Abstract
Human Epstein-Barr virus-immortalized lymphoblastoid B-cell lines tested positive by PCR for simian virus 40 (SV40) DNA (22 of 42 cell lines, 52.3%). B lymphocytes or tissues from which B-cell lines derived were also SV40 positive. In situ hybridization showed that SV40 DNA was present in the nucleus of a small fraction (1/250) of cells. SV40 T-antigen mRNA was detected by reverse transcription-PCR. Lymphoblastoid B-cell lines (n = 4) infected with SV40 remained SV40 positive for 4 to 6 months. SV40-positive B-cell lines were more tumorigenic in SCID mice than were SV40-negative cell lines (4 of 5 [80%] SV40-positive cell lines versus 2 of 4 [50%] SV40-negative cell lines). These results suggest that SV40 may play a role in the early phases of human lymphomagenesis.
Recent reports have provided evidence for the association of simian virus 40 (SV40) with human lymphomas (6, 13, 16, 18, 19) and other lymphoproliferative disorders (13). In this study, we investigated a series of Epstein-Barr virus (EBV)-immortalized lymphoblastoid cell lines (LCLs) for the presence of SV40 sequences encoding the large-T-antigen (Tag) N-terminal domain (2, 12, 13; F. Martini, M. De Mattei, L. Iaccheri, L. Lazzarin, G. Barbanti-Brodano, M. Gerosa, and M. Tognon, Letter, J. Natl. Cancer Inst. 87:1331, 1995). This conserved SV40 DNA region was detected in 22 of 42 (52.3%) LCLs analyzed, with similar prevalence in LCLs obtained by spontaneous in vitro outgrowth (7 of 15, 46.7%) and those induced with the prototype B95.8 EBV strain (15 of 27, 55.5%). No evidence of SV40 sequences was found in the marmoset B95.8 cell line from which infectious EBV virions were produced and in mock samples, thus ruling out laboratory contamination. SV40 Tag sequences were also detected in peripheral blood mononuclear cells (PBMCs) of two blood donors (DPPI-16 and DFM-17) from whom SV40-positive B95.8 LCLs were obtained, whereas no Tag sequences were found in PBMCs of two other donors from whom SV40-negative B95.8 LCLs were derived (Table 1).
TABLE 1.
Prevalence of SV40 DNA in EBV-immortalized LCLs
| Derivation of LCLsa | Source | Mean age (yr) of patient or donor (range) | No. of LCLs with SV40 large-Tag sequences/no. of LCLs examinedb |
|---|---|---|---|
| Obtained by spontaneous outgrowth | NHLc biopsy specimens | 72 (66-78) | 0/4 |
| HD biopsy specimens | 44 (23-77) | 6/8 | |
| Reactive lymphadenopathies | 51.5 (35-68) | 0/2 | |
| PBMCs of healthy donors | 59 | 1/1 | |
| Total | 52.1 (23-78) | 7/15 (46.7%) | |
| Induced with the B95.8 EBV strain | PBMCs of HD patients | 41.8 (17-77) | 9/14 |
| PBMCs of healthy donors | 33.5 (22-50) | 6/13 | |
| Total | 37.3 (17-77) | 15/27 (55.5%) |
The LCLs analyzed were established at different times over a 5-year period (1991 to 1996). Spontaneous LCLs were derived from biopsy specimens and PBMCs as previously described (7). Briefly, finely minced fragments from biopsy material were placed in fetal calf serum-coated 24-well plates and cultured in RPMI 1640 medium (GIBCO, Grand Island, N.Y.) supplemented with 10% heat-inactivated fetal calf serum, 2 mmol of l-glutamine/liter, 100 IU of penicillin/ml, and 100 IU of streptomycin/ml (complete medium). Purified PBMCs (106/ml), obtained by Ficoll-Hypaque gradient centrifugation, were seeded in 96-flat-well plates and cultured in 200 μl of complete medium. Cyclosporin A (0.1 μg/ml; Sigma, St. Louis, Mo.) was added to the medium to inhibit T-cell activation. LCLs carrying the B95.8 prototypic EBV strain (B95.8 LCLs) were established by infecting PBMCs from lymphoma patients or healthy donors with spent supernatant of the B95.8 marmoset cell line.
In all, 22 of 42 (52.3%) LCLs contained SV40 large-Tag sequences.
NHL, non-Hodgkin's lymphoma.
Analysis of B cells purified by immunomagnetic separation confirmed that SV40 was present in circulating CD19+ B lymphocytes of the DPPI-16 donor, consistent with the detection of SV40 sequences in the related B95.8-induced LCL. Moreover, all six lymph nodes (LN) from which the six SV40-negative LCLs were obtained were also SV40 negative, whereas in two cases, both the LN and the corresponding spontaneous LCLs were SV40 positive. Conversely, SV40 DNA was not found in two LN from which two SV40-positive spontaneous LCLs were obtained, probably because the viral DNA load in these specimens were below the detection limit of our PCR.
To further assess SV40 specificity, DNA sequence analysis was performed with all of the PCR products found to be positive for the SV40 sequences, i.e., Tag N-terminal region from nucleotides 4945 to 4403 (2, 12, 13; Martini et al., letter). In all cases, the SV40 DNA sequence was not distinguishable from that of the wild-type SV40 776 strain (GenBank accession no. AF3161397). This highly conserved SV40 Tag domain was repeatedly detected in human tumors and, to a lesser extent, in healthy human tissues by many investigators (1, 4, 10). Significantly, SV40 Tag sequences have also been detected in different samples from monkeys, who are considered to be the natural host of the virus (1, 2, 11, 17).
In situ hybridization revealed the presence of SV40 DNA in the nucleus of SV40-positive LCLs, but only in a small fraction (1/250) of the cells (Fig. 1). These findings are consistent with the low load of SV40 sequences detected in SV40-positive primary tumors and derived cell lines, as well as normal cells infected in vitro with SV40 (13). LCLs (n = 4) that were found to be negative for the SV40 sequences by PCR analysis were infected in vitro with the SV40 776 strain at a multiplicity of infection of 0.1 PFU/cell. As demonstrated by cocultivation experiments carried out with CV-1 permissive monkey cells, SV40-infected LCLs released infecting SV40 virions without apparent cytopathic effects, as has been described previously for SV40-infected human mesothelial cells (5). However, LCLs remained positive for SV40 for only 4 to 6 months.
FIG. 1.
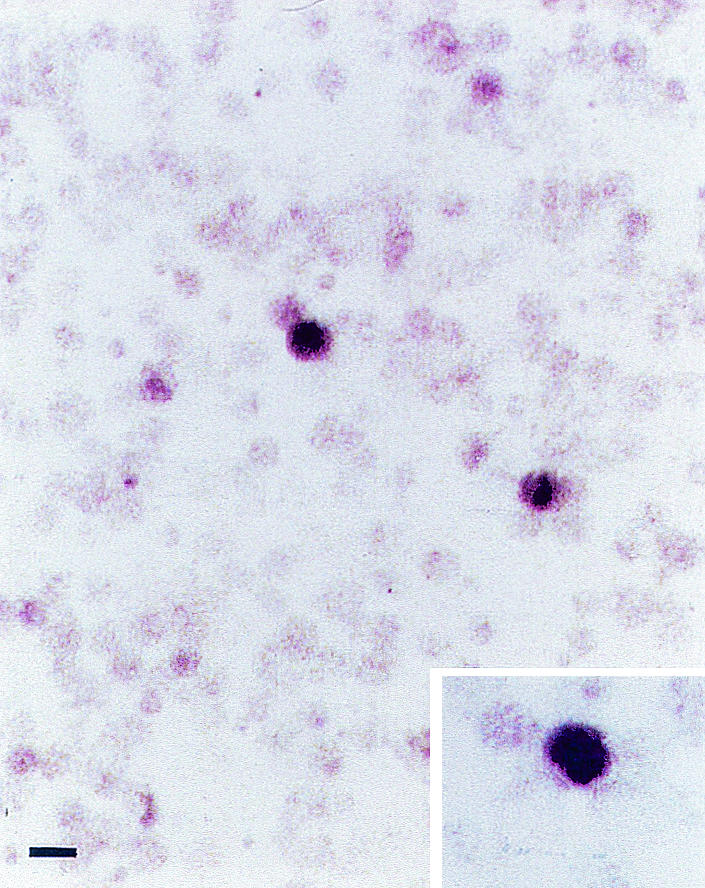
In situ hybridization for SV40 DNA in the DBA24 spontaneous LCL. A small fraction of cells (1 to 2 of every 500) was positive for SV40 DNA. At higher magnification (inset), the SV40 signal can be seen as dense grains over the nucleus. Bar = 20 μM (main panel) and 12.5 μM (inset).
The expression of the SV40 Tag in nine LCLs was investigated by reverse transcription (RT)-PCR. Interestingly, by using a specific RT-PCR (12, 14), SV40 Tag mRNA was detected in five LCLs which had previously been found by PCR to be positive for SV40 Tag sequences, whereas four LCLs that were negative for SV40 DNA also scored negative for Tag mRNA.
Since LCLs are heterogeneous with regard to their ability to grow in vivo following transplantation into SCID mice, we investigated possible relationships between SV40 infection and LCL tumorigenicity. Groups of five CB.17 scid/scid mice (4 weeks old; Harlan-Nossan, Milan, Italy) were inoculated subcutaneously in the right flank with a suspension of 107 cells in 200 μl of saline buffer and inspected weekly for the appearance of tumor masses. Five SV40-positive LCLs (three obtained from healthy donors and two from patients with Hodgkin's disease [HD]) and four SV40-negative LCLs (one derived from a healthy donor and three from lymphoma patients, one with HD and two with non-Hodgkin's lymphoma) were used in these experiments. Compared with SV40-negative LCLs, a higher percentage of SV40-positive cell lines were tumorigenic (4 of 5, 80%, versus 2 of 4, 50%). However, this difference is not statistically significant (P = 0.4 by Fisher's exact test). Tumors induced by SV40-positive and -negative LCLs appeared at similar times (in approximately 2 months) and were of comparable size. Interestingly, unlike what was observed with LCLs infected with SV40 in vitro, SV40 DNA was retained in tumors induced by SV40-positive LCLs, with a viral load similar to that of the parental cells.
These results confirm and extend previous findings indicating that SV40 is present in PBMCs from oncologic patients and blood donors (1, 6, 12-14, 20; Martini et al., letter) and provide additional evidence indicating that B lymphocytes may constitute a reservoir for SV40 or an SV40-like agent in humans. Our results also support the hypothesis that SV40 may be transmitted among humans, as has been suggested recently by the detection of SV40-neutralizing antibodies in human immunodeficiency virus-negative and -positive patients (9). Although we did not observe spontaneous SV40 production from our SV40-positive LCLs, probably because of the low number of SV40-carrying cells, we demonstrate here that LCLs are able to release SV40 virions upon infection with exogenous SV40. These results are consistent with the possibility that B lymphocytes may also be responsible for the spreading of the virus in vivo, at least in the early phases of infection.
The fact that in no instance did we observe the outgrowth of an SV40-negative LCL from SV40-positive tissues and the observation that SV40 DNA was markedly more prevalent in LCLs (52.3%) than in PBMCs from healthy donors (4.5 to 23%) (12, 20) are consistent with the possibility that EBV-induced B-cell immortalization may favor the outgrowth of SV40-infected cells. In this respect, EBV could behave like other herpesviruses, namely, herpes simplex virus and human cytomegalovirus, which complement and stimulate the SV40 origin of replication (3, 15), whereas human cytomegalovirus complements the replication of JC virus DNA in human fibroblasts, which are nonpermissive cells for this polyomavirus that is closely related to SV40 (8).
Our observation that SV40 may successfully infect and persist within human B cells, at least for 4 to 6 months, constitutes an important prerequisite for further assessment of whether the virus has a role in the development of B-cell lymphoproliferative disorders. In this respect, the detection of Tag mRNA expression and the slightly increased tumorigenicity of SV40-positive LCLs are consistent with the possibility that SV40 contributes to human lymphomagenesis. However, it should be noted that EBV per se is sufficient to immortalize B lymphocytes and sustain their growth when transplanted into SCID mice, thus limiting the possibility of disclosing and adequately evaluating the growth advantage possibly conferred by SV40 both in vitro and in vivo. Our findings, together with the recent demonstration of hepatocyte growth factor/MET autocrine and paracrine loops in SV40-transformed human mesothelial cells (5), should stimulate further studies aimed at elucidating whether SV40 has a contributory role in the development of human B-cell lymphomas.
Acknowledgments
We thank José Menezes, Laboratory of Immunovirology, Department of Microbiology and Immunology, University of Montreal, Montreal, Canada, for helpful discussions. Laura Iaccheri provided competent technical assistance.
This work was supported, in part, by grants from the Associazione Italiana per la Ricerca sul Cancro, Milan (R.D. and M.T.); Istituto Superiore di Sanità, AIDS Project, Rome (M.T.); and Ministero dell'Istruzione, Università e Ricerca, Rome (M.T.). L. Iaccheri is supported by a fellowship from Fondazione Cassa di Risparmío di Cento (FE).
REFERENCES
- 1.Barbanti-Brodano, G., F. Martini, M. De Mattei, L. Lazzarin, A. Corallini, and M. Tognon. 1998. BK and JC human polyomaviruses and simian virus 40: natural history of infection in humans, experimental oncogenicity and association with human tumors. Adv. Virus Res. 50:69-99. [DOI] [PubMed] [Google Scholar]
- 2.Bergsagel, D. J., M. J. Finegold, J. S. Butel, W. J. Kupsky, and R. L. Garcea. 1992. DNA sequences similar to those of simian virus SV40 in ependymomas and choroid plexus tumors of childhood. N. Engl. J. Med. 326:988-993. [DOI] [PubMed] [Google Scholar]
- 3.Blumel, J., S. Gasper, and B. Matz. 2000. Structure of simian virus 40 DNA replicated by herpes simplex virus type 1. Virology 276:445-454. [DOI] [PubMed] [Google Scholar]
- 4.Butel, J. S., and J. A. Lednicky. 1999. Cell and molecular biology of simian virus 40: implications for human infections and disease. J. Natl. Cancer Inst. 91:119-134. [DOI] [PubMed] [Google Scholar]
- 5.Cacciotti, P., R. Libener, P. Betta, F. Martini, C. Porta, A. Procopio, L. Strizzi, L. Penengo, M. Tognon, L. Mutti, and G. Gaudino. 2001. SV40 replication in human mesothelial cells induces HGF/Met receptor activation: a model for viral-related carcinogenesis of human malignant mesothelioma. Proc. Natl. Acad. Sci. USA 98:12032-12037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.David, H., S. Mendoza, T. Konishi, and C. W. Miller. 2001. Simian virus 40 is present in human lymphomas and normal blood. Cancer Lett. 162:57-64. [DOI] [PubMed] [Google Scholar]
- 7.Dolcetti, R., M. Quaia, A. Gloghini, V. De Re, P. Zancai, R. Cariati, L. Babuin, A. M. Cilia, S. Rizzo, A. Carbone, and M. Boiocchi. 1999. Biologically relevant phenotypic changes and enhanced growth properties induced in B lymphocytes by an EBV strain derived from a histologically aggressive Hodgkin's disease. Int. J. Cancer 80:240-249. [DOI] [PubMed] [Google Scholar]
- 8.Heilbronn, R., I. Albrecht, S. Stephan, A. Burkle, and H. zur Hausen. 1993. Human cytomegalovirus induces JC virus DNA replication in human fibroblasts. Proc. Natl. Acad. Sci. USA 90:11406-11410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jafar, S., M. Rodriguez-Barradas, D. Y. Graham, and J. Butel. 1998. Serological evidence of SV40 infections in HIV-infected and HIV-negative adults. J. Med. Virol. 54:276-284. [DOI] [PubMed] [Google Scholar]
- 10.Jasani, B., A. Cristaudo, S. A. Emri, A. F. Gazdar, A. Gibbs, B. Krynska, C. Miller, L. Mutti, C. Radu, M. Tognon, and A. Procopio. 2001. Association of SV40 with human tumors. Semin. Cancer Biol. 11:49-61. [DOI] [PubMed] [Google Scholar]
- 11.Lednicky, J. A., A. S. Arrington, A. R. Stewart, X. M. Dai, C. Wong, S. Jafar, M. Murphey-Corb, J. S. Butel. 1998. Natural isolates of simian virus 40 from immunocompromised monkeys display extensive genetic heterogeneity: new implications for polyomavirus disease. J. Virol. 72:3980-3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martini, F., L. Iaccheri, L. Lazzarin, A. Corallini, M. Gerosa, P. Iuzzolino, G. Barbanti-Brodano, and M. Tognon. 1996. Simian virus 40 early region and large T antigen in human brain tumors, peripheral blood cells and sperm fluids from healthy individuals. Cancer Res. 56:4820-4825. [PubMed] [Google Scholar]
- 13.Martini, F., R. Dolcetti, A. Gloghini, L. Iaccheri, A. Carbone, M. Boiocchi, and M. Tognon. 1998. Simian-virus-40 footprints in human lymphoproliferative disorders of HIV− and HIV+ patients. Int. J. Cancer 78:669-674. [DOI] [PubMed] [Google Scholar]
- 14.Martini, F., L. Lazzarin, L. Iaccheri, B. Vignocchi, G. Finocchiaro, I. Magnani, M. Serra, K. Scotlandi, G. Barbanti-Brodano, and M. Tognon. 2002. Different simian virus 40 genomic regions and sequences homologous with SV40 large T antigen in DNA of human brain and bone tumors and of leukocytes from blood donors. Cancer 94:1037-1048. [PubMed] [Google Scholar]
- 15.Pari, G. S., and S. C. St. Jeor. 1990. Effect of human cytomegalovirus on replication of SV40 origin and the expression of T antigen. Virology 177:824-828. [DOI] [PubMed] [Google Scholar]
- 16.Shivapurkar, N., K. Harada, J. Reddy, R. H. Scheuermann, Y. Xu, R. W. McKenna, S. Milchgrub, S. H. Kroft, Z. Feng, and A. F. Gazdar. 2002. Presence of simian virus 40 DNA sequences in human lymphomas. Lancet 359:851-852. [DOI] [PubMed] [Google Scholar]
- 17.Stewart, A. R., J. A. Lednicky, and J. S. Butel. 1998. Sequence analyses of human tumor-associated SV40 DNAs and SV40 viral isolates from monkeys and humans. J. Neurovirol. 4:182-193. [DOI] [PubMed] [Google Scholar]
- 18.Vilchez, R. A., J. A. Lednicky, S. J. Halvorson, Z. S. White, C. A. Kozinetz, and J. S. Butel. 2002. Detection of polyomavirus simian virus 40 tumor antigen DNA in AIDS-related systemic non-Hodgkin lymphoma. J. Acquir. Immune Defic. Syndr. 29:109-116. [DOI] [PubMed] [Google Scholar]
- 19.Vilchez, R. A., C. R. Madden, C. A. Kozinetz, S. J. Halvorson, Z. S. White, J. L. Jorgensen, C. J. Finch, and J. S. Butel. 2002. Association between simian virus 40 and non-Hodgkin lymphoma. Lancet 359:817-823. [DOI] [PubMed] [Google Scholar]
- 20.Yamamoto, H., T. Nakayama, H. Murakami, T. Hosaka, T. Nakamata, T. Tsuboyama, M. Oka, T. Nakamura, and J. Toguchida. 2000. High incidence of SV40-like sequences detection in tumour and peripheral blood cells of Japanese osteosarcoma patients. Br. J. Cancer 82:1677-1681. [DOI] [PMC free article] [PubMed] [Google Scholar]


