Abstract
Virus-specific T-cell immune responses are important in restraint of human immunodeficiency virus type 1 (HIV-1) replication and control of disease. Plasma viral load is a key determinant of disease progression and infectiousness in HIV infection. Although HIV-1 subtype C (HIV-1C) is the predominant virus in the AIDS epidemic worldwide, the relationship between HIV-1C-specific T-cell immune responses and plasma viral load has not been elucidated. In the present study we address (i) the association between the level of plasma viral load and virus-specific immune responses to different HIV-1C proteins and their subregions and (ii) the specifics of correlation between plasma viral load and T-cell responses within the major histocompatibility complex (MHC) class I HLA supertypes. Virus-specific immune responses in the natural course of HIV-1C infection were analyzed in the gamma interferon (IFN-γ)-enzyme-linked immunospot assay by using synthetic overlapping peptides corresponding to the HIV-1C consensus sequence. For Gag p24, a correlation was seen between better T-cell responses and lower plasma viral load. For Nef, an opposite trend was observed where a higher T-cell response was more likely to be associated with a higher viral load. At the level of the HLA supertypes, a lower viral load was associated with higher T-cell responses to Gag p24 within the HLA A2, A24, B27, and B58 supertypes, in contrast to the absence of such a correlation within the HLA B44 supertype. The present study demonstrated differential correlations (or trends to correlation) in various HIV-1C proteins, suggesting (i) an important role of the HIV-1C Gag p24-specific immune responses in control of viremia and (ii) more rapid viral escape from immune responses to Nef with no restraint of plasma viral load. Correlations between the level of IFN-γ-secreting T cells and viral load within the MHC class I HLA supertypes should be considered in HIV vaccine design and efficacy trials.
An essential role for virus-specific CD8+-T-cell responses in the control of simian immunodeficiency virus (SIV) and human immunodeficiency virus (HIV) infection has been documented (29, 34, 61, 70). Potent cytotoxic-T-lymphocyte (CTL) responses can apparently cause a dramatic drop of plasma viral load in acute HIV type 1 (HIV-1) infection (7, 38) and contain viral replication (54). The emergence of HIV-1-specific CTLs in acute infection coincides with a plasma viral load decline (7, 38). The presence of CD8+ T cells was directly correlated with the control of SIV infection in the macaque model (29, 61). Moreover, vaccine-induced CTL responses protected macaques against the development of AIDS (1, 3, 4, 64). Taken together, these data provide evidence for a protective role of virus-specific CD8+-T-cell responses. However, taken alone, CTL responses are unable in most cases to prevent HIV-1 infection or thwart viral escape from immune recognition (8, 25, 58), which necessitates a cooperation with CD4+-T-helper responses and neutralizing antibodies. An additional challenge for efficient CTL responses is the accumulating genetic diversity of HIV-1 in the global AIDS epidemic driven by a relatively high evolution rate of the virus (18, 36). HIV-1 subtype C (HIV-1C) is the most prevalent HIV-1 subtype worldwide (15, 55) and is responsible for the majority of new HIV-1 infections (16, 55). The emergent ascendancy of HIV-1C indicates a need for a comprehensive analysis of HIV-1C-specific immune responses, both CD4+ and CD8+, in the context of vaccine design.
The association between CTL responses and plasma viral load has been addressed in previous studies of HIV-1B infection (10, 27, 34, 59). An inverse correlation between virus-specific CTL responses and plasma viral load was observed for Gag (14, 53), Pol (6, 53), and Env (47). In contrast, an association with high viral load was reported for Env- and Nef- (5), Gag-, Pol-, and Nef-specific CTL responses (39) or for the total frequency of CD8+ T cells (5). No significant correlation between CTL responses and plasma viral load was found in a number of other recent studies (13, 14, 19, 42). A correlation between CTL responses and higher HIV production was observed during highly active antiretroviral therapy, but an inverse correlation was seen at rapid virus rebound (44) or in patients with relatively high CD4+ counts (37). The discrepancies in correlation between plasma viral load and virus-specific T-cell immune responses might be explained, at least in part, by differences in assay methodologies, the targeting of different epitopes across the viral genome, different disease stages, and differences between diverse population groups. Discrepancies have also been reported between CD8+-T-cell responses as assayed by tetramer staining and immune function indicators such as gamma interferon (IFN-γ) release (21, 26, 37).
The major histocompatibility complex (MHC) class I HLA alleles are involved in presenting foreign antigens to immune cells and are believed to be essential in host immune responses to viral infections. Some HLA alleles or haplotypes have been associated with altered HIV-1 susceptibility and/or progression to AIDS (9, 11, 12, 28, 31, 40, 60, 65-67). The B*35-Cw*04 haplotype was linked with rapid progression of HIV infection. In contrast, B*27 and B*57 were shown to have a protective effect on progression to AIDS (25, 43, 56). HLA-A29 and HLA-B22 were associated with rapid progression, whereas B14 and C8 were associated with slow progression. (28) Different levels of plasma viral load in HIV-1C infection were associated with several HLA class I alleles and haplotypes (66), suggesting favorable (B*57) and unfavorable (B*39, A*30-Cw*03, *02-Cw*16, A*23-B*14, and A*23-Cw*07) HLA alleles. Differences in frequencies of MHC class I HLA alleles within populations might be one of the reasons for disagreements between different studies that addressed associations between CTL responses and plasma viral load. An introduction of major HLA class I supertypes (62, 63) that grouped the MHC class I alleles on the basis of their functional properties allowed us to address associations between CTL responses and plasma viral load within the HLA supertypes.
In the present study, we addressed associations between virus-specific T-cell immune responses and plasma viral load in the course of natural HIV-1C infection in Botswana, an African country with a severe burden of HIV-1C infection. The virus-specific immune responses were measured in asymptomatic HIV-1-infected blood donors by using overlapping peptides that spanned HIV-1C proteins in the IFN-γ-enzyme-linked immunospot (ELISPOT) assay. Correlations between T-cell immune responses and plasma viral loads were estimated for each HIV-1C protein and subregions of the structural proteins Gag (p17, p24, and p2p7p1p6), Pol (protease, reverse transcriptase, RNase H, and integrase), and Env (gp120 and gp41) by analysis of unadjusted and adjusted (for the CD4 and CD8 counts) sets of data. Correlation was also assessed within five major HLA supertypes that were most common among the study population.
MATERIALS AND METHODS
Study subjects.
The sample set used in the present study was the same collection from asymptomatic donors who tested HIV seropositive at the National Blood Transfusion Centre in Gaborone, Botswana, as described previously (48, 50). Details on the HIV testing, sample collection, peripheral blood mononuclear cell (PBMC) isolation, and relevant information of the cohort were described previously (48, 50). The total number of study subjects was 105. Plasma viral load data was available for 103 subjects (median, 37,769 copies/ml; range, <400 to >750,000 copies/ml). CD4 (median count, 434; mean count, 451) and CD8 (median count, 984; mean count, 1,006) data were available for 98 study subjects. Plasma viral load determinations, CD4 and CD8 counts, and HLA typing were performed as described previously (48, 50).
Synthetic peptides.
PBMCs were screened for T-cell immune responses in the IFN-γ-ELISPOT assay within HIV-1C Gag, Pol, Vif, Vpr, Tat, Rev, Vpu, Env, and Nef by using overlapping peptides of 15 to 20 amino acids that overlapped by 10 amino acids based on the HIV-1C consensus sequence as described previously (48, 50). Peptides spanning variable regions were represented by two or three peptides that represented major viral variants based on our previous study of HIV-1C consensus sequence (51). The purity of peptides in most cases was >85%.
ELISPOT assay.
HIV-1C-specific T-cell responses were measured by quantification of the IFN-γ release in a screening ELISPOT assay as described previously (48, 50). The anti-IFN-γ monoclonal antibody (MAb) 1-D1K (Mabtech AB, Nacka, Sweden), biotinylated anti-IFN-γ MAb 7-B6-1 (Mabtech AB), and streptavidin-alkaline phosphatase conjugate (Mabtech AB) were used. IFN-γ-producing cells were counted by direct visualization or by using a stereo microscope and expressed as spot-forming cells per million PBMCs. Only responses with a magnitude >100 spot-forming cells/million PBMC were considered positive in all screening tests. Immune responses to a particular viral protein were expressed as a sum of IFN-γ-ELISPOT T-cell responses to individual peptides spanning the protein. The overall CD8+-T-cell specificity of the IFN-γ-ELISPOT-based responses to synthetic peptides was demonstrated previously by others (23, 24) and by us (48, 50) in a series of CD8 and CD4 depletion and enrichment experiments. However, the CD4+ or CD8+ origin of responses was not systematically quantified in the present study, and therefore the immune responses are presented as total T-cell immune responses.
Statistical analysis.
Statistical analyses and basic graphical delineations were performed by using SigmaPlot 2001 (SPSS, Inc.), Splus v.6.0 (Insightful Corp.), and Microsoft Excel 2000 (Microsoft Corp.) enhanced by the package Analyze-It (Analyze-It Software, Ltd.). For unadjusted analyses, the linear correlation (slope) between the log plasma viral load and the HIV-1C-specific T-cell response within each viral protein or protein subregion was assessed by using the Spearman rank correlation coefficient. Correlations adjusted for CD4 and CD8 counts were assessed by using linear regression models. Spearman rank correlation coefficients were used to compare the log plasma viral load and the HIV-1C-specific immune response within each of the five major HLA supertypes. Linear regression models were used to test whether there were interactions between HLA supertypes and T-cell responses in their affect on the log plasma viral load. All tests were two-tailed, and P values of <0.05 indicated statistical significance. No adjustments for multiple comparisons were made.
RESULTS
T-cell responses to HIV-1C proteins.
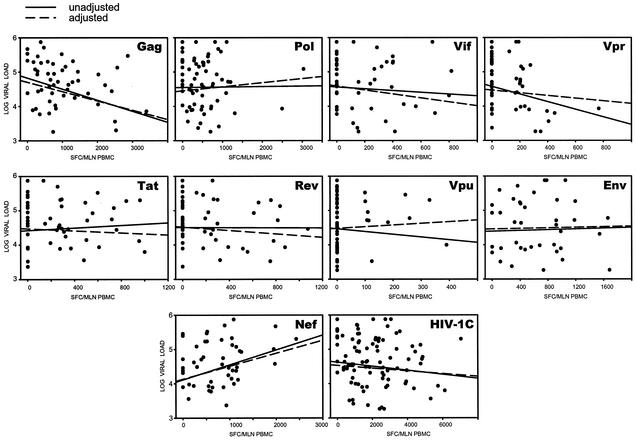
The association between virus-specific T-cell responses and plasma viral load for the HIV-1C Gag, Pol, Vif, Vpr, Tat, Rev, Vpu, Env, and Nef proteins was analyzed (Fig. 1). The opposite trends were revealed in the correlation between T-cell immune responses and viral load for the Gag and Nef proteins (Table 1). Increasing values for HIV-1C Gag-specific T-cell responses were correlated with decreasing levels of plasma viral load (P = 0.020, slope = −0.31, and 95% confidence interval [CI] = −0.52 to −0.05 in the unadjusted analysis [unadjusted], and P = 0.025, slope = −0.27, and 95% CI = −0.51 to −0.04 in the adjusted analysis [adjusted]). In contrast, higher HIV-1C Nef-specific T-cell responses were correlated with higher plasma viral load, although in the unadjusted analysis only a trend was observed (P = 0.079, slope = 0.24, and 95% CI = −0.03 to 0.48 [unadjusted], and P = 0.049, slope = 0.38, and 95% CI = 0.00 to 0.75 [adjusted]). No statistically significant correlation between T-cell immune responses and plasma viral load was found for other HIV-1C proteins or for total T-cell responses in either the unadjusted analysis or the adjusted analysis (Fig. 1 and Table 1).
FIG. 1.
Association between HIV-1C-specific T-cell responses and plasma viral load. Regression curves represent results of unadjusted and adjusted analyses. Corresponding statistics are shown in Table 1.
TABLE 1.
Association between HIV-1C-specific T-cell responses and plasma viral load
| Protein | Unadjusted analysis (Spearman rank correlation coefficient)
|
Adjusted analysis (linear regression)
|
||||
|---|---|---|---|---|---|---|
| Slope estimate | 95% CIa | P | Slope estimate | 95% CIa | P | |
| Gag | −0.31 | −0.52, −0.05 | 0.020 | −0.27 | −0.51, −0.04 | 0.025 |
| Pol | −0.01 | −0.25, 0.22 | 0.90 | 0.12 | −0.21, 0.45 | 0.47 |
| Vif | −0.10 | −0.36, 0.18 | 0.47 | −0.58 | −1.57, 0.41 | 0.24 |
| Vpr | −0.20 | −0.45, 0.07 | 0.15 | −0.39 | −1.90, 1.11 | 0.60 |
| Tat | 0.02 | −0.24, 0.29 | 0.86 | −0.15 | −0.88, 0.58 | 0.68 |
| Rev | −0.05 | −0.31, 0.23 | 0.75 | −0.25 | −0.96, 0.45 | 0.47 |
| Vpu | −0.04 | −0.30, 0.22 | 0.76 | 0.49 | −1.77, 2.75 | 0.66 |
| Env | 0.01 | −0.29, 0.31 | 0.94 | 0.04 | −0.39, 0.48 | 0.85 |
| Nef | 0.24 | −0.03, 0.48 | 0.079 | 0.38 | 0.00, 0.75 | 0.049 |
| HIV-1C | −0.16 | −0.35, 0.03 | 0.11 | −0.04 | −0.15, 0.07 | 0.45 |
Minimum, maximum.
Association between T-cell responses and viral load within the Gag, Pol, and Env.
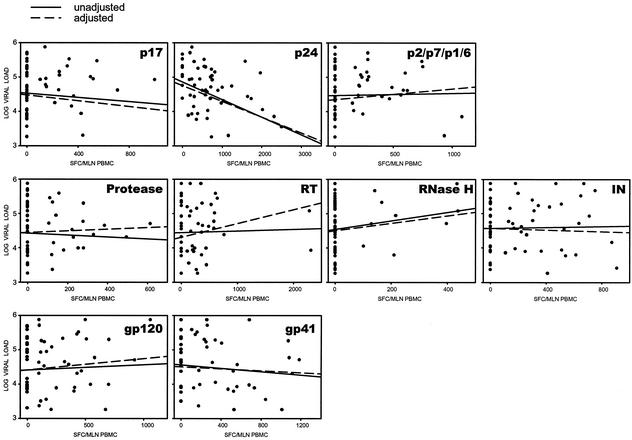
To address potential trends within the structural HIV-1C proteins, a similar analysis of association between the HIV-1C-specific T-cell responses and plasma viral load was performed for the p17, p24, and p2/p7/p1/p6 cleavage products of Gag; the protease, reverse transcriptase, RNase H, and integrase of Pol; and the gp120 and gp41 of Env (Fig. 2, accompanied by Table 2). Only Gag p24 demonstrated a strong inverse correlation between T-cell response and viral load (P = 0.0016, slope = −0.40, and 95% CI = −0.60 to −0.16 [unadjusted], and P = 0.005, slope = −0.43, and 95% CI = −0.72 to −0.14 [adjusted]).
FIG. 2.
Association between HIV-1C-specific T-cell responses and plasma viral load in subregions of Gag, Pol, and Env proteins. Regression curves represent results of unadjusted and adjusted analyses. Corresponding statistics are shown in Table 2.
TABLE 2.
Association between HIV-1C-specific T-cell responses and plasma viral load within the structural HIV-1C proteins
| Protein | Unadjusted analysis (Spearman rank correlation coefficient)
|
Adjusted analysis (linear regression)
|
||||
|---|---|---|---|---|---|---|
| Slope estimate | 95% CIa | P | Slope estimate | 95% CIa | P | |
| Gag p17 | 0.02 | −0.24, −0.27 | 0.90 | −0.43 | −1.32, 0.46 | 0.34 |
| Gag p24 | −0.40 | −0.60, −0.16 | 0.0016 | −0.43 | −0.72, −0.14 | 0.005 |
| Gag p2/p7/p1/p6 | 0.12 | −0.14, 0.37 | 0.35 | 0.33 | −0.44, 1.11 | 0.39 |
| Pol protease | −0.05 | −0.30, 0.22 | 0.73 | 0.26 | −1.15, 1.67 | 0.71 |
| Pol reverse transcriptase | −0.01 | −0.28, 0.26 | 0.96 | 0.37 | −0.07, 0.81 | 0.10 |
| Pol RNase H | 0.11 | −0.14, 0.35 | 0.40 | 0.90 | −1.06, 2.86 | 0.35 |
| Pol integrase | 0.02 | −0.23, 0.27 | 0.86 | 0.00 | −0.78, 0.78 | 0.90 |
| Env gp120 | 0.00 | −0.25, 0.26 | 0.97 | 0.31 | −0.43, 1.05 | 0.40 |
| Env gp41 | −0.15 | −0.41, 0.13 | 0.28 | −0.12 | −0.72, 0.48 | 0.69 |
Minimum, maximum.
For both HIV-1C proteins and their subregions, unadjusted and adjusted analyses resulted in similar associations and/or trends (Fig. 1 and 2, Tables 1 and 2).
T-cell responses and viral load in relation to MHC class I supertypes.
Previously, we identified HLA class I alleles and/or antigen specificities that are most common in the Botswana population (49, 50). This allowed us to determine how HLA alleles common in Botswana are represented within the major HLA class I supertypes (62, 63). The distribution of the Botswana HLA alleles within MHC class I supertypes highlighted specifics of the common HLA alleles in the Botswana population (Table 3). Based on a cumulative allele frequency, the A24 supertype included the HLA alleles that were most common in Botswana (the cumulative frequency of A23 and A30 antigen specificities was 37.2%), followed by the A2, B27, B44, and B58 supertypes that were accountable for a frequency of about 20% each in the Botswana population. However, four HLA class I supertypes—A1, A3, B7, and B62—included HLA alleles that were seen at a relatively low frequency in Botswana (cumulative frequency of <20%). Based on this analysis, we selected five of the most representative major HLA class I supertypes in Botswana, namely, A2, A24, B27, B44, and B58, and analyzed associations and trends between HIV-1C-specific T-cell responses and plasma viral load within these HLA supertypes. About 97.8% of the study population was covered by at least one HLA allele belonging to the A2, A24, B27, B44, or B58 HLA supertypes.
TABLE 3.
Major HLA class I supertypes and their representation in Botswana
| HLA supertypea | MHC class I HLA alleles; potential alleles | Frequency (total %) in Botswana |
|---|---|---|
| A2 | A*0201-0207, A*6802, A*6901 | 24 |
| A3 | A*0301, A*1101, A*3101, A*3301, A*6801 | 8 |
| B7 | B*0702, B*3501-03, B*51, B*5301, B*5401; B*0703-05, B*1508, B*5501-02, B*5601-02, B*6701; B*7801 | 12 |
| B44 | B*3701, B*4402-03, B60 (B*4001); B61 (B*4006); B*18, B*4101, B*4901, B*5001 | 20 |
| A1 | A*0101, A*2501, A*2601, A*2602, A*3201; A*0102; A*2604, A*3601, A*4301 | 14 |
| A24 | A*2301, A*2402-04, A*3001-03 | 37 |
| B27 | B*1401-02, B*1503, B*1509, B*1510; B*1518, B*2701-08, B*3801-02, B*3902-04, B*4801-02, B*7301 | 22 |
| B58 | B*1516, B*1517, B*5701, B*5702, B*58 | 21 |
| B62 | B*4601, B*52, B*1501 (B62), B*1502 (B75), B*1513 (B77); B*1301-02, B*1506, B*1512, B*1514, B*1519, B*1521 | 3 |
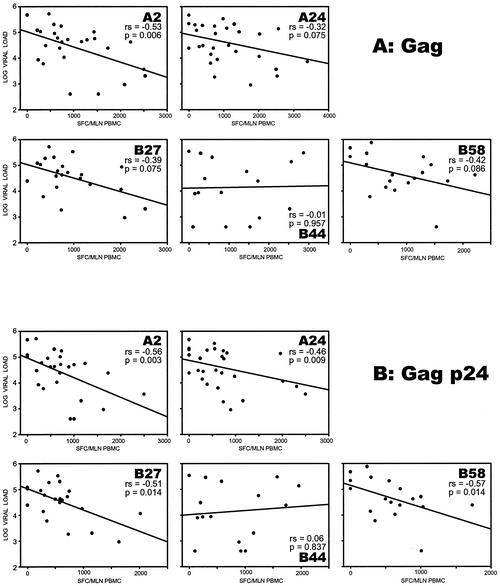
Distinct patterns of correlation were found within different MHC class I HLA supertypes (Fig. 3). The correlation patterns also differed across the HIV-1C proteins. Pronounced HLA supertype-based differences were seen in the HIV-1C Gag and Gag p24. Within HIV-1C Gag, a statistically significant correlation between strong T-cell responses and low viral load was found in the A2 supertype (P = 0.0058, slope = −0.53, and 95% CI = −0.76 to −0.17 [unadjusted], and P = 0.0024, slope = −0.53, and 95% CI = −0.87 to −0.19 [adjusted]). The A24, B27, and B58 supertypes demonstrated trends toward the same correlation in both unadjusted and adjusted analyses (P = 0.065, 0.059, and 0.066, respectively, in the adjusted analysis). Interestingly, there was no correlation within the B44 supertype (P = 0.95). The observed associations for Gag were more pronounced for HIV-1C Gag p24, revealing a significant correlation between higher T-cell responses and lower plasma viral loads for all but the B44 supertype (P = 0.0028 for A2, P = 0.0086 for A24, P = 0.0142 for B27, and P = 0.0144 for B58, but P = 0.84 for B44 [unadjusted]; P = 0.0009 for A2, P = 0.0053 for A24, P = 0.0073 for B27, P = 0.0064 for B58, but P = 0.81 for B44 [adjusted]).
FIG. 3.
Association between HIV-1C-specific T-cell responses and plasma viral load within the major HLA class I supertypes. Regression curves represent results of unadjusted analysis. (A) Gag associations; (B) Gag p24 associations.
If T-cell responses and viral load correlate inversely for some HLA supertypes but not for others, then T-cell responses may be HLA supertype specific. To assess this question statistically for HIV-1C Gag and for Gag p24, we used linear regression models to test whether HLA supertype and T-cell responses interacted in their effect on viral load. To test a potential interaction between HLA supertype and T-cell responses, we used linear regression models for the HIV-1C Gag and Gag p24 subregion. Two trends were found: (i) for Gag (HLA supertype A2 versus B44) there was a strong negative correlation for A2 but no correlation for B44 supertype, with an interaction test P value of 0.086; (ii) for Gag p24 (HLA supertype A2 versus B44) there was also a strong negative correlation for A2 but no correlation for B44 supertype, with an interaction test P value of 0.063. The strength of the observed correlation between the MHC class I HLA supertypes and the plasma viral load could be clarified by increasing the sample size in each HLA supertype.
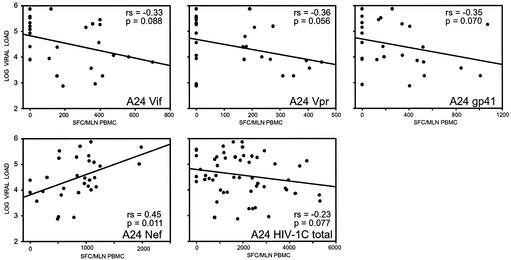
Within the A24 supertype, higher Nef-specific responses were correlated with higher plasma viral load (P = 0.011, slope = 0.45, 95% CI = 0.12 to 0.69 [unadjusted], and P = 0.006, slope = 0.45, and 95% CI = 0.13 to 0.77 [adjusted] [Fig. 4]). Trends for a correlation between higher virus-specific responses and lower viral load within the A24 supertype were seen for gp41 (P = 0.070, slope = −0.35, and 95% CI = −0.65 to 0.03 [unadjusted], and P = 0.091, slope = −0.32, and 95% CI = −0.69 to 0.05 [adjusted]), and the total HIV-1C (P = 0.077, slope = −0.23, and 95% CI = −0.46 to 0.03 [unadjusted], and P = 0.071, slope = −0.23, and 95% CI = −0.49 to 0.02 [adjusted]).
FIG. 4.
Association between HIV-1C-specific T-cell responses and plasma viral load within the HLA class I A24 supertype. Regression curves represent results of unadjusted analysis.
No other significant correlations were found between HIV-1C-specific T-cell responses and viral load.
DISCUSSION
In the present study correlation patterns between virus-specific immune responses and plasma viral load were found to vary at two different levels: viral and host. Specifically, differences in correlation were detected (i) between the viral proteins and (ii) between different MHC class I HLA supertypes. Although the results of the present study warrant further research in regards to different stages of disease (i.e., acute versus chronic), these findings have direct relevance for our understanding of HIV pathogenesis, as well as for the design of HIV vaccines and vaccine efficacy trials.
HIV-1C dominates in the global AIDS epidemic. However, associations between HIV-1C-specific immune responses and viral loads have not been analyzed previously. In addition, differences in geographical distribution of HLA alleles, taken in the context of MHC class I restriction of CTL epitope presentation, may imply another level of divergence between populations and/or ethnic groups. For example, the predominance of HIV-1C in southern Africa (15, 16, 51, 52, 68, 69) and specifics of MHC class I HLA allele frequencies in the Botswana population (49, 50) determined a unique immunodominant profile of cell-mediated immune responses (48, 50).
Patterns of correlation between HIV-1C-specific immune responses and plasma viral load for different viral proteins included an association between strong T-cell responses and lower viremia or an association between stronger T-cell responses and high viral load. In most instances the magnitude of T-cell response was not correlated with either a higher or a lower viral load. A correlation between stronger T-cell responses and lower plasma viral load in the Gag/Gag-p24 proteins coexisted with a trend toward an opposite correlation in the Nef, whereas there was no pattern of correlation between T-cell responses and plasma viral load for the other HIV-1C proteins (analyzed for the entire study group).
The correlation between virus-specific immune responses and plasma viral load in HIV-1C Gag, and particularly in Gag p24, suggests an important role of p24-specific T-cell responses in the control of viremia. The highly conserved p24 appears to have functional constraints and may not be flexible for accumulation of new mutations, which in turn would allow control of the virus by a capable Gag p24-specific T-cell response. It also raises the notion of relative importance of HIV proteins for vaccine design and suggests that the Gag p24 might be the most attractive region to include in vaccine candidates to induce T-cell immune responses that could contain viremia.
In contrast, the correlation (or trend) between increasing Nef-specific T-cell responses and increasing plasma viral load suggests that no benefit may be realized from including Nef or some Nef epitopes in such a vaccine. It also suggests that viral escape from immune recognition is more likely to occur rapidly for CTL epitopes of Nef. The notorious capability of HIV-1 to escape from CTL recognition in Nef was supported by several studies of HIV and SIV (17, 33, 35, 45, 46, 58). HIV-1 Nef is believed to downmodulate the expression of MHC class I molecules, which in turn impairs CTL responses, resulting in an inability to control virus replication (reviewed in references 2, 30, and 57). It is not clear whether Nef-mediated downmodulation of HLA class I molecules plays a role in the observed correlation between increased HIV-1C Nef-specific T-cell responses and elevated levels of plasma viral load.
Diversity in host genetics is likely to be manifested by assorted virus-specific CTL responses for carriers of different MHC class I HLA alleles or HLA supertypes. In the present study the correlation between increasing HIV-1C Gag/Gag p24 immune responses and decreasing viral load within the HLA supertypes A2, A24, B27, and B58 contrasted with the absence of such a correlation for the HLA B44 supertype. This finding demonstrates differences in virus-specific T-cell immune responses in subsets of the population according to expressed HLA class I alleles or HLA supertypes. It is apparent that carriers of the A2 supertype have a strong correlation between T-cell responses and plasma viral load, whereas carriers of B44 supertype do not have such a correlation. In fact, many previous studies that have described a correlation between increasing HIV-1-specific CTL responses and decreasing viral load for HIV-1B have used HLA-A02-Gag epitope tetramer complexes or Gag-specific CTL responses as markers of immune response.
The genetic background of the population might be an important factor for vaccine efficacy, particularly when limited epitope-specific vaccine designs are used. Kaslow et al. described a predictive power for particular HLA class I alleles for the outcome of vaccine trials, showing that individuals vaccinated with HLA-B*27 or HLA-B*57, HLA alleles associated with slower disease progression, had better responses to an ALVAC-HIV recombinant canarypox vaccine (32). The results of our study also point, although indirectly, to a potential difference in immune responses among carriers of different HLA supertypes. Assuming that differences in correlation patterns are related to control of viremia, differences between diverse MHC class I HLA supertypes should be taken into account in vaccine design to elicit optimal CTL responses within HLA supertypes, as well as to design vaccine efficacy trials for participants that represent the population in which such a vaccine would be used in the future. This information could also be used in sieve analyses of efficacy trial data (20) that assess whether the presence and number of HLA-restricted epitopes in the infecting strain relative to the vaccine strain(s) is associated with vaccine control of viral load.
Despite their usefulness for research analysis, the nine designated major HLA supertypes listed in Table 3 do not represent a final and comprehensive classification of MHC class I. Not all HLA alleles were assigned to the major HLA supertypes (62, 63), i.e., HLA-A29, HLA-A34, HLA-A36, HLA-A66, HLA-A74, HLA-B08, HLA-B42, and HLA-B45. Most of those unclassified HLA supertypes are HLA specificities common in African countries. For example, HLA supertypes did not include 18% of HLA-A and 23% of HLA-B antigen specificities that were found in the Botswana population (49). In addition, placing all HLA-B58 alleles into one supertype probably needs to be further tested because of known structural and binding differences between the HLA-B*5801 and HLA-B*5802 alleles (22, 41). Since new HIV vaccine trials are being designed for African populations where rates of HIV infection are high, it would be important to facilitate the assignment of missing alleles to the HLA supertypes.
In summary, we demonstrated different correlations between plasma viral load and T-cell immune responses in the course of natural HIV-1C infection that included increasing responses to Gag p24 and decreasing viral loads, as well as increasing T-cell responses to Nef and increases in viral load. Within the five major MHC class I HLA supertypes, a correlation between reduced viral load and Gag p24-specific T-cell responses within the A2, A24, B27, and B58 supertypes stood out against no correlation within the HLA B44 supertype. We suggest that the correlation within HIV-1C Gag p24 responses makes the Gag p24 region an attractive vaccine candidate. In contrast, the lack of such a correlation in Nef might suggest no control of viremia. Identified differences in association between virus-specific T-cell responses and plasma viral load should be considered in vaccine design and development, particularly as they relate to the populations of southern Africa that are severely impacted by the epidemic of HIV-1C, which has the highest rates of infection in the world (15, 16).
Acknowledgments
We thank the Botswana Ministry of Health for encouragement; S. Y. Chang, S. Gaseitsiwe, E. Sepako, G. Sebetso, N. Monametsi, A. Reich, and Y. Wu for sample processing and HIV-1 diagnostics; the personnel of the National Blood Transfusion Center in Botswana for collaboration; and Chanc E VanWinkle for editorial assistance.
This research was supported in part by grants AI47067, AI43255, and HD37793 from the National Institutes of Health (NIH) and grant TW00004 from the Fogarty International Center, NIH.
REFERENCES
- 1.Amara, R. R., F. Villinger, J. D. Altman, S. L. Lydy, S. P. O'Neil, S. I. Staprans, D. C. Montefiori, Y. Xu, J. G. Herndon, L. S. Wyatt, M. A. Candido, N. L. Kozyr, P. L. Earl, J. M. Smith, H.-L. Ma, B. D. Grimm, M. L. Hulsey, J. Miller, H. M. McClure, J. M. McNicholl, B. Moss, and H. L. Robinson. 2001. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science 292:69-74. [DOI] [PubMed] [Google Scholar]
- 2.Arora, V. K., B. L. Fredericksen, and J. V. Garcia. 2002. Nef: agent of cell subversion. Microbes Infect. 4:189-199. [DOI] [PubMed] [Google Scholar]
- 3.Barouch, D. H., S. Santra, M. J. Kuroda, J. E. Schmitz, R. Plishka, A. Buckler-White, A. E. Gaitan, R. Zin, J. H. Nam, L. S. Wyatt, M. A. Lifton, C. E. Nickerson, B. Moss, D. C. Montefiori, V. M. Hirsch, and N. L. Letvin. 2001. Reduction of simian-human immunodeficiency virus 89.6P viremia in rhesus monkeys by recombinant modified vaccinia virus Ankara vaccination. J. Virol. 75:5151-5158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barouch, D. H., S. Santra, J. E. Schmitz, M. J. Kuroda, T. M. Fu, W. Wagner, M. Bilska, A. Craiu, X. X. Zheng, G. R. Krivulka, K. Beaudry, M. A. Lifton, C. E. Nickerson, W. L. Trigona, K. Punt, D. C. Freed, L. Guan, S. Dubey, D. Casimiro, A. Simon, M. E. Davies, M. Chastain, T. B. Strom, R. S. Gelman, D. C. Montefiori, and M. G. Lewis. 2000. Control of viremia and prevention of clinical AIDS in rhesus monkeys by cytokine-augmented DNA vaccination. Science 290:486-492. [DOI] [PubMed] [Google Scholar]
- 5.Betts, M. R., D. R. Ambrozak, D. C. Douek, S. Bonhoeffer, J. M. Brenchley, J. P. Casazza, R. A. Koup, and L. J. Picker. 2001. Analysis of total human immunodeficiency virus (HIV)-specific CD4+ and CD8+ T-cell responses: relationship to viral load in untreated HIV infection. J. Virol. 75:11983-11991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Betts, M. R., J. F. Krowka, T. B. Kepler, M. Davidian, C. Christopherson, S. Kwok, L. Louie, J. Eron, H. Sheppard, and J. A. Frelinger. 1999. Human immunodeficiency virus type 1-specific cytotoxic T lymphocyte activity is inversely correlated with HIV type 1 viral load in HIV type 1-infected long-term survivors. AIDS Res. Hum. Retrovir. 15:1219-1228. [DOI] [PubMed] [Google Scholar]
- 7.Borrow, P., H. Lewicki, B. H. Hahn, G. M. Shaw, and M. B. A. Oldstone. 1994. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J. Virol. 68:6103-6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borrow, P., H. Lewicki, X. Wei, M. S. Horwitz, N. Peffer, H. Meyers, J. A. Nelson, J. E. Gairin, B. H. Hahn, M. B. Oldstone, and G. M. Shaw. 1997. Antiviral pressure exerted by HIV-1-specific cytotoxic T lymphocytes (CTLs) during primary infection demonstrated by rapid selection of CTL escape virus. Nat. Med. 3:205-211. [DOI] [PubMed] [Google Scholar]
- 9.Candore, G., G. C. Romano, C. D'Anna, G. DiLorenzo, F. Gervasi, D. Lio, M. A. Modica, M. Potestio, and C. Caruso. 1998. Biological basis of the HLA-B8,DR3-associated progression of acquired immune deficiency syndrome. Pathobiology 66:33-37. [DOI] [PubMed] [Google Scholar]
- 10.Cao, Y., L. Qin, L. Zhang, J. Safrit, and D. D. Ho. 1995. Virologic and immunologic characterization of long-term survivors of human immunodeficiency virus type 1 infection. N. Engl. J. Med. 332:201-208. [DOI] [PubMed] [Google Scholar]
- 11.Carrington, M., G. W. Nelson, M. P. Martin, T. Kissner, D. Vlahov, J. J. Goedert, R. Kaslow, S. Buchbinder, K. Hoots, and S. J. O'Brien. 1999. HLA and HIV-1: heterozygote advantage and B*35-Cw*04 disadvantage. Science 283:1748-1752. [DOI] [PubMed] [Google Scholar]
- 12.Chen, Y., R. Winchester, B. Korber, J. Gagliano, Y. Bryson, C. Hutto, N. Martin, G. McSherry, A. Petru, D. Wara, and A. Ammann. 1997. Influence of HLA alleles on the rate of progression of vertically transmitted HIV infection in children: association of several HLA-DR13 alleles with long-term survivorship and the potential association of HLA-A*2301 with rapid progression to AIDS. Long-Term Survivor Study. Hum. Immunol. 55:154-162. [DOI] [PubMed] [Google Scholar]
- 13.Dalod, M., M. Dupuis, J. C. Deschemin, D. Sicard, D. Salmon, J. F. Delfraissy, A. Venet, M. Sinet, and J. G. Guillet. 1999. Broad, intense anti-human immunodeficiency virus (HIV) ex vivo CD8+ responses in HIV type 1-infected patients: comparison with anti-Epstein-Barr virus responses and changes during antiretroviral therapy. J. Virol. 73:7108-7116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edwards, B. H., A. Bansal, S. Sabbaj, J. Bakari, M. J. Mulligan, and P. A. Goepfert. 2002. Magnitude of functional CD8+ T-cell responses to the Gag protein of human immunodeficiency virus type 1 correlates inversely with viral load in plasma. J. Virol. 76:2298-2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Esparza, J., and N. Bhamarapravati. 2000. Accelerating the development and future availability of HIV-1 vaccines: why, when, where, and how? Lancet 355:2061-2066. [DOI] [PubMed] [Google Scholar]
- 16.Essex, M., and S. Mboup. 2002. Regional variations in the African epidemics, p. 629-638. In M. Essex, S. Mboup, P. Kanki, R. Marlink, and S. Tlou (ed.), AIDS in Africa, 2nd ed. Kluwer Academic/Plenum Publishers, New York, N.Y.
- 17.Evans, D. T., D. H. O'Connor, P. Jing, J. L. Dzuris, J. Sidney, J. da Silva, T. M. Allen, H. Horton, J. E. Venham, R. A. Rudersdorf, T. Vogel, C. D. Pauza, R. E. Bontrop, R. DeMars, A. Sette, A. L. Hughes, and D. I. Watkins. 1999. Virus-specific cytotoxic T-lymphocyte responses select for amino-acid variation in simian immunodeficiency virus Env and Nef. Nat. Med. 5:1270-1276. [DOI] [PubMed] [Google Scholar]
- 18.Foley, B. T. 2000. An overview of the molecular phylogeny of lentiviruses, p. 35-43. In C. Kuiken, B. Foley, B. Hahn, P. Marx, F. McCutchan, J. W. Mellors, J. Mullins, S. Wolinsky, and B. Korber (ed.), HIV sequence compendium 2000: theoretical biology and biophysics. Los Alamos National Laboratory, Los Alamos, N.Mex.
- 19.Gea-Banacloche, J. C., S. A. Migueles, L. Martino, W. L. Shupert, A. C. McNeil, M. S. Sabbaghian, L. Ehler, C. Prussin, R. Stevens, L. Lambert, J. Altman, C. W. Hallahan, J. C. de Quiros, and M. Connors. 2000. Maintenance of large numbers of virus-specific CD8+ T cells in HIV-infected progressors and long-term nonprogressors. J. Immunol. 165:1082-1092. [DOI] [PubMed] [Google Scholar]
- 20.Gilbert, P., S. Self, M. Rao, A. Naficy, and J. Clemens. 2001. Sieve analysis: methods for assessing from vaccine trial data how vaccine efficacy varies with genotypic and phenotypic pathogen variation. J. Clin. Epidemiol. 54:68-85. [DOI] [PubMed] [Google Scholar]
- 21.Goepfert, P. A., A. Bansal, B. H. Edwards, G. D. J. Ritter, I. Tellez, S. A. McPherson, S. Sabbaj, and M. J. Mulligan. 2000. A significant number of human immunodeficiency virus epitope-specific cytotoxic T lymphocytes detected by tetramer binding do not produce gamma interferon. J. Virol. 74:10249-10255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goulder, P. J. 2000. Rapid characterization of HIV clade C-specific cytotoxic T lymphocyte responses in infected African children and adults. Ann. N. Y. Acad. Sci. 918:330-345. [DOI] [PubMed] [Google Scholar]
- 23.Goulder, P. J., M. M. Addo, M. A. Altfeld, E. S. Rosenberg, Y. Tang, U. Govender, N. Mngqundaniso, K. Annamalai, T. U. Vogel, M. Hammond, M. Bunce, H. M. Coovadia, and B. D. Walker. 2001. Rapid definition of five novel HLA-A*3002-restricted human immunodeficiency virus-specific cytotoxic T-lymphocyte epitopes by ELISPOT and intracellular cytokine staining assays. J. Virol. 75:1339-1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goulder, P. J., C. Brander, K. Annamalai, N. Mngqundaniso, U. Govender, Y. Tang, S. He, K. E. Hartman, C. A. O'Callaghan, G. S. Ogg, M. A. Altfeld, E. S. Rosenberg, H. Cao, S. A. Kalams, M. Hammond, M. Bunce, S. I. Pelton, S. A. Burchett, K. McIntosh, H. M. Coovadia, and B. D. Walker. 2000. Differential narrow focusing of immunodominant human immunodeficiency virus gag-specific cytotoxic T-lymphocyte responses in infected African and Caucasoid adults and children. J. Virol. 74:5679-5690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goulder, P. J., R. E. Phillips, R. A. Colbert, S. McAdam, G. Ogg, M. A. Nowak, P. Giangrande, G. Luzzi, B. Morgan, A. Edwards, A. J. McMichael, and S. Rowland-Jones. 1997. Late escape from an immunodominant cytotoxic T-lymphocyte response associated with progression to AIDS. Nat. Med. 3:212-217. [DOI] [PubMed] [Google Scholar]
- 26.Goulder, P. J., Y. Tang, C. Brander, M. R. Betts, M. Altfeld, K. Annamalai, A. Trocha, S. He, E. S. Rosenberg, G. Ogg, C. A. O'Callaghan, S. A. Kalams, R. E. McKinney, K. Mayer, R. A. Koup, S. I. Pelton, S. K. Burchett, K. McIntosh, and B. D. Walker. 2000. Functionally inert HIV-specific cytotoxic T lymphocytes do not play a major role in chronically infected adults and children. J. Exp. Med. 192:1819-1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greenough, T. C., D. B. Brettler, M. Somasundaran, D. L. Panicali, and J. L. Sullivan. 1997. Human immunodeficiency virus type 1-specific cytotoxic T lymphocytes (CTL), virus load, and CD4 T-cell loss: evidence supporting a protective role for CTL in vivo. J. Infect. Dis. 176:118-125. [DOI] [PubMed] [Google Scholar]
- 28.Hendel, H., S. Caillat-Zucman, H. Lebuanec, M. Carrington, S. O'Brien, J. M. Andrieu, F. Schachter, D. Zagury, J. Rappaport, C. Winkler, G. W. Nelson, and J. F. Zagury. 1999. New class I and II HLA alleles strongly associated with opposite patterns of progression to AIDS. J. Immunol. 162:6942-6946. [PubMed] [Google Scholar]
- 29.Jin, X., D. E. Bauer, S. E. Tuttleton, S. Lewin, A. Gettie, J. Blanchard, C. E. Irwin, J. T. Safrit, J. Mittler, L. Weinberger, L. G. Kostrikis, L. Zhang, A. S. Perelson, and D. D. Ho. 1999. Dramatic rise in plasma viremia after CD8+ T-cell depletion in simian immunodeficiency virus-infected macaques. J. Exp. Med. 189:991-998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson, W. E., and R. C. Desrosiers. 2002. Viral persistance: HIV's strategies of immune system evasion. Annu. Rev. Med. 53:499-518. [DOI] [PubMed] [Google Scholar]
- 31.Kaslow, R. A., M. Carrington, R. Apple, L. Park, A. Munoz, A. J. Saah, J. J. Goedert, C. Winkler, S. J. O'Brien, C. Rinaldo, R. Detels, W. Blattner, J. Phair, H. Erlich, and D. L. Mann. 1996. Influence of combinations of human major histocompatibility complex genes on the course of HIV-1 infection. Nat. Med. 2:405-411. [DOI] [PubMed] [Google Scholar]
- 32.Kaslow, R. A., C. Rivers, J. Tang, T. J. Bender, P. A. Goepfert, R. El Habib, K. Weinhold, M. J. Mulligan, et al. 2001. Polymorphisms in HLA class I genes associated with both favorable prognosis of human immunodeficiency virus (HIV) type 1 infection and positive cytotoxic-T-lymphocyte responses to ALVAC-HIV recombinant canarypox vaccines. J. Virol. 75:8681-8689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaur, A., L. Alexander, S. I. Staprans, L. Denekamp, C. L. Hale, H. M. McClure, M. B. Feinberg, R. C. Desrosiers, and R. P. Johnson. 2001. Emergence of cytotoxic T lymphocyte escape mutations in nonpathogenic simian immunodeficiency virus infection. Eur. J. Immunol. 31:3207-3217. [DOI] [PubMed] [Google Scholar]
- 34.Klein, M. R., C. A. van Baalen, A. M. Holwerda, S. R. Kerkhof-Garde, R. J. Bende, I. P. Keet, J. K. Eeftinck-Schattenkerk, A. D. Osterhaus, H. Schuitemaker, and F. Miedema. 1995. Kinetics of Gag-specific cytotoxic T lymphocyte responses during the clinical course of HIV-1 infection: a longitudinal analysis of rapid progressors and long-term asymptomatics. J. Exp. Med. 181:1365-1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koenig, S., A. J. Conley, Y. A. Brewah, G. M. Jones, S. Leath, L. J. Boots, V. Davey, G. Pantaleo, J. F. Demarest, C. Carter, C. Wannebo, J. R. Yannelli, S. A. Rosenberg, and H. C. Lane. 1995. Transfer of HIV-1-specific cytotoxic T lymphocytes to an AIDS patient leads to selection for mutant HIV variants and subsequent disease progression. Nat. Med. 1:330-336. [DOI] [PubMed] [Google Scholar]
- 36.Korber, B., M. Muldoon, J. Theiler, F. Gao, R. Gupta, A. Lapedes, B. H. Hahn, S. Wolinsky, and T. Bhattacharya. 2000. Timing the ancestor of the HIV-1 pandemic strains. Science 288:1789-1796. [DOI] [PubMed] [Google Scholar]
- 37.Kostense, S., G. S. Ogg, E. H. Manting, G. Gillespie, J. Joling, K. Vandenberghe, E. Z. Veenhof, D. van Baarle, S. Jurriaans, M. R. Klein, and F. Miedema. 2001. High viral burden in the presence of major HIV-specific CD8+ T-cell expansions: evidence for impaired CTL effector function. Eur. J. Immunol. 31:677-686. [DOI] [PubMed] [Google Scholar]
- 38.Koup, R. A., J. T. Safrit, Y. Cao, C. A. Andrews, G. McLeod, W. Borkowsky, C. Farthing, and D. D. Ho. 1994. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J. Virol. 68:4650-4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lubaki, N. M., M. E. Shepherd, R. S. Brookmeyer, H. Hon, T. C. Quinn, M. Kashamuka, M. Johnson, R. Gottle, J. Devers, H. M. Lederman, and R. C. Bollinger. 1999. HIV-1-specific cytolytic T-lymphocyte activity correlates with lower viral load, higher CD4 count, and CD8+ CD38− DR− phenotype: comparison of statistical methods for measurement. J. Acquir. Immune Defic. Syndr. 22:19-30. [DOI] [PubMed] [Google Scholar]
- 40.MacDonald, K. S., K. R. Fowke, J. Kimani, V. A. Dunand, N. J. D. Nagelkerke, T. B. Ball, J. Oyudi, E. Njagi, L. K. Gaur, R. C. Brunham, J. Wade, M. A. Luscher, P. Krausa, S. Rowland-Jones, E. Ngugi, J. J. Bwayo, and F. A. Pummer. 2000. Influence of HLA supertypes on susceptibility and resistance to human immunodeficiency virus type 1 infection. J. Infect. Dis. 181:1581-1589. [DOI] [PubMed] [Google Scholar]
- 41.Marsh, S. G. E., P. Parham, and L. D. Barber. 2000. The HLA facts book. Academic Press, London, United Kingdom.
- 42.Migueles, S. A., and M. Connors. 2001. Frequency and function of HIV-specific CD8+ T cells. Immunol. Lett. 79:141-150. [DOI] [PubMed] [Google Scholar]
- 43.Migueles, S. A., M. S. Sabbaghian, W. L. Shupert, M. P. Bettinotti, F. M. Marincola, L. Martino, C. W. Hallahan, S. M. Selig, D. Schwartz, J. Sullivan, and M. Connors. 2000. HLA B*5701 is highly associated with restriction of virus replication in a subgroup of HIV-infected long term nonprogressors. Proc. Natl. Acad. Sci. USA 97:2709-2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mollet, L., T. S. Li, A. Samri, C. Tournay, R. Tubiana, V. Calvez, P. Debre, C. Katlama, and B. Autran. 2000. Dynamics of HIV-specific CD8+ T lymphocytes with changes in viral load. J. Immunol. 165:1692-1704. [DOI] [PubMed] [Google Scholar]
- 45.Mortara, L., F. Letourneur, H. Gras-Masse, A. Venet, J. G. Guillet, and I. Bourgault-Villada. 1998. Selection of virus variants and emergence of virus escape mutants after immunization with an epitope vaccine. J. Virol. 72:1403-1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mortara, L., F. Letourneur, P. Villefroy, C. Beyer, H. Gras-Masse, J. G. Guillet, and I. Bourgault-Villada. 2000. Temporal loss of Nef-epitope CTL recognition following macaque lipopeptide immunization and SIV challenge. Virology 278:551-561. [DOI] [PubMed] [Google Scholar]
- 47.Musey, L., J. Hughes, T. Schacker, T. Shea, L. Corey, and M. J. McElrath. 1997. Cytotoxic-T-cell responses, viral load, and disease progression in early human immunodeficiency virus type 1 infection. N. Engl. J. Med. 337:1267-1274. [DOI] [PubMed] [Google Scholar]
- 48.Novitsky, V., H. Cao, N. Rybak, P. Gilbert, M. F. McLane, S. Gaolekwe, T. Peter, I. Thior, T. Ndung'u, R. Marlink, T. H. Lee, and M. Essex. 2002. Magnitude and frequency of CTL responses: identification of immunodominant regions across HIV-1C genome. J. Virol. 76:10155-10168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Novitsky, V., P. O. Flores-Villanueva, P. Chigwedere, S. Gaolekwe, H. Bussman, G. Sebetso, R. Marlink, E. J. Yunis, and M. Essex. 2001. Identification of most frequent HLA class I antigen specificities in Botswana: relevance for HIV vaccine design. Hum. Immunol. 62:146-156. [DOI] [PubMed] [Google Scholar]
- 50.Novitsky, V., N. Rybak, M. F. McLane, P. Gilbert, P. Chigwedere, I. Klein, S. Gaolekwe, S. Y. Chang, T. Peter, I. Thior, T. Ndung'u, F. Vannberg, B. T. Foley, R. Marlink, T. H. Lee, and M. Essex. 2001. Identification of human immunodeficiency virus type 1 subtype C Gag-, Tat-, Rev-, and Nef-specific ELISPOT-based cytotoxic-T-lymphocyte responses for AIDS vaccine design. J. Virol. 75:9210-9228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Novitsky, V., U. R. Smith, P. Gilbert, M. F. McLane, P. Chigwedere, C. Williamson, T. Ndung'u, I. Klein, S. Y. Chang, T. Peter, I. Thior, B. T. Foley, S. Gaolekwe, N. Rybak, S. Gaseitsiwe, F. Vannberg, R. Marlink, T. H. Lee, and M. Essex. 2002. HIV-1 subtype C molecular phylogeny: consensus sequence for an AIDS vaccine design? J. Virol. 76:5435-5451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Novitsky, V. A., M. A. Montano, M. F. McLane, B. Renjifo, F. Vannberg, B. T. Foley, T. P. Ndung'u, M. Rahman, M. Makhema, J., R. Marlink, and M. Essex. 1999. Molecular cloning and phylogenetic analysis of HIV-1 subtype C: a set of 23 full-length clones from Botswana. J. Virol. 73:4427-4432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ogg, G. S., X. Jin, S. Bonhoeffer, P. R. Dunbar, M. A. Nowak, S. Monard, J. P. Segal, Y. Cao, S. L. Rowland-Jones, V. Cerundolo, A. Hurley, M. Markowitz, D. D. Ho, D. F. Nixon, and A. J. McMichael. 1998. Quantitation of HIV-1-specific cytotoxic T lymphocytes and plasma load of viral RNA. Science 279:2103-2106. [DOI] [PubMed] [Google Scholar]
- 54.Ogg, G. S., S. Kostense, M. R. Klein, S. Jurriaans, D. Hamann, A. J. McMichael, and F. Miedema. 1999. Longitudinal phenotypic analysis of human immunodeficiency virus type 1-specific cytotoxic T lymphocytes: correlation with disease progression. J. Virol. 73:9153-9160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Osmanov, S., C. Pattou, N. Walker, B. Schwardlander, and J. Esparza. 2002. Estimated global distribution and regional spread of HIV-1 genetic subtypes in the year 2000. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 29:184-190. [DOI] [PubMed] [Google Scholar]
- 56.Phillips, R. E., S. Rowland-Jones, D. F. Nixon, F. M. Gotch, J. P. Edwards, A. O. Ogunlesi, J. G. Elvin, J. A. Rothbard, C. R. Bangham, C. R. Rizza, et al. 1991. Human immunodeficiency virus genetic variation that can escape cytotoxic T cell recognition. Nature 354:453-459. [DOI] [PubMed] [Google Scholar]
- 57.Piguet, V., and D. Trono. 1999. A structure-function analysis of the Nef protein of primate lentiviruses, p. 448-459. In C. Kuiken, B. Foley, B. Hahn, P. Marx, F. McCutchan, J. Mellors, J. Mullins, S. Wolinsky, and B. Korber (ed.), Human retroviruses and AIDS 1999: theoretical biology and biophysics. Los Alamos National Laboratory, Los Alamos, N.Mex.
- 58.Price, D. A., P. J. Goulder, P. Klenerman, A. K. Sewell, P. J. Easterbrook, M. Troop, C. Bangham, R., and R. E. Phillips. 1997. Positive selection of HIV-1 cytotoxic T lymphocyte escape variants during primary infection. Proc. Natl. Acad. Sci. USA 94:1890-1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rinaldo, C., X.-L. Huang, Z. Fan, M. Ding, L. Beltz, A. Logar, D. Panicali, G. Mazzara, J. Liebmann, M. Cottrill, and P. Gupta. 1995. High levels of anti-human immunodeficiency virus type 1 (HIV-1) memory cytotoxic T-lymphocyte activity and low viral load are associated with lack of disease in HIV-1 infected long-term nonprogressors. J. Virol. 69:5838-5842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Saah, A. J., D. R. Hoover, S. Weng, M. Carrington, J. Mellors, C. R. J. Rinaldo, D. Mann, R. Apple, J. P. Phair, R. Detels, S. O'Brien, C. Enger, P. Johnson, R. A. Kaslow, et al. 1998. Association of HLA profiles with early plasma viral load, CD4+ cell count and rate of progression to AIDS following acute HIV-1 infection. Multicenter AIDS Cohort Study. AIDS 12:2107-2113. [DOI] [PubMed]
- 61.Schmitz, J. E., M. J. Kuroda, S. Santra, V. G. Sasseville, M. A. Simon, M. A. Lifton, P. Racz, K. Tenner-Racz, M. Dalesandro, B. J. Scallon, J. Ghrayeb, M. A. Forman, D. C. Montefiori, E. P. Rieber, N. L. Letvin, and K. A. Reimann. 1999. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 283:857-860. [DOI] [PubMed] [Google Scholar]
- 62.Sette, A., and J. Sidney. 1998. HLA supertypes and supermotifs: a functional perspective on HLA polymorphism. Curr. Opin. Immunol. 10:478-482. [DOI] [PubMed] [Google Scholar]
- 63.Sette, A., and J. Sidney. 1999. Nine major HLA class I supertypes account for the vast preponderance of HLA-A and -B polymorphism. Immunogenetics 50:201-212. [DOI] [PubMed] [Google Scholar]
- 64.Shiver, J. W., T. M. Fu, L. Chen, D. R. Casimiro, M. E. Davies, R. K. Evans, Z. Q. Zhang, A. J. Simon, W. L. Trigona, S. A. Dubey, L. Huang, V. A. Harris, R. S. Long, X. Liang, L. Handt, W. A. Schleif, L. Zhu, D. C. Freed, N. V. Persaud, L. Guan, K. S. Punt, A. Tang, M. Chen, K. A. Wilson, K. B. Collins, G. J. Heidecker, V. R. Fernandez, H. C. Perry, J. G. Joyce, K. M. Grimm, J. C. Cook, P. M. Keller, D. S. Kresock, H. Mach, R. D. Troutman, L. A. Isopi, D. M. Williams, Z. Xu, K. E. Bohannon, D. B. Volkin, D. C. Montefiori, A. Miura, G. R. Krivulka, M. A. Lifton, M. J. Kuroda, J. E. Schmitz, N. L. Letvin, M. J. Caulfield, A. J. Bett, R. Youil, D. C. Kaslow, and E. A. Emini. 2002. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature 415:331-335. [DOI] [PubMed] [Google Scholar]
- 65.Tang, J., C. Costello, I. P. Keet, C. Rivers, S. Leblanc, E. Karita, S. Allen, and R. A. Kaslow. 1999. HLA class I homozygosity accelerates disease progression in human immunodeficiency virus type 1 infection. AIDS Res. Hum. Retrovir. 15:317-324. [DOI] [PubMed] [Google Scholar]
- 66.Tang, J., S. Tang, E. Lobashevsky, A. D. Myracle, U. Fideli, G. Aldrovandi, S. Allen, R. Musonda, and R. A. Kaslow. 2002. Favorable and unfavorable HLA class I alleles and haplotypes in Zambians predominantly infected with clade C human immunodeficiency virus type 1. J. Virol. 76:8276-8284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Thorsby, E. 1997. HLA-associated diseases. Hum. Immunol. 53:1-11. [DOI] [PubMed] [Google Scholar]
- 68.van Harmelen, J., C. Williamson, B. Kim, L. Morris, J. Carr, S. S. Abdool Karim, and F. McCutchan. 2001. Characterization of full length HIV-1 subtype C sequences from South Africa. AIDS Res. Hum. Retrovir. 17:1527-1531. [DOI] [PubMed] [Google Scholar]
- 69.van Harmelen, J. H., E. van der Ryst, A. S. Loubser, D. York, S. Madurai, S. Lyons, R. Wood, and C. Williamson. 1999. A predominantly HIV type 1 subtype C-restricted epidemic in South African urban populations. AIDS Res. Hum. Retrovir. 15:395-398. [DOI] [PubMed] [Google Scholar]
- 70.Walker, C. M., D. J. Moody, D. P. Stites, and J. A. Levy. 1986. CD8+ lymphocytes can control HIV infection in vitro by suppressing virus replication. Science 234:1563-1566. [DOI] [PubMed] [Google Scholar]