By 1968, the basic principles underlying cholesterol solubility in bile had been established (1, 2). Free cholesterol (C) had been found to be almost entirely insoluble in water and very poorly soluble in bile salt solutions. Cholesterol was however very soluble in phosphotidylcholine (PC) lamellar liquid crystals (bilayers), and bile salts could solubilize the PC/C liquid crystal bilayers into mixed micelles, thus carrying the C into solution. In 1970, I speculated (3) that the molecular mechanism for biliary lipid secretion was “that bile salts penetrate the cannilicular membrane from the interior of the liver cell and dissect out specifically lecithin and cholesterol leaving the membrane protein and other structural lipids intact.” This hypothesis, based on the physical chemistry of the lipid systems, has now been proven to be almost entirely incorrect. It is now clear that all biliary lipids are secreted in a controlled way by ABC transporters, and that the physical chemistry of the lipid interactions probably occurs within the lumen of the canniliculus and biliary ducts.
The first break came with the knock out of the gene encoding the multiple drug-resistant protein MDR-2 (ABCB4), which prevented the secretion of PC and C into bile and caused liver pathology (4). High rates of bile salt infusion could not restore biliary PC in bile (5, 6), and it was concluded that MDR-2 was the PC transporter. Second, the bile salt export pump (BSEP) is the canniliculus-located ABC B11 gene product and is responsible for most of the bile salt transport from the hepatocyte into the bile cannilicular lumen (7–10). Defects in this gene gave rise to progressive familiar intrahepatic cholestatis type 2 (PFCI-2; ref. 11), whereas defects in MDR-2 (MDR-3 in humans) result in PFCI-3 (12).
The final step in revealing the undeniable role of ABC proteins in the secretion of bile lipids appeared in a recent issue of PNAS (13). Helen Hobbs and coworkers disrupted ABCG5 and AC5G8 genes in mice and greatly inhibited C secretion into bile, showing that this ABC heterodimer is responsible for secretion of biliary C. Dr. Hobbs' group had originally discovered ABCG5/G8 in their studies on a rare and mysterious disease, sitosterolemia, a recessive disorder characterized by poor biliary excretion of dietary sterols, increased absorption of plant and dietary sterols, hypercholesterolemia, hypersitosterolemia, and early coronary artherosclerosis. They found mutations in either of the two ABC monomers (ABCG5, ABCG8) in nine patients with sitosterolemia and concluded that these transporters “cooperate to limit intestinal absorption and promote biliary excretion of sterols” (14). Hobbs and coworkers then showed that ABCG5 and ABCG8 are present on nearly contiguous genes, and that when expressed, the proteins form a heterodimer in the endoplasmic reticulum. Coexpression is required for their movement into the Golgi and onto the apical surface of the cell. ABCB5/8 are expressed in the liver and intestine but not in other tissues (15). Next, they showed that, in transgenic animals overexpressing ABCB5/8, bile C is markedly increased, and that the absorption of added diet cholesterol and plant sterols is markedly decreased (16).
In the study by Yu et al. (13), the genes for ABCG5/8 were disrupted, producing a G5G8−/− mouse. On a chow diet (0.02% C, 0.05% sitosterol), the plasma sitosterol was elevated 30-fold, but C was decreased by 50%. When challenged with a 2% C diet, the plasma C increased 2.4-fold in G5G8−/− but not in controls. Liver C increased 3-fold in controls but 18-fold in G5G8−/− mice. G5G8−/− animals had decreased neutral fecal sterols and increased absorption of plant sterols, in a fashion similar to patients with sitosterolemia.
Bile C was ≈5 nm/ml in controls, but was low (0.1–0.64 nm/ml) in G5G8−/− mice. Heterozygotes had about half the biliary C. Biliary phospholipids and bile salts were virtually normal in G5G8−/− animals. Yu et al. conclude “that the secretion of cholesterol does not couple quantitatively to the secretion of phospholipids.” On the high C diet, both control and G5G8−/− animals increased biliary cholesterol from 5 to 14 nm/ml in controls, and from ≈0.3 to 1.9 nm/ml in G5G8−/− mice. The increase in the G5G8−/− mice indicates that high liver C can modestly increase non-ABCG5G8-mediated C transport into bile.
How does ABCG5/G8 transport C into bile? In G5G8−/− animals, C in bile is decreased markedly (by 91% on average), even though the C acceptors, phospholipids, and bile salts are secreted normally. Transgenics overexpressing ABCG5G8 at moderate and high levels of transcript both greatly increase the C in bile without appreciably changing the bile salt and phospholipids (16). Wittenberg and Carey suggested that the lack of difference in excessive C secretion by transgenic mice expressing low and high amounts of G5G8 is caused by a saturation of the acceptors, PC and bile salt, in the bile canniliculus (17). The upper limit of C which could be secreted was between 1 and 1.4 mol of C per mol of phospholipids (16), which is quite similar to the maximum solubility of C in PC bilayers (1). Thus, ABCG5G8 moves C into acceptors until they are saturated.
In many previous studies, bile salt was infused at different rates, and the bile salt, C, and PC secreted in bile was measured. C and PC were thought to be coupled, because increasing rates of infusion of bile acids caused secretion of more PC and C. The study on MDR-2 (ABCB4)-null animals also suggested that cholesterol was coupled with phospholipid secretion; not only were phospholipids absent, but C was very low in bile of MDR-2−/− animals, whereas bile salt secretion was normal (4–6). This result could be related to the fact that PC is the major carrier of C, and when it is absent, C cannot move from the canniliculus into the aqueous phase even if murine bile salts are present. Infusion of hydrophilic bile salt tauroursodeoxycholete (TUDC) to reach secretion rates ≈1,200 nm/min per 100 g was able to release ≈1.5 nm/min per 100 g of C without any PC secretion (6). The ratio of 1:800 C:TUDC is well within the solubilizing capacity of TUDC alone. Perfusion of hydrophobic, human-like bile salts taurocholate and taurodeoxycholate to maximum levels produced a very small release of both PC and C into bile, <2 nm/min per 100 g (5, 6). This finding indicates that bile salts are not effective in moving membrane C and PC into bile. Many years ago, we showed (1) that bile salts are extremely poor solubilizers of cholesterol, and that PC was required for micellar solubilization of C in bile. However, if the ratio of bile salts to C is >100, then some C can be solubilized without PC. The importance of PC rather than bile salt as the major acceptor is bolstered by the fact that, in BSEP−/− mice, biliary PC is increased 2.5-fold and biliary C is increased 7-fold, whereas bile salts are greatly reduced. In BSEP−/− mice, liver bile salts are greatly increased, and thus may augment PC and C secretion (10). Finally, a cross of MDR-2−/− mice with a transgenic overexpressor of ABCG5G8 failed to show any significant increase in bile C over MDR-2−/− controls (H. Hobbs, personal communication). Thus, PC is, by far, the most important C acceptor in bile.
Is ABCG5/G8 simply a C flipase moving cholesterol from cytoplasmic to luminal leaflet? Although C resides in both leaflets of bile cannilicular membrane, it has been suggested that a flip to the outer leaflet of the cannilicular membrane must occur to move cholesterol into bile. It was postulated that ABCG5G8 facilitates the movement of C from inner to outer leaflet (17) and, therefore, acts in a fashion analogous to MDR-2 (ABCB4), which is known to flip PC from the inner to the outer leaflet, and also to BSEP, which flips bile salt from the cytoplasm into bile. However, translocation or flipping of C is probably not the whole story. First, C contains one equatorial hydroxyl group (−OH); the rest is hydrocarbon. It takes ≈4 kcal/mol (1 kcal = 4.18 kJ) of hydroxyl group to move it from an aqueous phase into an oil phase (18), which is not a large amount of energy. The protonated fatty acids carboxyl (−COOH) takes ≈4.7 kcal/mol to move from water to oil (18), and they flip rapidly in PC bilayers, relatively independently of hydrocarbon mass (t1/2 of C14–18 < 10 ms; t1/2 of C20–24 < 1 s; ref. 19). Therefore, the barrier to move a hydroxyl across a membrane is not adequate justification for suggesting that C flips slowly. Second, several recent studies have clearly shown that C and dehydroergosterol (a fluorescent molecule very similar to C) flip-flops in PC vesicles or PC:C vesicles (2:PC:1C) rapidly (<1.5 min to s). Even dehydroergosterol in DMPC small unilamellar vesicles at 10°C, where the lipid is in the ordered gel phase, has flip rates of 5–8 min (20). Third, Steck et al. (21) have shown that the flip-flop rate of cholesterol in red cells, a rather viscous membrane where there is no doubt that cholesterol is in both leaflets, is extremely fast (t1/2 < 1 s at 37°C). In fact, all of the cholesterol can be extracted from the red cells by using cyclodextrin in a few seconds.
Finally, flip and flop rates of 13C-labeled molecules in bilayers can be accurately measured by NMR techniques if they are in the ms to min range. For instance, two pools of protonated bile acids (22, 23) or diacylglycerols (24), one on the inner leaflet and one on the outer leaflet, can be identified and the exchange rates measured. Transbilayer exchange rates are about t1/2 = 10 s for 1,2 dilaurylglycerol (24); for the more polar cholic acid (22), t1/2 was 139 s. [13C]Cholesterol showed only one pool, suggesting very rapid exchange between sides of the bilayer. Therefore, energetic consideration and experimental evidence show that C flip-flop is rapid in PC vesicles, red cell membranes, and, presumably, in most other biological membranes. Only if the entire cannicular membrane were solid would a protein-catalyzed flip to the external leaflet be necessary.
So how does cholesterol get into bile if it is readily available on the external luminal leaf of the canniliculus? It is clearly related to the function of ABCG5/G8, which brings us to the off rate of cholesterol from membranes. Steck et al. (21) showed that when the C acceptor (cyclodextrin) to red cell ratio is held constant, the transfer rate from donor red blood cells (RBC) to acceptor cyclodextrin approached 0 at infinite dilution. This result is consistent with an extremely slow off rate into the aqueous phase from RBC, and led them to propose that the mechanism of transfer of RBC C to cyclodextrin was through an “activation–collision” mechanism, in which the C that is partly out of the membrane (activated) could be picked up by cyclodextrin (21). The greater the distance of the cyclodextrin is from the donor, i.e., the greater the dilution, the slower is the rate. A simple collision model fit less well, and a desorption into the aqueous phase did not fit the data at all. They showed that the transfer rate is directly proportional to the temperature being fastest at the highest temperature. This finding is consistent with temperature increasing membrane lipid free volume and fluidity, which leads to a greater proportion of C being in an activated (partly out of the membrane) state.
From an Arrhenius plot, Steck et al. (21) calculated an activation energy for the transfer reaction at ≈27–28 kcal/mol. A very similar value of 28 kcal/mol can be estimated for the transfer of C from oil to water. This amount is the energy required to move the large hydrocarbon part of C from the hydrocarbon membrane environment into water. A similar activation energy is found for very long chain fatty acids (19). A fatty acid of 26–28 carbons would have an activation energy about the same as C. The off rate of saturated fatty acids is a linear function of the number of −CH2− groups; the shorter the chain, the faster is the rate. The off rate t1/2 for C-18 fatty acid is 1.3 s; for C-24 fatty acid, it is 1,890 s (31.5 min). I estimate that the t1/2 for C-26 and C-28 fatty acids is 4.7 and 44 h, respectively. Thus, C should have a very slow off rate.
In studies using cyclodextrin as a shuttle to accelerate the transfer of cholesterol between large unilamellar vesicles, the first-order exchange constants are directly proportional to the concentration of cyclodextrin (20). At zero cyclodextrin, the exchange rate was extremely slow (t1/2 of several hours), showing that phospholipid vesicles are capable of exchanging cholesterol, but only very slowly. Bile salts can enhance the rate of ergosterol transfer from membrane-like donors to biliary-like PC (25), but apparently they are not very effective, because only 9% of C is found in G5/B8−/− animals that have normal bile salt secretion.
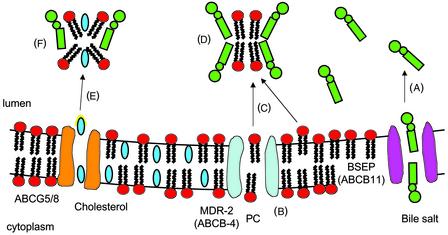
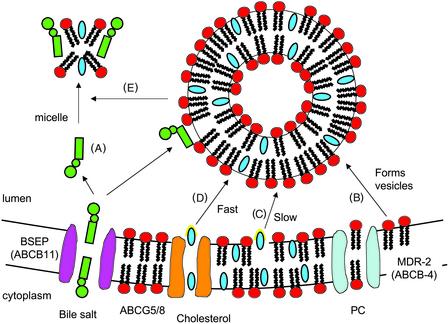
I speculate that ABCG5/G8 promotes an activated state of cholesterol so that acceptors (mixed bile salt-phospholipids micelles or vesicles) can easily pick it up (Fig. 1). ABCG5G8 probably forms a channel which binds C and then pushes it partly into the lumen (activates it) when ATP is hydrolyzed. Once C is activated, mixed bile salt-PC micelles can pick it up. With high bile salt secretion (Fig. 1), most of the PC is in micelles. When bile salt secretion is lower, as occurs when the enterohepatic circulation is interrupted, then bile becomes highly enriched in PC (Fig. 2), exceeds the capacity for micelle formation (26), and numerous PC vesicles form in the canniliculus (27). These PC vesicles also act as acceptors. In the extreme case, when BSEP is nonfunctional, PC is then virtually the only C acceptor. Although favoring a flipase mechanism for ABCG5/G8, Wittenburg and Carey (17) may have suggested something similar when they said “following ATP binding and hydrolysis the (ABCG5G8C) complex undergoes a conformational change flipping a cholesterol molecule into the outer membrane leaflet in a configuration that favors its release into the cannilicular space.” It may not be necessary that it releases the C into the aqueous phase (i.e., increases its off rate), but simply that it projects part of the C molecule, which can be easily recognized by the acceptor and picked up. This part of C could be the −OH end or the isooctyl tail. If C were moved from the cytoplasmic leaflet to an activated state, presenting the isooctyl tail, it would not be flipped but only translocated. In such a configuration, cholesterol could readily be transferred to micelles or vesicles on collision.
Figure 1.
(A) When the bile salt secretion rate is high, BSEP (ABCB11) pumps bile salt from the cytoplasm into the cannilicular lumen at secretion rates 5–10 times the rate of PC secretion, i.e., in great excess. (B) PC are flipped from the cytoplasmic leaflet to the luminal side by MDR-2 (ABCB4). (C) Bile salts pick up PC that have been flipped to the outer leaflet either directly in contact with MDR-2 or from projections of PC from the luminal leaflet. (D) Bile salts and PC in the lumen combine to form small mixed PC-bile salt micelles. (E) These micelles collide with the ABC5G8 transporter, which projects cholesterol partly into the aqueous phase (the activated state shown with yellow halo), and the micelle captures it. (F) Mixed bile salt-PC-cholesterol micelles move down the biliary tract into the gallbladder and duodenum.
Figure 2.
(A) Bile salt secretion is low because of physiologic interruption of the enteropatic circulation (e.g., fasting) or, in extreme cases, because of dysfunctional BSEP. (B) MDR-2 flips PC from the cytoplasmic to the luminal layer, where excess PC form projections into the lumen and then vesicles in close proximity to the cannilicular membrane (27). These vesicles may contain a small amount of cholesterol. (C) They could also pick up a small amount of activated cholesterol (yellow halo) from the membrane, but this process is very slow and probably unimportant. (D) Movement of cholesterol to the vesicle on collision with ABCG5G8 is greatly facilitated to the point where the vesicle becomes saturated at ≈1:1 cholesterol:PC. This result exceeds the micellar solubility of cholesterol (26) and creates a vesicular phase (liquid crystals) and the potential for cholesterol crystallization. (E) When the secretion rate of bile salts is relatively low, some micelles are formed, but most of the PC and cholesterol travels to the gallbladder in vesicular liquid crystalline form. In BSEP-null mice, where bile salt secretion is very low, the transfer of PC into vesicles and the movement of cholesterol into those vesicles from ABCG5G8 is enhanced, so that BSEP−/− mice actually secrete more PC and cholesterol than wild-type control (10).
Acknowledgments
I thank Donna Ross and Dr. Libo Wang for manuscript and figure preparation.
Footnotes
See companion article on page 16237 in issue 25 of volume 99.
References
- 1.Small D M, Bourges M, Dervichian D G. Nature. 1966;211:816–818. doi: 10.1038/211816a0. [DOI] [PubMed] [Google Scholar]
- 2.Admirand W H, Small D M. J Clin Invest. 1968;47:1045–1052. doi: 10.1172/JCI105794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Small D M. In: Advances in Internal Medicine. Stollermann G, editor. Vol. 16. Chicago: Yearbook Medical Publication; 1970. , 243–264. [Google Scholar]
- 4.Smit J J, Schinkel A H, Oude Elferink R P J, Groen A K, Wagenaar E, van Deemter L, Mol C A, Ottenhoff R, van der Lugt N M T, van Roon M A, et al. Cell. 1993;75:451–462. doi: 10.1016/0092-8674(93)90380-9. [DOI] [PubMed] [Google Scholar]
- 5.Oude Elferink R P J, Ottenhoff R, van Wijland M, Smit J J M, Schinkel A H, Groen A K. J Clin Invest. 1995;95:31–38. doi: 10.1172/JCI117658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oude Elferink R P J, Ottenhoff R, van Wijland M, Frijters M G, van Nieuwkerk C, Groen A K. J Lipid Res. 1996;37:1065–1075. [PubMed] [Google Scholar]
- 7.Strautnieks S S, Bull L N, Knisely A S, Kocoshis S A, Dahl N, Arnell H, Sokal E, Dahan K, Childs S, Ling V, et al. Nat Genet. 1998;20:233–238. doi: 10.1038/3034. [DOI] [PubMed] [Google Scholar]
- 8.Green R M, Hoda F, Ward K L. Gene. 2000;241:117–123. doi: 10.1016/s0378-1119(99)00460-6. [DOI] [PubMed] [Google Scholar]
- 9.Meier P J, Stieger B. Annu Rev Physiol. 2002;64:635–661. doi: 10.1146/annurev.physiol.64.082201.100300. [DOI] [PubMed] [Google Scholar]
- 10.Wang N, Salem M, Yousef I M, Tuchweber P, Lam P, Childs S J, Helgason C D, Ackerley C, Phillips M J, Ling V. Proc Natl Acad Sci USA. 2001;98:2011–2016. doi: 10.1073/pnas.031465498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jansen P L M, Strautnieks S S, Jacquemin E, Hadchouel M, Sokal E M, Hooiveld G J E J, Koning J H, De Jager-Krikken A, Kuipers F, Stellaard F, et al. Gastro. 1999;117:1370–1379. doi: 10.1016/s0016-5085(99)70287-8. [DOI] [PubMed] [Google Scholar]
- 12.de Vree J M, Jacquemin E, Sturm E, Cresteil D, Bosma P J. Proc Natl Acad Sci USA. 1998;95:282–287. doi: 10.1073/pnas.95.1.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu L, Hammer R E, Li-Hawkins J, von Bergmann K, Lutjohann D, Cohen J C, Hobbs H H. Proc Natl Acad Sci USA. 2002;99:16237–16242. doi: 10.1073/pnas.252582399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berge K E, Tian H, Graf G A, Yu L, Grishin N V, Schultz J, Kwiterovich P, Shan B, Barnes R, Hobbs H H. Science. 2000;290:1881–1775. doi: 10.1126/science.290.5497.1771. [DOI] [PubMed] [Google Scholar]
- 15.Graf G A, Li W-P, Gerard R D, Gelissen I, White A, Cohen J C, Hobbs H H. J Clin Invest. 2002;110:659–669. doi: 10.1172/JCI16000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu L, Li-Hawkins J, Hammer R E, Berge K E, Horton J D, Cohen J C, Hobbs H H. J Clin Invest. 2002;110:671–680. doi: 10.1172/JCI16001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wittenburg H, Carey M C. J Clin Invest. 2002;110:605–609. doi: 10.1172/JCI16548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tanford C. The Hydrophobic Effect: Formation of Micelles and Biological Membranes. New York: Wiley Interscience; 1980. [Google Scholar]
- 19.Zhang F, Kamp F, Hamilton J A. Biochemistry. 1996;35:16055–16060. doi: 10.1021/bi961685b. [DOI] [PubMed] [Google Scholar]
- 20.Leventis R, Silvius J R. Biophys J. 2001;81:2257–2267. doi: 10.1016/S0006-3495(01)75873-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steck T L, Ye J, Lange Y. Biophys J. 2002;83:2118–2125. doi: 10.1016/S0006-3495(02)73972-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cabral D J, Small D M, Lilly H S, Hamilton J A. Biochemistry. 1987;26:1801–1804. doi: 10.1021/bi00381a002. [DOI] [PubMed] [Google Scholar]
- 23.Cabral D J, Small D M. In: Handbook of Physiology. Schultz S G, Forte J G, Rauner B B, editors. Washington, DC: Am. Physiol. Soc.; 1989. , Sec. 6, Vol. III, pp. 621–662. [Google Scholar]
- 24.Hamilton J A, Bhamidipati S P, Kodali D R, Small D M. J Biol Chem. 1991;266:1177–1186. [PubMed] [Google Scholar]
- 25.van Erpecum K J, Carey M C. Biochim Biophys Acta. 1997;1245:269–282. doi: 10.1016/s0005-2760(97)00002-7. [DOI] [PubMed] [Google Scholar]
- 26.Redinger R N, Small D M. Arch Intern Med (Moscow) 1972;130:618–630. [PubMed] [Google Scholar]
- 27.Crawford J M, Gotthold-Mathias M, Crawford A R, Hagen S J, Hatch V C, Barnes S, Godleski J J, Carey M C. J Lipid Res. 1995;36:2147–2163. [PubMed] [Google Scholar]