Abstract
An efficient vaccine against human immunodeficiency virus (HIV) must induce good cellular immune responses. To do this, it must be processed and presented by dendritic cells, which are required for primary T-lymphocyte stimulation. We have previously shown that a model lipopeptide containing a short epitopic peptide from HIV-1 was endocytosed and presented in association with major histocompatibility complex class I molecules by human dendritic cells to specific CD8+ T lymphocytes, but the cross-presentation pathway needed to be precisely determined. We have studied a longer lipopeptide (Pol461-484) and another lipopeptide (Nef66-97) currently being used in vaccine trials. Like the shorter lipopeptide, the rhodamine-labeled Pol461-484 lipopeptide was internalized by endocytosis, as assessed by confocal microscopy. The lipopeptides were processed by dendritic cells and presented to CD8+ T cells specific for the HLA-A*0201-restricted Pol476-484 and the HLA-A*0301-restricted Nef73-82 epitope, respectively. Presentation of both lipopeptides was inhibited by brefeldin A. Presentation of the Pol lipopeptide was inhibited by epoxomycin, a proteasome-specific inhibitor, but not by monensin. This shows that it gained access to the cytosol to be digested by the proteasome. In contrast, presentation of the Nef lipopeptide was not inhibited by epoxomycin but was inhibited by monensin, a classical inhibitor of acid-dependent endosomal enzyme activity, indicating an endocytic processing pathway yielding to major histocompatibility complex class I-restricted presentation. Therefore, the two lipopeptides followed different cross-presentation pathways, both resulting in efficient presentation to CD8+ T lymphocytes.
CD8+ T lymphocytes recognize infected or tumoral cells through specific interaction of their T-cell receptors with viral or tumoral epitopes associated with major histocompatibility complex (MHC) class I molecules. Following this recognition, they either kill the target cells or secrete molecules with effector antiviral or antitumoral functions such as gamma interferon (IFN-γ). In human immunodeficiency virus (HIV) infection, they are essential to control viral replication (reviewed in reference 15). Therefore, they must be stimulated by vaccination.
Production of MHC class I-restricted epitopes in the antigen-presenting cells has been mostly documented as processing by the proteasome in the cytosol, followed by TAP-mediated transport into the endoplasmic reticulum and association with nascent MHC class I molecules (37, 38). Among antigen-presenting cells, only dendritic cells can stimulate naive T lymphocytes, and therefore they are required for primary immunization (6). Dendritic cells have apparently evolved specific cross-presentation mechanisms allowing them to prime CD8+ T lymphocytes for exogenous proteins that are not produced in their cytosol but that are first internalized by macropinocytosis, phagocytosis, or receptor-mediated endocytosis (1, 7, 8, 21, 22, 32, 34, 38). Dendritic cells process these antigens and associate them with their MHC class I molecules. Two major routes for cross-presentation have been described (6): exit from the endocytic compartment and processing in the cytosol (1, 7, 8, 21, 22, 28) and processing in the endosomal system and transfer of the peptides to recycling MHC class I molecules either in the endocytic pathway (14) or, after regurgitation, at the cell surface (26). These mechanisms are essential for the cells to become sensitive to and develop tolerance to antigens that are not endogenously synthesized in dendritic cells.
As a model to study cross-presentation pathways in dendritic cells, we have previously used a lipopeptide containing a short epitopic peptide from HIV-1 (Pol476-484 lipopeptide) (3). Lipopeptides containing class I-restricted T-cell epitopes can induce consistently strong cytotoxic CD8+ T-cell responses against different pathogens in mice, nonhuman primates, and humans without additional adjuvant (12, 13, 16, 20, 27, 35). They are very well defined at the molecular level and can be labeled with a fluorochrome at specific sites to study antigen presentation pathways. We found that the Pol476-484 lipopeptide was endocytosed and presented in association with MHC class I by human dendritic cells (3).
To define the cross-presentation pathway and to generalize these results to more than one MHC class I molecule, we used longer lipopeptides derived from the sequence of HIV-1 Lai (Pol461-484 and Nef66-97) by terminal addition of an N-Ε-palmytoyl-lysine. In contrast to the short lipopeptide model used to develop the system, these longer lipopeptides contain flanking sequences added to the epitopes for which processing is required before association with MHC class I molecules and presentation. To analyze lipopeptide entry into dendritic cells, we labeled the Pol461-484 lipopeptide unequivocally at position 482 with rhodamine for confocal microscopy. We next tested the capacity of dendritic cells pulsed with lipopeptides to present MHC class I-restricted epitopes (HLA-A*0201-restricted Pol476-484 and HLA-A*0301-restricted Nef73-82) to specific CD8+ T cells, using intracellular cytokine assays. To determine the intracellular pathways of lipopeptide presentation, different antigen-processing inhibitors were used.
MATERIALS AND METHODS
Peptide synthesis and characterization.
The HLA-A*0201-restricted peptides HTLV-1 Tax11-19 (LLFGYPVYV), HIV reverse transcriptase Pol476-484, the HLA-B*27 influenza virus nucleoprotein NP383-391 (SRYWAIRTR), and the HLA-A*0301-restricted peptide HIV Nef73-82 were synthesized by Neosystem (Strasbourg, France). The Nef66-97 lipopeptide was synthesized by Bachem Feinchemikalen (Budendorf, Switzerland). For confocal microscopy studies, the Pol461-484 lipopeptide was labeled by substitution of the histidine in position 482 by an N-Ε-rhodamine-lysine.
Labeled and nonlabeled Pol461-484 lipopeptides were synthesized as previously described (17). Briefly, lipopeptides were built up by solid-phase synthesis with the 9-fluorenylmethoxycarbonyl-tert-butyl strategy. The side chains of the lysine residues to be modified by palmitoylation or selective fluorescent labeling were protected with a 4-methyltrityl group (Novabiochem, Läufelfingen, Switzerland), allowing selective deprotection by 0.5% trifluoroacetic acid in dichloromethane, followed by on-resin acylation with palmitic acid or 5(6)-carboxytetramethylrhodamine (Fluka, Buchs, Switzerland). After deprotection and cleavage, the lipopeptides were purified by reversed-phase high-pressure liquid chromatography on a Zorbax C3 column. All lipopeptides were more than 95% pure. Identity was confirmed by determination of amino acid composition after total acid hydrolysis and molecular mass determination recorded on a Vision 2000 MALDI-TOF (matrix-assisted laser desorption ionization-time of flight) spectrometer (Finigan, Bremen, Germany). Peptide and lipopeptide sequences are shown in Table 1.
TABLE 1.
Sequences of HIV-1-derived peptides and lipopeptides
| Name | Sequencea |
|---|---|
| Lipopeptide Pol461-484 | NH2-K(Nɛ-Pam)PLTEEAELELAENREILKEPVHGV-COOH |
| Lipopeptide Pol461-484 (Rho) | NH2-K(Nɛ-Pam)PLTEEAELELAENREILKEPVK(Nɛ-Rho)GV-COOH |
| Lipopeptide Nef66-97 | NH2-GVFPVTPQVPLRPMTYKAAVDLSHFLKEKGGL(Nɛ-Pam)-COOH |
| Pol476-484 | NH2-ILKEPVHGV-COOH |
| Nef73-82 | NH2-QVPLRPMTYK-COOH |
The conventional one-letter code is used for amino acids. Pam and Rho, palmitoyl- and 5(6)carboxytetramethylrhodamine, respectively.
Binding of peptides on purified HLA molecules.
The binding of peptides on purified HLA class I molecules has been described previously (3, 11). HLA-A*0201 and HLA-A*0301 class I molecules were purified by affinity chromatography from Epstein-Barr virus-immortalized lymphoblastoid B-cell lines and denatured with NaOH and urea before being incubated with exogenous peptide (10−8 to 10−4 M) and 2 μg of β2 microglobulin (Sigma, St Quentin Fallavier, France) per ml for 1 h at 37°C. Reassembled HLA class I molecules were then dispatched in microtiter plates previously coated with anti-HLA-A*0201 BB7.2 (ATCC HB-82: American Type Culture Collection, Rockville, Md.) or anti-HLA-A*0301 (ATCC HB.164) monoclonal antibodies in the presence of protease inhibitors. Correctly folded HLA complexes were revealed with the anti-β2-microglobulin immunoglobulin M28 antibody (mouse immunoglobulin G3, a kind gift from G. Kalil) coupled to alkaline phosphatase and measured at 360/460 nm in a Microfluor reader (Victor 1420; Wallac, Turku, Finland).
Generation of dendritic cells.
Dendritic cells were differentiated from peripheral blood monocytes in the presence of 50 ng of human recombinant granulocyte-macrophage colony-stimulating factor (Schering-Plough, Dardilly, France) and 500 U of human recombinant interleukin-4 (PeproTech, distributed by Tebu, Le-Perray-en-Yvelines, France) per ml for 7 or 8 days as previously described, and their differentiation was followed in each experiment by flow cytometry analysis of different surface markers (3, 9, 29). Dendritic cells expressed relatively high levels of the MHC class II molecules (HLA-DR), MHC class I molecules (HLA-A, -B, and -C), and CD1a. They were immature, as they expressed intermediate levels of CD40 and CD80 and low levels of CD86 and did not express CD83, a marker of mature dendritic cells. They did not express CD3, CD14, or CD16.
In some experiments, they were infected with recombinant Copenhagen vaccinia viruses (VV) from Transgène (Strasbourg, France): Nef-VV (VV.TG.1147, expressing the HIV-1 Lai Nef protein), or RT-VV (VV.TG.4163, expressing the HIV-1 Lai reverse transcriptase protein). Dendritic cells were infected at a multiplicity of infection of 5 in RPMI medium supplemented with 10% fetal calf serum. After incubation for 18 h at 37°C in 5% CO2, they were washed before use.
Generation of HIV-specific CD8+ T-cell lines.
HIV-1 Lai Pol476-484- and Nef73-82-specific-CD8+ T-cell lines were generated as previously described from the peripheral blood mononuclear cells of HIV-seropositive HLA-A*0201- and HLA-A*0301-positive individuals from a cohort study established with the approval of the ethics committee of the Cochin Hospital (2, 3, 9).
Flow cytometric detection of intracellular IFN-γ production in antigen-specific CD8+ T cells.
Dendritic cells were incubated with the peptides or lipopeptides at 10−6 M or infected with recombinant Copenhagen vaccinia viruses. When used, brefeldin A (Sigma) or epoxomycin (Alexis Biochemicals, distributed by Coger, Paris, France) was added 1 h before the peptides or lipopeptides at concentrations of 10 μg/ml and 10 μM, respectively, and then diluted at 2 μg/ml and 2 μM for the overnight incubation (31). Monensin (Sigma) was added only 10 min after peptide or lipopeptide addition (in an attempt to avoid preventing their endocytosis) at a concentration of 50 μM. In all cases, after overnight incubation, dendritic cells were then washed and added to the antigen-specific CD8+ T cells (ratio, 1:1) in the presence of brefeldin A (10 μg/ml) for 5 h at 37°C. CD8+ T cells incubated in the presence of phorbol myristate acetate (50 ng/ml) and ionomycin (500 ng/ml) (both from Sigma) were used as positive controls.
The cells were first surface stained for 30 min at room temperature with peridinin chlorophyll protein-conjugated anti-CD8 monoclonal antibody (SK7 clone) and sometimes with phycoerythrin-conjugated anti-CD3 monoclonal antibody (SK1 clone), both from Becton Dickinson (Le Pont de Claix, France). After fixation in 1× fluorescence-activated cell sorting lysing solution and permeabilization with the 1× fluorescence-activated cell sorting permeabilization solution (both from Becton Dickinson) according to the manufacturer's procedures, cells were subjected to intracellular IFN-γ staining with fluorescein isothiocyanate-conjugated anti-IFN-γ monoclonal antibody (25723.11 clone; Becton Dickinson) for 30 min in the dark at room temperature. The cells were washed and resuspended in 1% paraformaldehyde-phosphate-buffered saline (PBS) prior to analysis on the flow cytometer. Samples were acquired on a FACScan flow cytometer, and data were analyzed with the Lysis software (Becton Dickinson). For each sample, 10,000 events, gated on CD8 expression and on a scatter gate designed to include only viable lymphocytes, were acquired.
Confocal microscopy.
The rhodamine-labeled Pol461-484 lipopeptide (2 × 10−6 M) and dextran-fluorescein isothiocyanate (1 mg/ml, 40 kDa; Sigma) were incubated at the same time with the dendritic cells in culture medium. After incubation for from 2 min to 4 h at 37°C, the cells were washed in phosphate-buffered saline, then fixed in 4% paraformaldehyde-phosphate-buffered saline for 1 h at 4°C, washed again in phosphate-buffered saline, and mounted in Fluoromount-G (Southern Biotechnology Associates, Inc., distributed by CliniSciences, Montrouge, France). Dendritic cells were examined by confocal microscopy with a Bio-Rad MRC-1024 device mounted on a Nikon Diaphot 300 inverted microscope (Nikon France, Champigny-sur-Marne, France) equipped with a 60× objective (plan apo, NA 1.4). Sets of optical sections at 0.5-μm intervals were recorded. The data were processed and presented with the Laser Sharp software, version 3.2 (Bio-Rad, Ivry-Sur-Seine, France).
RESULTS
Entry of rhodamine-labeled lipopeptide into dendritic cells.
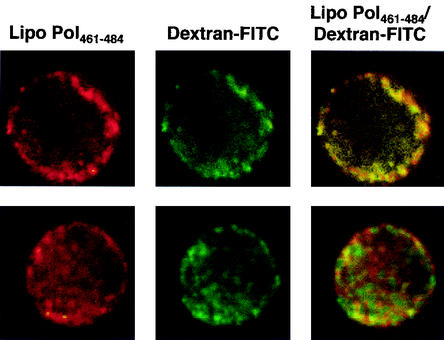
For confocal microscopy, the Pol461-484 lipopeptide was labeled by substitution of the histidine in position 482 by an N-Ε-rhodaminyl-lysine (Table 1). This lipopeptide [Pol461-484(Rho)] was rapidly internalized at 37°C by dendritic cells as early as 2 min of incubation and seemed to accumulate in endocytic vesicles, under the plasma membrane (Fig. 1). This was confirmed by colocalization of the lipopeptide with dextran-fluorescein isothiocyanate, a marker of fluid phase endocytosis (18) (Fig. 1). Moreover, entry occurred at 37°C but was inhibited at 4°C (data not shown). Therefore, the major entry of the lipopeptide occurred in an endocytic compartment, as previously observed for the Pol476-484 lipopeptide (3). Upon further incubation at 37°C (30 min [Fig. 1] or 2 or 4 h [data not shown]), diffuse cytosolic staining was observed in addition to vesicular labeling, which suggests that a fraction of the internalized lipopeptide may have gained access to the cytosol.
FIG. 1.
Endocytosis of Pol461-484(Rho) lipopeptide by human dendritic cells. Monocyte-derived dendritic cells were incubated at 37°C with rhodamine-modified Pol461-484 lipopeptide in the presence of dextran-fluorescein isothiocyanate (FITC), a marker of fluid-phase endocytosis, for 2 min (top row) or 30 min (bottom row) and then washed and fixed. Original magnification, ×600.
FIG. 2.
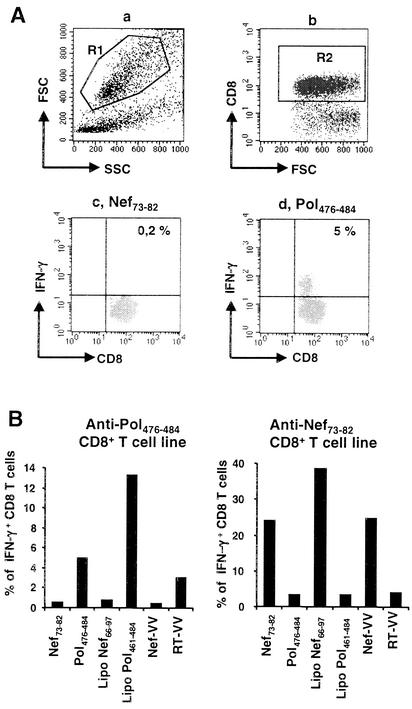
Lipopeptides are presented by human dendritic cells to specific CD8+ T lymphocytes. (A) Flow cytometric analysis of surface CD8 and intracellular IFN-γ expression by Pol476-484-specific CD8+ T lymphocytes. A Pol476-484-specific CD8+ T-cell line was stimulated by HLA-A*0201 dendritic cells previously pulsed overnight with the irrelevant peptide Nef73-82 or with the peptide Pol476-484. Sample acquisition was performed on 10,000 events, gated on a scatter gate (R1) designed to include only viable lymphocytes (a) and a gate (R2) designed to include only CD8+ T cells (b). IFN-γ expression in viable, CD8+ T lymphocytes (R1*R2) is shown in dot plots c (irrelevant Nef peptide) and d (Pol peptide). (B) HLA-A*0201, HLA-A*0301-positive dendritic cells were incubated overnight with lipopeptides, peptides, or recombinant vaccinia viruses and then washed before being incubated with either the anti-Pol476-484 or the anti-Nef73-82CD8+ T-cell line. Expression of surface CD8-peridinin chlorophyll protein and intracellular IFN-γ-fluorescein isothiocyanate was quantified by flow cytometry analysis. The results show the ratio of IFN-γ-expressing cells in viable, CD8+ T cells. This experiment is representative of more than 10 independent experiments.
FIG. 4.
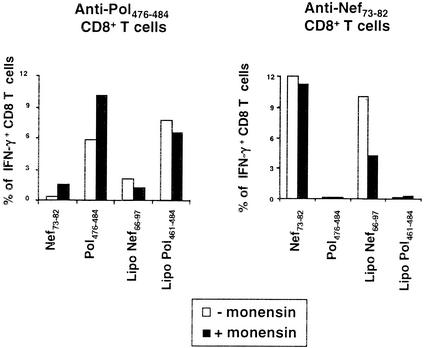
Lipopeptide presentation in the presence of monensin. HLA-A*0201 and HLA-A*0301 dendritic cells were pulsed with lipopeptides or peptides, and monensin was added 10 min later. IFN-γ production by peptide-specific CD8+ T cells was tested as in Fig. 2. Results are representative of three independent experiments. The average response in the presence of monensin compared to the uninhibited control was, for anti-Nef73-82 responses, 88% ± 15% for Nef73-82 versus 32% ± 18% for lipopeptide Nef66-97 (P < 0.05); for anti-Pol476-484 responses, 133% ± 45% for Pol476-484 versus 87% ± 6% for lipopeptide Pol461-484 (not significant).
Long lipopeptide presentation by human dendritic cells to specific CD8+ T cells.
We next investigated the capacity of dendritic cells pulsed with the Pol461-484 and Nef66-97 lipopeptides to present the HLA-A*0201-restricted HIV-1 Pol476-484 and the HLA-A*0301-restricted HIV-1 Nef73-82 epitopes, respectively, to specific CD8+ T cells. HLA-A*0201, HLA-A*0301-positive dendritic cells were pulsed overnight with lipopeptides and then washed before being added to the anti-Pol476-484 or the anti-Nef73-82 CD8+ T-cell line. As positive controls, optimal epitopes (Pol476-484 and Nef73-82) or recombinant vaccinia viruses encoding the Nef protein (Nef-VV) or the reverse transcriptase (RT-VV) were used. Each specific T-cell line was used as a control for the other.
After 5 h in the presence of brefeldin A, CD8+ T-cell activation was detected by intracellular measurement of IFN-γ expression, as shown in Fig. 2A. The Pol461-484 lipopeptide specifically stimulated the anti-Pol476-484 CD8+ T-cell line, and the Nef66-97 lipopeptide specifically stimulated the anti-Nef73-82 CD8+ T-cell line (Fig. 2B). Therefore, these lipopeptides were processed and presented in association with MHC class I molecules by human dendritic cells to specific CD8+ T lymphocytes. In both cases, responses to the long lipopeptides were higher than to the minimal epitope (Fig. 2B), but this was not always reproduced (see Fig. 4 to 6).
FIG. 6.
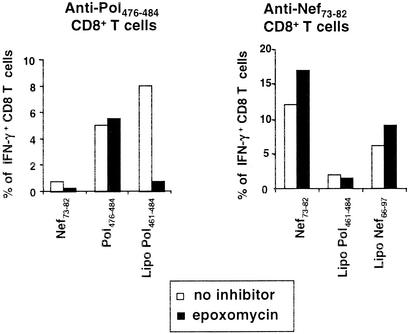
Lipopeptide presentation in the presence of epoxomycin. The experiment was conducted like that shown in Fig. 5 except that epoxomycin was used instead of brefeldin A. Results are representative of three independent experiments. The average response in the presence of epoxomycin compared to the uninhibited control was, for anti-Nef73-82 responses, 133% ± 10% for Nef73-82 versus 130 ± 33% for lipopeptide Nef66-97 (not significant); for anti-Pol476-484 responses, 120% ± 51% for Pol476-484 versus 34% ± 26% for lipopeptide Pol461-484 (P < 0.03).
Absence of binding of long lipopeptides to purified HLA class I molecules.
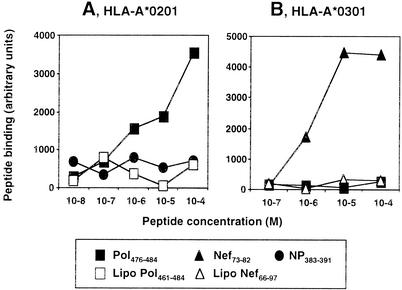
To control for the possibility that direct binding to surface MHC class I molecules was responsible for this functional presentation, we tested the capacity of the lipopeptides to bind to purified HLA class I molecules with an enzyme-linked immunosorbent assay. Conversely to the Pol476-484 and Nef73-82 epitopic peptides, neither Pol461-484 nor the Nef66-97 lipopeptide bound significantly to HLA-A*0201 or HLA-A*0301 molecules, respectively (Fig. 3). Since lipopeptides cannot be presented to CD8+-specific T-cell lines after direct binding to surface HLA class I molecules, they first need processing.
FIG. 3.
Lipopeptides did not bind to purified HLA molecules. Purified HLA-A*0201 (A) or HLA-A*0301 (B) molecules were incubated in the presence of β2-microglobulin and different peptides or lipopeptides. They were incubated on plates previously coated with anti-HLA-A*0201 or -HLA-A*0301 monoclonal antibodies. Correctly formed HLA complexes were revealed with an anti-β2-microglobulin antibody coupled to alkaline phosphatase. Results are expressed as arbitrary fluorescence units. HLA-B27-restricted influenza virus NP383-391 and HLA-A*0201 restricted Pol476-484 were used as negative controls in A and B, respectively.
Differential effect of monensin on presentation.
To identify the processing pathway followed by the lipopeptides after their endocytosis by dendritic cells, we used different antigen-processing inhibitors. First, after internalization by endocytosis, processing could occur partially or totally in the endosomal pathway. To study this hypothesis, we used monensin, which inhibits endosomal acidification and therefore enzymatic degradation in the lysosomal compartments (33). Internalization of the lipopeptides was allowed for 10 min before addition of monensin, which might otherwise also disturb endocytosis (33).
As shown in Fig. 4, monensin did not inhibit control epitope presentation, showing that it was not toxic to dendritic cells at this concentration. Monensin inhibited Nef66-97 lipopeptide presentation, indicating at least partial processing in the endosomal compartment and/or recycling through monensin-sensitive endocytic vesicles. Conversely, presentation of the Pol461-484 lipopeptide was not inhibited, showing that monensin did not inhibit its entry when it was added after 10 min and that this lipopeptide did not depend on endosomal acidification for its processing.
Inhibition by brefeldin A of lipopeptide presentation.
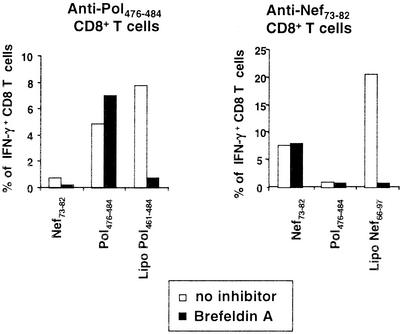
The presentation of both lipopeptides but not of the control epitopes was inhibited by brefeldin A (Fig. 5). This indicates either a passage from the endoplasmic reticulum to the Golgi and to the exocytic pathway (24, 36) or an inhibition at the level of MHC class I molecule recycling (25).
FIG. 5.
Lipopeptide presentation in the presence of brefeldin A. HLA-A*0201 and HLA-A*0301 dendritic cells were incubated alone or in the presence of brefeldin A for 1 h, and peptides or lipopeptides were added for overnight incubation in the presence or absence of brefeldin A. IFN-γ production by peptide-specific CD8+ T cells was tested as in Fig. 2. Results are representative of three independent experiments. The average response in the presence of brefeldin A compared to the uninhibited control was, for anti-Nef73-82 responses, 119% ± 36% for Nef73-82 versus 25% ± 23% for lipopeptide Nef66-97 (P < 0.03); for anti-Pol476-484 responses, 114% ± 27% for Pol476-484 versus 7% ± 4% for lipopeptide Pol461-484 (P < 0.05).
Differential effect of epoxomycin on presentation.
To determine if processing of the lipopeptides required proteasomal degradation, we repeated similar experiments in the presence of epoxomycin, a specific proteasome inhibitor (19). Epoxomycin has an inhibition profile similar to that of lactacystin but is about 100-fold more potent and can be used at nontoxic doses (30). It inhibits the chymotrypsin-like activity and to a lesser extent the trypsin-like and peptidyl-glutamyl peptide-hydrolyzing activities of the proteasome. It is very specific for the proteasome and does not inhibit nonproteasomal proteases such as trypsin, chymotrypsin, papaïn, cathepsin B, calpain, or tripeptidyl peptidase II (19, 30).
Epoxomycin completely blocked the presentation of the Pol461-484 lipopeptide but not of the control epitope to the specific CD8 cell line (Fig. 6). Therefore, the Pol461-484 lipopeptide gained access to the cytosol after its endocytosis to be processed by the proteasome. In contrast to the Pol461-484 lipopeptide, the Nef66-97 lipopeptide was still processed and presented in the presence of epoxomycin (Fig. 6). This result shows that production of the HLA-A*0301-restricted Nef73-82 epitope after lipopeptide internalization is independent of the proteasome.
DISCUSSION
Like the shorter model lipopeptide used in our previous studies, the long Pol461-484 lipopeptide was internalized by endocytosis. It was then degraded by the proteasome. The Pol476-484 epitope was also found to be produced by proteasomal degradation after expression from an HIV-1 reverse transcriptase-vaccinia vector recombinant in the cytosol (31). The experiments presented here show that the Pol461-484 lipopeptide followed an endosome-to-cytosol pathway, which is becoming a classical cross-presentation pathway. This pathway seems to be restricted to dendritic cells, filling a requirement for presentation of exogenous antigens to CD8+ effector T lymphocytes to generate sensitization or tolerance of the immune system without exposing nondendritic cells to destruction by these effectors (1, 7, 8, 22, 23, 34, 38). The molecular mechanisms leading to egress from the endosomal system are still unknown. They might be elucidated with lipopeptides, which are well-defined molecules and can be labeled precisely on one residue.
Surprisingly, the pathway used by the Nef66-97 lipopeptide was different. Indeed, this lipopeptide did not depend on the proteasome for processing. Absence of inhibition by epoxomycin was controlled by simultaneous experiments with the Pol system as a control, where presentation of the Pol461-484 lipopeptide was inhibited. Processing of the Nef73-82 epitope from a peptide precursor (Nef66-100 without a lipid moiety) was already found not to be achieved by 20S proteasome preparations, either by mass spectrometric and sequence analysis or by functional analysis with HIV-specific CD8+ T cells (10). Instead, the proteasome seems to preferentially cleave the sequence between residues 81 and 82, destroying the Nef73-82 epitope (10). Accordingly, epoxomycin seemed in several cases to enhance presentation of the epitope as in Fig. 6, but this was not always reproducible.
Our lipopeptide and the long peptide precursor used previously (10) extend on the N-and C-terminal sides of the minimal Nef epitope and therefore require cleavage on both sides. The Pol lipopeptide conversely ends at the C terminus of the Pol476-484 epitope. It might be expected that the Pol lipopeptide, which needs trimming only on the N-terminal side, would be more easily processed in the endosome, whereas the Nef lipopeptide might not. However, this prediction is exactly the opposite of what was observed, since only presentation of the Nef epitope was sensitive to monensin. Another difference between the two lipopeptides was that the palmytoyl lipid moiety was attached at the N terminus of the Pol peptide but at the C terminus of the Nef construct. This might direct the lipopeptides to different processing sites, either by attachment to lipid receptors or chaperones or by passive transport through the membranes in certain pH conditions. Using constructs with flanking regions and palmitoylation on either side of the epitopes might help discover the general rules for guiding such constructs to different processing pathways. This was only partly addressed previously (17). However, the independence from the proteasome of the Nef73-82 epitope seems to be an intrinsic property of the polypeptide sequence, since it was also found after whole Nef protein expression in antigen-presenting cells from recombinant vaccinia virus infection (U. Seifert et al., submitted for publication).
Our data are compatible with a processing pathway involving endocytosis of the lipopeptide, followed by digestion and loading of the epitopic peptides on MHC class I molecules in the endocytic pathway. Indeed, presentation was sensitive to monensin, which inhibits lysosomal acidification. It was also sensitive to brefeldin A, which inhibits the function of small GTP-binding proteins of the ADP ribosylation factor family and therefore probably recycling of MHC class I molecules from some of the late endosomal compartments to the cell surface, as well as transport from the endoplasmic reticulum to the Golgi (24, 25, 36). In the endosomal system, this lipopeptide may be protected from the predominant cleavage by the proteasome that would destroy the Nef73-82 epitope in the cytosol and may find a suitable enzyme for generation of this epitope. The nature of this enzyme is still unknown. Association with MHC class I molecules is possible in the late endosomes in dendritic cells, which were found to contain MHC class I as well as MHC class II molecules (5, 14, 34). Alternatively, the lipopeptide might also egress to the cytosol from a different, monensin-sensitive compartment than the Pol461-484 lipopeptide and be digested by an enzymatic activity that is insensitive to epoxomycin, like the Nef protein after recombinant vaccinia virus infection (Seifert et al., submitted). How is it then protected from proteasomal digestion may depend on various factors. Kinetic competition between the proteasome and another enzyme probably plays a role. Association with a chaperone may be involved. These results point again to the importance of compartmentalization in antigen processing. The processing pathway of the antigen depends not only on the presence of cleavage and binding sites for the proteasomes or for other antigen-processing enzymes, but also on the availability to these enzymes (endosome/lysosomes versus cytosol, endoplasmic reticulum, or nucleus) (4, 8, 26, 34, 37, 38). Although the Pol476-484 peptide was first located in the endosomal system because of its association with a lipid moiety, its presentation seemed to be exclusively proteasome dependent, showing that no endosomal enzyme could replace the proteasome, at least in the conditions used here, to generate the Pol476-484 epitope, but that transport into the cytosol was achieved.
A phase I study (Agence Nationale de Recherche sur le SIDA VAC 04) on healthy, HIV-1-uninfected human volunteers given a mixture of six lipopeptides derived from regulatory or structural HIV-1 proteins (Nef, Gag, reverse transcriptase, and gp160) and containing more than 50 specific cytotoxic T lymphocytes epitopes restricted by different HLA molecules showed that lipopeptides are able to induce strong multiepitopic B- and CD4 and CD8 T-cell responses (13, 27). Therefore, lipopeptides are promising vectors for an AIDS vaccine. They are currently being evaluated as a therapeutic vaccine in chronically HIV-infected patients (Lipthera assay). Our results allow better understanding of the mechanisms of their immunogenicity, since they show that lipopeptides address epitopic sequences to cross-presentation pathways, which are particularly efficient in dendritic cells to induce protective CD8+ T lymphocyte responses.
Acknowledgments
We thank I. Bouchaert for expert technical assistance in confocal microscopy, M.-.P. Kieny (Transgène, Strasbourg, France) for recombinant vaccinia viruses, A. Dautry and D. Ojcius for helpful advice, and A. Dautry, C. Marañón, and J. Choppin for reviewing the manuscript.
This work was supported by INSERM and by grants from the Fondation pour la Recherche Médicale (FRM), the Agence Nationale de Recherche sur le SIDA (ANRS), Ensemble contre le SIDA, and the Etablissement Français du Sang-Alsace.
REFERENCES
- 1.Albert, M. L., B. Sauter, and N. Bhardwaj. 1998. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature 392:86-89. [DOI] [PubMed] [Google Scholar]
- 2.Andrieu, M., D. Chassin, J. F. Desoutter, I. Bouchaert, M. Baillet, D. Hanau, J. G. Guillet, and A. Hosmalin. 2001. Downregulation of major histocompatibility class I on human dendritic cells by HIV nef impairs antigen presentation to HIV-specific CD8+ T lymphocytes. AIDS Res. Hum. Retroviruses 17:1365-1370. [DOI] [PubMed] [Google Scholar]
- 3.Andrieu, M., E. Loing, J. F. Desoutter, F. Connan, J. Choppin, H. Gras-Masse, D. Hanau, A. Dautry-Varsat, J. G. Guillet, and A. Hosmalin. 2000. Endocytosis of an HIV-derived lipopeptide into human dendritic cells followed by class I-restricted CD8+ T lymphocyte activation. Eur. J. Immunol. 30:3256-3265. [DOI] [PubMed] [Google Scholar]
- 4.Anton, L. C., U. Schubert, I. Bacik, M. F. Princiotta, P. A. Wearsch, J. Gibbs, P. M. Day, C. Realini, M. C. Rechsteiner, J. R. Bennink, and J. W. Yewdell. 1999. Intracellular localization of proteasomal degradation of a viral antigen. J. Cell. Biol. 146:113-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arnold-Schild, D., D. Hanau, D. Spehner, C. Schmid, H. G. Rammensee, H. de la Salle, and H. Schild. 1999. Cutting edge: receptor-mediated endocytosis of heat shock proteins by professional antigen-presenting cells. J. Immunol. 162:3757-3760. [PubMed] [Google Scholar]
- 6.Banchereau, J., F. Briere, C. Caux, J. Davoust, S. Lebecque, Y. J. Liu, B. Pulendran, and K. Palucka. 2000. Immunobiology of dendritic cells. Annu. Rev. Immunol. 18:767-811. [DOI] [PubMed] [Google Scholar]
- 7.Carbone, F. R., C. Kurts, S. R. Bennett, J. F. Miller, and W. R. Heath. 1998. Cross-presentation: a general mechanism for cytotoxic T lymphocytes immunity and tolerance. Immunol. Today 19:368-373. [DOI] [PubMed] [Google Scholar]
- 8.Castellino, F., P. E. Boucher, K. Eichelberg, M. Mayhew, J. E. Rothman, A. N. Houghton, and R. N. Germain. 2000. Receptor-mediated uptake of antigen/heat shock protein complexes results in major histocompatibility complex class I antigen presentation via two distinct processing pathways. J. Exp. Med. 191:1957-1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chassin, D., M. Andrieu, W. Cohen, B. Culmann-Penciolelli, M. Ostankovitch, D. Hanau, and J. G. Guillet. 1999. Dendritic cells transfected with the nef genes of HIV-1 primary isolates specifically activate cytotoxic T lymphocytes from seropositive subjects. Eur. J. Immunol. 29:196-202. [DOI] [PubMed] [Google Scholar]
- 10.Choppin, J., W. Cohen, A. Bianco, J. P. Briand, F. Connan, M. Dalod, and J. G. Guillet. 2001. Characteristics of HIV-1 Nef regions containing multiple CD8+ T-cell epitopes: wealth of HLA-binding motifs and sensitivity to proteasome degradation. J. Immunol. 166:6164-6169. [DOI] [PubMed] [Google Scholar]
- 11.Connan, F., F. Hlavac, J. Hoebeke, J. G. Guillet, J. Choppin, M. Dalod, M. Harzic, I. Pellegrin, B. Dumon, B. Hoen, D. Sereni, J. C. Deschemin, J. P. Levy, A. Venet, and E. Gomard. 1994. A simple assay for detection of peptides promoting the assembly of HLA class I molecules. Eur. J. Immunol. 24:777-780. [DOI] [PubMed] [Google Scholar]
- 12.Deres, K., H. Schild, K. H. Wiesmuller, G. Jung, and H. G. Rammensee. 1989. In vivo priming of virus-specific cytotoxic T lymphocytes with synthetic lipopeptide vaccine. Nature 342:561-564. [DOI] [PubMed] [Google Scholar]
- 13.Gahéry-Ségard, H., G. Pialoux, B. Charmeteau, S. Sermet, H. Poncelet, M. Raux, A. Tartar, J. P. Lévy, H. Gras-Masse, and J. G. Guillet. 2000. Multiepitopic B- and T-cell responses induced in humans by a human immunodeficiency virus type 1 lipopeptide vaccine, J. Virol. 74:1694-1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gromme, M., F. G. Uytdehaag, H. Janssen, J. Calafat, R. S. van Binnendijk, M. J. Kenter, A. Tulp, D. Verwoerd, and J. Neefjes. 1999. Recycling MHC class I molecules and endosomal peptide loading, Proc. Natl. Acad. Sci. USA 96:10326-10331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hosmalin, A., A. Samri, M. J. Dumaurier, Y. Dudoit, E. Oksenhendler, M. Karmochkine, B. Autran, S. Wain-Hobson, and R. Cheynier. 2001. HIV-specific effector cytotoxic T lymphocytes and HIV-producing cells colocalize in white pulps and germinal centers from infected patients. Blood 97:2695-2701. [DOI] [PubMed] [Google Scholar]
- 16.Livingston, B. D., C. Crimi, H. Grey, G. Ishioka, F. V. Chisari, J. Fikes, H. Grey, R. W. Chesnut, and A. Sette. 1997. The hepatitis B virus-specific cytotoxic T lymphocytes responses induced in humans by lipopeptide vaccination are comparable to those elicited by acute viral infection. J. Immunol. 159:1383-1392. [PubMed] [Google Scholar]
- 17.Loing, E., M. Andrieu, K. Thiam, D. Schorner, K. H. Wiesmuller, A. Hosmalin, G. Jung, and H. Gras-Masse. 2000. Extension of HLA-A*0201-restricted minimal epitope by N-epsilon-palmitoyl-lysine increases the life span of functional presentation to cytotoxic T cells. J. Immunol. 164:900-907. [DOI] [PubMed] [Google Scholar]
- 18.Lutz, M. B., P. Rovere, M. J. Kleijmeer, M. Rescigno, C. U. Assmann, V. M. Oorschot, H. J. Geuze, J. Trucy, D. Demandolx, J. Davoust, and P. Ricciardi-Castagnoli. 1997. Intracellular routes and selective retention of antigens in mildly acidic cathepsin D/lysosome-associated membrane protein-1/MHC class II-positive vesicles in immature dendritic cells. J. Immunol. 159:3707-3716. [PubMed] [Google Scholar]
- 19.Meng, L., R. Mohan, B. H. Kwok, M. Elofsson, N. Sin, and C. M. Crews. 1999. Epoxomicin, a potent and selective proteasome inhibitor, exhibits in vivo anti-inflammatory activity. Proc. Natl. Acad. Sci. USA 96:10403-10408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mortara, L., H. Gras-Masse, C. Rommens, A. Venet, J. G. Guillet, and I. Bourgault-Villada. 1999. Type 1 CD4+ T-cell help is required for induction of antipeptide multispecific cytotoxic T lymphocytes by a lipopeptidic vaccine in rhesus macaques. J. Virol. 73:4447-4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nair, S., F. Zhou, R. Reddy, L. Huang, and B. T. Rouse. 1992. Soluble proteins delivered to dendritic cells via pH-sensitive liposomes induce primary cytotoxic T lymphocyte responses in vitro. J. Exp. Med. 175:609-612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Norbury, C. C., B. J. Chambers, A. R. Prescott, H. G. Ljunggren, and C. Watts. 1997. Constitutive macropinocytosis allows TAP-dependent major histocompatibility complex class I presentation of exogenous soluble antigen by bone marrow-derived dendritic cells. Eur. J. Immunol. 27:280-288. [DOI] [PubMed] [Google Scholar]
- 23.Norbury, C. C., M. F. Princiotta, I. Bacik, R. R. Brutkiewicz, P. Wood, T. Elliott, J. R. Bennink, and J. W. Yewdell. 2001. Multiple antigen-specific processing pathways for activating naive CD8+ T cells in vivo. J. Immunol. 166:4355-4362. [DOI] [PubMed] [Google Scholar]
- 24.Nuchtern, J. G., J. S. Bonifacino, W. E. Biddison, and R. D. Klausner. 1989. brefeldin A implicates egress from endoplasmic reticulum in class I restricted antigen presentation. Nature 339:223-226. [DOI] [PubMed] [Google Scholar]
- 25.Peyroche, A., B. Antonny, S. Robineau, J. Acker, J. Cherfils, and C. L. Jackson. 1999. brefeldin A acts to stabilize an abortive ARF-GDP-Sec7 domain protein complex: involvement of specific residues of the Sec7 domain. Mol. Cell 3:275-285. [DOI] [PubMed] [Google Scholar]
- 26.Pfeifer, J. D., M. J. Wick, R. L. Roberts, K. Findlay, S. J. Normark, and C. V. Harding. 1993. Phagocytic processing of bacterial antigens for class I MHC presentation to T cells. Nature 361:359-362. [DOI] [PubMed] [Google Scholar]
- 27.Pialoux, G., H. Gahery-Segard, S. Sermet, H. Poncelet, S. Fournier, L. Gerard, A. Tartar, H. Gras-Masse, J. P. Levy, and J. G. Guillet. 2001. Lipopeptides induce cell-mediated anti-HIV immune responses in seronegative volunteers. AIDS 15:1239-1249. [DOI] [PubMed] [Google Scholar]
- 28.Rodriguez, A., A. Regnault, M. Kleijmeer, P. Ricciardi-Castagnoli, and S. Amigorena. 1999. Selective transport of internalized antigens to the cytosol for MHC class I presentation in dendritic cells. Nat. Cell Biol. 1:362-368. [DOI] [PubMed] [Google Scholar]
- 29.Sallusto, F., and A. Lanzavecchia. 1994. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J. Exp. Med. 179:1109-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schwarz, K., R. de Giuli, G. Schmidtke, S. Kostka, M. van den Broek, K. B. Kim, C. M. Crews, R. Kraft, and M. Groettrup. 2000. The selective proteasome inhibitors lactacystin and epoxomicin can be used to either up- or down-regulate antigen presentation at nontoxic doses. J. Immunol. 164:6147-6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sewell, A. K., D. A. Price, H. Teisserenc, B. L. Booth, Jr., U. Gileadi, F. M. Flavin, J. Trowsdale, R. E. Phillips, and V. Cerundolo. 1999. IFN-gamma exposes a cryptic cytotoxic T lymphocyte epitope in HIV-1 reverse transcriptase. J. Immunol. 162:7075-7079. [PubMed] [Google Scholar]
- 32.Svensson, M., and M. J. Wick. 1999. Classical MHC class I peptide presentation of a bacterial fusion protein by bone marrow-derived dendritic cells. Eur. J. Immunol. 29:180-188. [DOI] [PubMed] [Google Scholar]
- 33.Takahashi, H., K. B. Cease, and J. A. Berzofsky. 1989. Identification of proteases that process distinct epitopes on the same protein. J. Immunol. 142:2221-2229. [PubMed] [Google Scholar]
- 34.Thery, C., and S. Amigorena. 2001. The cell biology of antigen presentation in dendritic cells. Curr. Opin. Immunol. 13:45-51. [DOI] [PubMed] [Google Scholar]
- 35.Vitiello, A., G. Ishioka, H. M. Grey, R. Rose, P. Farness, R. La Fond, L. Yuan, F. V. Chisari, J. Furze, R. Bartholomeuz, et al. 1995. Development of a lipopeptide-based therapeutic vaccine to treat chronic HBV infection. I. Induction of a primary cytotoxic T lymphocyte response in humans. J. Clin. Investig. 95:341-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yewdell, J. W., and J. R. Bennink. 1989. Brefeldin A specifically inhibits presentation of protein antigens to cytotoxic T lymphocytes. Science 244:1072-1075. [DOI] [PubMed] [Google Scholar]
- 37.Yewdell, J. W., and J. R. Bennink. 2001. Cut and trim: generating MHC class I peptide ligands. Curr. Opin. Immunol. 13:13-18. [DOI] [PubMed] [Google Scholar]
- 38.York, I. A., A. L. Goldberg, X. Y. Mo, and K. L. Rock. 1999. Proteolysis and class I major histocompatibility complex antigen presentation. Immunol. Rev. 172:49-66. [DOI] [PubMed] [Google Scholar]