Abstract
CAD (Cath.a-differentiated) cells, a mouse neuronal cell line, were subjected to electrohydrodynamic jetting at a field strength of 0.47–0.67 kV/mm, corresponding to an applied voltage of 7–10 kV. After jetting, the cells appeared normal and continued to divide at rates similar to those shown by control samples. Jetted cells, when placed in serum-free medium, underwent differentiation that was sustained for at least 1 month. Some of the droplets produced by jetting contained only a few cells. These results indicate that the process of jetting does not significantly perturb neuronal cells and that this novel approach might in the future be a useful way to deposit small numbers of living nerve cells on to surfaces.
Keywords: electrohydrodynamic jetting (EHDJ), electrospraying, inkjet printing (IJP), neurons
Abbreviations: CAD, Cath.a-differentiated; DMEM, Dulbecco's modified Eagle's medium; EHDJ, electrohydrodynamic jetting; IJP, inkjet printing
INTRODUCTION
The phenomenon whereby a liquid medium is charged and fragmented, forming droplets, is of widespread occurrence and is known as electrospraying or EHDJ (electrohydrodynamic jetting). This physical phenomenon has been researched for over 125 years [1–3]. The size and distribution of the droplets can be controlled on the basis of the applied potential difference, flow rate of the medium and the medium's liquid properties [4,5]. EHDJ is emerging as an important tool for biotechnology [6,7] and is one of a range of jetting techniques that are currently being used to process cells and biological materials [8–10].
IJP (inkjet printing) is also a jet-based technology. However, it has its limitations, especially for the deposition of submicrometre-size droplets from concentrated suspensions. This is because the technology of IJP is governed largely by the size of the jetting needle, leading to a droplet diameter approximately double the size of the needle's orifice. In practice, this limits the resolution of IJP on surfaces to around 100 μm for the processing of concentrated suspensions, because, to produce this kind of resolution, droplets of approx. 60 μm diameter need to be generated, requiring a needle orifice of <30 μm. In turn this means that the needle can easily become blocked. EHDJ technology does not suffer from this severe disadvantage; it can be used to process concentrated suspensions from needles with internal diameters of millimetres, yet it is still capable of generating submicrometre-size droplets, giving the technique a resolution down to a few nanometres [11].
IJP has been successfully employed to process living cells [9] and biological tissues [12], although, as a result of the above-described limitations, rather coarse structures were generated.
Recently we have shown that EHDJ can be used to deposit living cells [13]. This new jetting technology may have considerable potential in the future for processing a variety of living cellular materials, because, in this case, and unlike the situation with IJP, the minimum droplet size, and hence potential resolution, will be determined by the size of the particulate matter and not by the technology. In this respect, EHDJ could have important uses in the fabrication of biological tissues and the production of biochips and biosensors – techniques that are destined to revolutionize the biotechnology industry [14–16].
We wanted to explore further the range of cells that EHDJ might be useful for, and one of the most important to study would be neuronal cells, because patterning of these cells would enable studies on neuronal networking, interactions and biochemical communications to be initiated that could prelude investigations on tissue construction. A good model cell system for this is the CAD (Cath.a-differentiated) cell, which can differentiate and behave like a primary neuron when it is deprived of serum [17,18].
We show here, for the first time, that neuronal cells can be electrosprayed and remain viable at applied voltages of between 7 and 10 kV, that these cells have the ability to differentiate and that they exhibit long-term survival after spraying.
MATERIALS AND METHODS
Electrospraying
The equipment and procedures used for EHDJ of Jurkat cells have been described previously in detail [13] and were not modified for neuronal cells. Briefly, the stainless-steel jetting needle had an internal diameter of ≈500 μm and the ring-shaped ground electrode was positioned 15 mm centrally below the needle. The needle and ground electrode were housed in a sterile tissue-culture hood, and jetting was carried out at an electric field strength ranging from 0.47 to 0.67 kV/mm at a flow rate of 10−8 m3·s−1. After electrospraying the droplets were collected in a sterile Petri dish if the cells were to be cultured, or on glass slides or nylon membranes (Biodyne B transfer membrane; FlowGen, Wilford, Ruddington, Nottingham, U.K.) if the droplet relics were to be analysed by microscopy for their size or cell content.
Cell culture
CAD cells (a gift from Dr Matthias Krause, King's College London, University of London, Randall Division of Cell and Molecular Biophysics, London, U.K.) were grown for 96 h in 100 ml of DMEM (Dulbecco's modified Eagle's medium)/Ham's F-12 growth medium (Sigma) with 10% (v/v) foetal-calf serum (Invitrogen) in a Heraeus BB16 incubator at 37 °C and 4% (v/v) CO2. Cell viability was assessed by staining cells with Trypan Blue (Sigma), then counting them using a haemocytometer (Sigma) under a phase-contrast microscope (Zeiss ID 02) employing a 10× objective. A 2–5 ml portion of a cell suspension containing (1–2)×106 cells/ml were used for each electrospray experiment. For the control, similar volumes of the suspension were passed through the needle with no applied voltage and collected in a Petri dish. The cells were counted immediately after electrospraying and after a period of incubation. For analysis of droplet size distribution and content, electrosprayed samples were also collected on glass microscope slides or on nylon membranes. Microscope slides were cleaned in chromic acid then water-washed before use.
Labelling of cells and medium with fluorescent dyes
Rhodamine 6G (Sigma) was used to label cells. For labelling, 5 μl of Rhodamine 6G solution (3 mg/ml in water) was added to cells in a volume of 10 ml and left for 10 min. The cells were then spun down on a bench-top centrifuge at ≈200 g for 10 min and then the pellet was resuspended in DMEM/Ham's F-12 medium containing 10% foetal-calf serum. This procedure was repeated three times, and the final pellet resuspended in 10 ml of fresh medium.
DMEM/Ham's F-12 medium containing 10% foetal calf serum was labelled with FITC. A 3 μl portion of FITC (10 mg/ml in DMSO) was added to 10 ml of the DMEM and left for 20 min. Cells took up FITC medium over time. All procedures were carried out at room temperature (23 °C).
Microscopy
Phase-contrast microscopy and viewing of fluorescence staining was undertaken on a Zeiss Axiovert 200 microscope employing a 2.5× or 10× objective with Rhodamine or FITC filter sets. Digitized images were recorded on computer and measurements taken using standard software. A 1 mm graticule (Ziess), subdivided into 100 divisions, was used to calibrate the system. After each experiment a sample of between 300–400 droplet relics were analysed for their size (diameter in μm), frequency, and number of cells that they contained, and histograms were plotted from the results.
RESULTS
Table 1 shows the results of a typical experiment where cells were jetted, counted and incubated under conditions that promoted cell division. Cells were alive and viable after jetting and divided normally over 48 h, compared with control samples. After spraying, cells placed in serum-free medium differentiated.
Table 1. Cell counts for jetted CAD cells.
Cell counts for the control cells and cells jetted at 10 kV at zero time (immediately after jetting) and after 24 and 48 h. Results shown are the means±S.D. for three separate experiments.
| 10−5×Cell count for electrosprayed CAD cells (cells/ml) | ||
|---|---|---|
| Time (h) | Control CAD cells | 10 kV jetted CAD cells |
| 0 | 5.0±0.24 | 4.7±0.24 |
| 24 | 5.6±0.31 | 5.9±0.31 |
| 48 | 6.3±0.29 | 6.9±0.29 |
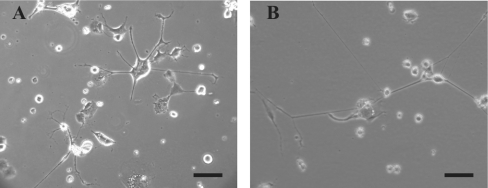
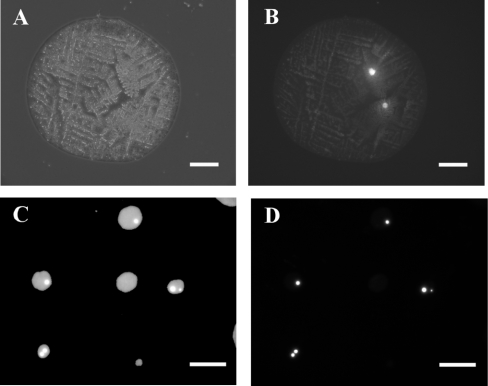
A sample of these cells was incubated for 4 weeks, passaged, and then placed in serum-free medium. This is illustrated in Figure 1, which shows control cells (Figure 1A) and cells that had been jetted at 7 kV (Figure 1B) after 1 month's incubation, demonstrating the point that sprayed cells retain the ability to elaborate processes some time after spraying. The process of electrospraying generates droplets of various sizes containing a variable number of cells. To get some idea of the size distribution and cell content of the droplets, cell suspensions were electrosprayed on to cleaned glass slides. The droplets, when deposited on to slides (‘droplet relics’), quickly dried, initiating crystallization of the medium (Figure 2A), which caused difficulties in identifying cells. To overcome this we decided to label the cells with Rhodamine 6G, a cationic dye with high permeability to cell membranes. It was chosen to label the CAD cells because this dye has been used extensively in the past for cellular studies and does not harm cells significantly [19,20]. Figure 2(B) shows the same relic as in Figure 2(A), but, under fluorescence optics, clearly showing the presence of two cells. To aid in the measurement of droplet sizes, FITC was used to label the medium in which the Rhodamine-labelled cells were suspended.
Figure 1. Optical micrographs of jetted and control CAD cells.
Optical micrographs obtained using a phase-contrast microscope with a 10× objective are shown. The scale bar represents 100 μm. (A) Control CAD cells; (B) CAD cells that were jetted at 7 kV. The pictures were taken approx. 1 month after the cells were jetted.
Figure 2. Optical micrographs of droplet relics containing CAD cells.
The optical micrographs shown here were obtained with phase-contrast and fluorescence optics. Cell suspensions containing Rhodamine-stained CAD cells in fluorescein-labelled medium were jetted at 10 kV on to glass slides (A and B) or on to nylon membrane (C and D). (A) shows a dried droplet relic seen under phase-contrast, and (B) shows the same relic using Rhodamine filters, demonstrating that two cells were present in the droplet. The scale bars in (A) and (B) represent 100 μm. (C) and (D) show an example of droplet relics after the cell suspension was jetted on to nylon membrane. (C) shows a group of spots observed using fluorescein filters and (D) shows the same spots using Rhodamine filters. The scale bars for (C) and (D) represent 500 μm.
Relics of droplets containing labelled cells were also collected on nylon membranes. This has been shown to be a good way of collecting fluorescently labelled Jurkat cells in droplets, enabling accurate estimations of numbers of cells in droplets and the sizes of droplets (results not shown). Figures 2(C) and 2(D) show examples of relics collected on nylon and illustrate the point that spread droplet relics ranged in size from about 50 μm upwards, and that some contained only a few cells when electrosprayed under the conditions described here.
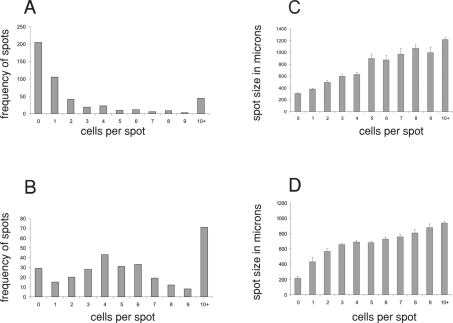
An estimation of relic sizes and their contents was made on droplets deposited on glass and nylon, and similar results were obtained in both cases. Figure 3 shows results from typical experiments at two applied voltages, 7 and 10 kV, showing histograms of relic sizes and number of cells that the droplets originally contained. For both voltages the average diameters of the spots ranged from about 300 μm to about 1000 μm with larger droplets containing more cells. At 7 kV there was a greater proportion of droplets with either no cells or small numbers of cells (Figure 3A) compared with results obtained at 10 kV (Figure 3B). In addition, of the droplets that contained cells at 7 kV, 54% of them had either one or two cells. Similar results to these have also been obtained with Jurkat cells after electrospraying at similar voltages (results not shown).
Figure 3. Histograms of droplet relic sizes and their cell content after jetting at 7 and 10 kV.
The histograms in (A) and (B) show the frequency of droplet relics plotted against cell content of the relic, and the histograms in (C) and (D) show the size distribution of droplet relics plotted against the cell content of the relic. The cells used in (A) and (C) were sprayed at 7 kV and the cells used in (B) and (D) were sprayed at 10 kV.
We have shown in the present study that EHDJ can be used to deposit neuronal cells in droplets having sizes in the micrometre range. In addition, the cells are viable, are able to differentiate after deposition and exhibit long-term survival. We find that the droplets produced by jetting contain variable numbers of cells, although many can be found that contain only a few cells. It will clearly be important to establish a medium that will allow for a controlled droplet size, to determine the conditions that are required for droplets to be deposited at desired locations, and to determine whether this technique is also suitable for processing primary neurons; we hope to investigate these aspects in the future. In summary, the findings of the present study open up the possibility of being able to deposit single neuronal cells for a range of bioengineering applications.
Acknowledgments
P. A. M. E. gratefully acknowledges King's College London for supporting these trial studies. S. N. J. gratefully acknowledges the seed corn equipment fund provided by The Royal Society, which has allowed this exploratory investigation. We thank Dr Matthias Krause for CAD cells, and Ms Patricia Lei of King's College London for technical help.
References
- 1.Rayleigh L. On the instability of jets. Proc. R. Soc. London. 1878;10:4–13. [Google Scholar]
- 2.Taylor G. I. Disintegration of water droplets in an electric field. Proc. R. Soc. London Ser. A. 1964;280:383–397. [Google Scholar]
- 3.Hayati I., Bailey A. I., Tadros T. F. Mechanism of stable jet formation in electrohydrodynamic atomization. Nature (London) 1996;319:41–43. [Google Scholar]
- 4.Zeleny J. Instability of electrified liquid surfaces. Phys. Rev. 1917;10:1–6. [Google Scholar]
- 5.Cloupeau M., Prunet-Foch B. Electrostatic spraying of liquids. Main functioning modes. J. Electrost. 1990;25:165–184. [Google Scholar]
- 6.Jaworek A., Krupa A. Classification of the modes of EHD spraying. J. Aerosol Sci. 1999;30:873–893. [Google Scholar]
- 7.Jayasinghe S. N., Edirisinghe M. J., Wang D. Z. Controlled deposition of nanoparticle clusters by electrohydrodynamic atomization. Nanotechnology. 2004;15:1519–1523. [Google Scholar]
- 8.Sanjana N. E., Fuller S. B. A fast flexible ink-jet printing method for patterning dissociated neurons in culture. J. Neurosci. Methods. 2004;136:151–163. doi: 10.1016/j.jneumeth.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 9.Xu Y., Jin J., Gregory C., Hickman J. J., Boland T. Inkjet printing of viable mammalian cells. Biomaterials. 2005;26:93–99. doi: 10.1016/j.biomaterials.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 10.Tsang V. L., Bhatia S. N. Three-dimensional tissue fabrication. Adv. Drug Rev. 2004;56:1635–1647. doi: 10.1016/j.addr.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 11.Chen D.-R., Pui D. Y. H., Kaufmann S. L. Electrospraying of conducting liquids for monodisperse aerosol generation in the 4 nm to 1.8 μm diameter range. J. Aerosol Sci. 1995;26:963–977. [Google Scholar]
- 12.Turcu F., Tratsk-Nitz K., Thanos S., Schumann W., Heiduschka P. Inkjet printing for micropattern generation of lamin for neuronal adhesion. J. Neurosci. Methods. 2003;131:141–148. doi: 10.1016/j.jneumeth.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 13.Jayasinghe S., Qureshi A., Eagles P. Electrohydrodynamic jet processing: an advanced electric field-driven jetting phenomenon for processing living cells. Small. 2006;2:216–219. doi: 10.1002/smll.200500291. [DOI] [PubMed] [Google Scholar]
- 14.Roth E. A., Xu T., Das M., Gregory C., Hickman J. J., Boland T. Inkjet printing for high-throughput cell patterning. Biomaterials. 2004;25:3707–3715. doi: 10.1016/j.biomaterials.2003.10.052. [DOI] [PubMed] [Google Scholar]
- 15.Tsang V. L., Bhatia S. N. Three-dimensional tissue fabrication. Adv. Drug Rev. 2004;56:1635–1647. doi: 10.1016/j.addr.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 16.Pancrazio J. L., Whelan J. P., Borkholder D. A., Ma W., Stenger D. A. Development and application of cell-based biosensors. Ann. Biomed. Eng. 1999;27:697–711. doi: 10.1114/1.225. [DOI] [PubMed] [Google Scholar]
- 17.Qi Y., Wang J. K. T., McMillan M., Chikaraishi D. M. Characterization of a CNS cell line, CAD, in which morphological differentiation is initiated by serum deprivation. J. Neurosci. 1997;17:1217–1225. doi: 10.1523/JNEUROSCI.17-04-01217.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang H., Oxford G. S. Voltage-dependent ion channels in CAD cells: a catecholaminergic neuronal line that exhibits inducible differentiation. J. Neurophysiol. 2000;84:2888–2895. doi: 10.1152/jn.2000.84.6.2888. [DOI] [PubMed] [Google Scholar]
- 19.Mandala M., Serck-Hanssen G., Martino G., Helle K. B. The fluorescent cationic dye Rhodamine 6G as a probe for membrane potential in bovine aortic endothelial cells. Anal. Biochem. 1999;274:1–6. doi: 10.1006/abio.1999.4253. [DOI] [PubMed] [Google Scholar]
- 20.Berns M. W., Siemens A. F., Walter R. J. Mitochondrial fluorescence patterns in rhodamine 6G-stained myocardial cells in vitro: analysis by real time computer video microscopy and laser microspot excitation. Cell Biophys. 1984;6:263–277. doi: 10.1007/BF02788632. [DOI] [PubMed] [Google Scholar]