Abstract
To better understand the immunological mechanisms that permit prolonged shedding of murine cytomegalovirus (MCMV) from the salivary gland, the phenotypic and functional characteristics of leukocytes infiltrating the submaxillary gland (SMG) were analyzed in infected BALB/c mice. A robust innate immune response, comprised of CD11c+ major histocompatibility complex class II+ CD11b− CD8α+ dendritic cells and γ/δ T-cell receptor-bearing CD3+ T cells was prominent through at least 28 days postinfection. Concurrently, a dramatic increase in pan-NK (DX5+) CD3+ and CD8+ T cells was observed, while CD4+ T cells, known to be essential for viral clearance from this tissue, increased slightly. The expression particularly of gamma interferon but also of interleukin-10 and CC chemokines was extraordinarily high in the SMG in response to MCMV infection. The gamma interferon was produced primarily by CD4+ and CD8+ T lymphocytes and DX5+ CD3+ T cells. The SMG CD8+ T cells were highly cytolytic ex vivo, and a significant proportion of these cells were specific to an immunodominant MCMV peptide. These peptide-specific clones were not exhausted by the presence of high virus titers, which persisted in the SMG despite the strength of the cell-mediated responses. In contrast, MCMV replication was efficiently cleared from the draining cervical and periglandular lymph nodes, a tissue displaying a substantially weaker antiviral response. Our data indicated that vigorous innate and acquired immune responses are elicited, activated, and retained in response to mucosal inflammation from persistent MCMV infection of the submaxillary gland.
Human cytomegalovirus (HCMV) is a ubiquitous betaherpesvirus that is transmitted vertically (transplacentally) and horizontally to susceptible hosts. In the latter case, transmission occurs primarily through contaminated saliva and/or genital secretions. Following an acute infection, HCMV persists in its host for life either as a low-level chronic infection or as viral latency, from which reactivation can periodically occur. In these instances, virus is excreted in mucosal secretions for extended periods of time and in the absence of clinical disease. It is estimated that asymptomatic shedding of HCMV in saliva, cervical secretions, semen, and breast milk occurs in 10 to 30% of infected individuals (9). Therefore, in order to develop strategies or vaccines to reduce the transmission of HCMV, the mechanisms that permit prolonged shedding of virus from mucosal tissues must first be defined.
Because CMV infections are species specific, infection of mice with murine CMV (MCMV) has been used extensively as an animal model for human disease (10). Studies in this mouse model have demonstrated that infectious virus replicates to high titers in the salivary gland long after it is cleared from systemic organs, including the spleen, lung, liver, kidney, adrenal glands, and lymph nodes (31, 32). In the mouse salivary gland, MCMV replicates primarily in the acinar glandular epithelial cells (20, 31). Typically, in mice infected by the intraperitoneal route, virus titers in the salivary gland are undetectable until approximately 5 to 6 days after infection, reach peak levels between 14 and 21 days, and are still detectable for months following infection.
Immunological studies in the mouse demonstrate the requirement for T lymphocytes in protection against MCMV infection (reviewed in reference 35). In adoptive transfer experiments utilizing immunodepleted mice as recipients, virus-specific CD4− CD8+ T lymphocytes prevent fatal disease when given both prophylactically and therapeutically (53, 55). These and additional studies verify that CD8+ T lymphocytes are necessary and sufficient for clearing infectious virus from all target organs except the salivary gland (31, 32, 38, 52). Although CD8+ T cells (31) and possibly other perforin- or granzyme-containing cytolytic cells (56) function to clear MCMV from salivary gland fibroblasts, elimination of virus from glandular epithelial cells requires CD4+ cells (31), as well as production of the cytokines gamma interferon (IFN-γ) (38) and tumor necrosis factor alpha (TNF-α) (52).
In CD4-depleted mice, a persistent MCMV infection is established in the salivary glands and is confined to the acinar glandular epithelial cells (31), the same cell type that harbors chronic MCMV infection (20). Because antibodies are not essential for clearance of primary MCMV infection of the salivary gland (33), it is unlikely that CD4+ T cells provide antiviral function through cooperation with B lymphocytes. Questions remain as to the quality of the CD8+ T cells within the submaxillary gland (SMG), why the SMG epithelial cells are exempt from CD8+ T-cell-mediated control, how CD4+ cells exert antiviral function, and which immunoregulatory and effector cells are responsible for the induction and production of IFN-γ and TNF-α.
To date, the immune response to MCMV in the salivary gland has not been fully characterized. However, immune cells residing in the uninfected mouse salivary gland have been examined by Kiyono and coworkers (16, 21, 43). These studies reveal that the mouse salivary gland possesses unique immunological features. For example, roughly one-half of the mononuclear cells isolated from the salivary glands of C3H mice are CD3+, with nearly equal proportions of CD4+ and CD8+ T-cell subsets (43). These mononuclear cells contain large numbers of CD4+ T cells that spontaneously produce interleukin-5 (IL-5) and IL-6 (Th2) or IFN-γ (Th1), with the frequency of Th2-type cells being three times greater than that of Th1-type cells (21). In addition, large numbers of γ/δ T-cell receptor (TCR)-bearing CD8+ T cells and CD4−CD8− (double negative) γ/δ T cells, cell types rarely found in other tissues, are detected in the salivary gland (43).
In contrast to this organ, mononuclear cells from the periglandular and cervical lymph nodes draining the salivary gland display quite different phenotypic and cytokine profiles. Approximately 80% of the lymph node cells are CD3+, with five times as many CD4+ as CD8+ cells, very few γ/δ TCR+ cells, and many fewer cytokine-producing cells, most of which represent the Th1 type (21, 43). Given these findings, the salivary gland, like other mucosal tissues, is poised for Th2 responses important for immunoglobulin A (IgA) production and the regulation of oral tolerance. However, it is well recognized that Th1 cytokines are induced in mucosa in response to pathogenic microorganisms (27, 28). Currently unknown is how a Th1 response is induced and regulated in MCMV-infected salivary gland tissue, where virus production and inflammation likely influence the response of resident and recruited mononuclear cells. Defining the effector cells and molecules that mediate antiviral activity at this mucosal site is a prerequisite for designing strategies to prevent horizontal transmission of HCMV.
The purpose of this study was to examine the phenotype and function of mononuclear cells elicited in the SMG of mice following an acute MCMV infection. The response in this mucosal tissue was compared to that found in a systemic lymphoid tissue that is efficiently cleared of replicating MCMV, the periglandular and cervical lymph nodes draining the SMG. Our results revealed a striking difference in the immune response to MCMV infection between the SMG and draining lymph nodes. The number of immune cells infiltrating the SMG increased dramatically following MCMV infection, particularly that of dendritic cells, DX5+ CD3+ cells, γ/δ TCR-bearing CD3+ T cells, and CD8+ T lymphocytes. Cytokine and chemokine profiles demonstrated that the expression of IFN-γ, IL-10, and CC chemokines was significantly greater in immune cells of the SMG than in spleen and lymph node cells. Furthermore, an unexpectedly high proportion of the CD8+ T lymphocytes isolated from the SMG were virus specific and cytotoxic and produced IFN-γ in response to MCMV infection. These data indicated that MCMV infection of the SMG persists in the face of robust innate and acquired Th1-type cellular immune responses that are likely regulated by mucosal inflammation.
MATERIALS AND METHODS
Virus.
The Smith strain of MCMV (VR-194) was purchased from the American Type Culture Collection (Manassas, Va.). For in vitro infections, a tissue culture-passaged virus stock, prepared by propagating virus in NIH 3T3 fibroblasts, was used. For in vivo infections, salivary gland-passaged virus was used. Salivary gland MCMV stocks were prepared by intraperitoneal inoculation of 3-week-old male BALB/c weanlings as previously described (39). Titers of all virus stocks were determined on NIH 3T3 cells by standard plaque assay.
Cells.
NIH 3T3 fibroblasts were purchased from the American Type Culture Collection (CRL 1658) and maintained in complete medium (Dulbecco's modified Eagle's medium, 10% bovine calf serum, and 1% l-glutamine). mKSA cells (H-2d) and WT-19 cells (H-2b) are simian virus 40-transformed fibroblast cell lines and were a kind gift from Satvir Tevethia (Pennsylvania State University College of Medicine, Hershey, Pa.). These cells were maintained in Dulbecco's modified Eagle's medium containing 5% bovine calf serum and 1% l-glutamine. CD3ɛ hybridoma cells (clone 145-2C11) were purchased from the American Type Culture Collection (CRL-1975) and maintained, as directed, at a density of 105 to 106 cells per milliliter of medium (Iscove's medium with 10% fetal bovine serum and 4 mM l-glutamine). All cell lines were maintained in their respective media without antibiotics.
Mice.
Male BALB/c mice (H-2d haplotype) were purchased from Harlan Laboratories (Indianapolis, Ind.) and housed in our animal facility in sterile microisolator cages, with sterile food, water, and bedding. All animal procedures were approved by the Animal Care and Use Committee at Eastern Virginia Medical School and adhered to the guidelines established by the U.S. Animal Welfare Act. In all experiments, adult mice (6 weeks of age) were infected intraperitoneally with 4 × 103 PFU of virulent, salivary gland-passaged MCMV. Groups of 25 mice were sacrificed on days 0 (representing uninfected control animals), 3, 6, 14, 21, and 28 days after infection, and their spleens, SMG, and cervical and periglandular lymph nodes were harvested. These tissues were then processed for virus titers, immune cell quantitation and phenotyping, and cytokine and chemokine expression (as described below).
Male C57BL/6 mice (H-2b haplotype) were also purchased from Harlan Laboratories and used to obtain immune cells as a negative haplotype control in the tetramer staining experiment (described below). Because this strain of mice is more resistant to MCMV infection, they were infected intraperitoneally with 105 PFU of salivary gland-passaged MCMV.
Quantitation of infectious virus in organs.
The amount of infectious MCMV in each tissue at each time point following infection was determined by preparing 20% (wt/vol) tissue homogenates and determining virus titers in these homogenates by standard plaque assay on subconfluent NIH 3T3 cells. Tissue titers were determined for five individual mice at each time point after MCMV infection. The results are presented as the log10 of infectious MCMV per milliliter of tissue homogenate. The limit of detection was 102 PFU/ml.
Histopathology.
Submaxillary glands and periglandular lymph nodes were harvested at days 0, 7, and 14 postinfection and fixed in 3% paraformaldehyde in phosphate-buffered saline (pH 7.4). Tissues were sectioned (4 μm) and stained with hematoxylin and eosin. Images were photographed with a SPOT RT digital camera.
Isolation of tissue leukocytes.
Leukocytes were isolated from the SMG by an enzymatic dissociation procedure described by Mega and coworkers (43) with some modifications. In brief, SMG were minced into small fragments with a scalpel, pooled, and dissociated into single cells by four sequential incubations in digestion medium (RPMI 1640 containing 1 mg of collagenase type H [Sigma Chemical Co., St. Louis, Mo.] per ml, 5 mM CaCl2, 5% fetal bovine serum, and 50 μg of DNase I [Sigma Chemical Co.] per ml) with continuous stirring, the first three at room temperature and the last at 37°C. Following these four digestions, the dissociated cells were washed in complete medium and passed over a glass wool column to remove dead cells and tissue debris. The leukocytes were then purified by a reverse, discontinuous Percoll gradient as described (43) and passed through a 40-μm-pore-size nylon cell strainer (Falcon 2340) to remove any cell clusters prior to counting. Mononuclear cells were harvested from the cervical and periglandular lymph nodes as previously described (11).
Quantitation and phenotyping of tissue leukocytes.
The number and phenotype of leukocytes isolated from the SMG and draining lymph nodes of MCMV-infected mice were determined by flow cytometry on days 0, 14, and 28 following infection. The panel of fluorochrome-conjugated monoclonal antibodies to mouse cell surface markers used in this study included the following: anti-mouse CD3ɛ conjugated to fluorescein isothiocyanate (FITC), phycoerythrin (PE), peridinin chlorophyll protein (PerCP), and allophycocyanin (APC) (all clone 145-2C11); anti-mouse CD4-FITC (clone GK1.5); anti-mouse CD8α-FITC (clone 53-6.7); anti-mouse TCR β chain-PE (clone H57-597); anti-mouse TCR γδ-FITC and -PE (both clone GL3); anti-mouse CD11c-PE (clone HL3); anti-mouse pan-NK-PE (CD49b; clone DX5); anti-mouse I-Ad-FITC (clone AMS-32.1); anti-mouse F4/80-PE (clone CI:A3-1), anti-mouse Ly-6G/C-FITC or -APC (clone RB6-8C5), anti-mouse CD45R/B220-PerCP (clone RA3-6B2), and anti-mouse CD11b-APC (clone M1/70). Isotype-matched control antibodies included rat IgMκ (clone R4-22), mouse IgG2bκ (clone MPC-11 or A95-1), and hamster IgG2 (clone Ha4/8 or B81-3). All monoclonal antibodies were purchased from BD Pharmingen (San Diego, Calif.) except for anti-F4/80, which was purchased from Caltag Laboratories (Burlingame, Calif.).
Freshly isolated leukocytes were stained for surface marker expression according to standard protocols. Cells isolated from the SMG or draining lymph nodes were resuspended to a density of 2 × 107 cells/ml in stain buffer (BD Pharmingen; phosphate-buffered saline containing 2% fetal bovine serum and 0.09% NaN3). A total of 106 cells (in 50 μl of stain buffer) were added to 50 μl of stain buffer containing the indicated monoclonal antibodies at the recommended dilutions. Cells were incubated with monoclonal antibodies for 30 min on ice in the dark, washed three times with stain buffer, and resuspended in a final volume of 250 μl per sample. Stained cells were immediately analyzed on a FACStarPlus flow cytometer (Becton Dickinson), and the data acquired (approximately 100,000 events/sample) were analyzed with CellQuest software. All FL1, FL2, FL3, and FL4 gates were set at each time postinfection with the CD3-conjugated fluorochromes.
Because experiments were performed with SMG and lymph node cells pooled from 25 mice, each experiment was performed a minimum of five times. Less than 10% variation in the numbers of cells and percentages of cells staining positive for each antibody at each time postinfection was observed among experiments.
Isolation of RNA from tissue leukocytes.
RNA was isolated from tissue leukocytes on days 0, 3, 6, 14, 21, and 28 following infection. Total RNA was extracted with Tri reagent solution (Molecular Research Center, Inc., Cincinnati, Ohio) according to the manufacturer's instructions and stored in 10-μg aliquots in ethanol at −80°C.
Quantitation of cytokine and chemokine expression.
In order to determine the cytokine and chemokine profiles of leukocytes isolated from the spleen, SMG, and lymph nodes following MCMV infection, RNase protection assays were used to quantitate the expression of RNA species of various Th1 and Th2 cytokines as well as for numerous chemokines. For these assays, we used the RiboQuant RPA system (BD Pharmingen) according to the manufacturer's instructions. For some experiments, we employed the services of BD Pharmingen's Custom Technology Team for RNA analysis, also with the RiboQuant RPA system. RNase-protected hybrids were purified, resolved on denaturing polyacrylamide gels, and quantified by phosphorimaging. The multiprobe template sets to mouse cytokines and chemokines included in these analyses were the following: mCK-1, mCK-2b, mCK-3b, and mCK-5 (BD Pharmingen).
The relative expression levels of cytokines and chemokines were quantitated by calculating band density ratios from the phosphorimage. The band density ratios for each RNA species was calculated by dividing the band density of the cytokine or chemokine by the band density of L32 (a housekeeping gene) in the same lane.
Detection of intracellular IFN-γ.
Four-color cytofluorometric analysis was performed with BD Pharmingen's Cytofix/Cytoperm Plus kit with GolgiPlug (containing brefeldin A) and the panel of monoclonal antibodies to cell surface antigens to identify the cell type producing IFN-γ in the SMG of MCMV-infected mice. Leukocytes were isolated from the SMG and draining lymph nodes of MCMV-infected BALB/c mice on day 14 after infection. According to the manufacturer's instructions, the cells were incubated in brefeldin A, stained for surface markers, fixed and permeabilized, and stained for intracellular IFN-γ (APC-conjugated rat anti-mouse IFN-γ, clone XMG1.2, isotype rat IgG1). An APC-conjugated isotype-matched control monoclonal antibody (clone R3-34) served as a negative control. Approximately 125,000 events per sample were acquired. With CellQuest software, IFN-γ+ cells were analyzed for the presence of various surface markers. Because these cells were not stimulated in vitro with any polyclonal activators, the IFN-γ+ cells identified in this study represent those stimulated exclusively in vivo.
IFN-γ-based ELISPOT assay.
An enzyme-linked immunospot (ELISPOT) assay kit for mouse IFN-γ (BD Pharmingen) was used to determine the number of T cells capable of producing IFN-γ upon polyclonal or MCMV-specific activation. The antigen-presenting cells and conditions used in this assay have been described by Holtappels and coworkers (24), and the manufacturer's suggested protocol was followed. Leukocytes isolated from the SMG and draining lymph nodes of MCMV-infected mice on day 14 after infection were seeded in serial 10-fold dilutions into the microwells of 96-well nylon membrane-backed plates (triplicate wells per test) and stimulated for 16 h at 37°C with 105 antigen-presenting cells.
To enumerate polyclonal activation through the TCR-CD3 complex, hybridoma cells producing anti-CD3ɛ monoclonal antibody (clone 145-2C11) were used as antigen-presenting cells. To determine the frequency of cells secreting IFN-γ in response to MCMV antigens, mKSA cells (H-2d haplotype) infected with MCMV (3 PFU/cell) were washed and used as antigen-presenting cells. These cells were incubated with recombinant mouse IFN-γ (BD Pharmingen; 50 U/ml) for 24 h prior to MCMV infection, during MCMV infection, and for 24 h following MCMV infection in order to maintain high expression levels of class I molecules (11) and optimize processing of viral peptides (8). Mock-infected, IFN-γ-treated mKSA cells were used as a negative control and to enumerate the frequency of IFN-γ-secreting cells stimulated in vivo. Following incubation with these antigen-presenting cells, the leukocytes were washed off, the membrane-bound IFN-γ was labeled, and the red spots, representing individual IFN-γ-secreting effector cells, were counted under a dissecting microscope.
Cytotoxicity assay.
Total mononuclear cells isolated from the SMG or periglandular and cervical lymph nodes (pooled) at day 14 postinfection were assayed for cytolytic activity against MCMV-infected syngeneic and allogeneic target cells. The effector cells were assayed ex vivo, directly from the tissues and without secondary in vitro stimulation. Cytotoxicity assays were performed using a standard chromium release assay in microtiter wells essentially as described (65), except that target and effector cells were coincubated overnight in consideration of the primary response. Target cells were treated with IFN-γ as above and either mock infected or infected with 1.5 PFU/cell for 24 h. The cells were labeled overnight with 25 μCi of 51Cr (DuPont NEN, Wilmington, Del.) per ml.
Tetramer staining of tissue leukocytes.
In order to enumerate MCMV-specific CD8+ T lymphocytes, flow cytometric analyses for direct TCR binding with an MHC class I-peptide tetramer were conducted. A tetrameric complex of mouse H-2Ld and an immunodominant nonapeptide from the major immediate-early protein (m123, IE1, or pp89) of MCMV (54) was produced by the National Institute of Allergy and Infectious Disease Tetramer Facility and the National Institutes of Health AIDS Research and Reference Reagent Program (Emory University Vaccine Center, Yerkes Regional Primate Research Center, Atlanta, Ga.). The Ld-IE1 tetramer incorporated the nonapeptide H2N-168YPHFMPTNL176-COOH (synthesized by Genemed Synthesis, Inc., San Francisco, Calif.) and was directly conjugated to the APC fluorochrome. MCMV IE1-specific T cells were identified by four-color cytofluorometric analyses with monoclonal antibodies to mouse surface markers (listed above) and the tetramer at a 1:50 dilution and following the protocol recommended by the tetramer facility. Leukocytes from MCMV-infected C57BL/6 mice were included as a negative haplotype control. Stained cells were immediately acquired (300,000 events/sample), and the data were analyzed with CellQuest software.
RESULTS
MCMV clearance from salivary glands of infected mice is delayed.
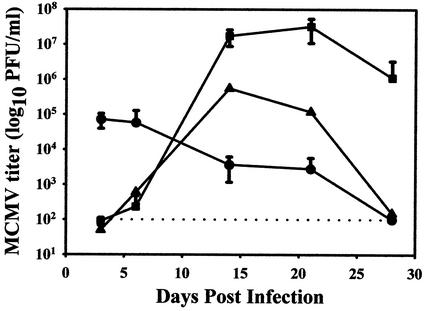
MCMV replication persists in the salivary gland long after it is cleared from all other target organs of infected mice. To verify this pattern of viral clearance at the dose of virus used in our experiments, virus titers were determined in the spleen, SMG, and draining cervical and periglandular lymph nodes at 3, 6, 14, 21, and 28 days following infection with 4 × 103 PFU of virulent, salivary gland-passaged MCMV (Fig. 1). This inoculum represents a high dose in the genetically susceptible BALB/c strain of mice, thereby ensuring an acute level of infection in each animal. As shown in Fig. 1, in the spleen, titers quickly rose to peak levels of approximately 105 PFU/ml of tissue homogenate by day 3 after infection. MCMV titers in the spleen remained detectable through day 21 and subsequently declined to undetectable levels by day 28. In the draining lymph nodes, MCMV titers were undetectable until day 6 after infection, peaked to nearly 106 PFU/ml by day 14, and then gradually declined to undetectable levels by day 28. Efficient replication of MCMV in the lymph nodes of BALB/c mice has been previously reported by us (10) and others (58). In the SMG, titers remained undetectable until day 6, peaked to 107 to 108 PFU/ml between days 14 and 21 after infection, and then began to decline. As expected, by day 28 following acute MCMV infection, virus was cleared from the spleen and draining lymph nodes; however, virus persisted in the SMG at high levels through at least 4 weeks postinfection.
FIG. 1.
MCMV titers in tissues of infected mice. Infectious MCMV was quantitated in the spleen (•), SMG (▪), and draining lymph nodes (▴) of mice on days 0 (uninfected control group), 3, 6, 14, 21, and 28 after infection. Titers were determined by preparing 20% (wt/vol) tissue homogenates and titering these homogenates by standard plaque assay on NIH 3T3 fibroblasts. Titers were determined for five individual mice at each time point, with the exception of the lymph nodes, which were pooled from all five animals at each time point. The results are presented as the log10 of infectious MCMV per milliliter of tissue homogenate, and standard error bars are shown for the spleen and SMG. The limit of detection was 102 PFU/ml and is illustrated as a dashed line.
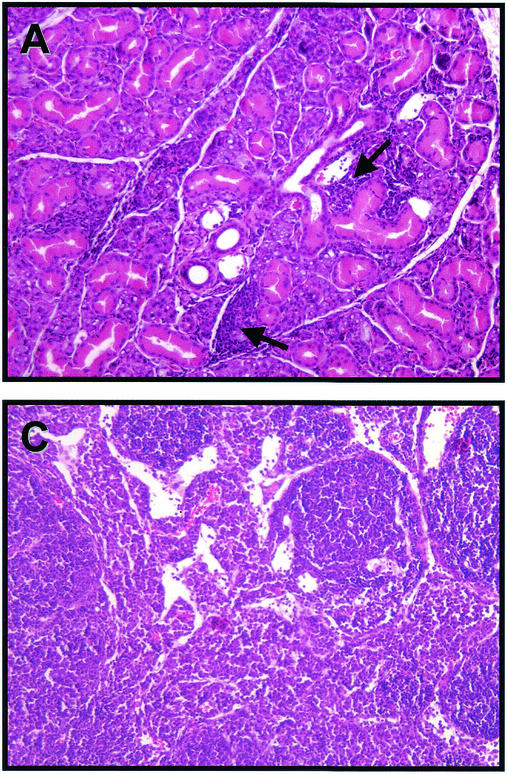
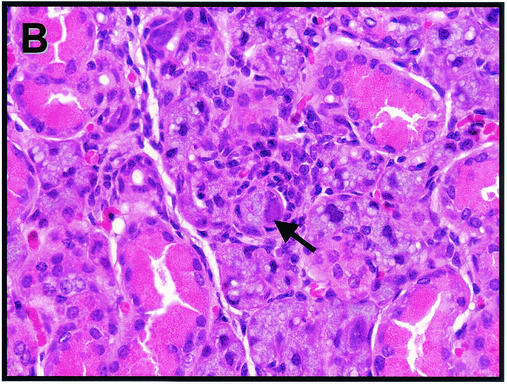
Sections of salivary gland and adjacent periglandular lymph node from mice on day 14 postinfection showed scattered clusters of interstitial mononuclear inflammatory cells (Fig. 2A). Intranuclear eosinophilic inclusions indicative of viral replication could be identified in many mucous gland alveolar epithelial cells of both the submaxillary (Fig. 2B) and sublingual glands (data not shown). Inclusion formation was accompanied by nuclear enlargement and peripheral margination of chromatin. Viral inclusions were not seen in granulated secretory epithelium of intercalated tubules or in parotid gland epithelia. Inclusions could also be identified, though less abundantly, in day 7 postinfection submaxillary glands (data not shown). Adjacent lymph nodes exhibited marked dilatation of medullary sinuses and expansion of both medullary cords and paracortex by plasma cells and activated lymphocytes (Fig. 2C).
FIG. 2.
Virus-induced histological changes in the SMG and periglandular lymph node. Hematoxylin- and eosin-stained tissues from mice on day 14 post-MCMV infection are shown. (A) Submaxillary salivary gland, with arrows indicating interstitial mononuclear inflammation (×400). (B) Submaxillary salivary gland, with arrow depicting a cytomegalic cell with an enlarged nucleus (×600). (C) Periglandular lymph node, revealing dilatation of medullary sinuses and expansion of medullary cords and paracortex by plasma cells and lymphocytes (×400).
Numbers of CD8+ and DX5+ T cells infiltrating the SMG increase dramatically following MCMV infection.
As a first step in characterizing the immune response to MCMV infection in the salivary gland, the numbers and phenotypes of infiltrating mononuclear cells were determined by flow cytometry on days 0 (uninfected controls), 14 (peak virus titers), and 28 (SMG-specific persistence) following infection. Leukocytes isolated from these tissues were characterized by four-color cytofluorometric analyses with a panel of monoclonal antibodies to the following mouse surface molecules: CD3ɛ, CD4, CD8α, TCR β chain, TCRγ/δ, DX5, I-Ad (MHC class II alloantigen), F4/80 (macrophage cell marker), and CD11c (dendritic cell marker).
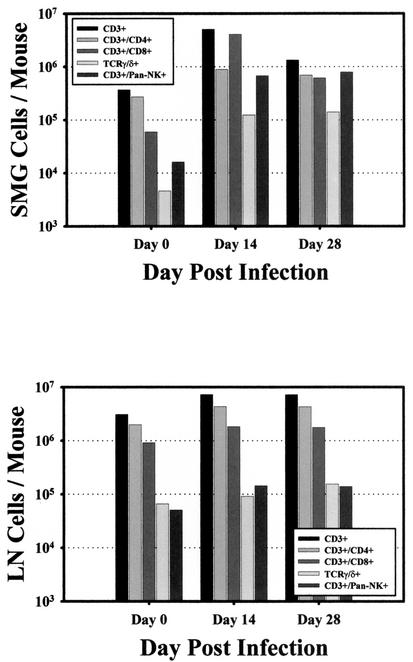
As shown in Fig. 3, the absolute number of CD3+ lymphocytes increased significantly in the SMG following MCMV infection, from 3.7 × 105 cells/mouse in uninfected animals to 5.1 × 106 cells/mouse at the peak of infection, a nearly 14-fold increase. By day 28 after infection, CD3+ cell numbers in the SMG dropped slightly, to 1.3 × 106 cells/mouse. Comparatively, in the draining lymph nodes, the absolute number of CD3+ lymphocytes increased only twofold after MCMV infection, from 3.1 × 106 cells/mouse on day 0 to 7.2 × 106 cells/mouse on day 14, and remained at this frequency through day 28.
FIG. 3.
Absolute numbers of T-cell subsets in the SMG and draining lymph nodes of MCMV-infected mice. The number and phenotype of CD3+ cells isolated from the SMG (upper panel) and draining lymph nodes (LN, lower panel) of MCMV-infected mice were determined on days 0 (uninfected control group), 14, and 28 after infection. Surface expression of mouse CD3ɛ in combination with CD4, CD8α, TCR γ/δ, or DX5 was determined by three-color cytofluorometric analysis. The absolute numbers of CD3+ T-cell subsets was calculated by multiplying the resulting percentage of each subset (corrected for isotype control background staining) by the total number of leukocytes isolated from each tissue at each time point. The results are represented as the log10 of absolute cell numbers per mouse. These results are representative of one experiment that was repeated three times with similar results.
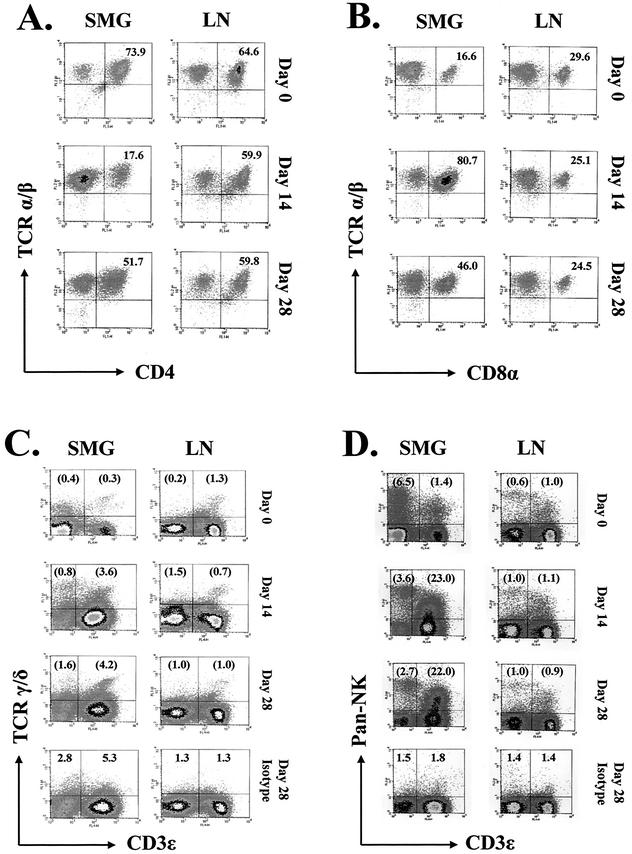
CD3+ T cells isolated from the SMG and draining lymph nodes of infected mice were analyzed further for the expression of CD4, CD8, TCR, and NK surface molecules (Fig. 3 and 4). At the peak of MCMV infection on day 14, the absolute number of CD4+ T cells in the SMG was three times (8.9 × 105 cells/mouse) that observed in the glands of uninfected mice (Fig. 3). Nevertheless, density plots of gated CD3+ cells revealed that the overall proportion of CD4+ cells dropped sharply from 74% in the uninfected mouse to only 18% on day 14 after infection (Fig. 4A). This precipitous decline was due to a remarkable rise in the number of CD8+ T lymphocytes in the infected salivary gland. The number of CD8+ T cells in this tissue increased dramatically after infection to 4.1 × 106 cells/mouse on day 14, nearly 69 times the number found in uninfected mice. Just as striking, density plot analysis of the CD3+ T-cell population demonstrated that the proportion of CD8+ T cells increased from 17% on day 0 to 81% on day 14 (Fig. 4B).
FIG. 4.
CD3+ T-cell subpopulations in the SMG and draining lymph nodes of MCMV-infected mice. Leukocytes isolated from the salivary glands (SMG) and draining lymph nodes (LN) of MCMV-infected mice were analyzed for the surface expression of mouse CD3ɛ, CD4, CD8α, TCR α/β, TCR γ/δ, and DX5 by three-color cytofluorometry on days 0 (uninfected control group), 14, and 28 after infection. The percentages of CD3+ T-lymphocyte populations expressing CD4 (FL1, x axis) and TCR α/β (FL2, y axis) (A), and CD8α (FL1, x axis) and TCR α/β (FL2, y axis) (B) were determined on gated CD3+ (FL4) cells. The percentages of populations expressing TCR γ/δ (FL1, y axis) and/or CD3ɛ (FL4, x axis) (C) and the percentages of populations expressing DX5 (FL2, y axis) and/or CD3ɛ (FL4, x axis) (D) were determined on ungated leukocytes. The results are illustrated as two-dimensional density plots of 20,000 gated cells or 100,000 ungated events. In panels C and D, nonspecific staining by isotype-matched controls for TCR γ/δ (C) or DX5 (D) is shown for the day 28 samples, representing the maximal level of nonspecific staining by these antibodies. The percentages of each population are indicated in the appropriate quadrants. In panels C and D, percentages in parentheses are adjusted numbers corrected for nonspecific staining of the isotype-matched control antibodies at each corresponding time postinfection. The data shown here are representative of one experiment that was repeated three times with comparable results.
In the draining lymph nodes of infected mice, the total number of CD4+ cells doubled (to 4.3 × 106 cells/mouse), yet the proportion of these cells remained between 60 and 65% over the entire course of MCMV infection. In stark contrast to the SMG, the number of CD8+ cells in the draining lymph nodes only doubled, to 1.8 × 106 cells/mouse, and the proportion of CD8+ cells remained between 25 and 30% and did not change significantly over the course of infection. Collectively, these data indicate that although the absolute numbers of both CD4+ and CD8+ T-cell subsets increased in both tissues following MCMV infection, there was an extraordinary influx of CD8+ T lymphocytes into the infected SMG.
Since mucosal tissues are enriched for T lymphocytes bearing the γ/δ TCR (1, 17, 37, 43), we determined the proportion of CD3+ T cells expressing this TCR in infected SMG and lymph nodes. The data (Fig. 3 and 4C) indicate that the absolute numbers of CD3+ γ/δ TCR in the SMG increased 30-fold (from 4.6 × 103 to 1.4 × 105 cells/mouse) in response to infection, with a 10-fold increase in the percentage of these cells. In contrast, the number (1.0 × 105 cells/mouse) and percentage (1.0%) of CD3+ γ/δ TCR in the lymph nodes remained constant throughout infection. Thus, although the SMG contained fewer resident CD3+ γ/δ TCR than the adjacent lymph node, T cells within this tissue responded more vigorously to MCMV infection.
NK cells mediate early host defense against MCMV infection, primarily by controlling virus replication in target organs via cytolytic mechanisms and the production of antiviral cytokines (3, 7, 62, 67, 68, 71). In the BALB/c mouse strain, which lacks the prototypical NK marker NK1.1, pan-NK (DX5, CD49b) is used to identify NK populations.
In this study, DX5+ CD3− NK (non-T) cells and those coexpressing DX5 and CD3 were identified in the SMG and draining lymph nodes of MCMV-infected mice. Surprisingly, the percentage of NK cells did not increase in response to MCMV infection of the SMG. However, the numbers and percentages of DX5+ CD3+ T cells increased dramatically in this tissue (Fig. 3 and 4D). The absolute numbers of DX5+ CD3+ T cells increased sharply from 1.6 × 104 cells/mouse salivary gland on day 0 to 6.7 × 105 cells/mouse on day 14 postinfection and to 7.9 × 105 on day 28 (a 49-fold increase).
Density plots of ungated leukocytes from these tissues (Fig. 4D) clearly illustrated the striking influx or expansion of DX5+ CD3+ T cells in the infected SMG, to 23% of all mononuclear cells on day 14 postinfection. In contrast to the SMG, there were no significant changes in the proportion of either NK or DX5+ CD3+ T cells in the lymph nodes of the infected animals (Fig. 4D). It is important to note that the identity of the DX5+ CD3+ T cells was not revealed during these studies. At least some of these cells may be NK T cells; however, a significant proportion may be a subpopulation of antigen-specific CD4+ and/or CD8+ T cells activated during viral infection and coexpressing NK markers (41, 63).
Interestingly, the cellular composition of the SMG from uninfected (day 0) BALB/c mice differed from those of the C3H mice used by Mega et al. (43). For example, while C3H SMG contained approximately equal numbers of CD4+ and CD8+ T cells, the SMG of BALB/c mice contained five times as many CD4+ as CD8+ T cells (Fig. 3). In addition, a negligible number of SMG CD8+ T cells from BALB/c mice expressed TCR γ/δ (Fig. 4), as opposed to 25% in C3H mice (43).
MCMV infection recruits antigen-presenting cells to the SMG.
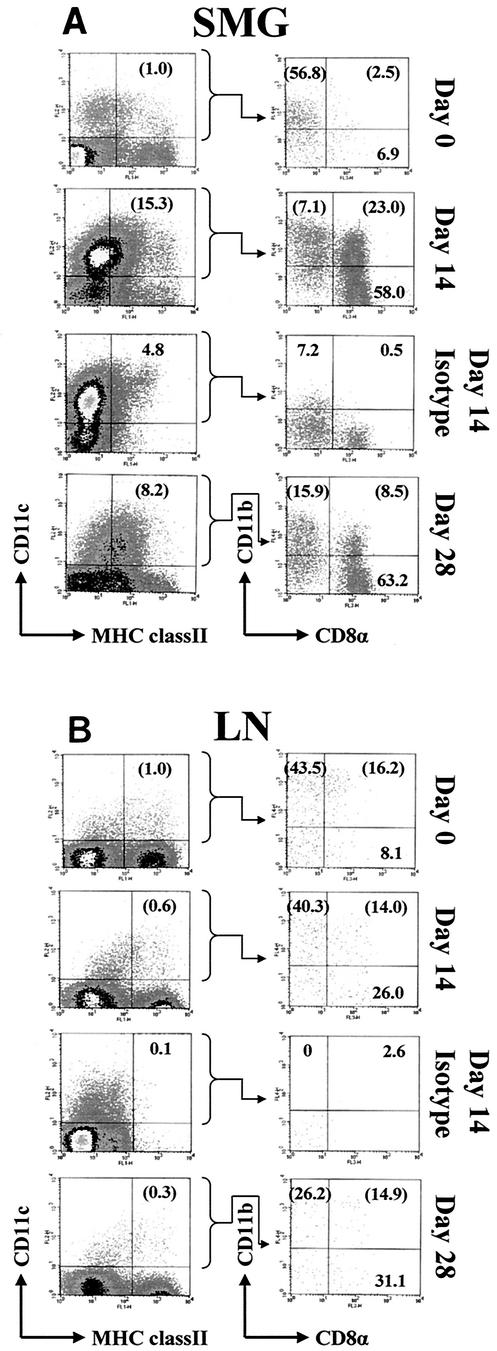
The relative response of tissue macrophages and dendritic cells to MCMV infection was also compared between the SMG and lymph nodes with time after infection. Tissue macrophages, identified by the I-Ad+ F4/80+ phenotype, increased by similar levels in both organs, to 4.4 × 105 cells/mouse by day 14 in the SMG (a 15-fold increase) and to 1.2 × 106 cells/mouse in the draining lymph nodes (a 13-fold increase). However, the percentage and total numbers of tissue dendritic cells, defined by the I-Ad+ CD11c+ phenotype, increased to much greater levels in the infected SMG compared to the lymph nodes. By day 14 postinfection, the dendritic cells in the SMG increased to 15% of the total mononuclear cells (Fig. 5), or 4.5 × 105 cells/mouse, a 39-fold increase over those found in uninfected mice. In contrast, the lymph nodes displayed a negligible increase in the total number of dendritic cells (from 5.1 × 104 to 7.8 × 104 cells/mouse).
FIG. 5.
CD11c+ MHC class II+ dendritic cells and their subpopulations within salivary glands (SMG) (A) and draining lymph nodes (LN) (B) of MCMV-infected mice. Ungated mononuclear cells, isolated from each tissue at the indicated times postinfection, were analyzed for expression of I-Ad (FL1, x axis) and CD11c (FL2, y axis). CD11c+ MHC class II+ cells were gated and analyzed further for expression of CD8α (FL3, x axis) and CD11b (FL4, y axis). Isotype-matched controls for I-Ad (left column, FL1, x axis) and CD11b (right column, FL4, y axis) are shown for the day 14 samples, representing the maximal level of nonspecific staining by these antibodies. Percentages in parentheses are adjusted numbers corrected for nonspecific staining of the isotype-matched control antibodies at each corresponding time postinfection.
To authenticate and further subtype these dendritic cells, the CD11c+ cells expressing low to high levels of MHC class II were gated and further analyzed for expression of the dendritic cell subset markers CD11b and CD8α (Fig. 5). In uninfected mice, the majority of the SMG (57%) and lymph node (44%) CD11c+ MHC class II+ cells were CD11b+ CD8α−, a finding similar to previous observations of lymph node and splenic dendritic cells in BALB/c mice (45). After infection of the SMG, the CD11b− CD8α+ subpopulation predominated (58%), and 23% of the dendritic cells were double positive for both CD11b and CD8α (Fig. 5A). The skewing toward the CD11b− CD8α+ phenotype remained through 28 days postinfection. This shift also occurred, although to a lesser extent, in the lymph nodes of MCMV-infected mice, such that by day 28 postinfection, CD11b+ CD8α− and CD11b− CD8α+ subpopulations each represented 27 to 31% of CD11c+ MHC class II+ cells (Fig. 5B). Plasmacytoid-like dendritic cells expressing both B220 and Ly6G/C also increased in infected SMG, from 0.05% of CD11c+ MHC class II+ cells to 2.2% on day 14 postinfection (data not shown). Thus, dendritic cells are a major cell type infiltrating inflamed, MCMV-infected SMG tissue, where they likely regulate antiviral T-cell responses.
Expression of IFN-γ, IL-10, and CC chemokines is significantly increased in SMG mononuclear cells following MCMV infection.
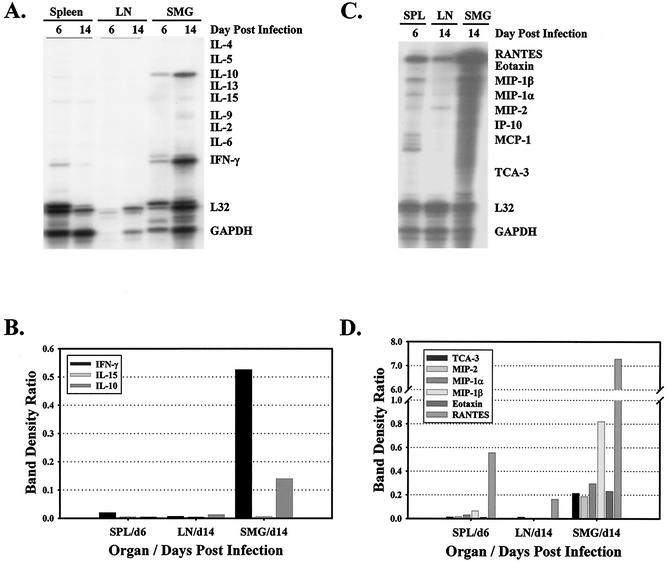
One could conclude from the above data that replication of MCMV in SMG epithelial cells is refractory to the antiviral activities of the various cell types identified above as responders of MCMV infection, activities exerted through target cell lysis or antiviral cytokines. However, it is possible that in the environment of the SMG, these effector cells are rendered nonfunctional due to inactivation or clonal exhaustion (50, 70). Therefore, with the aim of assessing the functionality of immune cells infiltrating the SMG and draining lymph nodes of MCMV-infected mice, cytokine profiles were determined for mononuclear cells isolated from each organ at various times after infection (Fig. 6). RNase protection assays were used to determine the expression of RNA species of various Th1 (including IFN-γ and IL-2) and Th2 (including IL-4, IL-5, and IL-10) cytokines (Fig. 6A), as well as numerous chemokines (Fig. 6C).
FIG. 6.
Expression of cytokines and chemokines in the spleen, SMG, and draining lymph nodes of MCMV-infected mice. RNA purified from leukocytes isolated from the spleen, SMG, and draining lymph nodes (LN) of MCMV-infected mice on days 6 and 14 after infection were used in RNase protection assays to quantitate the expression of various cytokines (A) and chemokines (C). The relative expression levels of cytokines (B) and chemokines (D) were determined by calculating band density ratios from the phosphorimage of each gel. The band density ratio for each RNA species was calculated by dividing the band density of the cytokine or chemokine by the band density of L32 in the same lane.
The expression of IFN-γ and IL-10 was significantly greater in the SMG compared to secondary lymphoid organs at the peak of MCMV infection on day 14. By comparing band density ratios, the expression of IFN-γ was 77-fold higher and that of IL-10 was 11-fold higher in leukocytes isolated from the SMG than in those from the draining lymph nodes on day 14 after infection (Fig. 6B). TNF-α was detectable in the SMG, lymph nodes, and spleen following MCMV infection, although expression in the SMG was only twofold higher than in the draining lymph node and threefold higher than in the spleen (data not shown). The strength of these cytokine responses in the SMG indicated that the leukocytes infiltrating this tissue were functionally capable of responding to MCMV infection. However, the dichotomy of the cytokine production pattern in the salivary gland was unexpected, as IFN-γ and TNF-α are Th1 cytokines known to promote viral clearance, whereas IL-10 is a Th2 cytokine known to antagonize antiviral cytotoxic T-lymphocyte responses.
When the same RNA samples from MCMV-infected mice were hybridized with a chemokine probe set, the expression of several chemokines was found to be extraordinarily high in the SMG (Fig. 6C). When the band density ratios for each chemokine were calculated, the expression of TCA-3 (T-cell activation gene 3), MIP-1β (macrophage inflammatory protein 1β), eotaxin, MIP-1α, RANTES (regulated upon activation, normal T-cell expressed and secreted), and MIP-2 in the SMG on day 14 was 306-fold, 130-fold, 85-fold, 57-fold, 44-fold, and 16-fold higher, respectively, than in the draining lymph nodes. Although not easily discernible from Fig. 6C, CC chemokine MCP-1 and CXC chemokine IP-10 were not elevated in response to MCMV infection. Given that the CC chemokines are produced largely by activated T cells, mononuclear phagocytes, and NK cells, this indicated that the leukocytes infiltrating the infected SMG were activated and functionally responsive to MCMV infection. The strong expression of these molecules in the infected salivary gland likely contributed to the dramatic infiltration and retention of leukocytes in this tissue.
IFN-γ produced in the SMG following MCMV infection is secreted by CD4+, CD8+, or DX5+ T cells.
To identify the cellular sources of the vigorous IFN-γ response detected in the SMG at the peak of MCMV infection, leukocytes were isolated from infected mice on day 14 and stained for determination of the intracellular accumulation of IFN-γ by four-color cytofluorometric analysis (Fig. 7). It is important to note that the leukocytes used in this assay did not receive in vitro stimulation before analysis; therefore, IFN-γ+ cells represent those stimulated exclusively in vivo in response to virus infection. An isotype-matched control for the anti-IFN-γ monoclonal antibody was included in each analysis to ensure the specificity of IFN-γ synthesis.
FIG. 7.
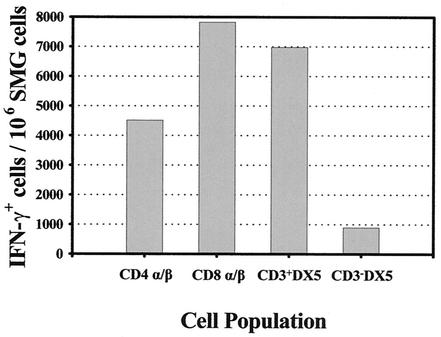
Identification of IFN-γ-producing effector cells in the SMG of MCMV-infected mice. Leukocytes were isolated from the SMG of MCMV-infected mice on day 14 after infection, and intracellular IFN-γ was measured by four-color cytofluorometric analysis. Cells were incubated in brefeldin A, stained for surface markers, fixed and permeabilized, and stained for the intracellular accumulation of IFN-γ. Each cell subpopulation was identified by the presence of CD3ɛ (FL3), CD4 (FL1), CD8α (FL1), TCR α/β (FL2), and DX5 (FL2) surface molecules, gated, and further analyzed for IFN-γ production (FL4). The number of IFN-γ+ cells within each cell subpopulation was calculated by multiplying the proportion (of the total cells) each subpopulation comprised with the percentage of IFN-γ+ cells within each subpopulation. The results are expressed as the number of IFN-γ+ cells per 106 SMG cells. An isotype-matched control monoclonal antibody served as a negative control and showed virtually no reactivity (<0.35%) in any of the samples.
On day 14 postinfection, 2.2% of the total cells isolated from the SMG were producing IFN-γ. This percentage represented roughly 1.4 × 105 IFN-γ+ cells/salivary gland. As shown in Fig. 7, most of the IFN-γ-producing cells were CD8+ TCR α/β+ T cells, followed by DX5+ CD3+ cells and CD4+ TCR α/β+ T cells.
In contrast to the SMG, no IFN-γ-producing cells were detected in the draining lymph nodes of MCMV-infected mice. Apparently, in the absence of in vitro stimulation, the amount of IFN-γ produced in the lymph nodes was below the level of detection in this assay. Intracellular IL-10 was undetectable in mononuclear cells isolated from SMG or lymph nodes. In the absence of in vitro stimulation and/or affinity matrix technology, the quantity of IL-10 protein produced was likely below the level of detection under our assay conditions.
IFN-γ-producing cells in the SMG and draining lymph nodes of MCMV-infected mice are virus specific.
Cytokine secretion is a highly relevant method of assessing the effector function of virus-specific T lymphocytes in MCMV-infected mice (22-25) and in HCMV-infected patients (4, 19). Therefore, an IFN-γ-based ELISPOT assay, modified by Reddehase and coworkers (22, 24), was used to quantitate the frequency of leukocytes specific for MCMV antigens and functionally capable of responding to infection with the secretion of IFN-γ. In this assay, leukocytes were isolated from the SMG and draining lymph nodes of MCMV-infected mice on day 14 and seeded in graded numbers into ELISPOT microwells with each of three types of antigen-presenting stimulator cells: mock-infected mKSA cells, MCMV-infected mKSA cells, and anti-CD3ɛ monoclonal antibody-producing hybridoma cells.
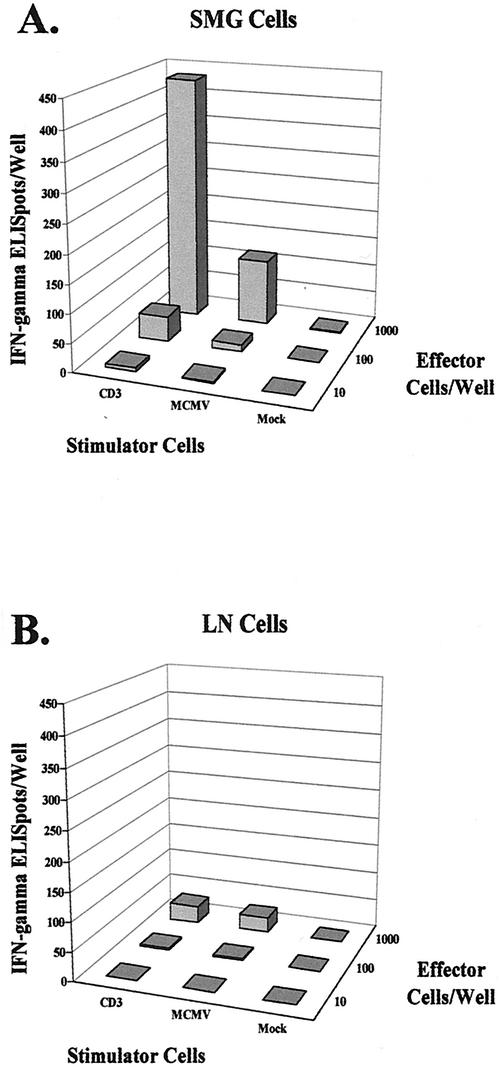
Roughly 43% (432 of 1,000 cells) of SMG cells were capable of producing IFN-γ upon polyclonal activation via the CD3ɛ molecule of the TCR-CD3 complex, as shown in Fig. 8A. When SMG cells were stimulated with MCMV-infected cells, the frequency of IFN-γ-producing cells was 11.5% (115 of 1,000 cells). Therefore, in the SMG, approximately one-fourth of all cells capable of producing IFN-γ upon stimulation were virus-specific effector cells. Stimulation with mock-infected cells resulted in less than two IFN-γ-secreting cells out of 432 functionally competent cells (0.4%), demonstrating the virus specificity of the response. A clear dose response was evident in this assay, with 10-fold serial dilutions of the effector cell population leading to 10-fold differences in the numbers of polyclonal and MCMV-activated responder cells.
FIG. 8.
Frequencies of virus-specific effector cells in the SMG and draining lymph nodes of MCMV-infected mice. An IFN-γ-based ELISPOT assay was performed to determine the number of effector cells capable of producing IFN-γ upon polyclonal or MCMV-specific activation. Effector cells used in this assay were leukocytes isolated from the SMG (A) and draining lymph nodes (LN) (B) of MCMV-infected mice on day 14 after infection, and stimulator cells were mock-infected mKSA cells, MCMV-infected mKSA cells, and anti-CD3ɛ-producing hybridoma cells. Effector cells were seeded in serial 10-fold dilutions into microwells and stimulated for 16 h with 105 antigen-presenting stimulator cells. Mock-infected stimulators served as a negative control as well as to enumerate the frequency of IFN-γ-secreting cells stimulated in vivo. MCMV-infected stimulators were used to determine the frequency of cells secreting IFN-γ in response to MCMV antigens, and anti-CD3ɛ hybridoma cells were used to deter-mine the frequency of cells capable of producing IFN-γ upon polyclonal activation. The data are shown as the average of triplicate wells for 10, 100, and 1,000 effector cells seeded with each of the three types of stimulator cells.
Analysis of cells isolated from the draining lymph nodes of MCMV-infected mice (Fig. 8B) revealed that 30 of 1,000 seeded cells were capable of producing IFN-γ upon polyclonal activation with anti-CD3ɛ monoclonal antibody. Although this fraction represented only 3% of the isolated lymph node cells (significantly less than the 43% fraction responding in the SMG), there are several possible explanations for this difference. First, the number of CD3+ cells represented approximately 42% of the total cells isolated from the draining lymph nodes on day 14 after MCMV infection, whereas this fraction represented 84% of the total cells isolated from the SMG. Second, the NK cell populations (DX5+) comprised roughly 2% of lymph node cells on day 14 but nearly 27% of SMG cells (see Fig. 4C). Finally, the proportion of activated as opposed to resting CD3+ cells is likely much higher in the SMG compared to the lymph node.
Taken together, the CD3+ T lymphocyte populations that represent the bulk of IFN-γ-producing cells were present in significantly greater numbers in the SMG than in the draining lymph nodes. When cells from the draining lymph nodes were stimulated with MCMV-infected cells, 26 of the 30 functionally capable cells responded by secreting IFN-γ, indicating that 87% of the responding cells were directed against MCMV antigens. Therefore, despite the overall lower frequency of lymph node cells capable of responding to polyclonal activation, the majority of these IFN-γ-secreting cells were MCMV specific.
Mononuclear SMG cells are cytolytic for MCMV-infected fibroblast cells.
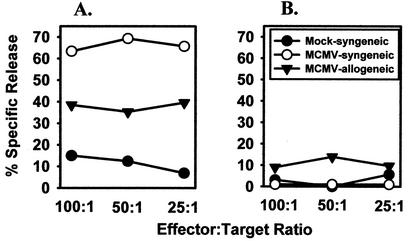
The production of IFN-γ by CD3+ T cells (CD4+, CD8+, or DX5+) indicated that SMG mononuclear cells were activated in response to infection to exert effector functions. To confirm this hypothesis, the cytolytic activity of the SMG mononuclear cells isolated at 14 days postinfection was also quantitated as a second functional assay. As above, these cells were assayed ex vivo, without in vitro restimulation. The results shown in Fig. 9A revealed a high degree of cytolytic activity of MHC class I-restricted cytotoxic T lymphocytes and MHC class I nonrestricted cells (presumably NK cells) for infected fibroblast cells. Therefore, the virus-specific cytotoxic T lymphocytes possessed potent cytolytic activity. However, nonfibroblast cells such as epithelial cells may be refractory to their lytic mechanisms, as virus persisted in high titers in the SMG beyond day 14 in spite of this vigorous cytolytic response. In the lymph nodes, which also supported high levels of MCMV replication at this time, the magnitude of cytolytic activity in the absence of further clonal expansion by antigen restimulation was negligible (Fig. 9B); yet virus was cleared from this site 1 week later.
FIG. 9.
Cytolytic activity of SMG (A) and lymph node (B) mononuclear cells. Mononuclear cells were harvested from the SMG and lymph nodes of mice on day 14 postinfection and incubated overnight in microtiter wells with 51Cr-labeled target cells at the indicated effector-to-target cell ratios. Syngeneic target cells were mKSA fibroblasts from BALB/c mice; allogeneic target cells were WT-19 fibroblasts from C57BL/6 mice. Cytotoxicity is expressed as percent specific release above spontaneous release values.
Frequency of CD8+ T cells specific for MCMV IE1 is sustained during the course of MCMV infection.
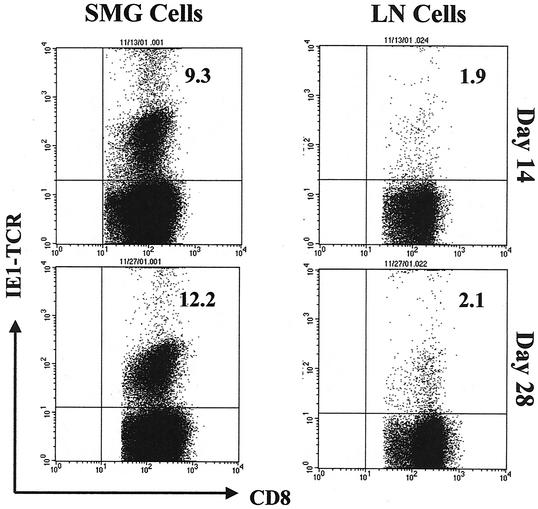
Viral persistence in the salivary glands of MCMV-infected mice could be explained by clonal exhaustion of the activated MCMV-specific T lymphocytes in the presence of a persistent high viral load. To test for this possibility, MCMV-specific T cells were enumerated in the SMG of virus-infected mice on day 14 and again on day 28 postinfection and compared to those found in the draining lymph nodes. A tetrameric complex of mouse H-2Ld and an immunodominant peptide within the MCMV IE1 protein (54) was used in a cytofluorometric analysis to determine the frequency of cytotoxic T lymphocytes directed against this peptide in MCMV-infected mice.
At the peak of MCMV infection on day 14, 9.3% of CD8+ T cells isolated from the SMG were IE1 specific, whereas 1.9% of lymph node CD8+ T cells were directed against this viral peptide (Fig. 10). On day 28, when there was still a high virus load in the salivary glands, the proportion of IE1-specific cytotoxic T lymphocytes increased to 12.2% of the SMG CD8+ population. On day 28 in the draining lymph nodes, when virus infection had resolved, the proportion of IE1-specific cells remained unchanged at 2.1% of the CD8+ population. Since CD8+ T cells comprised 64% of the total leukocytes isolated from the SMG on day 14, roughly 6% of all SMG leukocytes were specific to MCMV IE1 (an absolute number of 3.8 × 105 cells/mouse). Likewise, CD8+ T cells comprised 30% of the total leukocytes isolated from the SMG on day 28, and therefore 3.7% of these cells were IE1 specific (an absolute number of 7.5 × 104 cells/mouse). In the draining lymph nodes, CD8+ T cells represented 11% of the total leukocytes on day 14 as well as on day 28, and therefore the overall frequency of IE1-specific T cells represented 0.21% of all lymph node leukocytes on day 14 (an absolute number of 3.5 × 104 cells/mouse) and 0.22% on day 28 (an absolute number of 3.7 × 104 cells/mouse).
FIG. 10.
Tetramer staining of CD8+ T lymphocytes from the SMG and draining lymph nodes of MCMV-infected mice. A tetrameric complex of mouse H-2Ld and pp89 (the major immediate-early protein of MCMV and an immunodominant antigen for the antiviral immune response in BALB/c mice) was used in a four-color cytofluorometric analysis to determine the frequency of MCMV-specific CD8+ T lymphocytes in the SMG and draining lymph nodes (LN) of infected mice. The tetramer incorporated the nonapeptide H2N-168YPHFMPTNL176-COOH and was directly conjugated to the APC fluorochrome. Leukocytes were isolated from the SMG and draining lymph nodes of MCMV-infected BALB/c mice on days 14 and 28 after infection. Peptide-specific CD8+ T cells were identified with monoclonal antibodies to mouse surface molecules (CD3ɛ [FL3], CD4 [FL1], CD8a [FL1], TCR α/β [FL2], and TCR γ/δ [FL2]) and the pp89 tetramer (FL4). The data are represented as dot plots of gated CD8+ lymphocytes (CD8+ and TCR α/β+ population) expressing CD8α (FL1, x axis) and pp89 (FL4, y axis). The percentages of peptide-specific CD8+ T cells in each tissue at each time point are indicated in the upper right quadrants. Approximately 150,000 total events were acquired per sample, and dot plots display between 16,000 (for lymph node cells) and 100,000 (for SMG cells) gated cells. As a negative control, leukocytes from MCMV-infected C57BL/6 mice were included (data not shown) and showed minimal staining of CD8+ T cells (<1%).
These data indicate that the frequency of MCMV IE1-specific cytotoxic T lymphocytes was significantly higher in the salivary glands than in the draining lymph nodes of infected mice. In addition, because the proportion of CD8+ T lymphocytes directed against this viral peptide actually increased slightly over the course of infection, clonal exhaustion is likely not the cause of virus persistence in the salivary gland. Although the absolute number of IE1-specific CD8+ T cells in the SMG decreased fivefold between days 14 and 28 after infection, the number of these cells in the SMG on day 28 was still twice that found in the draining lymph node, which was efficiently cleared of replicating virus.
DISCUSSION
This study is the first to characterize the phenotype and function of mononuclear cells that respond to high-titer MCMV infection within the salivary glands of acutely infected BALB/c mice. Our results demonstrated that the antiviral response to MCMV in the submaxillary salivary gland is comprised of both innate and acquired regulatory and effector cells that are sustained in higher numbers throughout the chronic phase of infection.
The acquired immune response to MCMV at this mucosal site was dominated by CD8+ T lymphocytes. The number of these cells increased almost 70-fold to represent four-fifths of all CD3+ cells in the infected salivary gland. Notably, these CD8+ T cells were functionally activated; their production of IFN-γ and cytotoxicity were readily detected ex vivo, in the absence of in vitro restimulation. Importantly, the numbers of MCMV IE1-specific T cells were sustained in spite of the high virus load; thus, clonal exhaustion did not contribute to persistent virus infection in this model. This cellular response to acute MCMV infection of the SMG mimics that seen in infected lungs of BALB/c mice recovering from bone marrow transplantation, where a strong CD8+, α/β TCR, cytolytic T-cell infiltrate predominates over CD4+ T cells (23). However, unlike the SMG, the CD8+ T-cell response in infected lung tissue coincides with resolution of pulmonary infection.
CD4+ T lymphocytes, a cell population essential for viral clearance from the salivary glands (31), also responded to infection by producing IFN-γ. Nevertheless, due to the striking increases in the numbers of CD8+ T cells, as well as the infiltration of DX5+ CD3+ and dendritic cells, the proportional level of CD4+ T cells was quite low, representing less than one-fifth of all CD3+ T cells at the peak of MCMV infection in the SMG. It is important to note that, although CD4+ T cells were present in considerably lower numbers than CD8+ T cells at the peak of MCMV infection in the salivary gland, both T-cell subsets were present in roughly equal numbers by day 28 after infection, when virus titers in this organ began to decline. Hence, the reciprocal increase in CD4+ T cells and decrease in CD8+ T cells between days 14 and 28 after infection coincided with declining virus titers in the salivary gland, suggesting that the presence of progressively greater numbers of CD4+ T cells is associated with the eventual clearance of replicating virus from this tissue. It is unknown whether the CD4+ T cells directly mediate clearance of MCMV in the SMG or whether the CD4+ T cells induce another antiviral effector cell, as was previously demonstrated in the spleen and liver of MCMV-infected BALB/c mice (32).
In addition to this acquired immune response to MCMV infection of the SMG, innate immunity was also vigorously elicited at this site. Dendritic cells (CD11c+ MHC class II+) increased substantially in response to virus infection. The vast majority of these cells were mature, expressing differentiation markers CD11b and/or CD8α (45), and approximately 2% of the dendritic cells in infected tissue expressed the plasmacytoid-like phenotype CD11c+ B220+ Ly6G/C+ (5, 45). Infection of the SMG caused a dramatic shift from a predominance of CD11b+ CD8α− dendritic cells to CD8α+ CD11b− and CD11b+ CD8α+ cells. A major role for the CD8α+ dendritic cell subpopulation in regulating the immune response to MCMV infection has been reported previously (13). However, it is possible that at least some of the CD8α+ CD11c+ cells are in fact CD8+ T cells rather than dendritic cells.
CD11c has been identified on mucosal intestinal intraepithelial CD8+ T cells in response to microbial antigens of the gut and is likely a hallmark of persistent antigen-specific T-cell activation in the intestinal epithelium (26). It will therefore be important to further define the CD11c+ CD8α+ population to decipher the relative roles of dendritic cells and this potential subpopulation of T cells in the mucosal response to MCMV infection. Finally, the extent to which potentially permissive dendritic cells in the SMG are infected with MCMV was not determined; however, there was no functional evidence of virus-induced immunosuppression in the context of the SMG (2, 44).
Dendritic cell infiltration from the blood into inflamed, infected SMG tissue was likely triggered and perhaps sustained by virus-induced expression of chemokines from dendritic cells or SMG epithelial cells (12, 15, 28, 42, 51, 69). In our studies, the CC chemokines RANTES, MIP-1α, and MIP-1β were expressed (at least at the level of RNA) at high levels in SMG mononuclear cells. MIP-1α specifically triggers NK-mediated resistance to MCMV in the liver of C57BL/6 mice (59, 60). Other effector molecules likely produced by activated dendritic cells include the cytokines IFN-α and IL-12, not detected in our studies but likely expressed transiently very early after MCMV infection, and IL-10 (29, 34, 40), which was abundantly expressed by an as yet unidentified mononuclear cell infiltrating the infected SMG. Although this cytokine is generally regarded as an inhibitor of Th1-mediated cytotoxic T-lymphocyte development, IL-10 did not appear to dampen the activation of virus-specific, IFN-γ-secreting T cells in the SMG. Continued studies are aimed at assessing in more detail the role of specific dendritic cell subsets, parenchymal and mononuclear cell chemokines, and IL-10 in regulation of the immune response to MCMV in the SMG.
A significant increase in the number of DX5+ CD3+ cells was also observed in the SMG in response to MCMV infection. Although NK non-T cells function as early mediators of defense against MCMV infection (3, 7, 66) and produce the vast majority of IFN-γ early in MCMV infection in the spleen and liver of C57BL/6 mice (7, 14, 48, 49, 59, 67), our study revealed that in the BALB/c mouse SMG, there is a disproportionate increase in the IFN-γ-producing DX5+ CD3+ cells at late times after infection. Whether BALB/c SMG NK and DX5+ CD3+ cells possess the same regulatory functions as liver or splenic NK and NK T cells in MCMV-infected C57BL/6 mice remains to be determined (6, 57). The NK and/or DX5+ CD3+ cells may contribute to the robust expansion in the SMG of CD8+ T cells and their production of IFN-γ; however, inhibitory effects of NK and NK T cells on virus-specific cytotoxic T-lymphocyte activity have been reported (30, 64).
It will be of utmost importance to determine if the DX5+ T cells are of a true NK lineage or if they are virus-specific CD8+ T cells that coexpress NK markers (41, 63). We are currently addressing this question through use of CD1d tetramers, as CD1d restriction is a better identifier of NK T cells than DX5, especially in BALB/c mice (18). A further, particularly intriguing question is whether the NK cells in the SMG, which did not expand in response to infection, are subject to the m152- or m02 gene family-mediated downregulation of NK cell activation (36, 47).
T lymphocytes bearing a γ/δ TCR responded to MCMV infection of the SMG as well. Previous studies of MCMV-infected mice documented that depletion of γ/δ T cells results in significant increases in virus titers and decreases in IFN-γ in the liver on day 3 after infection (46), suggesting the importance of γ/δ T cells in early protection against MCMV infection. In contrast to their documented role early after infection, we report here a significant (30-fold) increase in the number of γ/δ T cells in the salivary gland late after MCMV infection. Preliminary data indicate that, on average, 54% of the γ/δ T cells in the SMG also express DX5, while 25% of lymph node γ/δ T cells coexpress this marker. Thus, at least a quantitative but perhaps also a qualitative difference in the γ/δ T-cell response is induced in the SMG compared to the draining lymph nodes of MCMV-infected mice. Interestingly, a recent report documents a role for IFN-γ-producing and NK1.1+ γ/δ T cells early in innate resistance to vaccinia virus infection (61).
In summary, our results suggest that both innate and adaptive effector cells possessing cytolytic activity and/or producing antiviral cytokines are vigorously recruited and subsequently maintained throughout the later stages of MCMV infection in the salivary gland. This copious response endures in parallel with and is perhaps driven by persistent infectious virus. Virus replication within glandular epithelial cells may be resistant to cell-mediated cytotoxicity but eventually controlled by antiviral cytokines. However, now that a unique cellular environment comprised of highly reactive CD8+ and CD4+ T cells, DX5+CD3+ cells, and CD11c+ cells has been identified within the infected SMG, additional factors contributing to persistent infection and the eventual clearance of virus from this major target organ can be considered and further pursued.
Acknowledgments
We thank Richard Ciavarra and Dan Holterman of the Department of Microbiology and Molecular Cell Biology, Eastern Virginia Medical School, for technical advice and helpful discussions. We also acknowledge Noeline Guillaume and Ellen Jing of the Glennan Center for Geriatrics and Gerontology Flow Cytometry Laboratory for excellent technical assistance. We are grateful to the NIAID Tetramer Facility and the NIH AIDS Research and Reference Reagent Program (Emory University Vaccine Center, Yerkes Regional Primate Research Center, Atlanta, Ga.) for production of the tetramer used in this study. We also recognize the valuable input of Hiroshi Kiyono and Stephen Jennings in the early, developmental stages of this project.
This study was supported by Public Health Service grant R03 AI45084 from the National Institutes of Health.
REFERENCES
- 1.Aicher, W. K., K. Fujihashi, M. Yamamoto, H. Kiyono, A. M. Pitts, and J. R. McGhee. 1992. Effects of the lpr/lpr mutation on T and B cell populations in the lamina propria of the small intestine, a mucosal effector site. Int. Immunol. 4:959-968. [DOI] [PubMed] [Google Scholar]
- 2.Andrews, D. M., C. E. Andoniou, F. Granucci, P. Ricciardi-Castagnoli, and M. A. Degli-Esposti. 2001. Infection of dendritic cells by murine cytomegalovirus induces functional paralysis. Nat. Immunol. 2:1077-1084. [DOI] [PubMed] [Google Scholar]
- 3.Andrews, D. M., H. E. Farrell, E. H. Densley, A. A. Scalzo, G. R. Shellam, and M. A. Degli-Esposti. 2001. NK1.1+ cells and murine cytomegalovirus infection: what happens in situ? J. Immunol. 166:1796-1802. [DOI] [PubMed] [Google Scholar]
- 4.Appay, V., D. F. Nixon, S. M. Donahoe, G. M. Gillespie, T. Dong, A. King, G. S. Ogg, H. M. Spiegel, C. Conlon, C. A. Spina, D. V. Havlir, D. D. Richman, A. Waters, P. Easterbrook, A. J. McMichael, and S. L. Rowland-Jones. 2000. HIV-specific CD8+ T cells produce antiviral cytokines but are impaired in cytolytic function. J. Exp. Med. 192:63-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asselin-Paturel, C., A. Boonstra, M. Dalod, I. Durand, N. Yessaad, C. Dezutter-Dambuyant, A. Vicari, A. O'Garra, C. Biron, F. Briere, and G. Trinchieri. 2001. Mouse type I IFN-producing cells are immature APCs with plasmacytoid morphology. Nat. Immunol. 2:1144-1150. [DOI] [PubMed] [Google Scholar]
- 6.Biron, C. A., and L. Brossay. 2001. NK cells and NKT cells in innate defense against viral infections. Curr. Opin. Immunol. 13:458-464. [DOI] [PubMed] [Google Scholar]
- 7.Biron, C. A., K. B. Nguyen, G. C. Pien, L. P. Cousens, and T. P. Salazar-Mather. 1999. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu. Rev. Immunol. 17:189-220. [DOI] [PubMed] [Google Scholar]
- 8.Boes, B., H. Hengel, T. Ruppert, G. Multhaup, U. H. Koszinowski, and P. M. Kloetzel. 1994. Interferon gamma stimulation modulates the proteolytic activity and cleavage site preference of 20S mouse proteasomes. J. Exp. Med. 179:901-909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Britt, W. J. and. C. A. Alford. 1996. Cytomegalovirus, p. 2493-2523. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology, vol. 2. Lippincott-Raven Publishers, Philadelphia, Pa.
- 10.Campbell, A. E. 1999. Murine cytomegalovirus, p. 447-466. In R. Ahmed and I. Chen (ed.), Persistent viral infections. John Wiley & Sons Ltd., New York, N.Y.
- 11.Campbell, A. E., J. S. Slater, V. J. Cavanaugh, and R. M. Stenberg. 1992. An early event in murine cytomegalovirus replication inhibits presentation of cellular antigens to cytotoxic T lymphocytes. J. Virol. 66:3011-3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caux, C., B. Vanbervliet, C. Massacrier, S. Ait-Yahia, C. Vaure, K. Chemin, N. Dieu, Mc, and A. Vicari. 2002. Regulation of dendritic cell recruitment by chemokines. Transplantation 73:S7-S11. [DOI] [PubMed] [Google Scholar]
- 13.Dalod, M., T. P. Salazar-Mather, L. Malmgaard, C. Lewis, C. Asselin-Paturel, F. Briere, G. Trinchieri, and C. A. Biron. 2002. Interferon alpha/beta and interleukin 12 responses to viral infections: pathways regulating dendritic cell cytokine expression in vivo. J. Exp. Med. 195:517-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daniels, K. A., G. Devora, W. C. Lai, C. L. O'Donnell, M. Bennett, and R. M. Welsh. 2001. Murine cytomegalovirus is regulated by a discrete subset of natural killer cells reactive with monoclonal antibody to ly49h. J. Exp. Med. 194:29-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fischer, F. R., Y. Luo, M. Luo, L. Santambrogio, and M. E. Dorf. 2001. RANTES-induced chemokine cascade in dendritic cells. J. Immunol. 167:1637-1643. [DOI] [PubMed] [Google Scholar]
- 16.Fujihashi, K., J. R. McGhee, M. N. Kweon, M. D. Cooper, S. Tonegawa, I. Takahashi, T. Hiroi, J. Mestecky, and H. Kiyono. 1996. gamma/delta T-cell-deficient mice have impaired mucosal immunoglobulin A responses. J. Exp. Med. 183:1929-1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goodman, T., and L. Lefrancois. 1988. Expression of the gamma-delta T-cell receptor on intestinal CD8+ intraepithelial lymphocytes. Nature 333:855-858. [DOI] [PubMed] [Google Scholar]
- 18.Hammond, K. J., D. G. Pellicci, L. D. Poulton, O. V. Naidenko, A. A. Scalzo, A. G. Baxter, and D. I. Godfrey. 2001. CD1d-restricted NKT cells: an interstrain comparison. J. Immunol. 167:1164-1173. [DOI] [PubMed] [Google Scholar]
- 19.Hassan-Walker, A. F., A. L. Vargas Cuero, F. M. Mattes, P. Klenerman, F. Lechner, A. K. Burroughs, P. D. Griffiths, R. E. Phillips, and V. C. Emery. 2001. CD8+ cytotoxic lymphocyte responses against cytomegalovirus after liver transplantation: correlation with time from transplant to receipt of tacrolimus. J. Infect. Dis. 183:835-843. [DOI] [PubMed] [Google Scholar]
- 20.Henson, D., and A. J. Strano. 1972. Mouse cytomegalovirus. Necrosis of infected and morphologically normal submaxillary gland acinar cells during termination of chronic infection. Am. J. Pathol. 68:183-202. [PMC free article] [PubMed] [Google Scholar]
- 21.Hiroi, T., K. Fujihashi, J. R. McGhee, and H. Kiyono. 1994. Characterization of cytokine-producing cells in mucosal effector sites: CD3+ T cells of Th1 and Th2 type in salivary gland-associated tissues. Eur. J. Immunol. 24:2653-2658. [DOI] [PubMed] [Google Scholar]
- 22.Holtappels, R., M. F. Pahl-Seibert, D. Thomas, and M. J. Reddehase. 2000. Enrichment of immediate-early 1 (m123/pp89) peptide-specific CD8 T cells in a pulmonary CD62Llo memory-effector cell pool during latent murine cytomegalovirus infection of the lungs. J. Virol. 74:11495-11503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holtappels, R., J. Podlech, G. Geginat, H. P. Steffens, D. Thomas, and M. J. Reddehase. 1998. Control of murine cytomegalovirus in the lungs: relative but not absolute immunodominance of the immediate-early 1 nonapeptide during the antiviral cytolytic T-lymphocyte response in pulmonary infiltrates. J. Virol. 72:7201-7212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holtappels, R., J. Podlech, N. K. Grzimek, D. Thomas, M. F. Pahl-Seibert, and M. J. Reddehase. 2001. Experimental preemptive immunotherapy of murine cytomegalovirus disease with CD8 T-cell lines specific for ppM83 and pM84, the two homologs of human cytomegalovirus tegument protein ppUL83 (pp65). J. Virol. 75:6584-6600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holtappels, R., D. Thomas, J. Podlech, and M. J. Reddehase. 2002. Two antigenic peptides from genes m123 and m164 of murine cytomegalovirus quantitatively dominate CD8 T-cell memory in the H-2d haplotype. J. Virol. 76:151-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huleatt, J. W., and L. Lefrancois. 1995. Antigen-driven induction of CD11c on intestinal intraepithelial lymphocytes and CD8+ T cells in vivo. J. Immunol. 154:5684-5693. [PubMed] [Google Scholar]
- 27.Iwasaki, A., and B. L. Kelsall. 1999. Freshly isolated Peyer's patch, but not spleen, dendritic cells produce interleukin 10 and induce the differentiation of T helper type 2 cells. J. Exp. Med. 190:229-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iwasaki, A., and B. L. Kelsall. 2000. Localization of distinct Peyer's patch dendritic cell subsets and their recruitment by chemokines macrophage inflammatory protein (MIP)-3α, MIP-3β, and secondary lymphoid organ chemokine. J. Exp. Med. 191:1381-1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iwasaki, A., and B. L. Kelsall. 2001. Unique functions of CD11b+, CD8α+, and double-negative Peyer's patch dendritic cells. J. Immunol. 166:4884-4890. [DOI] [PubMed] [Google Scholar]
- 30.Johnson, T. R., S. Hong, L. Van Kaer, Y. Koezuka, and B. S. Graham. 2002. NK T cells contribute to expansion of CD8+ T cells and amplification of antiviral immune responses to respiratory syncytial virus. J. Virol. 76:4294-4303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jonjic, S., W. Mutter, F. Weiland, M. J. Reddehase, and U. H. Koszinowski. 1989. Site-restricted persistent cytomegalovirus infection after selective long-term depletion of CD4+ T lymphocytes. J. Exp. Med. 169:1199-1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jonjic, S., I. Pavic, P. Lucin, D. Rukavina, and U. H. Koszinowski. 1990. Efficacious control of cytomegalovirus infection after long-term depletion of CD8+ T lymphocytes. J. Virol. 64:5457-5464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jonjic, S., I. Pavic, B. Polic, I. Crnkovic, P. Lucin, and U. H. Koszinowski. 1994. Antibodies are not essential for the resolution of primary cytomegalovirus infection but limit dissemination of recurrent virus. J. Exp. Med. 179:1713-1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jotwani, R., A. K. Palucka, M. Al-Quotub, M. Nouri-Shirazi, J. Kim, D. Bell, J. Banchereau, and C. W. Cutler. 2001. Mature dendritic cells infiltrate the T-cell-rich region of oral mucosa in chronic periodontitis: in situ, in vivo, and in vitro studies. J. Immunol. 167:4693-4700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koszinowski, U. H., M. Del Val, and M. J. Reddehase. 1990. Cellular and molecular basis of the protective immune response to cytomegalovirus infection. Curr. Top. Microbiol. Immunol. 154:189-220. [DOI] [PubMed] [Google Scholar]
- 36.Krmpotic, A., D. H. Busch, I. Bubic, F. Gebhardt, H. Hengel, M. Hasan, A. A. Scalzo, U. H. Koszinowski, and S. Jonjic. 2002. MCMV glycoprotein gp40 confers virus resistance to CD8+ T cells and NK cells in vivo. Nat. Immunol. 3:529-535. [DOI] [PubMed] [Google Scholar]
- 37.Kyes, S., E. Carew, S. R. Carding, C. A. Janeway, Jr., and A. Hayday. 1989. Diversity in T-cell receptor gamma gene usage in intestinal epithelium. Proc. Natl. Acad. Sci. USA 86:5527-5531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lucin, P., I. Pavic, B. Polic, S. Jonjic, and U. H. Koszinowski. 1992. Gamma interferon-dependent clearance of cytomegalovirus infection in salivary glands. J. Virol. 66:1977-1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.MacDonald, M. R., X. Y. Li, R. M. Stenberg, A. E. Campbell, and H. W. Virgin. 1998. Mucosal and parenteral vaccination against acute and latent murine cytomegalovirus (MCMV) infection by with an attenuated MCMV mutant. J. Virol. 72:442-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McGuirk, P., C. McCann, and K. H. Mills. 2002. Pathogen-specific T regulatory 1 cells induced in the respiratory tract by a bacterial molecule that stimulates interleukin 10 production by dendritic cells: A novel strategy for evasion of protective T helper type 1 responses by Bordetella pertussis. J. Exp. Med. 195:221-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McMahon, C. W., A. J. Zajac, A. M. Jamieson, L. Corral, G. E. Hammer, R. Ahmed, and D. H. Raulet. 2002. Viral and bacterial infections induce expression of multiple NK cell receptors in responding CD8+ T cells. J. Immunol. 169:1444-1452. [DOI] [PubMed] [Google Scholar]
- 42.McWilliam, A. S., S. Napoli, A. M. Marsh, F. L. Pemper, D. J. Nelson, C. L. Pimm, P. A. Stumbles, T. N. Wells, and P. G. Holt. 1996. Dendritic cells are recruited into the airway epithelium during the inflammatory response to a broad spectrum of stimuli. J. Exp. Med. 184:2429-2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mega, J., J. R. McGhee, and H. Kiyono. 1992. Cytokine- and Ig-producing T cells in mucosal effector tissues: analysis of IL-5- and IFN-gamma-producing T cells, T-cell receptor expression, and IgA plasma cells from mouse salivary gland-associated tissues. J. Immunol. 148:2030-2039. [PubMed] [Google Scholar]
- 44.Moutaftsi, M., A. M. Mehl, L. K. Borysiewicz, and Z. Tabi. 2002. Human cytomegalovirus inhibits maturation and impairs function of monocyte-derived dendritic cells. Blood 99:2913-2921. [DOI] [PubMed] [Google Scholar]
- 45.Nakano, H., M. Yanagita, and M. D. Gunn. 2001. CD11c+B220+Gr-1+ cells in mouse lymph nodes and spleen display characteristics of plasmacytoid dendritic cells. J. Exp. Med. 194:1171-1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ninomiya, T., H. Takimoto, G. Matsuzaki, S. Hamano, H. Yoshida, Y. Yoshikai, G. Kimura, and K. Nomoto. 2000. Vγ1+ γδ T cells play protective roles at an early phase of murine cytomegalovirus infection through production of interferon-gamma. Immunology 99:187-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oliveira, S. A., S. H. Park, P. Lee, A. Bendelac, and T. E. Shenk. 2002. Murine cytomegalovirus m02 gene family protects against natural killer cell-mediated immune surveillance. J. Virol. 76:885-894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Orange, J. S., and C. A. Biron. 1996. An absolute and restricted requirement for IL-12 in natural killer cell IFN-gamma production and antiviral defense. Studies of natural killer and T-cell responses in contrasting viral infections. J. Immunol. 156:1138-1142. [PubMed] [Google Scholar]
- 49.Orange, J. S., B. Wang, C. Terhorst, and C. A. Biron. 1995. Requirement for natural killer cell-produced interferon gamma in defense against murine cytomegalovirus infection and enhancement of this defense pathway by interleukin 12 administration. J. Exp. Med. 182:1045-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ou, R., S. Zhou, L. Huang, and D. Moskophidis. 2001. Critical role for alpha/beta and gamma interferons in persistence of lymphocytic choriomeningitis virus by clonal exhaustion of cytotoxic T cells. J. Virol. 75:8407-8423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pan, J., E. J. Kunkel, U. Gosslar, N. Lazarus, P. Langdon, K. Broadwell, M. A. Vierra, M. C. Genovese, E. C. Butcher, and D. Soler. 2000. A novel chemokine ligand for CCR10 and CCR3 expressed by epithelial cells in mucosal tissues. J. Immunol. 165:2943-2949. [DOI] [PubMed] [Google Scholar]
- 52.Pavic, I., B. Polic, I. Crnkovic, P. Lucin, S. Jonjic, and U. H. Koszinowski. 1993. Participation of endogenous tumour necrosis factor alpha in host resistance to cytomegalovirus infection. J. Gen Virol. 74:2215-2223. [DOI] [PubMed] [Google Scholar]
- 53.Reddehase, M. J., S. Jonjic, F. Weiland, W. Mutter, and U. H. Koszinowski. 1988. Adoptive immunotherapy of murine cytomegalovirus adrenalitis in the immunocompromised host: CD4-helper-independent antiviral function of CD8-positive memory T lymphocytes derived from latently infected donors. J. Virol. 62:1061-1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reddehase, M. J., J. B. Rothbard, and U. H. Koszinowski. 1989. A pentapeptide as minimal antigenic determinant for MHC class I-restricted T lymphocytes. Nature 337:651-653. [DOI] [PubMed] [Google Scholar]
- 55.Reddehase, M. J., F. Weiland, K. Munch, S. Jonjic, A. Luske, and U. H. Koszinowski. 1985. Interstitial murine cytomegalovirus pneumonia after irradiation: characterization of cells that limit viral replication during established infection of the lungs. J. Virol. 55:264-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Riera, L., M. Gariglio, G. Valente, A. Mullbacher, C. Museteanu, S. Landolfo, and M. M. Simon. 2000. Murine cytomegalovirus replication in salivary glands is controlled by both perforin and granzymes during acute infection. Eur. J. Immunol. 30:1350-1355. [DOI] [PubMed] [Google Scholar]
- 57.Robbins, S. H., K. B. Nguyen, N. Takahashi, T. Mikayama, C. A. Biron, and L. Brossay. 2002. Cutting edge: inhibitory functions of the killer cell lectin-like receptor G1 molecule during the activation of mouse NK cells. J. Immunol. 168:2585-2589. [DOI] [PubMed] [Google Scholar]
- 58.Saederup, N., S. A. Aguirre, T. E. Sparer, D. M. Bouley, and E. S. Mocarski. 2001. Murine cytomegalovirus CC chemokine homolog MCK-2 (m131-129) is a determinant of dissemination that increases inflammation at initial sites of infection. J. Virol. 75:9966-9976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Salazar-Mather, T. P., T. A. Hamilton, and C. A. Biron. 2000. A chemokine-to-cytokine-to-chemokine cascade critical in antiviral defense. J. Clin. Investig. 105:985-993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Salazar-Mather, T. P., J. S. Orange, and C. A. Biron. 1998. Early murine cytomegalovirus (MCMV) infection induces liver natural killer (NK) cell inflammation and protection through macrophage inflammatory protein 1α (MIP-1α)-dependent pathways. J. Exp. Med. 187:1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Selin, L. K., P. A. Santolucito, A. K. Pinto, E. Szomolanyi-Tsuda, and R. M. Welsh. 2001. Innate immunity to viruses: control of vaccinia virus infection by gamma delta T cells. J. Immunol. 166:6784-6794. [DOI] [PubMed] [Google Scholar]
- 62.Shanley, J. D. 1990. In vivo administration of monoclonal antibody to the NK 1.1 antigen of natural killer cells: effect on acute murine cytomegalovirus infection. J. Med. Virol. 30:58-60. [DOI] [PubMed] [Google Scholar]
- 63.Slifka, M. K., R. R. Pagarigan, and J. L. Whitton. 2000. NK markers are expressed on a high percentage of virus-specific CD8+ and CD4+ T cells. J. Immunol. 164:2009-2015. [DOI] [PubMed] [Google Scholar]
- 64.Su, H. C., K. B. Nguyen, T. P. Salazar-Mather, M. C. Ruzek, M. Y. Dalod, and C. A. Biron. 2001. NK cell functions restrain T-cell responses during viral infections. Eur. J. Immunol. 31:3048-3055. [DOI] [PubMed] [Google Scholar]
- 65.Tanaka, Y., and S. S. Tevethia. 1988. Differential effect of adenovirus 2 E3/19K glycoprotein on the expression of H-2Kb and H-2Db class I antigens and H-2Kb- and H-2Db-restricted simian virus 40-specific cytotoxic T lymphocytes-mediated lysis. Virology 165:357-366. [DOI] [PubMed] [Google Scholar]
- 66.Tay, C. H., E. Szomolanyi-Tsuda, and R. M. Welsh. 1998. Control of infections by NK cells. Curr. Top. Microbiol. Immunol. 230:193-220. [DOI] [PubMed] [Google Scholar]
- 67.Tay, C. H., and R. M. Welsh. 1997. Distinct organ-dependent mechanisms for the control of murine cytomegalovirus infection by natural killer cells. J. Virol. 71:267-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tay, C. H., R. M. Welsh, and R. R. Brutkiewicz. 1995. NK cell response to viral infections in beta 2-microglobulin-deficient mice. J. Immunol. 154:780-789. [PubMed] [Google Scholar]
- 69.Wang, W., H. Soto, E. R. Oldham, M. E. Buchanan, B. Homey, D. Catron, N. Jenkins, N. G. Copeland, D. J. Gilbert, N. Nguyen, J. Abrams, D. Kershenovich, K. Smith, T. McClanahan, A. P. Vicari, and A. Zlotnik. 2000. Identification of a novel chemokine (CCL28), which binds CCR10 (GPR2). J. Biol. Chem. 275:22313-22323. [DOI] [PubMed] [Google Scholar]
- 70.Welsh, R. M. 2001. Assessing CD8 T-cell number and dysfunction in the presence of antigen. J. Exp. Med. 193:F19-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Welsh, R. M., C. L. O'Donnell, and L. D. Shultz. 1994. Antiviral activity of NK 1.1+ natural killer cells in C57BL/6 scid mice infected with murine cytomegalovirus. Nat. Immunol. 13:239-245. [PubMed] [Google Scholar]