. Rosenberg, and J. B. Domachowske. 2001. Epithelial cells infected with respiratory syncytial virus are resistant to the anti-inflammatory effects of hydrocortisone. Cell. Immunol. 213:134-140. [DOI] [PubMed] [Google Scholar]
- 5.Brandt, C. D., H. W. Kim, and J. O. Arrobio. 1973. Epidemiology of respiratory syncytial virus infection in Washington, D.C. III. Composite analysis of eleven consecutive yearly epidemics. Am. J. Epidemiol. 98:355-364. [DOI] [PubMed] [Google Scholar]
- 6.Byrd, L. G., and G. Prince. 1997. Animal models of respiratory syncytial virus infection. Clin. Infect. Dis. 25:1363-1368. [DOI] [PubMed] [Google Scholar]
- 7.Committee on Infectious Disease, American Academy of Pediatrics. 1996. Reassessment of the indications for ribavirin therapy in respiratory syncytial virus infections. Pediatrics 97:137-140. [PubMed] [Google Scholar]
- 8.Cononico, P. G. 1985. Efficacy, toxicology and clinical applications of ribavirin against virulent RNA viral infections. Antiviral Res. Suppl. 1:75-81. [DOI] [PubMed] [Google Scholar]
- 9.Cook, D. N., M. A. Beck, T. M. Coffman, S. L. Kirby, J. F. Sheridan, I. B. Pragnell, and O. Smithies. 1995. Requirement of MIP-1α for an inflammatory response to viral infection. Science 269:1583-1585. [DOI] [PubMed] [Google Scholar]
- 10.Cook, P. M., R. P. Eglin, and A. J. Easton. 1998. Pathogenesis of pneumovirus infections in mice: detection of pneumonia virus of mice and human respiratory syncytial virus mRNA in lungs of infected mice by in situ hybridization. J. Gen. Virol. 79:2411-2417. [DOI] [PubMed] [Google Scholar]
- 11.Diab, A., H. Abdalla, H. L. Li, F. D. Shi, J. Zhu, B. Hojberg, L. Lindquist, B. Wretlind, M. Bakhiet, and H. Link. 1999. Neutralization of macrophage inflammatory protein 2 (MIP-2) and MIP-1α attenuates neutrophil recruitment in the central nervous system during experimental bacterial meningitis. Infect. Immun. 67:2590-2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Domachowske, J. B., C. A. Bonville, and H. F. Rosenberg. 2001. Gene expression in epithelial cells in response to pneumovirus infection. Respir. Res. 2:225-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Domachowske, J. B., C. A. Bonville, D. Ali-Ahmad, K. D. Dyer, A. J. Easton, and H. F. Rosenberg. 2001. Glucocorticoid administration accelerates mortality of pneumovirus-infected mice. J. Infect. Dis. 184:1518-1523. [DOI] [PubMed] [Google Scholar]
- 14.Domachowske, J. B., C. A. Bonville, J. L. Gao, P. M. Murphy, A. J. Easton, and H. F. Rosenberg. 2000. The chemokine macrophage-inflammatory protein-1α and its receptor CCR1 control pulmonary inflammation and antiviral host defense in paramyxovirus infection. J. Immunol. 165:2677-2682. [DOI] [PubMed] [Google Scholar]
- 15.Domachowske, J. B., C. A. Bonville, K. D. Dyer, A. J. Easton, and H. F. Rosenberg. 2000. Pulmonary eosinophilia and production of MIP-1α are prominent responses to infection with pneumonia virus of mice. Cell. Immunol. 200:98-104. [DOI] [PubMed] [Google Scholar]
- 16.Garofalo, R. P., J. Patti, K. A. Hintz, V. Hill, P. L. Ogra, and R. C. Welliver. 2001. Macrophage inflammatory protein-1α (not T helper type 2 cytokines) is associated with severe forms of respiratory syncytial virus bronchiolitis. J. Infect. Dis. 184:393-399. [DOI] [PubMed] [Google Scholar]
- 17.Garrison, M. M., D. A. Christakis, E. Harvey, P. Cummings, and R. L. Davis. 2000. Systemic corticosteroids in infant bronchiolitis: a meta-analysis. Pediatrics 105:324-330. [DOI] [PubMed] [Google Scholar]
- 18.Haeberle, H. A., W. A. Kuziel, H. J. Dieterich, A. Casola, Z. Gatalica, and R. P. Garofalo. 2001. Inducible expression of inflammatory chemokines in respiratory syncytial virus-infected mice: role of MIP-1α in lung pathology. J. Virol. 75:878-890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hall, C. B., J. T. McBride, and E. E. Walsh. 1983. Aerosolized ribavirin treatment of infants with respiratory syncytial virus infection. N. Engl. J. Med. 308:1443-1447. [DOI] [PubMed] [Google Scholar]
- 20.Harrington, R. D., T. M. Hooten, R. C. Hackman, G. A. Storch, B. Osborne, C. A. Gleaves, A. Benson, and J. D. Meyers. 1992. An outbreak of respiratory syncytial virus in a bone marrow transplant center. J. Infect. Dis. 165:987-993. [DOI] [PubMed] [Google Scholar]
- 21.Harrison, A. M., C. A. Bonville, H. F. Rosenberg, and J. B. Domachowske. 1999. Respiratory syncytial virus-induced chemokine expression in the lower airways: eosinophil recruitment and degranulation. Am. J. Respir. Crit. Care Med. 159:1918-1924. [DOI] [PubMed] [Google Scholar]
- 22.Holberg, C. J., A. L. Wright, F. D. Martinez, C. G. Ray, L. M. Taussig, and M. A. Leibowitz. 1991. Risk factors for respiratory syncytial virus-associated lower respiratory illnesses in the first year of life. Am. J. Epidemiol. 133:1135-1151. [DOI] [PubMed] [Google Scholar]
- 23.Impact-RSV Study Group. 1998. Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. Pediatrics 102:531-537. [PubMed] [Google Scholar]
- 24.Matsuse, H., A. K. Behera, M. Kumar, R. F. Lockey, and S. S. Mohapatra. 2000. Differential cytokine mRNA expression in Dermatophagoides farinae allergen-sensitized and respiratory-syncytial virus infected mice. Microbes Infect. 2:753-759. [DOI] [PubMed] [Google Scholar]
- 25.Parrot, R. H., H. W. Kim, and J. O. Arrobio. 1973. Epidemiology of respiratory syncytial virus infection in Washington D.C. II. Infection and disease with respect to age, immunologic status, race and age. Am. J. Epidemiol. 98:289-300. [DOI] [PubMed] [Google Scholar]
- 26.Peebles, R. S., Jr., K. Hashimoto, R. D. Collins, K. Jarzecka, J. Furlong, D. B. Mitchell, J. R. Sheller, and B. S. Graham. 2001. Immune interaction between respiratory syncytial virus infection and allergen sensitization critically depends on timing of challenges. J. Infect. Dis. 184:1374-1379. [DOI] [PubMed] [Google Scholar]
- 27.Prince, G. A., A. Mathews, S. J. Curtis, and D. D. Porter. 2000. Treatment of respiratory syncytial virus bronchiolitis and pneumonia in a cotton rat model with systemically administered monoclonal antibody (palivizumab) and glucocorticosteroid. J. Infect. Dis. 182:1326-1330. [Google Scholar]
- 28.Prince, G. A., and D. D. Porter. 1996. Treatment of parainfluenza virus type 3 bronchiolitis and pneumonia in a cotton rat model using topical antibody and glucocorticosteroid. J. Infect. Dis. 173:598-608. [DOI] [PubMed] [Google Scholar]
- 29.Randolph, A. G., and E. E. Wang. 1996. Ribavirin for respiratory syncytial virus lower respiratory tract infection: a systematic overview. Arch. Pediatr. Adolesc. Med. 150:942-947. [DOI] [PubMed] [Google Scholar]
- 30.Rosenberg, H. F., and J. I. Gallin. 1996. Inflammation, p. 1051-1067. In W. E. Paul (ed.), Fundamental immunology. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 31.Schwarze, J., G. Cieslewicz, E. Hamelmann, A. Joetham, L. D. Shultz, M. C. Lamers, and E. W. Gelfand. 1999. IL-5 and eosinophils are essential for the development of airway hyperresponsiveness following acute respiratory syncytial virus infection. J. Immunol. 162:2997-3004. [PubMed] [Google Scholar]
- 32.Smith, D. W., L. R. Frankel, L. H. Mathers, A. T. Tang, R. L. Ariagno, and C. G. Prober. 1991. A controlled trial of aerosolized ribavirin in infants receiving mechanical ventilation for severe respiratory syncytial virus infection. N. Engl. J. Med. 325:24-29. [DOI] [PubMed] [Google Scholar]
- 33.Uzel, G., A. Premkumar, H. L. Malech, and S. M. Holland. 2000. Respiratory syncytial virus infection in patients with phagocyte defects. Pediatrics 106:835-837. [DOI] [PubMed] [Google Scholar]
- 34.van Schaik, S. M., R. C. Welliver, and J. L. L. Kimpen. 2000. Novel pathways in the pathogenesis of respiratory syncytial virus disease. Pediatr. Pulmonol. 30:131-138. [DOI] [PubMed] [Google Scholar]